Potential Role of the Yeast Papiliotrema terrestris Strain PT22AV in the Management of the Root-Knot Nematode Meloidogyne incognita
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Vitro Bioassay
2.3. Experiment in Pot
2.4. Experiments in Greenhouse
2.5. Statistical Analysis
3. Results
3.1. In Vitro Bioassay
3.2. Experiment in Pots
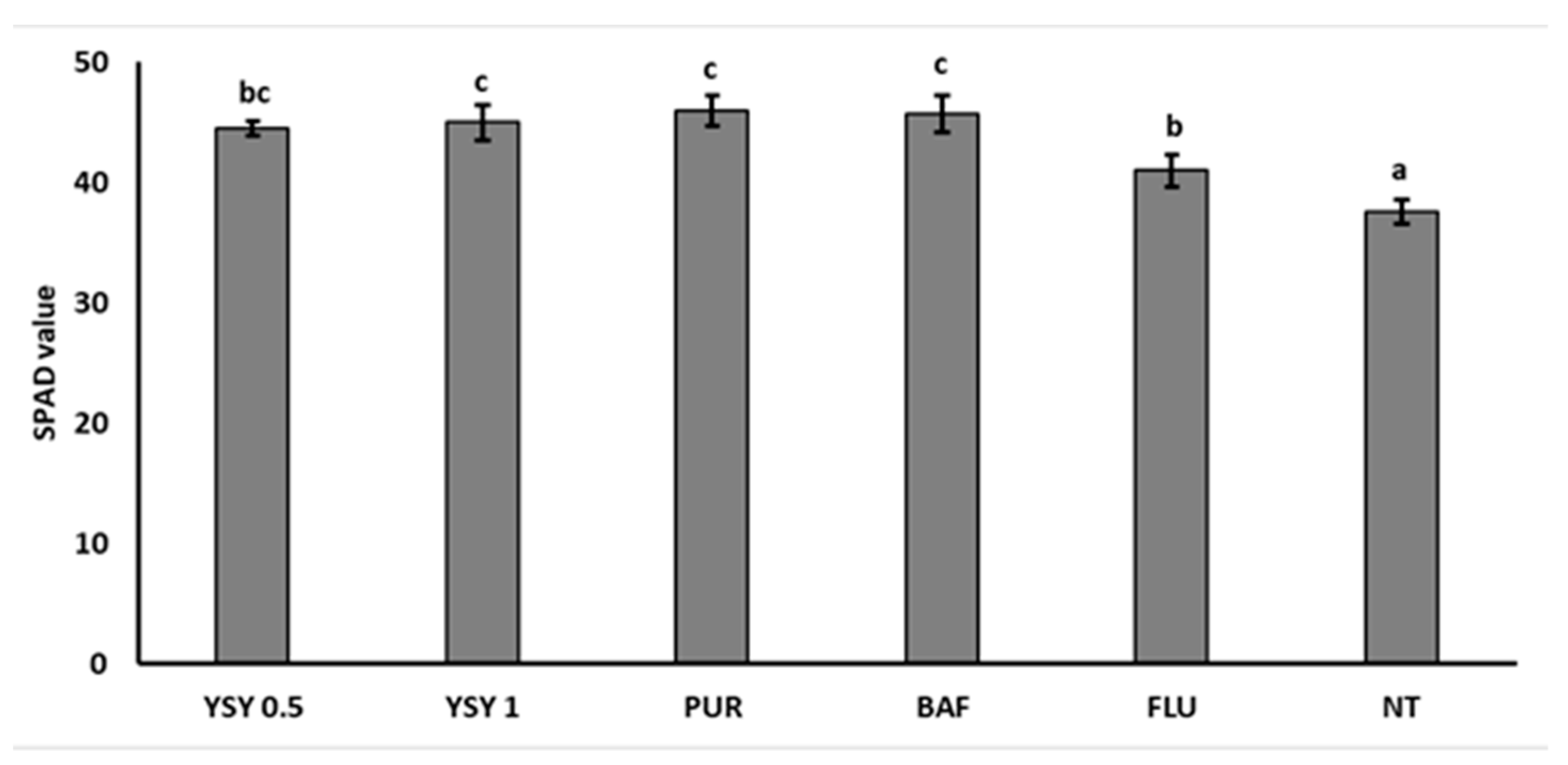
3.3. Greenhouse Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perry, R.N.; Moens, M. Introduction to plant-parasitic nematodes; modes of parasitism. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 3–20. [Google Scholar] [CrossRef]
- Palomares-Rius, J.E.; Escobar, C.; Cabrera, J.; Vovlas, A.; Castillo, P. Anatomical alterations in plant tissues induced by plant-parasitic nematodes. Front. Plant Sci. 2017, 8, 288614. [Google Scholar] [CrossRef] [PubMed]
- Ragozzino, A.; d’Errico, G. Interactions between nematodes and fungi: A concise review. Redia 2011, 94, 123–125. [Google Scholar]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-Lopez, R.; Palomares-Rius, S.J.E.; Wesemael, W.M.L.; et al. Top ten plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) of 25 May 2011 implementing Regulation (EC) No1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. No. 540/2011. Off. J. Eur. Union 2011, L153, 1–186.
