Potential Use of Compatible Osmolytes as Drought Tolerance Indicator in Local Watermelon (Citrullus lanatus) Landraces
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Materials
2.2. Water Deficit Stress Experiment
2.3. Irrigation Schedule and Drought Stress Initiation
2.4. Physiological Data Collection
2.5. Assays of Proline, Citrulline, and Arginine under Water Deficit Conditions
2.5.1. Sample Preparation
2.5.2. Extraction and Quantification of Citrulline and Arginine
2.5.3. Extraction and Quantification of Proline
2.6. Relative Expression of Citrulline- and Arginine-Related Genes under Drought Stress
2.7. Data Analysis
3. Results
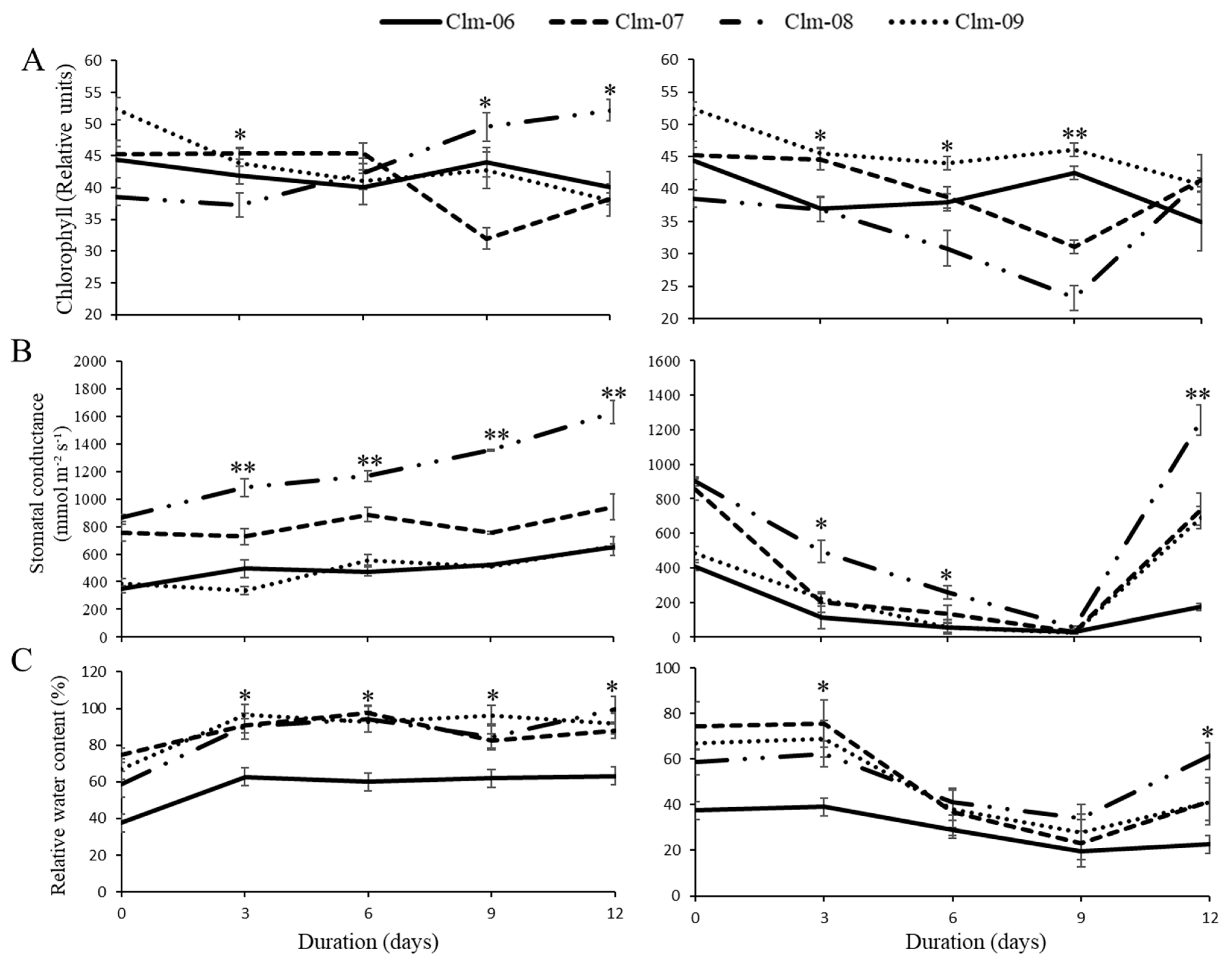
3.1. Soil Moisture Content
3.2. Physiological Parameters
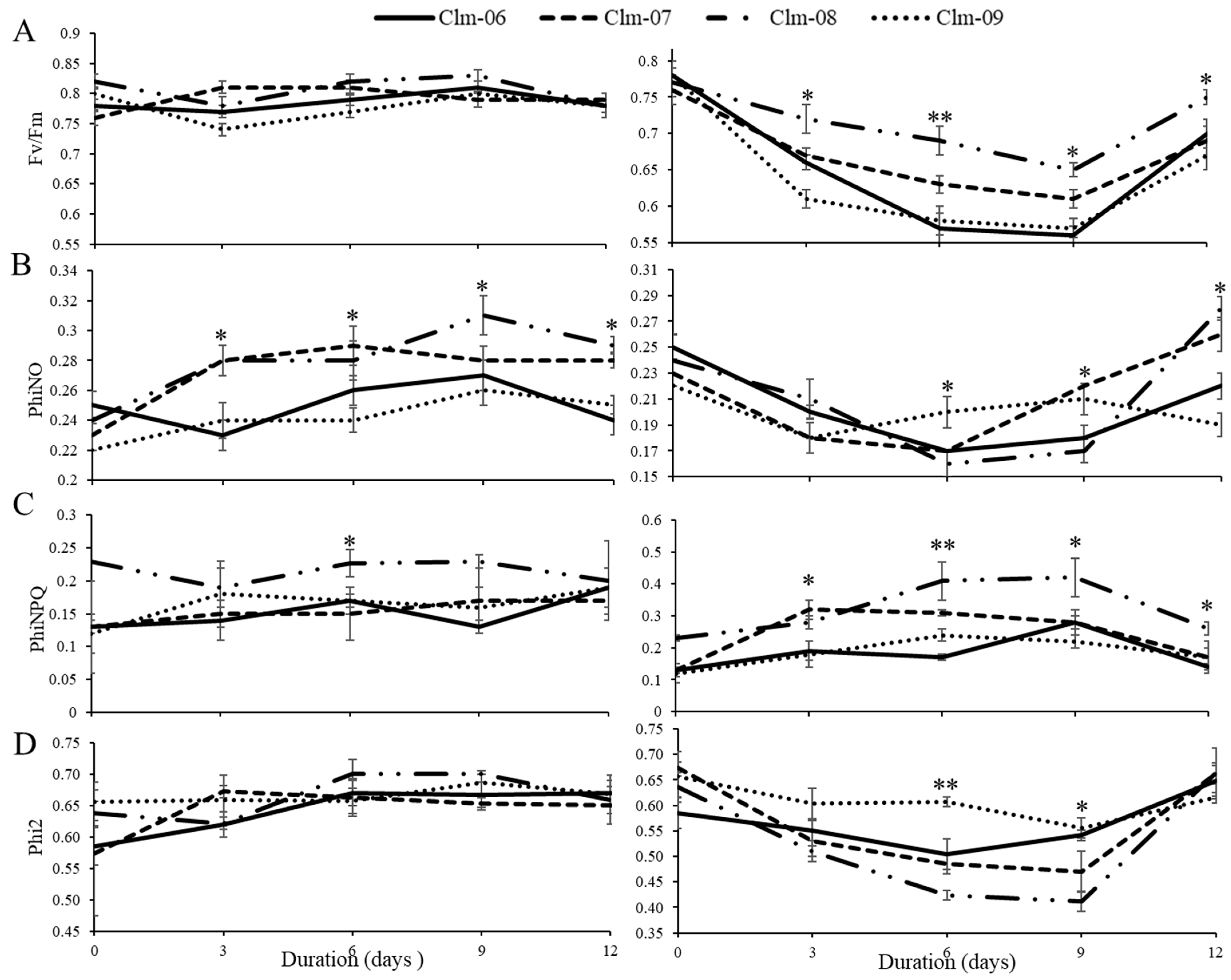
3.3. Response of Fluorescence Parameters to Water Deficit
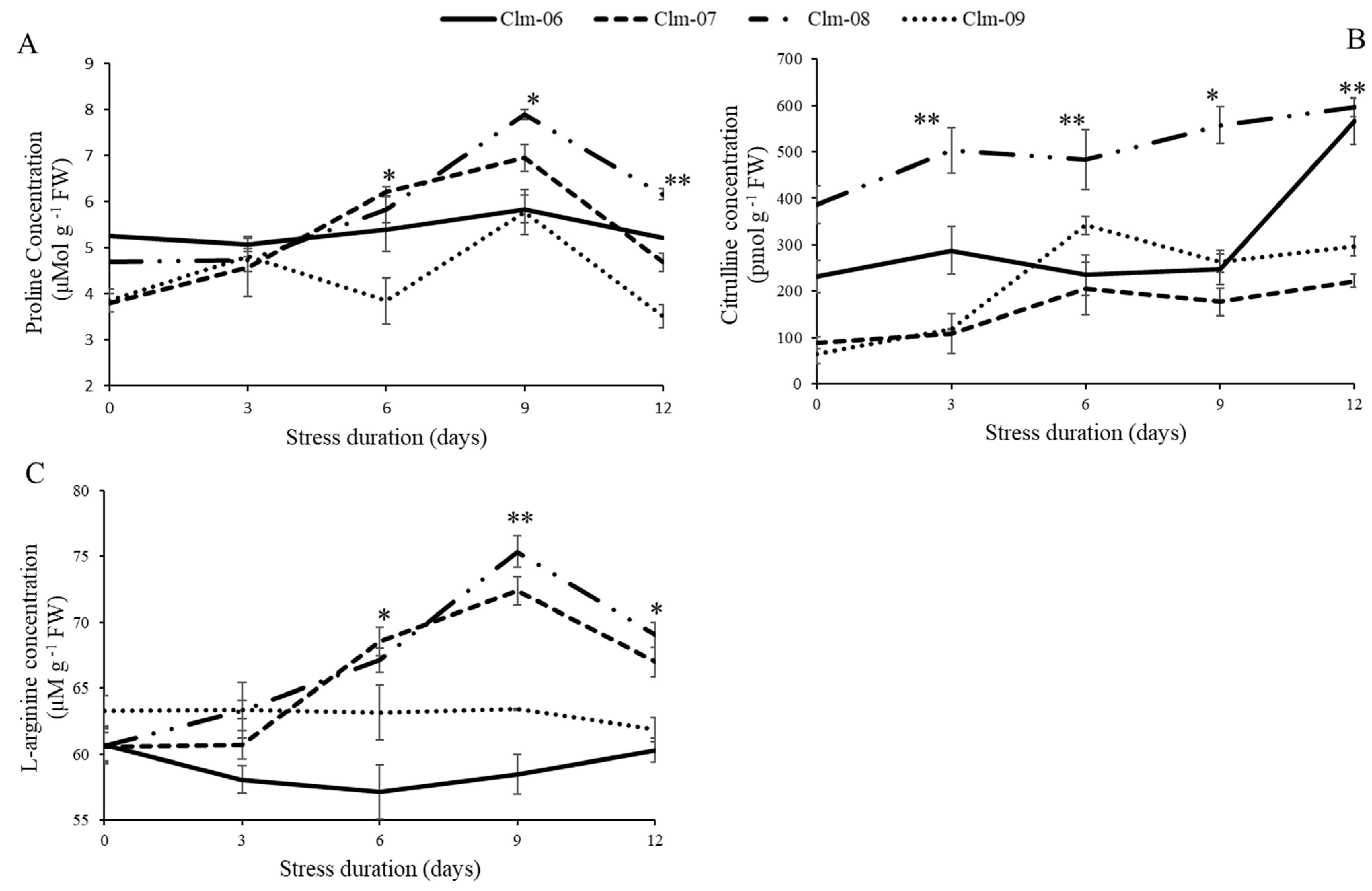
3.4. Quantification of Proline, Citrulline, and Arginine Concentrations
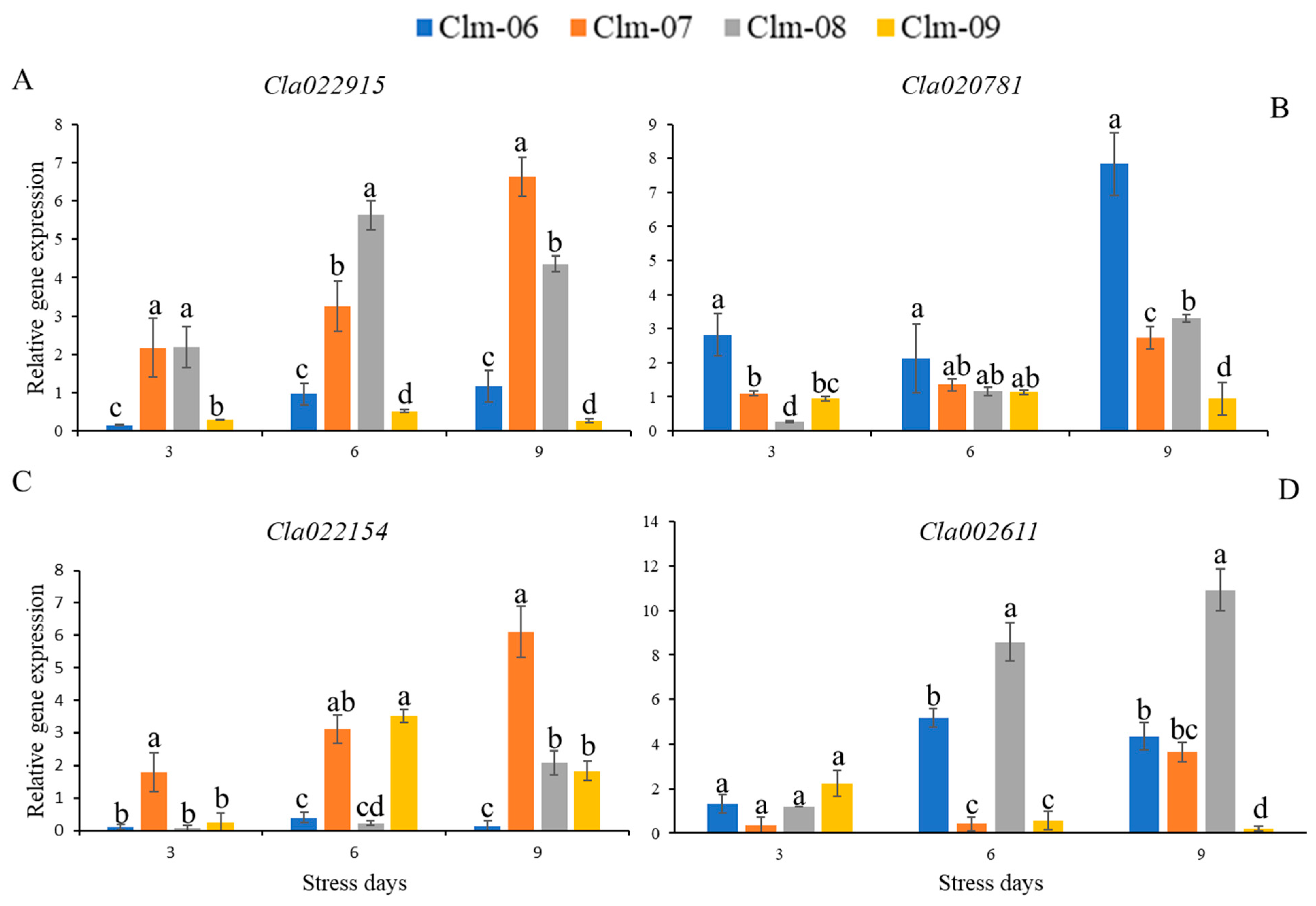
3.5. Expression Analysis of Target Genes Involved in the Citrulline and Arginine Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Habib-ur-Rahman, M.; Ahmad, A.; Raza, A.; Hasnain, M.U.; Alharby, H.F.; Alzahrani, Y.M.; Bamagoos, A.A.; Hakeem, K.R.; Ahmad, S.; Nasim, W.; et al. Impact of climate change on agricultural production; Issues, challenges, and opportunities in Asia. Front. Plant Sci. 2022, 13, 925548. [Google Scholar] [CrossRef] [PubMed]
