Eco-Sustainability of Soils in Baby-Leaf Crop Systems under Tunnel through the Application of C-Rich Inputs: Towards Combating Soil Degradation
Abstract
1. Introduction
- (a)
- After a two-year period, the SOC change and conversion efficiency of C input were assessed in relation to the organic C sources and two levels of supply.
- (b)
- In the second year of organic amendment, the repeated measurement (three times) of soil microbial biomass and some enzymatic activities linked to C, N, P and S biogeochemical cycles were carried out.
- (c)
- In the second year, the yield of rocket (fresh and dry matter) as a response to the repeated amendment was evaluated.
- (d)
- In the second year, as already measured in the first year, the possible influence of total N applied by organic amendments on the uptake of nitrate in leaves of rocket, which, as is known, is a nitrate hyper-accumulating species, was verified [9].
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Crop Management
2.3. Soil C Balance
2.4. Microbial Biomass and Soil Enzyme Activities
2.5. Yield Measurements and Concentration of Nitrate in Leaves
2.6. Statistical Analysis
3. Results
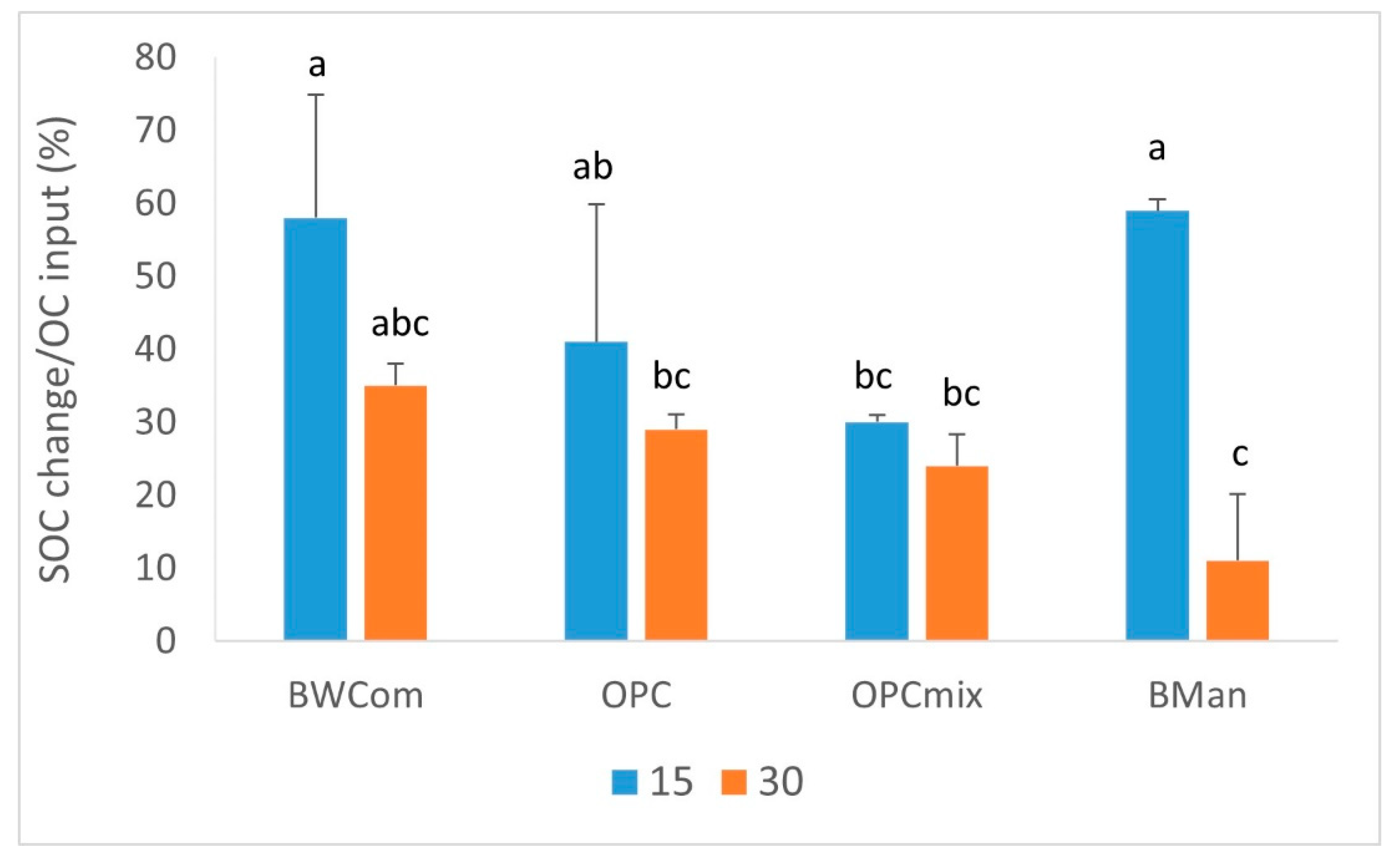
3.1. Soil C Stock Changes
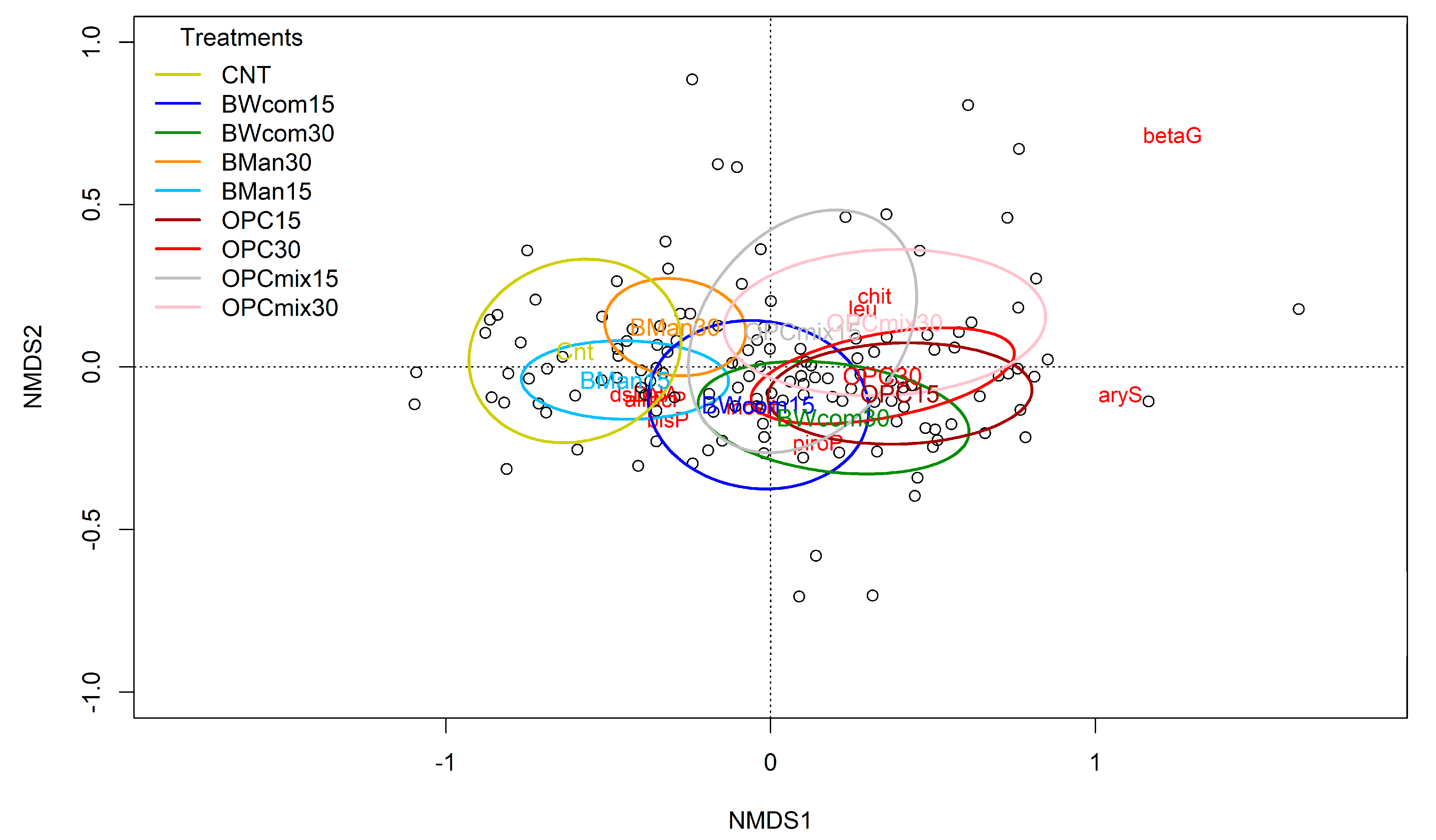
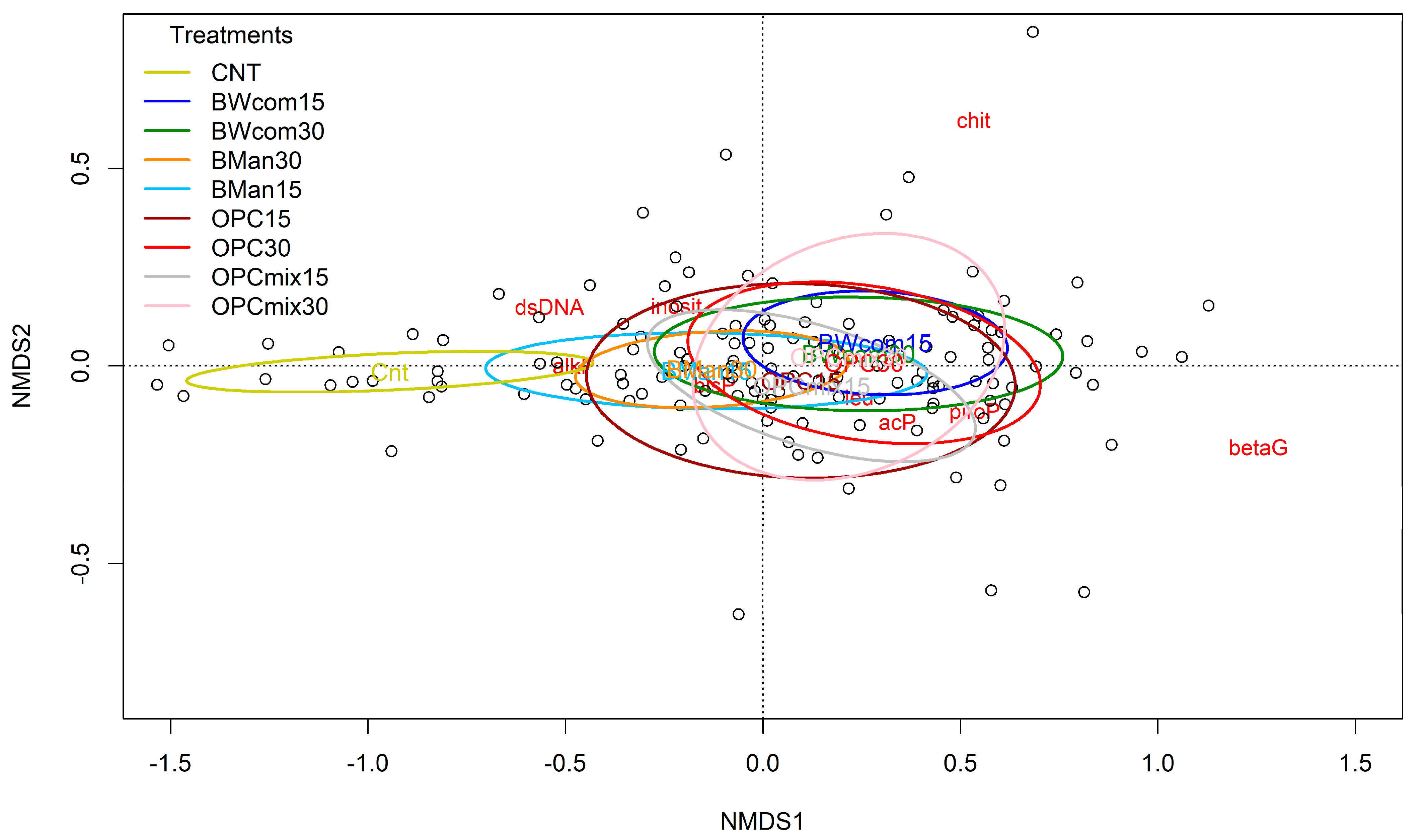
3.2. Microbial Biomass and Soil Enzyme Activities
3.3. Yield Measurements and Concentration of Nitrate in Leaves
4. Discussion
4.1. Soil C Stock Changes
4.2. Microbial Biomass and Soil Enzyme Activities
4.3. Yield Measurements and Concentration of Nitrate in Leaves
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Penati, M.; Ferrante, A.; Martinetti, L.; Quattrini, E.; Marino, P.; Salamone, F.; Sari, M.; Quattrini, L.O.E.; Marino, P.; Ferrante, A. Asportazioni di Elementi Nutritivi ed Ottimizzazione delle Colture da Foglia Destinate alla IV Gamma; Quaderni della Ricerca della Regione Lombardia: Milan, Italy, 2009; Volume 107, p. 48. [Google Scholar]
- Scotti, R.; D’Ascoli, R.; Gonzalez Caceres, M.; Bonanomi, G.; Sultana, S.; Cozzolino, L.; Scelza, R.; Zoina, A.; Rao, M.A. Combined use of compost and wood scraps to increase carbon stock and improve soil quality in intensive farming systems. Eur. J. Soil Sci. 2015, 66, 463–475. [Google Scholar] [CrossRef]
- Ferrante, A. Ortaggi per la IV gamma. In La Concimazione Azotata Degli Ortaggi; Incrocci, L., Dimauro, B., Santamaria, P., Pardossi, A., Eds.; Barone e Bella & C.: Ragusa, Italy, 2014; pp. 165–180. [Google Scholar]
- CSO Centro Servizi Ortofrutticoli. 2020. Available online: www.csoservizi.com/prodotto/presentazione-i-consumi-in-italia-di-iv-gamma/ (accessed on 10 May 2020).