- Stirling, G.R. Biological control of plant-parasitic nematodes. In Diseases of Nematodes; Poinar, G.O., Jansson, H.B., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 103–150. [Google Scholar] [CrossRef]
- Timper, P. Utilization of biological control for managing plant-parasitic nematodes. In Biological Control of Plant-Parasitic Nematodes: Building Coherence between Microbial Ecology and Molecular Mechanisms; Davies, K., Spiegel, Y., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 259–289. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Xiang, N.; Lawrence, K.S.; Donald, P.A. Biological control potential of plant growth-promoting rhizobacteria suppression of Meloidogyne incognita on cotton and Heterodera glycines on soybean: A review. J. Phytopathol. 2018, 166, 449–458. [Google Scholar] [CrossRef]
- Schouten, A. Mechanisms involved in nematode control by endophytic fungi. Annu. Rev. Phytopathol. 2016, 4, 121–142. [Google Scholar] [CrossRef]
- Botha, A. The importance and ecology of yeasts in soil. Soil Biol. Biochem. 2011, 43, 1–8. [Google Scholar] [CrossRef]
- Cloete, K.J.; Valentine, A.J.; Stander, M.A.; Blomerus, L.M.; Botha, A. Evidence of symbiosis between the soil yeast Cryptococcus laurentii and a sclerophyllous medicinal shrub, Agathosma betulina (Berg.) Pillans. Microb. Ecol. 2009, 57, 624–632. [Google Scholar] [CrossRef]
- El-Tarabily, K.A. Suppression of Rhizoctonia solani diseases of sugar beet by antagonistic and plant growth-promoting yeasts. J. Appl. Microbiol. 2004, 96, 69–75. [Google Scholar] [CrossRef]
- Abokorah, M.S.; Fathalla, A.M. The nematicidal efficacy of fulvic acid, yeast fungus (Saccharomyces cerevisiae) and L-tryptophan on plant parasitic nematodes, growth, and yield of banana plants. Egypt. J. Crop Prot. 2022, 17, 27–37. [Google Scholar] [CrossRef]
- Hamouda, R.; Al-Saman, M.; El-Ansary, M. Effect of Saccharomyces cerevisiae and Spirulina platensis on suppressing root-knot nematode, Meloidogyne incognita infecting banana plants under greenhouse conditions. Egypt. J. Agronematol. 2019, 18, 90–102. [Google Scholar] [CrossRef]
- Karajeh, M.R. Efficacy of Saccharomyces cerevisiae on controlling the root-knot nematode (Meloidogyne javanica) infection and promoting cucumber growth and yield under laboratory and field conditions. Arch. Phytopathol. Plant Prot. 2013, 46, 2492–2500. [Google Scholar] [CrossRef]
- Sampaio, J.P.; Weiß, M.; Gadanho, M.; Bauer, R. New taxa in the Tremellales: Bulleribasidium oberjochense gen. Et sp. Nov., Papiliotrema bandonii gen. Et sp. Nov. And Fibulobasidium murrhardtense sp. Nov. Mycologia 2002, 94, 873–887. [Google Scholar] [CrossRef]