- Spinoni, J.; Barbosa, P.; De Jager, A.; McCormick, N.; Naumann, G.; Vogt, J.V.; Mazzeschi, M. A new global database of meteorological drought events from 1951 to 2016. J. Hydrol.-Reg. Stud. 2019, 22, 100593. [Google Scholar] [CrossRef]
- Orimoloye, I.R. Agricultural Drought and Its Potential Impacts: Enabling Decision- Support for food Security in Vulnerable Regions. Front. Sustain. Food Syst. 2022, 6, 838824. [Google Scholar] [CrossRef]
- Xu, Z.; Lai, X.; Ren, Y.; Yang, H.; Wang, H.; Wang, C.; Xia, J.; Wang, Z.; Yang, Z.; Geng, H.; et al. Impact of Drought Stress on Yield-Related Agronomic Traits of Different Genotypes in Spring Wheat. Agronomy 2023, 13, 2968. [Google Scholar] [CrossRef]
- Nuccio, M.L.; Paul, M.; Bate, N.J.; Cohn, J.; Cutler, S.R. Where are the drought tolerant crops? An assessment of more than two decades of plant biotechnology effort in crop improvement. Plant Sci. 2018, 273, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.S.; Hussain, A.; Hussain, S.J.; Wani, O.A.; Nabi, S.Z.; Dar, N.A.; Baloch, F.S.; Mansoor, S. Plant drought stress tolerance: Understanding its physiological, biochemical, and molecular mechanisms. Biotechnol. Biotechnol. Equip. 2021, 35, 191–1925. [Google Scholar] [CrossRef]
- Li, S.; Kupriyanovich, Y.; Wagg, C.; Zheng, F.; Hann, S. Water Deficit Duration Affects Potato Plant Growth, Yield and Tuber Quality. Agriculture 2023, 13, 2007. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant. Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms, and management. In Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2009; pp. 153–188. [Google Scholar]
