Kaempferia sakolchaii sp. nov. and K. phuphanensis var. viridans var. nov. (Zingiberaceae), Two New Taxa from Northeastern Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Experimental Site
2.2. Taxonomic Parameters Analysis
3. Results
3.1. Kaempferia sakolchaii P. Saensouk, Saensouk & Boonma sp. Nov. (Figure 2 and Figure 3), Subgenus Kaempferia
- Type: Thailand, Northeastern, Sakon Nakhon Province, Saensouk Kaemp. 2, 12 July 2020 (holo KKU!, iso BK!, FOF!)
- Vernacular name: Proh Ajarn Sumon.
- Etymology: The specific epithet “sakolchaii” was bestowed in honor of Professor Emeritus Dr. Sumon Sakolchai, who has a high level of knowledge and skill in the field of pharmacy. Furthermore, he has made significant contributions to the field of pharmaceutical botany, both in Thailand and across the world. In addition, he makes essential contributions as a behind-the-scenes person, providing consistent assistance to researchers in botany and pharmaceutical sciences. Previously, he was the President of Khon Kaen University in Thailand, the President of the Pharmacy Council of Thailand, and currently acts as the President of Burapha University Council in Thailand.
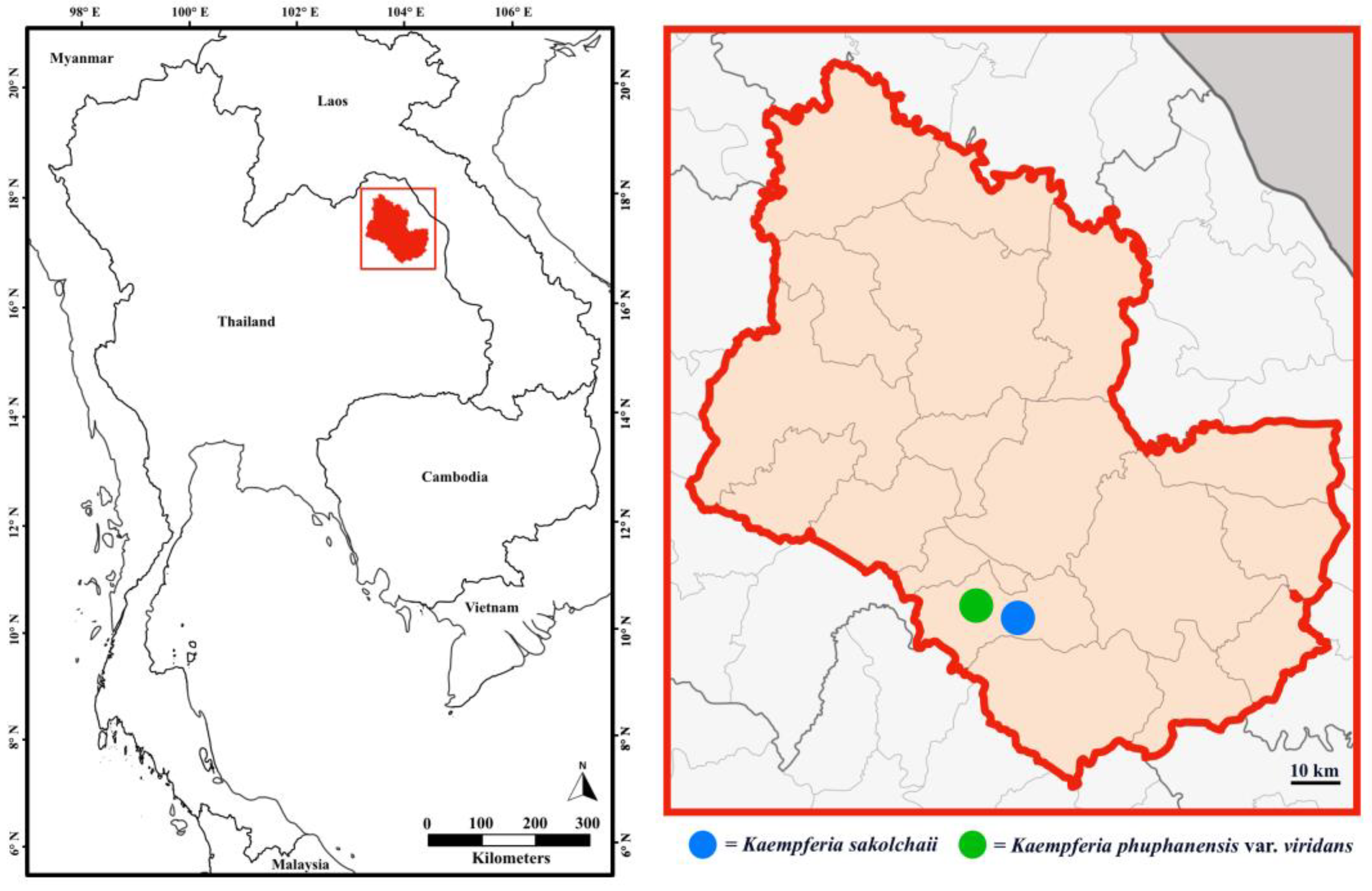
- Distribution: Endemic to Thailand; northeastern, Sakon Nakhon Province (Figure 1).