- Vadkertiova, R.; Dudasova, H.; Stratilova, H.; Balaskakova, M. Diversity of yeasts in the soil adjacent to fruit trees of the Rosaceae family. Yeast 2019, 36, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Crestani, J.; Fontes Landell, M.; Faganello, J.; Henning Vainstein, M.; Simpson Vishniac, H.; Valente, P. Cryptococcus terrestris sp. Nov., a tremellaceous, anamorphic yeast phylogenetically related to Cryptococcus flavescens. Int. J. Syst. Evol. Micr. 2009, 59, 631–636. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palmieri, D.; Ianiri, G.; Conte, T.; Castoria, R.; Lima, G.; De Curtis, F. Influence of biocontrol and integrated strategies and treatment timing on plum brown rot incidence and fungicide residues in fruits. Agriculture 2022, 12, 1656. [Google Scholar] [CrossRef]
- d’Errico, G.; Crescenzi, A.; Landi, S. First report of the southern root-knot nematode Meloidogyne incognita on the invasive weed Araujia sericifera in Italy. Plant Dis. 2014, 98, 1593. [Google Scholar] [CrossRef]
- Hussey, R.S.; Barker, K.R. A comparison of methods of collecting inocula of Meloidogyne spp. Including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- d’Errico, G.; Giacometti, R.; Roversi, P.F.; d’Errico, F.P.; Woo, S.L. Mode of action and efficacy of iprodione against the root-knot nematode Meloidogyne incognita. Ann. Appl. Biol. 2017, 171, 506–510. [Google Scholar] [CrossRef]
- Finney, D.J. Statistical Method in Biological Assay, 3rd ed.; Charles Griffin & Company Ltd.: High Wycombe, UK, 1978; pp. 1–508. [Google Scholar]
- Sasanelli, N. Tables of nematode-pathogenicity. Nematol. Medit. 1994, 22, 153–157. [Google Scholar]
- Zeck, W.M. A rating scheme for field evaluation of root-knot infestations. Pflanzenschutz-Nachrichten 1971, 24, 141–144. [Google Scholar]
- van Bezooijen, J. Methods and Techniques for Nematology; Wageningen University: Wageningen, The Netherlands, 2006; pp. 1–112. [Google Scholar]
- El-Sagheer, A.; El-Mesalamy, A.; Anany, A.M.; Mahmoud, N. Nematicidal properties of some yeast culture filtrates against Meloidogyne javanica infecting squash plants (in vitro and in vivo). Pak. J. Nematol. 2021, 39, 111–121. [Google Scholar] [CrossRef]
- Mioranza, T.M.; Schwan-Estrada, K.R.F.; Zubek, L.; Miamoto, A.; Hernandes, I.; Rissato, B.B.; Mizuno, M.S.; Schwan, R.F.; Dias-Arieira, C.R. Effects of yeast fermentation broths on the Meloidogyne incognita population in soybean. Trop. Plant Pathol. 2020, 45, 112–121. [Google Scholar] [CrossRef]
- Jothi, G.; Poornima, K.; Prasad, G. In vitro studies of antagonistic yeast against Meloidogyne incognita. Ann. Plant Prot. Sci 2019, 27, 146–151. [Google Scholar] [CrossRef]
- Vyshali, G.; Jothi, G.; Poornima, K. Effect of antigonistic yeast on penetration and biology of Meloidogyne incognita in tomato. Ann. Plant Prot. Sci. 2019, 27, 390–393. [Google Scholar] [CrossRef]
- Hashem, M.; Omran, Y.A.; Sallam, N.M. Efficacy of yeasts in the management of root-knot nematode Meloidogyne incognita, in flame seedless grape vines and the consequent effect on the productivity of the vines. Biocontr. Sci. Technol. 2008, 18, 357–375. [Google Scholar] [CrossRef]
- Shawky, S.M.; El-Shennawy, R.Z.; Shady, A.M. Biological control of Meloidogyne javanica on tomato plants with some isolated bioagents in Egypt. J. Plant Prot. Pathol. 2006, 31, 6049–6063. [Google Scholar] [CrossRef]