- Renzi, J.P.; Coyne, C.J.; Berger, J.; von Wettberg, E.; Nelson, M.; Ureta, S.; Hernández, F.; Smýkal, P.; Brus, J. How Could the Use of Crop Wild Relatives in Breeding Increase the Adaptation of Crops to Marginal Environments? Front. Plant Sci. 2022, 13, 886162. [Google Scholar] [CrossRef] [PubMed]
- Bohra, A.; Kilian, B.; Sivasankar, S.; Caccamo, M.; Mba, C.; McCouch, S.R.; Varshney, R.K. Reap the crop wild relatives for breeding future crops. Trends Biotechnol. 2021, 40, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, R.; Hodgkin, T. The use of wild relatives in crop improvement: A survey of developments over the last 20 years. Euphytica 2007, 156, 1–13. [Google Scholar] [CrossRef]
- Yang, Y.; Mo, Y.; Yang, X.; Zhang, H.; Wang, Y.; Li, H.; Wei, C.; Zhang, X. Transcriptome Profiling of Watermelon Root in Response to Short-Term Osmotic Stress. PLoS ONE 2016, 11, e0166314. [Google Scholar] [CrossRef] [PubMed]
- Morales, C.; Riveros-Burgos, C.; Espinoza Seguel, F.; Maldonado, C.; Mashilo, J.; Pinto, C.; Contreras-Soto, R.I. Rootstocks Comparison in Grafted Watermelon under Water Deficit: Effects on the Fruit Quality and Yield. Plants 2023, 12, 509. [Google Scholar] [CrossRef]
- Dube, L.; Ddamulira, G.; Maphosa, M. Watermelon production in Africa: Challenges and opportunities. Int. J. Veg. Sci. 2020. [Google Scholar] [CrossRef]