- Ecology: Found in sandy loam soil mixed with rocks, in dipterocarp and deciduous forests, at an altitude ranging from 300 to 400 m above sea level.
- Phenology: Flowering from late May to July; anthesis in the morning.
- Utilization: This beautiful plant is utilized for ornamental purposes.
- Conservation status: This newly discovered species is located exclusively in its type locality, with limited information available regarding its distribution range. The species potentially exists in nearby unexplored areas. Presently classified as Data Deficient (DD) following the IUCN guidelines of 2022 [38], we advise considering it as an endangered taxon unless additional evidence indicates otherwise.
| 1a. Shoot usually with a solitary leaf, both at anthesis and after anthesis | 2 |
| 1b. Shoot sometimes with a solitary leaf at anthesis, two or more leaves after anthesis | 8 |
| 2a. Labellum and staminodes in the same plane | K. picheansoonthonii |
| 2b. Labellum and staminodes not in the same plane | 3 |
| 3a. Anther crest and ovary white without reddish dots | 4 |
| 3b. Anther crest and ovary white with reddish dots | 7 |
| 4a. Lamina adaxially with light grey markings | K. pseudoparviflora |
| 4b. Lamina adaxially without markings | 5 |
| 5a. Epigynous glands 4 mm long | K. unifolia |
| 5b. Epigynous glands 8 mm long | 6 |
| 6a. Anther crest bilobed, each lobe apex acute | K. isanensis |
| 6b. Anther crest bilobed, each lobe apex rounded | K. gigantiphylla |
| 7a. Lamina adaxially plain dark green | K. siamensis |