- Hashem, M.; Abo-Elyousr, K.A. Management of the root-knot nematode Meloidogyne incognita on tomato with combinations of different biocontrol organisms. Crop Prot. 2011, 30, 285–292. [Google Scholar] [CrossRef]
- Fialho, M.B.; Bessi, R.; Inomoto, M.M.; Pascholati, S.F. Nematicidal effect of volatile organic compounds (VOCs) on the plant-parasitic nematode Meloidogyne javanica. Summa Phytopathol. 2012, 38, 152–154. [Google Scholar] [CrossRef][Green Version]
- Osman, H.A.; Ameen, H.H.; Mohamed, M.; Elkelany, U.S. Efficacy of integrated microorganisms in controlling root-knot nematode Meloidogyne javanica infecting peanut plants under field conditions. Bull. Nat. Res. Cent. 2020, 44, 134. [Google Scholar] [CrossRef]
- El-Nuby, A.S.M. Effect of some amino acids and yeast on root-knot disease on tomato plants. Egypt. J. Agronematol. 2021, 20, 17–33. [Google Scholar] [CrossRef]
- Cabrera, J.A.; Menjivar, R.D.; Dababat, A.E.F.A.; Sikora, R.A. Properties and nematicide performance of avermectins. J. Phytopathol. 2013, 161, 65–69. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Yang, L.; Zhang, T.; Jin, Y.; Liu, L.; Du, J.; Zhang, D.; Li, B.; Gao, C.; et al. The effect of abamectin application in combination with agronomic measures on the control efficacy of cucumber root-knot nematodes and the cucumber yield. Pest Manag. Sci. 2023, 79, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- d‘Errico, G.; Marra, R.; Vinale, F.; Landi, S.; Roversi, P.F.; Woo, S.L. Nematicidal efficacy of new abamectin-based products used alone and in combination with indolebutyric acid against the root-knot nematode Meloidogyne incognita. Redia 2017, 100, 95–101. [Google Scholar] [CrossRef]
- Qiao, K.; Liu, X.; Wang, H.; Xia, X.; Ji, X.; Wang, K. Effect of abamectin on root-knot nematodes and tomato yield. Pest Manag. Sci. 2012, 68, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Mukhtar, T.; Ahmed, R.; Ahmad, T.; Iqbal, M.A. Suppression of Meloidogyne javanica infection in peach (Prunus persica (L.) Batsch) using fungal biocontrol agents. Sustainability 2023, 15, 13833. [Google Scholar] [CrossRef]
- Santos, C.H.B.; De Andrade, L.A.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C.C. Purpureocillium lilacinum for biocontrol, bioremediation and biofertilization. Preprints 2023, 2023051926. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, R.K.; Goswami, B.K. Bio-control activity of Purpureocillium lilacinum strains in managing root-knot disease of tomato caused by Meloidogyne incognita. Biocontr. Sci. Technol. 2013, 23, 1469–1489. [Google Scholar] [CrossRef]
- Ghahremani, Z.; Escudero, N.; Beltrán-Anadón, D.; Saus, E.; Cunquero, M.; Andilla, J.; Loza-Alvarez, P.; Gabaldón, T.; Javier Sorribas, F. Bacillus firmus Strain I-1582, a nematode antagonist by itself and through the plant. Front. Plant Sci. 2020, 11, 796. [Google Scholar] [CrossRef]
- d‘Errico, G.; Marra, R.; Crescenzi, A.; Davino, S.W.; Fanigliulo, A.; Woo, S.L.; Lorito, M. Integrated management strategies of Meloidogyne incognita and Pseudopyrenochaeta lycopersici on tomato using a Bacillus firmus-based product and two synthetic nematicides in two consecutive crop cycles in greenhouse. Crop Prot. 2019, 122, 159–164. [Google Scholar] [CrossRef]