- Latella, M. Piana del Sele Polo Europeo Degli Ortaggi di IV Gamma. Corriere Ortofrutticolo. 2019. Available online: https://www.corriereortofrutticolo.it/piana-del-sele-polo-europeo-degli-ortaggi-iv-gamma/ (accessed on 10 May 2020).
- Bonanomi, G.; D’Ascoli, R.; Antignani, V.; Capodilupo, M.; Cozzolino, L.; Marzaioli, R.; Puopolo, G.; Rutigliano, F.A.; Scelza, R.; Scotti, R.; et al. Assessing soil quality under intensive cultivation and tree orchards in Southern Italy. Appl. Soil Ecol. 2011, 47, 184–194. [Google Scholar] [CrossRef]
- Cardarelli, M.; El Chami, A.; Iovieno, P.; Rouphael, Y.; Bonini, P.; Colla, G. Organic Fertilizer Sources Distinctively Modulate Productivity, Quality, Mineral Composition, and Soil Enzyme Activity of Greenhouse Lettuce Grown in Degraded Soil. Agronomy 2023, 13, 194. [Google Scholar] [CrossRef]
- Intergovernmental Technical Panel on Soils towards a Definition of Soil Health. FAO. 2020. Available online: http://www.fao.org/3/cb1110en/cb1110en.pdf (accessed on 12 May 2020).
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mori, M. Plant-based biostimulants influence the agronomical, physiological, and qualitative responses of baby rocket leaves under diverse nitrogen conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef]
- Caruso, G.; El-Nakhel, C.; Rouphael, Y.; Comite, E.; Lombardi, N.; Cuciniello, A.; Woo, S.L. Diplotaxis tenuifolia (L.) DC. yield and quality as influenced by cropping season, protein hydrolysates, and Trichoderma applications. Plants 2020, 9, 697. [Google Scholar] [CrossRef]
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; Woo, S.L.; Fagnano, M.; Rouphael, Y. Can Trichoderma-based biostimulants optimize N use efficiency and stimulate growth of leafy vegetables in greenhouse intensive cropping systems? Agronomy 2020, 10, 121. [Google Scholar] [CrossRef]
- Morra, L.; Cerrato, D.; Bilotto, M.; Baiano, S. Introduction of sorghum [Sorghum bicolor (L.) Moench] green manure in rotations of head salads and baby leaf crops under greenhouse. Ital. J. Agron. 2017, 12, 40–46. [Google Scholar] [CrossRef]
- Libutti, A.; Trotta, V.; Rivelli, A.R. Biochar, vermicompost, and compost as soil organic amendments: Influence on Growth Parameters, Nitrate and Chlorophyll Content of Swiss Chard (Beta vulgaris L. var. cycla). Agronomy 2020, 10, 346. [Google Scholar] [CrossRef]
- Kebalo, L.F.; Garnier, P.; Gonod, L.V.; Houot, S. Using bio-based fertilizer derived from peri-urban wastes affects soil properties and lettuce yield and quality. Sci. Hortic. 2024, 324, 112599. [Google Scholar] [CrossRef]
- Bonanomi, G.; De Filippis, F.; Zotti, M.; Idbella, M.; Cesarano, G.; Al-Rowaily, S.; Abd-ElGawad, A. Repeated applications of organic amendments promote beneficial microbiota, improve soil fertility and increase crop yield. Appl. Soil Ecol. 2020, 156, 103714. [Google Scholar] [CrossRef]
- Iovieno, P.; Morra, L.; Leone, A.; Pagano, L.; Alfani, A. Effect of organic and mineral fertilizers on soil respiration and enzyme activities of two Mediterranean horticultural soils. Biol. Fertil. Soils 2009, 45, 555–561. [Google Scholar] [CrossRef]
- Gianfreda, L.; Ruggiero, P. Enzyme activities in soil. In Soil Biology. Nucleic Acids and Proteins in Soil; Nannipieri, P., Smalla, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 257–297. [Google Scholar]
- Gianfreda, L.; Rao, M.A. Enzymes in Agricultural Sciences; OMICS Group: Foster, CA, USA, 2014. [Google Scholar]