| 7b. Lamina adaxially dark green with silver or white longitudinal stripes | K. sakolchaii |
| 8a. Rhizome dark purple; labellum and staminodes in the same plane; stigma white | K. parviflora |
| 8b. Rhizome yellowish; labellum and staminodes not in the same plane; stigma red | K. elegans |

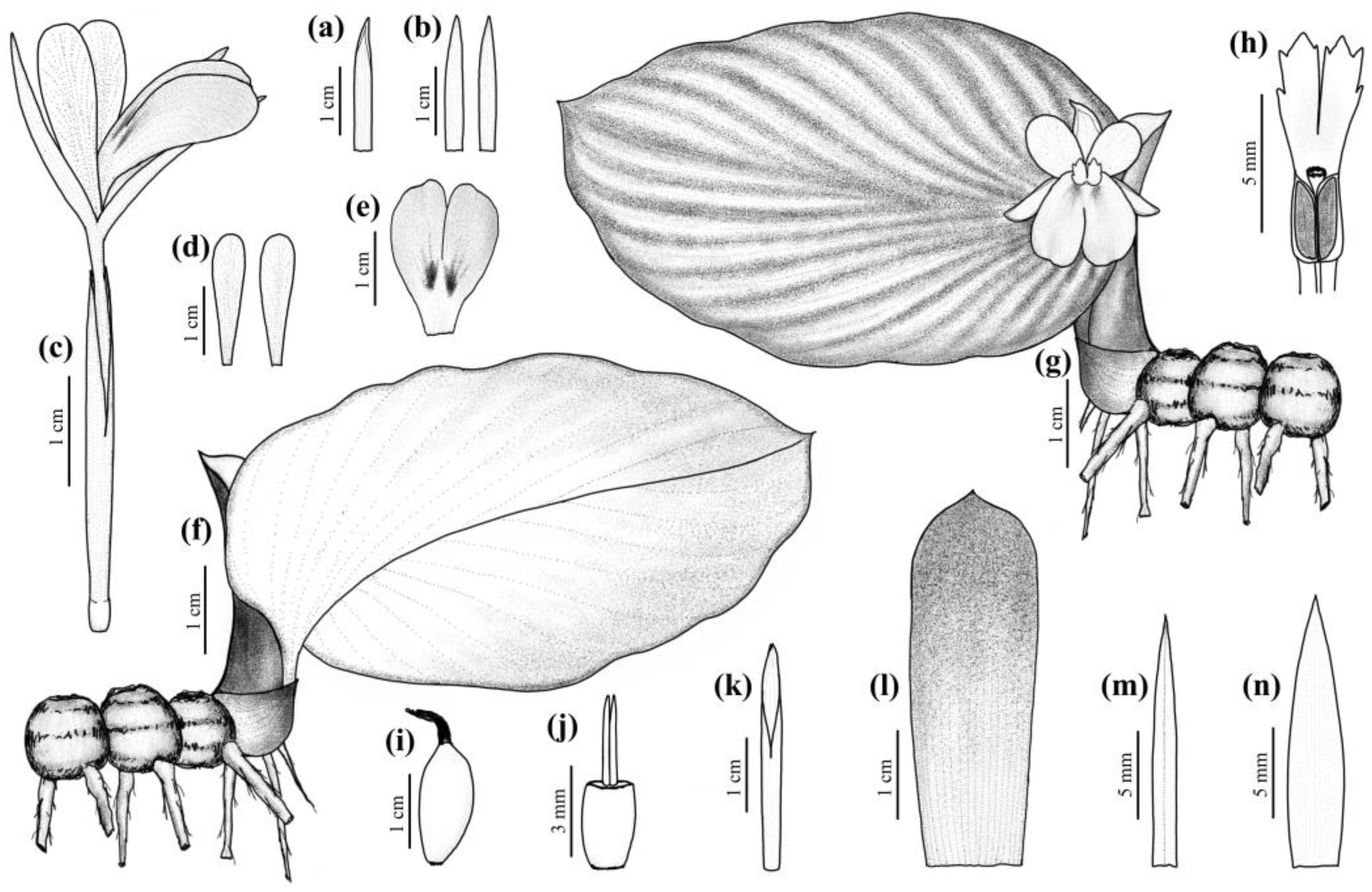
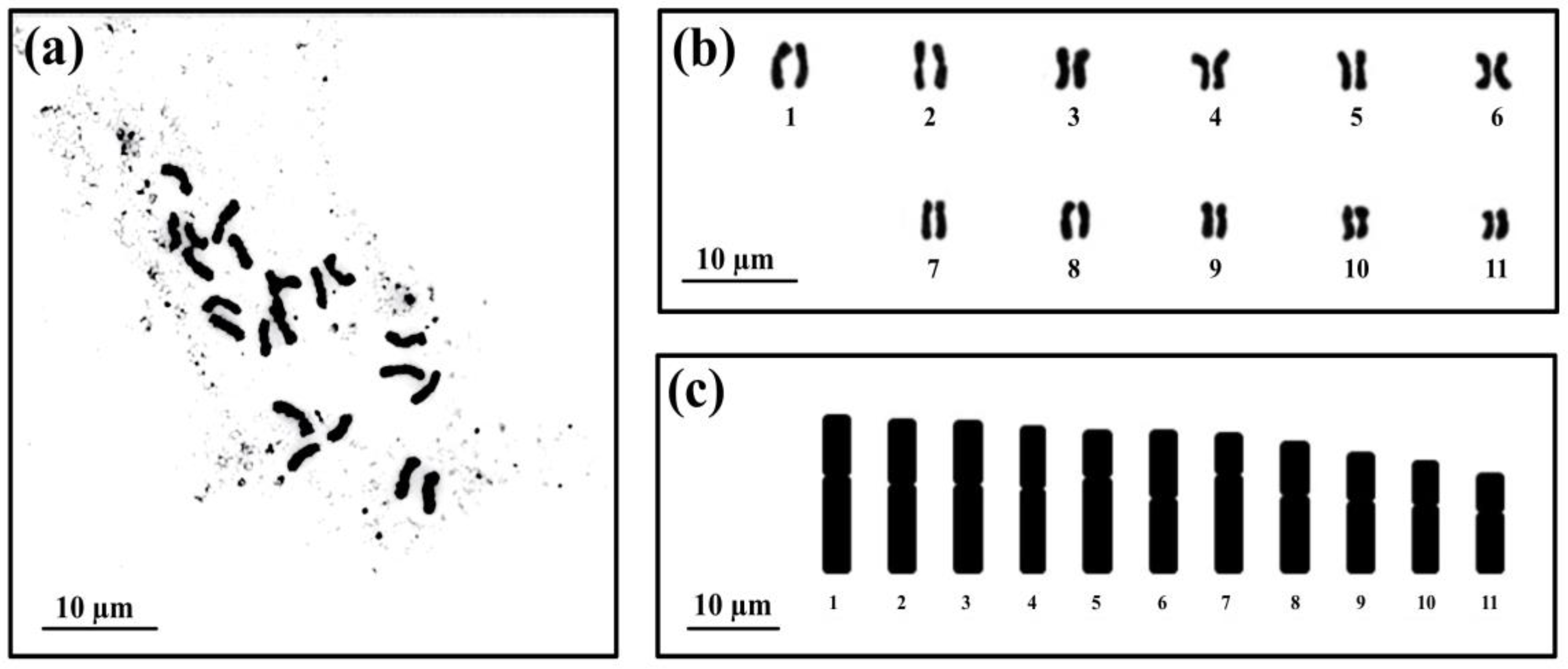
3.1.1. Chromosomes and Karyotype Formula of Kaempferia sakolchaii
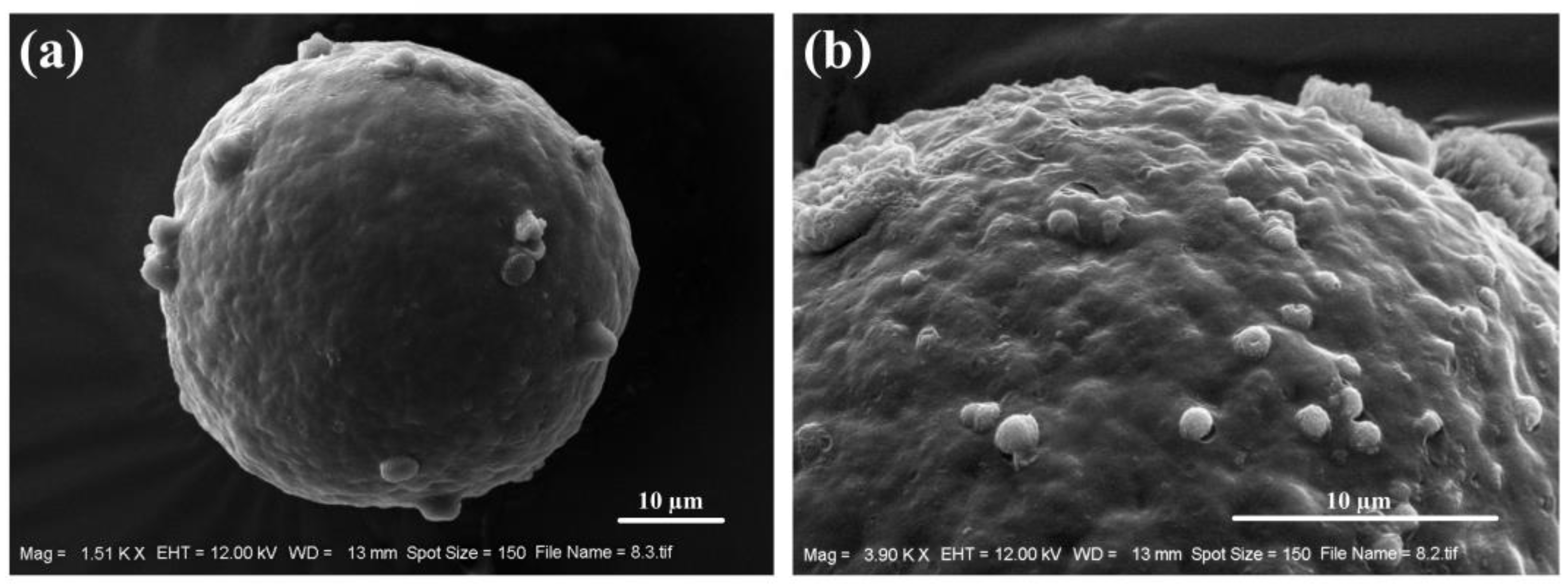
3.1.2. Palynology of Kaempferia sakolchaii (Figure 5)
3.2. Kaempferia phuphanensis var. viridans P. Saensouk, Saensouk & Boonma var. nov. (Figure 6, Figure 7, Figure 8 and Figure 9), Subgenus Kaempferia
- Type: Thailand, Sakon Nakhon Province, Phu Phan District, Saensouk Kaemp. 01, 10 June 2022 (holo KKU!, iso BK!, FOF!)