- Giannakou, I.O.; Karpouzas, D.G.; Prophetou-Athanasiadou, D. A novel non-chemical nematicide for the control of root-knot nematodes. Appl. Soil. Ecol. 2004, 26, 69–79. [Google Scholar] [CrossRef]
- Giffard, B.; Winter, S.; Guidoni, S.; Nicolai, A.; Castaldini, M.; Cluzeau, D.; Coll, P.; Cortet, J.; LeCadre, E.; d’Errico, G.; et al. Vineyard management and its impacts on soil biodiversity, functions, and ecosystem services. Front. Ecol. Evol. 2022, 10, 850272. [Google Scholar] [CrossRef]
- Lombardi, N.; Salzano, A.M.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Caira, S.; Lorito, M.; d’Errico, G.; et al. Effect of Trichoderma bioactive metabolite treatments on the production, quality, and protein profile of strawberry fruits. J. Agric. Food Chem. 2020, 68, 7246–7258. [Google Scholar] [CrossRef] [PubMed]
- Marra, R.; Coppola, M.; Pironti, A.; Grasso, F.; Lombardi, N.; d’Errico, G.; Sicari, A.; Bolletti Censi, S.; Woo, S.L.; Rao, R.; et al. The application of Trichoderma strains or metabolites alters the olive leaf metabolome and the expression of defense-related genes. J. Fungi 2020, 6, 369. [Google Scholar] [CrossRef]
- D’Addabbo, T.; Laquale, S.; Perniola, M.; Candido, V. Biostimulants for plant growth promotion and sustainable management of phytoparasitic nematodes in vegetable crops. Agronomy 2019, 9, 616. [Google Scholar] [CrossRef]
- Jones, J.G.; Kleczewski, N.M.; Desaeger, J.; Meyer, S.L.; Johnson, G.C. Evaluation of nematicides for southern root-knot nematode management in lima bean. Crop Prot. 2017, 96, 151–157. [Google Scholar] [CrossRef]
- Faske, T.R.; Hurd, K. Sensitivity of Meloidogyne incognita and Rotylenchulus reniformis to Fluopyram. J. Nematol. 2015, 47, 316–321. [Google Scholar] [PubMed]
- Labancová, E.; Šípošová, K.; Kučerová, D.; Horváthová, Á.; Schusterová, H.; Vivodová, Z.; Vadkertiová, R.; Kollárová, K. The Tremellaceous Yeast: Papiliotrema terrestris–As the Growth Stimulant of Maize Plants. J. Plant Growth Regul. 2023, 42, 3835–3850. [Google Scholar] [CrossRef]
- Ignatova, L.V.; Brazhnikova, Y.V.; Berzhanova, R.Z.; Mukasheva, T.D. Plant growth-promoting and antifungal activity of yeasts from dark chestnut soil. Microbiol. Res. 2015, 175, 78–83. [Google Scholar] [CrossRef]
- Kozacki, D.; Soika, G.; Skwiercz, A.; Malusà, E. Microbial-based products and soil management practices to control nematodes in organic horticultural crops. In Sustainable Management of Nematodes in Agriculture, Vol. 2: Role of Microbes-Assisted Strategies; Chaudhary, K.K., Meghvansi, M.K., Siddiqui, S., Eds.; Springer International Publishing: Cham, Switzerland, 2024; Volume 19, pp. 3–31. [Google Scholar] [CrossRef]


| Treatment | Dose | Eggs and J2 100 mL−1 Soil | Root Gall Infestation (0–10) | Plant Height (cm) | Plant Top Weight (g) | Plant Root Weight (g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| YSY | 0.5 1 | 137 ± 7.2 | a | 6.1 ± 0.5 | a | 69.2 ± 1.9 | b | 56.2 ± 3.3 | a | 8.1 ± 0.1 | b |