- Proietti, P.; Federici, E.; Fidati, L.; Scargetta, S.; Massaccesi, L.; Nasini, L.; Regni, L.; Ricci, A.; Cenci, G.; Gigliotti, G. Effects of amendment with oil mill waste and its derived-compost on soil chemical and microbiological characteristics and olive (Olea europaea L.) productivity. Agric. Ecosyst. Environ. 2015, 207, 51–60. [Google Scholar] [CrossRef]
- Innangi, M.; Niro, E.; D’Ascoli, R.; Danise, T.; Proietti, P.; Nasini, L.; Regni, L.; Castaldi, S.; Fioretto, A. Effects of olive pomace amendment on soil enzyme activities. Appl. Soil Ecol. 2017, 119, 242–249. [Google Scholar] [CrossRef]
- Altieri, R.; Esposito, A. Evaluation of the fertilizing effect of olive mill waste compost in short-term crops. Int. Biodeterior. Biodegrad. 2010, 64, 124–128. [Google Scholar] [CrossRef]
- Morra, L.; Pizzolongo, G.; Baiano, S.; Pentangelo, A. Comparison of olive pomace and biowaste composts in a vegetable cropping system. Ital. J. Agron. 2013, 8, 206–216. [Google Scholar] [CrossRef]
- CIC (Consorzio Italiano Compostatori). Rapporto Annuale 2019 Consorzio Italiano Compostatori. Available online: https://www.compost.it/rapporti-cic/rapporto-annuale-2019/ (accessed on 15 July 2020).
- Hargreaves, J.C.; Adl, M.S.; Warman, P.R. A review of the use of composted municipal solid waste in agriculture. Agric. Ecosyst. Environ. 2008, 123, 1–14. [Google Scholar] [CrossRef]
- Memoli, V.; De Marco, A.; Baldantoni, D.; De Nicola, F.; Maisto, G. Short- and long-term effects of a single application of two organic amendments. Ecosphere 2017, 8, e02009. [Google Scholar] [CrossRef]
- Morra, L. Role of compost in the organic amendment of vegetable crops. Italus Hortus 2019, 26, 27–39. [Google Scholar] [CrossRef]
- EIP-AGRI, Research & Innovation. Soil Organic Matter in Mediterranean Regions—EIP-AGRI Focus Group Soil Organic Matter in Mediterranean Regions. 2015; p.38. Available online: https://ec.europa.eu/eip/agriculture/sites/default/files/eip-agri_fg_soil_organic_matter_final_report_2015_en_0.pdf (accessed on 12 May 2020).
- Morra, L.; Bilotto, M.; Baiano, S.; Saviello, G.; Cerrato, D. Annual effects of different organic fertilisers in a baby leaf crops system under tunnel in Southern Italy. Ital. J. Agron. 2015, 10, 144–150. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–577. [Google Scholar]
- FAO. A Protocol for Measurement, Monitoring, Reporting and Verification of Soil Organic Carbon in Agricultural Landscapes—GSOC-MRV Protocol; FAO: Rome, Italy, 2020; ISBN 9789251331262. [Google Scholar]
- Fornasier, F.; Ascher, J.; Ceccherini, M.T.; Tomat, E.; Pietramellara, G. A simplified rapid, low-cost and versatile DNA-based assessment of soil microbial biomass. Ecol. Indic. 2014, 45, 75–82. [Google Scholar] [CrossRef]
- Bragato, G.; Fornasier, F.; Brus, D.J. Characterization of soil fertility and soil biodiversity with dsDNA as a covariate in a regression estimator for mean microbial biomass C. Eur. J. Soil Sci. 2016, 67, 827–834. [Google Scholar] [CrossRef]
- Fornasier, F.; Margon, A. Bovine serum albumin and Triton X-100 greatly increase phosphomonoesterases and arylsulphatase extraction yield from soil. Soil Biol. Biochem. 2007, 39, 2682–2684. [Google Scholar] [CrossRef]