- Mujaju, C.; Fatih, M. Distribution patterns of cultivated watermelon forms in Zimbabwe using DIVA-GIS. Int. J. Biodivers. Conserv. 2011, 3, 474–481. [Google Scholar]
- Malambane, G.; Batlang, U.; Ramolekwa, K.; Tsujimoto, H.; Akashi, K. Growth chamber and field evaluation of physiological factors of two watermelon genotypes. Plant Stress 2021, 2, 100017. [Google Scholar] [CrossRef]
- Akashi, K.; Miyake, C.; Yokota, A. Citrulline, a novel compatible solute in drought tolerant wild watermelon leaves, is an efficient hydroxyl radical scavenger. FEBS Lett. 2001, 508, 438–442. [Google Scholar] [CrossRef]
- Heuer, B. Role of Proline in Plant Response to Drought and Salinity. In Hand Book of Plant And Crop Stress; CRC Press: Boca Raton, FL, USA, 2010; pp. 213–238. [Google Scholar]
- Chun, S.C.; Paramasivan, M.; Chandrasekaran, M. Proline Accumulation Influenced by Osmotic Stress in Arbuscular Mycorrhizal Symbiotic Plants. Front. Microbiol. 2018, 9, 2525. [Google Scholar] [CrossRef]
- Furlan, A.L.; Bianucci, E.; Giordano, W.; Castro, S.; Becker, D.F. Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol. Biochem. 2020, 151, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Spormann, S.; Nadais, P.; Sousa, F.; Pinto, M.; Martins, M.; Sousa, B.; Fidalgo, F.; Soares, C. Accumulation of Proline in Plants under Contaminated Soils—Are We on the Same Page? Antioxidants 2023, 12, 666. [Google Scholar] [CrossRef] [PubMed]