- Vernacular name: Proh Tamu-Tami Bai Keaw.
- Etymology: The specific epithet ‘viridans’ in the variety name ‘Kaempferia phuphanensis var. viridans’ is derived from the Latin term signifying ‘green’ or ‘of a green color’. The distinguishing feature of this new variety lies in its uniform greenness, which lacks the reddish tinge and red margin observed in the typical variety, K. phuphanensis.
- Distribution: Endemic to Thailand; northeastern, Sakon Nakhon Province (Figure 1).
- Ecology: Found in sandy loam soil mixed with rocks in semi-open deciduous forest areas, at an altitude ranging from 300 to 400 m above sea level.
- Phenology: Flowering from June to September; anthesis in the morning.
- Utilization: This beautiful plant is utilized for ornamental purposes.
- Conservation status: After the identification and publishing of the typical species of Kaempferia phuphanensis in 2019 [30], the green variety was discovered a year later. Subsequent monitoring revealed that the mature plants in this green population amounted to less than 50, lacking the reddish tinge characteristic of the typical species. Consequently, additional samples were collected on June 10, 2022, for taxonomic treatments, and are described here as a new variety. However, there is insufficient information about its distribution range, and this variety may exist in nearby unexplored locations. Currently categorized as Data Deficient (DD) following the IUCN guidelines of 2022 [38], we nonetheless recommend considering it as an endangered taxon unless further evidence suggests otherwise.
| 1a. Leaves green with dark red margin; abaxially dark red; sheaths dark red | var. phuphanensis |
| 1b. Leaves green with green margin; abaxially green; sheaths green | var. viridans |


3.2.1. Chromosomes and Karyotype Formula of Kaempferia phuphanensis var. viridans
3.2.2. Palynology of Kaempferia phuphanensis var. viridans

4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kress, W.J.; Prince, L.M.; Williams, K.J. The phylogeny and a new classification of the gingers (Zingiberaceae): Evidence from molecular data. Am. J. Bot. 2002, 89, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- POWO. Plant of the World Online, Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 1 March 2024).