| YSY | 1 1 | 111 ± 8.4 | b | 4.8 ± 0.5 | b | 78.8 ± 2.0 | c | 77.7 ± 2.4 | b | 8.3 ± 0.2 | b |
| PUR | 0.75 2 | 109 ± 6.9 | b | 4.4 ± 0.4 | b | 75.5 ± 1.1 | bc | 75.4 ± 1.5 | b | 8.4 ± 0.1 | b |
| FLU | 0.625 2 | 72 ± 7.6 | c | 2.7 ± 0.1 | c | 76.9 ± 1.2 | c | 74.4 ± 3.9 | b | 8.5 ± 0.2 | b |
| NT | - | 153 ± 7.8 | a | 6.9 ± 0.4 | a | 61.3 ± 3.9 | a | 53.2 ± 2.8 | a | 7.3 ± 0.3 | a |
| Treatment | Dose | Eggs and J2 100 mL−1 Soil | Reproduction Rate (Pf/Pi) | Root Gall Infestation (0–10) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Initial (Pi) (11/08/22) | Final (Pf) (17/10/22) | ||||||||
| YSY | 0.5 1 | 87 ± 2.5 | a | 204 ± 4.9 | c | 2.3 ± 0.1 | cd | 2.5 ± 0.3 | cd |
| YSY | 1 1 | 86 ± 1.4 | a | 164 ± 6.2 | d | 1.9 ± 0.1 | e | 1.9 ± 0.2 | d |
| ERG | 6 + 3 2 | 89 ± 4.3 | a | 230 ± 18.7 | bc | 2.6 ± 0.1 | bc | 3.2 ± 0.5 | bc |
| FLU | 0.625 2 | 84 ± 1.8 | a | 162 ± 6.8 | d | 1.9 ± 0.1 | de | 1.9 ± 0.1 | d |
| NT | - | 87 ± 4.8 | a | 368 ± 20.0 | a | 4.2 ± 0.3 | a | 5.2 ± 0.3 | a |
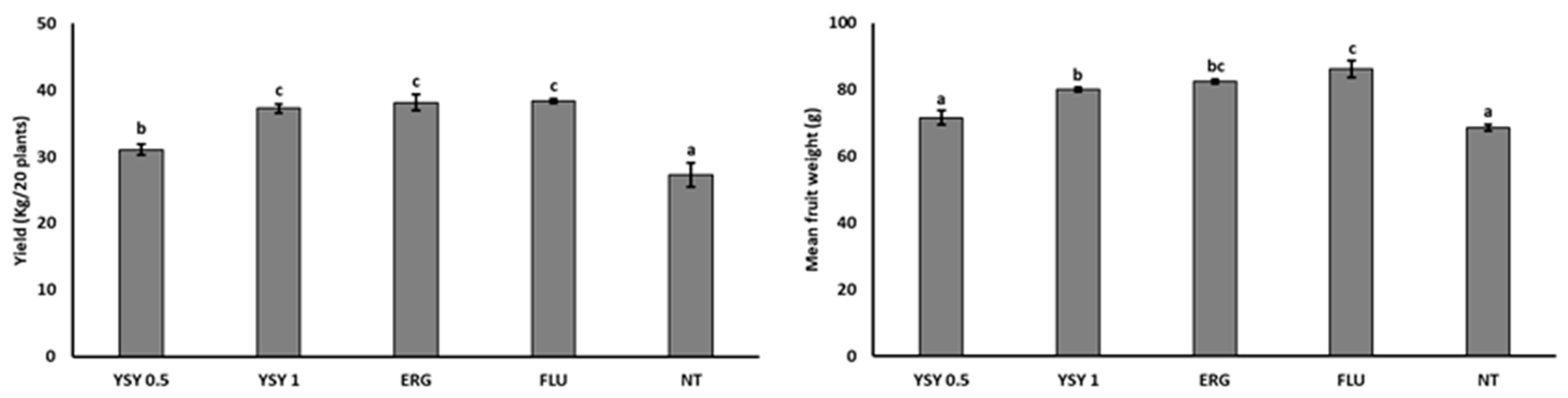
| Treatment | Dose | Yield of 20 Sample Plants | Mean Fruit Weight (g) | ||||
|---|---|---|---|---|---|---|---|
| Weight (kg) | No of Fruits | ||||||
| YSY | 0.5 1 | 15.8 ± 0.8 | ab | 69.0 ± 2.5 | b | 21.1 ± 0.5 | ab |
| YSY | 1 1 | 18.0 ± 0.7 | cd | 71.8 ± 2.7 | bc | 22.9 ± 0.6 | c |
| PUR | 0.75 2 | 19.1 ± 0.8 | d | 76.5 ± 3.1 | c | 24.1 ± 0.4 | cd |
| BAF | 40 1 | 19.3 ± 0.3 | d | 77.5 ± 1.3 | c | 26.4 ± 0.1 | e |
| FLU | 0.625 2 | 16.5 ± 0.4 | bc | 66.0 ± 1.6 | b | 21.4 ± 0.6 | b |
| NT | - | 14.6 ± 0.6 | a | 58.5 ± 2.2 | a | 20.0 ± 0.3 | a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Addabbo, T.; Landi, S.; Palmieri, D.; Piscitelli, L.; Caprio, E.; Esposito, V.; d’Errico, G. Potential Role of the Yeast Papiliotrema terrestris Strain PT22AV in the Management of the Root-Knot Nematode Meloidogyne incognita. Horticulturae 2024, 10, 472. https://doi.org/10.3390/horticulturae10050472
D’Addabbo T, Landi S, Palmieri D, Piscitelli L, Caprio E, Esposito V, d’Errico G. Potential Role of the Yeast Papiliotrema terrestris Strain PT22AV in the Management of the Root-Knot Nematode Meloidogyne incognita. Horticulturae. 2024; 10(5):472. https://doi.org/10.3390/horticulturae10050472
Chicago/Turabian StyleD’Addabbo, Trifone, Silvia Landi, Davide Palmieri, Lea Piscitelli, Elena Caprio, Vincenzo Esposito, and Giada d’Errico. 2024. "Potential Role of the Yeast Papiliotrema terrestris Strain PT22AV in the Management of the Root-Knot Nematode Meloidogyne incognita" Horticulturae 10, no. 5: 472. https://doi.org/10.3390/horticulturae10050472
APA StyleD’Addabbo, T., Landi, S., Palmieri, D., Piscitelli, L., Caprio, E., Esposito, V., & d’Errico, G. (2024). Potential Role of the Yeast Papiliotrema terrestris Strain PT22AV in the Management of the Root-Knot Nematode Meloidogyne incognita. Horticulturae, 10(5), 472. https://doi.org/10.3390/horticulturae10050472