- Bauduin, S.; Latini, M.; Belleggia, I.; Migliore, M.; Biancucci, M.; Mattioli, R.; Francioso, A.; Mosca, L.; Funck, D.; Trovato, M. Interplay between Proline Metabolism and ROS in the Fine Tuning of Root-Meristem Size in Arabidopsis. Plants 2022, 11, 1512. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I. Role of Osmolytes in Alleviation of Oxidative Stress. In Reactive Oxygen Species in Plants; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Khan, M.A.R. Understanding the roles of osmolytes for acclimatizing plants to changing environment: A review of potential mechanism. Plant Signal Behav. 2021, 16, 1913306. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.-G. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environl. Rev. 2010, 18, 309–319. [Google Scholar] [CrossRef]
- Machacha, S. Comparison of laboratory pH buffer methods for predicting lime requirements for acidic soils of Eastern Botswana. Commun. Soil Sci. Plant Anal. 2011, 35, 2675–2687. [Google Scholar] [CrossRef]
- Kawasaki, S.; Miyake, C.; Kohchi, T.; Fujii, S.; Uchida, M.; Yokota, A. Responses of wild watermelon to drought stress: Accumulation of an ArgE homologue and citrulline in leaves during water deficits. Plant Cell Physiol. 2000, 41, 864–873. [Google Scholar] [CrossRef]
- Tiger-F1 Factsheet. Available online: https://www.starkeayres.com/products/commercial-farming-seed/watermelon/watermelon-tiger-f1. (accessed on 15 July 2023).