- Linnaeus, C. Kaempferia galanga. In Species Plantarum; Laurentius Salvius: Stockholm, Sweden, 1753; Volume 1, pp. 2–3. [Google Scholar]
- Sirirugsa, P. Thai Zingiberaceae: Species diversity and their uses. In Proceedings of the International Conference on Biodiversity and Bioresources: Conservation and Utilization, Phuket, Thailand, 23–27 November 1997. [Google Scholar]
- Larsen, K.; Ibrahim, H.; Wong, K.M. Gingers of Peninsular Malaysia and Singapore; Natural History Pub.: Kota Kinabalu, Malaysia, 1999. [Google Scholar]
- Chiramongkolgarn, U. Study on Diversity and Uses of Plants in Tao Dam Forest, Changwat Kanchanaburi. Master’s Thesis, Kasetsart University, Bangkok, Thailand, 2001. [Google Scholar]
- Larsen, K.; Larsen, S.S. Ginger of Thailand; Queen Sirikit Botanic Garden, The Botanical Garden Organization: Chiang Mai, Thailand, 2006. [Google Scholar]
- Leong-Škorničková, J.; Newman, M.F. Gingers of Cambodia, Laos & Vietnam; Oxford Graphic Printers Pte Ltd.: Singapore, 2015. [Google Scholar]
- Sirirugsa, P. The genus Kaempferia (Zingiberaceae) in Thailand. Nord. J. Bot. 1989, 9, 257–260. [Google Scholar] [CrossRef]
- Kumar, A. Phytochemistry, pharmacological activities and uses of traditional medicinal plant Kaempferia galanga L.—An overview. J. Ethnopharmacol. 2020, 253, 112667. [Google Scholar] [CrossRef]
- Suksri, S.; Premcharoen, S.; Thawatphan, C.; Sangthongprow, S. Ethnobotany in Bung Khong Long non-hunting area, northeast Thailand. Agric. Nat. Resour. 2005, 39, 519–533. [Google Scholar]
- Ma, Q.; Fan, X.-D.; Liu, X.-C.; Qiu, T.-Q.; Jiang, J.-G. Ultrasound-enhanced subcritical water extraction of essential oils from Kaempferia galangal L. and their comparative antioxidant activities. Sep. Purif. Technol. 2015, 150, 73–79. [Google Scholar] [CrossRef]
- Tangjitman, K.; Wongsawad, C.; Kamwong, K.; Sukkho, T.; Trisonthi, C. Ethnomedicinal plants used for digestive system disorders by the Karen of northern Thailand. J. Ethnobiol. Ethnomed. 2015, 11, 27. [Google Scholar] [CrossRef]
- Bhadra, S.; Mondal, S.; Bandyopadhyay, M. An empirical study on the underutilized medicinal genus Kaempferia from India revealed cytological and genetic variability. Nucleus 2020, 63, 257–270. [Google Scholar] [CrossRef]
- Panyakaew, J.; Chalom, S.; Sookkhee, S.; Saiai, A.; Chandet, N.; Meepowpan, P.; Thavornyutikarn, P.; Mungkornasawakul, P. Kaempferia sp. Extracts as UV protecting and antioxidant agents in sunscreen. J. Herbs Spices Med. Plants 2021, 27, 37–56. [Google Scholar] [CrossRef]
- Subositi, D.; Kurnianingrum, N.; Mujahid, R.; Widiyastuti, Y. Kaempferia galanga L. A medicinal plant used by Indonesian ethnic groups: Genetic diversity based on inter-simple sequence repeats (ISSR). AGRIVITA J. Agric. Sci. 2020, 42, 45–52. [Google Scholar] [CrossRef]
- Ridtitid, W.; Sae-wong, C.; Reanmongkol, W.; Wongnawa, M. Antinociceptive activity of the methanolic extract of Kaempferia galanga Linn. in experimental animals. J. Ethnopharmacol. 2009, 118, 225–230. [Google Scholar] [CrossRef]
- Song, L.; Wu, X.; Xie, J.; Zhang, H.; Yang, H.; Zeng, Q.; Yang, X.; Xie, W. Kaempferia galanga Linn. Extract—A potential antibacterial agent for preservation of poultry products. LWT 2021, 147, 111553. [Google Scholar] [CrossRef]
- Srivastava, N.; Ranjana; Singh, S.; Gupta, A.C.; Shanker, K.; Bawankule, D.U.; Luqman, S. Aromatic ginger (Kaempferia galanga L.) extracts with ameliorative and protective potential as a functional food, beyond its flavor and nutritional benefits. Toxicol. Rep. 2019, 6, 521–528. [Google Scholar] [CrossRef]