- EU. Reg. n. 1258/2011 Regulation (EC) No. 1881/2006 as Regards Maximum Levels for Nitrates in Foodstuffs; EU: Brussels, Belgium, 2011; pp. 2010–2012. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Anderson, M.; Braak, C. Ter Permutation tests for multi-factorial analysis of variance. J. Stat. Comput. Simul. 2003, 73, 85–113. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.1.2; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Prout, J.M.; Shepherd, K.D.; McGrath, S.P.; Kirk, G.J.D.; Haefele, S.M. What is a good level of soil organic matter? An index based on organic carbon to clay ratio. Eur. J. Soil Sci. 2020, 72, 2493–2503. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Gattinger, A.; Gimeno, B.S. Managing soil carbon for climate change mitigation and adaptation in Mediterranean cropping systems: A meta-analysis. Agric. Ecosyst. Environ. 2013, 168, 25–36. [Google Scholar] [CrossRef]
- Tiefenbacher, A.; Sandén, T.; Haslmayr, H.P.; Miloczki, J.; Wenzel, W.; Spiegel, H. Optimizing carbon sequestration in croplands: A synthesis. Agronomy 2021, 11, 882. [Google Scholar] [CrossRef]
- Chen, S.; Arrouays, D.; Angers, D.A.; Martin, M.P.; Walter, C. Soil carbon stocks under different land uses and the applicability of the soil carbon saturation concept. Soil Tillage Res. 2019, 188, 53–58. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Balesdent, J.; Chenub, C.; Balabane, M. Relationship of soil organic matter dynamics to physical protection and tillage. Soil Tillage Res. 2000, 53, 215–230. [Google Scholar] [CrossRef]
- Chenu, C.; Angers, D.A.; Barré, P.; Derrien, D.; Arrouays, D.; Balesdent, J. Increasing organic stocks in agricultural soils: Knowledge gaps and potential innovations. Soil Tillage Res. 2019, 188, 41–52. [Google Scholar] [CrossRef]
- Stewart, C.E.; Paustian, K.; Conant, R.T.; Plante, A.F.; Six, J. Soil carbon saturation: Concept, evidence and evaluation. Biogeochemistry 2007, 86, 19–31. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Campbell, C.A.; McConkey, B.G.; Gameda, S.; Izaurralde, R.C.; Liang, B.C.; Zentner, R.P.; Sabourin, D. Efficiencies of conversion of residue C to soil C. In Agricultural Practices and Policies for Carbon Sequestration in Soil; Lewis Publishers: Boca Raton, FL, USA, 2016; pp. 305–313. ISBN 9781420032291. [Google Scholar]
- Shahbaz, M.; Kuzyakov, Y.; Heitkamp, F. Decrease of soil organic matter stabilization with increasing inputs: Mechanisms and controls. Geoderma 2017, 304, 76–82. [Google Scholar] [CrossRef]
- Morra, L.; Pagano, L.; Iovieno, P.; Baldantoni, D.; Alfani, A. Soil and vegetable crop response to addition of different levels of municipal waste compost under Mediterranean greenhouse conditions. Agron. Sustain. Dev. 2010, 30, 701–709. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.N.; Parra-Saldívar, R. Soil carbon sequestration—An interplay between soil microbial community and soil organic matter dynamics. Sci. Total Environ. 2022, 815, 152928. [Google Scholar] [CrossRef]
- Assirelli, A.; Fornasier, F.; Caputo, F.; Manici, L.M. Locally available compost application in organic farms: 2-year effect on biological soil properties. Renew. Agric. Food Syst. 2023, 38, e16. [Google Scholar] [CrossRef]
- Wang, D.; Lin, J.Y.; Sayre, J.M.; Schmidt, R.; Fonte, S.J.; Rodrigues, J.L.M.; Scow, K.M. Compost amendment maintains soil structure and carbon storage by increasing available carbon and microbial biomass in agricultural soil—A six-year field study. Geoderma 2022, 427, 116117. [Google Scholar] [CrossRef]