- Maggs-Kölling, G.L.; Madsen, S.; Christiansen, J.L. A phenetic analysis of morphological variation in Citrullus lanatusin Namibia. Genet. Resour. Crop Evol. 2000, 47, 385–389. [Google Scholar] [CrossRef]
- Maggs-Kölling, G.L.; Christiansen, J.L. Variability in Namibian landraces of watermelon (Citrullus lanatus). Euphytica 2003, 132, 251–258. [Google Scholar] [CrossRef]
- Malambane, G.; Madumane, K.; Sewelo, L.T.; Batlang, U. Drought stress tolerance mechanisms and their potential common indicators to salinity, insights from the wild watermelon (Citrullus lanatus): A review. Front. Plant Sci. 2023, 13, 1074395. [Google Scholar] [CrossRef] [PubMed]
- Madumane, K.; Sewelo, L.T.; Nkane, M.N.; Batlang, U.; Malambane, G. Morphological, Physiological And Molecular Stomatal Responses Of Wild Watermelon As A Drought Tolerance Mechanism. Horticulturae 2024, 10, 123. [Google Scholar] [CrossRef]
- Cui, Q.; Xiong, H.; Yufeng, Y.; Eaton, S.; Imamura, S.; Santamaria, J.; Ravelombola, W.; Mason, R.E.; Wood, L.; Mozzoni, S.A. Evaluation of drought tolerance in Arkansas cowpea lines at seedling stage. Hortic. Sci. 2020, 55, 1132–1143. [Google Scholar] [CrossRef]
- Negin, B.; Yaaran, A.; Kelly, G.; Zait, Y.; Moshelion, M. Mesophyll Abscisic Acid Restrains Early Growth and Flowering But Does Not Directly Suppress Photosynthesis. Plant Physiol. 2019, 180, 910–925. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.; Gonzalez-Vilar, M. Determination of relative water content. In Handbook of Plant Ecophysiology Techniques; Regoisa Roger, M.J., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 207–212. ISBN 978-0-7923-6. [Google Scholar]
- Hartman, J.L.; Wehner, T.C.; Ma, G.; Perkins-veazie, P. Citrulline and Arginine Content of Taxa of Cucurbitaceae. Horticulture 2019, 5, 22. [Google Scholar] [CrossRef]
- Kim, D.R.; Martin, S.; Desai, K. The effects of a comparatively higher dose of 1000 mg/kg/d of oral L- or D-arginine on the L-arginine metabolic pathways in male Sprague-Dawley rats. PLoS ONE 2023, 18, e0289476. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies’. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kong, Q.; Yuan, J.; Gao, L.; Zhao, L.; Cheng, F.; Huang, Y.; Bie, Z. Evaluation of Appropriate Reference Genes for Gene Expression Normalization during Watermelon Fruit Development. PLoS ONE 2015, 10, e0130865. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, J.; Sun, H.; Salse, J.; Lucas, W.J.; Zhang, H.; Zheng, Y.; Mao, L.; Ren, Y.; Wang, Z.; et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat. Genet. 2013, 45, 51–58. [Google Scholar] [CrossRef]
- Lalonde, L.F.; Reyes, J.; Gajadhar, A.A. Application of a qPCR assay with melting curve analysis for detection and differentiation of protozoan oocysts in human fecal samples from Dominican Republic. Am. J. Trop. Med. Hyg. 2013, 89, 892–898. [Google Scholar] [CrossRef][Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta-DeltaC (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Ravelombola, W.; Shi, A. Investigation on various above ground traits to identify drought tolerance in cowpea seedlings. Hortscience 2018, 53, 1757–1765. [Google Scholar] [CrossRef]
- Vennam, R.R.; Ramamoorthy, P.; Poudel, S.; Reddy, K.R.; Henry, W.B.; Bheemanahalli, R. Developing Functional Relationships between Soil Moisture Content and Corn Early-Season Physiology, Growth, and Development. Plants 2023, 12, 2471. [Google Scholar] [CrossRef] [PubMed]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Yolcu, S.; Alavilli, H.; Ganesh, P.; Panigrahy, M.; Song, K. Salt, and Drought Stress Responses in Cultivated Beets (Beta vulgaris L.) and Wild Beet (Beta maritima L.). Plants 2021, 10, 1843. [Google Scholar] [CrossRef]
- Feller, U. Drought stress and carbon assimilation in a warming climate: Reversible and irreversible impacts. J. Plant Physiol. 2016, 203, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Giménez, C.; Gallardo, M.; Thompson, R.B. Plant–Water Relations, Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Lv, X.; Li, Y.; Chen, R.; Rui, M.; Wang, Y. Stomatal Responses of Two Drought-Tolerant Barley Varieties with Different ROS Regulation Strategies under Drought Conditions. Antioxidants 2023, 12, 790. [Google Scholar] [CrossRef]
- Ribeiro, I.A.; Voltolini, T.V.; Simões, W.L.; Da Fonseca Ferreira, M.A.J.; Sobreira, A.M.; Gois, G.C. Responses of forage watermelon genotypes submitted to different water supply. Biol. Rhythm Res. 2021, 52, 293–306. [Google Scholar] [CrossRef]