- Shu, X.; He, J.; Zhou, Z.; Xia, L.; Hu, Y.; Zhang, Y.; Zhang, Y.; Luo, Y.; Chu, H.; Liu, W.; et al. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 2022, 829, 154627. [Google Scholar] [CrossRef]
- Gomez, E.; Ferreras, L.; Toresani, S. Soil bacterial functional diversity as influenced by organic amendment application. Bioresour. Technol. 2006, 97, 1484–1489. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ni, T.; Li, Y.; Xiong, W.; Ran, W.; Shen, B.; Shen, Q.; Zhang, R. Responses of bacterial communities in arable soils in a rice-wheat cropping system to different fertilizer regimes and sampling times. PLoS ONE 2014, 9, e85301. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ni, T.; Li, J.; Lu, Q.; Fang, Z.; Huang, Q.; Zhang, R.; Li, R.; Shen, B.; Shen, Q. Effects of organic-inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice-wheat cropping system. Appl. Soil Ecol. 2016, 99, 1–12. [Google Scholar] [CrossRef]
- López-Piñeiro, A.; Albarrán, A.; Rato Nunes, J.M.; Peña, D.; Cabrera, D. Long-term impacts of de-oiled two-phase olive mill waste on soil chemical properties, enzyme activities and productivity in an olive grove. Soil Tillage Res. 2011, 114, 175–182. [Google Scholar] [CrossRef]
- Roberto, G.R.; Ochoa, M.V.; Hinojosa, M.B.; Beatriz, G.M. Improved soil quality after 16 years of olive mill pomace application in olive oil groves. Agron. Sustain. Dev. 2012, 32, 803–810. [Google Scholar] [CrossRef]
- Nicoletti, R.; Raimo, F.; Miccio, G. Diplotaxis tenuifolia: Biology, Production and Properties. Eur. J. Plant Sci. Technol. 2007, 1, 36–43. [Google Scholar]
- Colla, G.; Kim, H.J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Herencia, J.F.; García-Galavís, P.A.; Dorado, J.A.R.; Maqueda, C. Comparison of nutritional quality of the crops grown in an organic and conventional fertilized soil. Sci. Hortic. 2011, 129, 882–888. [Google Scholar] [CrossRef]


| Amendments | Total Organic C g kg−1 d.m. | Total N g kg−1 d.m. | C/N | Dry Matter g kg−1 Fresh Matter |
|---|---|---|---|---|
| 2013 | ||||
| BMan | 31.5 | 2.50 | 12.6 | 400 |
| OPCmix | 36.7 | 2.18 | 16.8 | 760 |
| OPC | 38.3 | 1.25 | 31 | 740 |
| BWCom | 26.4 | 1.65 | 16 | 890 |
| 2014 | ||||
| BMan | 33.8 | 1.54 | 21.9 | 212 |
| OPCmix | 24.7 | 1.75 | 14.1 | 840 |
| OPC | 38.3 | 1.26 | 30.4 | 835 |
| BWCom | 21.2 | 1.57 | 13.5 | 863 |
| Soil Organic Amendment | Dry Matter (Mg ha−1) | TOC (Mg ha−1) | N Total (kg ha−1) |
|---|---|---|---|
| BMan 15 | 4.4 | 1.5 | 100 |
| BMan 30 | 8.8 | 3 | 199 |
| BWCom 15 | 13 | 3.1 | 211 |
| BWCom 30 | 26 | 6.2 | 423 |
| OPCmix 15 | 12 | 3.6 | 234 |
| OPCmix 30 | 24 | 7.3 | 469 |
| OPC 15 | 12 | 4.5 | 148 |
| OPC 30 | 24 | 9 | 297 |
| Soil Tillage | Organic Fertilization | Crop Sequence | Seeding | Number of Cuts and Last Harvest Time | |
|---|---|---|---|---|---|
| 2013–2014 | Rotavator to bury amendments along the 0–30 cm soil layer | Compost and manure distribution on 28 April 2013 | Rocket (Diplotaxis tenuifolia), cv. Reset | 05/02/13 | (3) 06/17/13 |
| Before each short cycle: Rotavator cultivation at 20 cm + raised seedbed preparation | Rocket (not monitored) | July | |||
| Basil (Ocimum basilicum), cv. Compatto | 08/28/13 | (2) 10/10/13 | |||
| Before each long cycle: Chisel cultivation at 50 cm depth + rotavator cultivation at 20 cm + raised seedbed preparation | Rocket (D. tenuifolia), cv. Winter | 11/7/13 | (7) 05/08/14 | ||
| 2014–2015 | Compost and manure distribution on 19 June 2014 | Rocket (D. tenuifolia), cv. Reset | 07/31/14 | (2) 08/27/14 | |
| Tillage as above | Rocket (D. tenuifolia), cv. Winter | 11/04/14 | (5) 04/01/15 |
| Treatments | Initial SOC Amount | Total C Input by Compost/Manure | Total C Input by Crop Residues | Final SOC Amount |
|---|---|---|---|---|
| (Mg ha−1) | (Mg ha−1) | (Mg ha−1) | (Mg ha−1) | |
| BWCom 15 | 21.6 | 6.2 | 5.1 | 28.1 (±1.11) a |
| BWCom 30 | 21.6 | 12.5 | 5.1 | 27.8 (±0.35) a |
| OPC 15 | 21.6 | 9.1 | 5.2 | 26.8 (±1.30) a |
| OPC 30 | 21.6 | 18.1 | 5.1 | 27.4 (±0.21) a |
| OPCmix 15 | 21.6 | 7.3 | 4.9 | 25.7 (±0.12) ab |
| OPCmix 30 | 21.6 | 14.6 | 4.9 | 27.1 (±0.62) a |
| BMan 15 | 21.6 | 3 | 5.2 | 26.4 (±0.13) a |
| BMan 30 | 21.6 | 5.9 | 5.1 | 22.8 (±0.58) b |
| Source of Variation | SOC Change | SOC Losses | C Conversion Efficiency | C Sequestr. Rate | |
|---|---|---|---|---|---|
| (Mg C ha−1) | (Mg C ha−1) | (%) | (Mg C ha−1 y−1) | ||
| Organic Amendment (OA) | |||||
| BWCom | 6.3 | −8.0 a | 47 | 3.2 | |
| OPC | 5.5 | −13.2 b | 35 | 2.7 | |
| OPCmix | 4.8 | −11.0 b | 27 | 2.4 | |
| BMan | 3.0 | −6.5 a | 35 | 1.5 | |
| p | n.s. | *** | n.s. | n.s. | |
| Dose (D) | |||||
| 15 | 5.1 | −6.2 | 47 | 2.6 | |
| 30 | 4.7 | −13.0 | 25 | 2.3 | |
| p | n.s. | *** | *** | n.s. | |
| OA × D | p | ** | n.s. | *** | * |
| Source | Fresh Marketable Yield | Dry Biomass Yield | Nitrate Content |
|---|---|---|---|
| (Mg ha−1) | (mg kg−1 f. m.) | ||
| Organic amendment (OA) | |||
| BWCom | 44.6 a | 4.3 a | 4408 a |
| OPC | 35.3 b | 3.7 b | 2681 bc |
| OPCmix | 37.9 b | 3.7 b | 2251 c |
| BMan | 43.4 a | 4.1 ab | 3156 b |
| p | *** | *** | *** |
| Dose | |||
| 15 | 40.0 | 4.35 | 3466 |
| 30 | 40.6 | 4.28 | 2643 |
| p | n.s. | n.s. | *** |
| Time of cut | |||
| 9 January 2015 | 4211 a | ||
| 2 February 2015 | 4532 a | ||
| 13 March 2015 | 3293 a | ||
| 2 April 2015 | 1335 b | ||
| p | *** | ||
| OA × Dose | n.s. | * | n.s. |
| OA × Time of cut | *** | ||
| Dose × Time of cut | * | ||
| OA × Dose × Time of cut | n.s. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picariello, E.; Fornasier, F.; Bilotto, M.; Mignoli, E.; Baiano, S.; Morra, L. Eco-Sustainability of Soils in Baby-Leaf Crop Systems under Tunnel through the Application of C-Rich Inputs: Towards Combating Soil Degradation. Horticulturae 2024, 10, 476. https://doi.org/10.3390/horticulturae10050476
Picariello E, Fornasier F, Bilotto M, Mignoli E, Baiano S, Morra L. Eco-Sustainability of Soils in Baby-Leaf Crop Systems under Tunnel through the Application of C-Rich Inputs: Towards Combating Soil Degradation. Horticulturae. 2024; 10(5):476. https://doi.org/10.3390/horticulturae10050476
Chicago/Turabian StylePicariello, Enrica, Flavio Fornasier, Maurizio Bilotto, Emiliana Mignoli, Salvatore Baiano, and Luigi Morra. 2024. "Eco-Sustainability of Soils in Baby-Leaf Crop Systems under Tunnel through the Application of C-Rich Inputs: Towards Combating Soil Degradation" Horticulturae 10, no. 5: 476. https://doi.org/10.3390/horticulturae10050476
APA StylePicariello, E., Fornasier, F., Bilotto, M., Mignoli, E., Baiano, S., & Morra, L. (2024). Eco-Sustainability of Soils in Baby-Leaf Crop Systems under Tunnel through the Application of C-Rich Inputs: Towards Combating Soil Degradation. Horticulturae, 10(5), 476. https://doi.org/10.3390/horticulturae10050476