- Chowdhury, M.K.; Hasan, M.A.; Bahadur, M.M.; Islam, M.R.; Hakim, M.A.; Iqbal, M.A.; Javed, T.; Raza, A.; Shabbir, R.; Sorour, S.; et al. Evaluation of drought tolerance of some wheat (Triticum aestivum L.) genotypes through phenology, growth, and physiological indices. Agronomy 2021, 11, 1792. [Google Scholar] [CrossRef]
- Efeoǧlu, B.; Ekmekçi, Y.; Çiçek, N. Physiological responses of three maize cultivars to drought stress and recovery. South Afr. J. Bot. 2009, 75, 34–42. [Google Scholar] [CrossRef]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Anjun, S.A.; Xie, X.; Wang, L.W.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 69, 2026–2032. [Google Scholar] [CrossRef]
- Hajibarat, Z.; Saidi, A. Senescence-associated proteins and nitrogen remobilization in grain filling under drought stress condition. J. Genet. Eng. Biotechnol. 2022, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, J. Effects of water stress on photosystem II photochemistry and its thermostability in wheat plants. J. Exp. Bot. 1999, 50, 1199–1206. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Chen, J.-H.; He, N.-Y.; Guo, F.-Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [PubMed]
- Tohamo, W. Significance of Water Splitting in Photosynthesis and its Impact on the Environment. J. Biol. Energ. 2023, 11, 197. [Google Scholar]
- Mishra, A.; Heyer, A.G.; Mishra, K.B. Chlorophyll fluorescence emission can screen cold tolerance of cold acclimated Arabidopsis thaliana accessions. Plant Methods 2010, 10, 38. [Google Scholar] [CrossRef]
- Banks, J.M. Chlorophyll fluorescence as a tool to identify drought stress in Acer genotypes. Environ. Exp. Bot. 2018, 155, 118–127. [Google Scholar] [CrossRef]
- Li, P.; Yang, H.; Wang, L.; Liu, H.; Huo, H.; Zhang, C.; Liu, A.; Zhu, A.; Hu, J.; Lin, Y.; et al. Physiological and Transcriptome Analyses Reveal Short-Term Responses and Formation of Memory Under Drought Stress in Rice. Front. Genet. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Sun, D.; Cen, H.; Xu, H.; Weng, H.; Yuan, F.; He, Y. Phenotyping of Arabidopsis drought stress response using kinetic chlorophyll fluorescence and multicolor fluorescence imaging. Front. Plant Sci. 2018, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Ruban, A.V. Dynamic non-photochemical quenching in plants: From molecular mechanism to productivity. Plant J. 2020, 101, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Bano, H.; Athar, H.R.; Zafar, Z.; Kalaji, H.M.; Ashraf, M. Linking changes in chlorophyll a fluorescence with drought stress susceptibility in mung bean [Vigna radiata (L.) Wilczek]. Physiol. Plant. 2021, 172, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, H.; Yu, F.; Hu, B.; Jia, Y.; Sha, H.; Zhao, H. Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci. Rep. 2019, 9, 8543. [Google Scholar] [CrossRef] [PubMed]
- Yokota, A.; Kawasaki, S.; Iwano, M.; Nakamura, C.; Miyake, C.; Akashi, K. Citrulline and DRIP-1 Protein (ArgE Homologue) in Drought Tolerance of Wild Watermelon. Ann. Bot. 2002, 89, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline Accumulation in Plants: Roles in Stress Tolerance and Plant Development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., Khan, N.A., Eds.; Springer: New Delhi, India, 2016. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pitchel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Ibrahim, A.E.-A.; El Mageed, T.; Abohamid, Y.; Abdallah, H.; El-Saadony, M.; AbuQamar, S.; El-Tarabily, K.; Abdou, N. Exogenously Applied Proline Enhances Morph-Physiological Responses and Yield of Drought-Stressed Maize Plants Grown Under Different Irrigation Systems. Front. Plant Sci. 2022, 13, 897027. [Google Scholar] [CrossRef]
- Song, Q.; Joshi, M.; DiPiazza, J.; Joshi, V. Functional relevance of citrulline in vegetative tissues of watermelon during abiotic stress. Plant Sci. 2020, 11, 512. [Google Scholar]
- Rimando, A.M.; Perkins-Veazie, P.M. Determination of Citrulline in Watermelon Rind. J. Chromatogr. A 2005, 1078, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Kusvuran, S.; Dasgan, H.Y.; Abak, K. Citrulline is an important biochemical indicator in tolerance to saline and drought stress in melon. Sci. World J. 2013, 2013, 253414. [Google Scholar] [CrossRef] [PubMed]
- Sircar, S.; Parekht, N. Meta-analysis of drought-tolerant genotypes in Oryza sativa: A network-based approach. PLoS ONE 2019, 14, e0216068. [Google Scholar] [CrossRef] [PubMed]
- Song, Q. Metabolic and Transcriptional Regulation of Citrulline in Watermelon. Master’s Thesis, Texas A&M University, College Station, TX, USA, 2020. Available online: https://hdl.handle.net/1969.1/191875 (accessed on 22 April 2024).
- Xu, S.; Zhou, J.; Liu, L.; Chen, J. Arginine: A novel compatible solute to protect Candida glabrata against hyperosmotic stress. Process Biochem. 2011, 46, 1230–1235. [Google Scholar] [CrossRef]
- Ragaey, M.M.; Sadak, M.S.; Dawood, M.F.A.; Mousa, N.H.S.; Hanafy, R.S.; Latef, A.A.H.A. Role of Signaling Molecules Sodium Nitroprusside and Arginine in Alleviating Salt-Induced Oxidative Stress in Wheat. Plants 2022, 11, 1786. [Google Scholar] [CrossRef] [PubMed]
- Nasibi, F.; Yaghoobi, M.M.; Kalantari, K.M. Effect of exogenous arginine on alleviation of oxidative damage in tomato plant underwater stress. J. Plant Interact. 2011, 6, 291–296. [Google Scholar] [CrossRef]
- Sun, Y.; Miao, F.; Wang, Y.; Liu, H.; Wang, X.; Wang, H.; Guo, J.; Shao, R.; Yang, Q. L-Arginine Alleviates the Reduction in Photosynthesis and Antioxidant Activity Induced by Drought Stress in Maize Seedlings. Antioxidants 2023, 12, 482. [Google Scholar] [CrossRef]
- Silveira, N.M.; Ribeiro, R.V.; de Morais, S.F.N.; de Souza, S.C.R.; da Silva, S.F.; Seabra, A.B.; Hancock, J.T.; Machado, E.C. Leaf arginine spraying improves leaf gas exchange under water deficit and root antioxidant responses during the recovery period. Plant Physiol. Biochem. 2021, 162, 315–326. [Google Scholar] [CrossRef]
- You, J.; Zhang, Y.; Liu, A.; Li, D.; Wang, X.; Dossa, K.; Zhou, R.; Yu, J.; Zhang, Y.; Wang, L.; et al. Transcriptomic and metabolomic profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol. 2019, 19, 267. [Google Scholar] [CrossRef]



| Code | Accession Type | Source | Type of Use | Flesh Color | Reference |
|---|---|---|---|---|---|
| Clm-06 | Hybrid | Agric shops | Fresh fruit | Red | [32] |
| Clm-07 | Landrace | BNPGRC | Cooking | Yellow | [33,34] |
| Clm-08 | Wild | BNPGRC | Feed | White | [35] |
| Clm-09 | Landrace | BNPGRC | Fresh fruit | Red | [36] |
| GOI | Enzyme | Pathway | Primer Sequences for GOIs |
|---|---|---|---|
| Cla020781 | ornithine carbamoyl-transferase (OTC), | Citrulline | F_CTCTACTCACTTCTACTCCGGTACG R_CTAAGATCTTCAAGAGGGTGGATTT |
| Cla022154 | arginosuccinate lyase (ASL) | Arginine | F_ACATCTTCATGCACTAAACAGAGTG R_GATCCATTAGAGATGCTTGTGATCT |
| Cla002611 | arginosuccinate synthases (ASS) | Arginine | F_GTGGAAGAAGCTCTACAAAGTCAAC R_AAGAGTGTCTTCTTCCTGGTTGTAA |
| Cla022915 | carbamoyl phosphate synthase (CPS) | Citrulline | F_TCTCTACACTGTTCCTGAAAATTCC R_TGTTCTGACCAAAACCTAATCTCTC |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | F_CTGGCAGTACTTTGCCAACA R_AGGATTGGAGAGGAGGTCGT | ||
| Actin | F_CCATGTATGTTGCCATCCAG R_GGATAGCATGGGGTAGAGCA | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sewelo, L.T.; Madumane, K.; Nkane, M.N.; Tait, M.; Malambane, G. Potential Use of Compatible Osmolytes as Drought Tolerance Indicator in Local Watermelon (Citrullus lanatus) Landraces. Horticulturae 2024, 10, 475. https://doi.org/10.3390/horticulturae10050475
Sewelo LT, Madumane K, Nkane MN, Tait M, Malambane G. Potential Use of Compatible Osmolytes as Drought Tolerance Indicator in Local Watermelon (Citrullus lanatus) Landraces. Horticulturae. 2024; 10(5):475. https://doi.org/10.3390/horticulturae10050475
Chicago/Turabian StyleSewelo, Lesego T., Kelebogile Madumane, Metseyabeng N. Nkane, Motlalepula Tait, and Goitseone Malambane. 2024. "Potential Use of Compatible Osmolytes as Drought Tolerance Indicator in Local Watermelon (Citrullus lanatus) Landraces" Horticulturae 10, no. 5: 475. https://doi.org/10.3390/horticulturae10050475
APA StyleSewelo, L. T., Madumane, K., Nkane, M. N., Tait, M., & Malambane, G. (2024). Potential Use of Compatible Osmolytes as Drought Tolerance Indicator in Local Watermelon (Citrullus lanatus) Landraces. Horticulturae, 10(5), 475. https://doi.org/10.3390/horticulturae10050475