- Amuamuta, A.; Plengsuriyakarn, T.; Na-Bangchang, K. Anticholangiocarcinoma activity and toxicity of the Kaempferia galanga Linn. Rhizome ethanolic extract. BMC Complement. Altern. Med. 2017, 17, 213. [Google Scholar] [CrossRef]
- Nontasit, N.; Kanlayanapaphon, C.; Mekanawakul, M.; Nualmangsar, O. Taxonomic studies and traditional uses of Zingiberaceae in Khao Luang National Park, Nakhon Si Thammarat Province, Thailand. Walailak J. Sci. Technol. 2015, 12, 643–658. [Google Scholar]
- Preetha, T.S.; Hemanthakumar, A.S.; Krishnan, P.N. A comprehensive review of Kaempferia galanga L. (Zingiberaceae): A high sought medicinal plant in Tropical Asia. J. Med. Plants Stud. 2016, 4, 270–276. [Google Scholar]
- Shetu, H.; Trisha, K.T.; Sikta, S.A.; Anwar, R.; Rashed, S.S.B.; Dash, P.R. Pharmacological importance of Kaempferia galanga (Zingiberaceae): A mini review. Int. J. Pharm. Sci. Res. 2018, 3, 32–39. [Google Scholar]
- Sirirugsa, P. Taxonomy of the genus Kaempferia (Zingiberaceae) in Thailand. Thai For. Bull. 1992, 19, 1–15. [Google Scholar]
- Picheansoonthon, C.; Koonterm, S. A new species of Kaempferia L. (Zingiberaceae) from Northeastern Thailand. Taiwania 2009, 54, 52–56. [Google Scholar]
- Picheansoonthon, C. Kaempferia lopburiensis (Zingiberaceae), a new species from Central Thailand. J. Jpn. Bot. 2010, 85, 148–152. [Google Scholar]
- Picheansoonthon, C. Two new Kaempferia (Zingiberaceae) from Thailand. J. Jpn. Bot. 2011, 86, 1–8. [Google Scholar]
- Phokham, B.; Wongsuwan, P.; Picheansoonthon, C. Three new species of Kaempferia (Zingiberaceae) from Thailand and Laos. J. Jpn. Bot. 2013, 88, 297–308. [Google Scholar]
- Wongsuwan, P.; Prasarn, S.; Picheansoonthon, C. Kaempferia koontermii (Zingiberaceae)—A new species from Thailand. J. Jpn. Bot. 2015, 90, 29–33. [Google Scholar]
- Saensouk, S.; Saensouk, P. Kaempferia phuphanensis (Zingiberaceae), a new species from Thailand. J. Jpn. Bot. 2019, 94, 149–152. [Google Scholar]
- Boonma, T.; Saensouk, S.; Saensouk, P. Two new species of Kaempferia L. (Zingiberaceae) from Thailand. Taiwania 2020, 65, 371–381. [Google Scholar]
- Jenjittikul, T.; Larsen, K. Two new species of Kaempferia (Zingiberaceae) from Thailand. Nat. Hist. Bull. Siam Soc. 2020, 64, 17–23. [Google Scholar]
- Nopporncharoenkul, N.; Somnoo, T.; Tanming, W.; Maknoi, C. Kaempferia jenjittikuliae (Kaempferia subg. Protanthium: Zingiberaceae), a new, endangered species endemic to Thailand. Edinb. J. Bot. 2021, 78, 1–13. [Google Scholar] [CrossRef]
- Saensouk, P.; Saensouk, S. Two new species of Kaempferia (Zingiberaceae) from Thailand. J. Jpn. Bot. 2021, 96, 193–198. [Google Scholar]
- Saensouk, P.; Saensouk, S.; Boonma, T. Two new species of Kaempferia subgenus Kaempferia (Zingiberaceae: Zingibereae) from Thailand. Biodiversitas 2022, 23, 4343–4354. [Google Scholar] [CrossRef]
- Nopporncharoenkul, N.; Jenjittikul, T. Taxonomic Revision of some taxa in Kaempferia subgenus Protanthium (Zingiberaceae) revealing a new species from Thailand and two new synonyms. Blumea 2024, 69, 16–26. [Google Scholar] [CrossRef]
- Wongsuwan, P.; Meechonkit, P.; Phokham, B.; Sangnark, S.; Yupparach, P.; Picheansoonthon, C. A new species of Kaempferia (Zingiberaceae) from Northern Thailand. J. Jpn. Bot. 2020, 95, 34–38. [Google Scholar]
- IUCN Standards and Petitions Committee. Guidelines for Using the IUCN Red List Categories and Criteria; Version 15.1; IUCN Standards and Petitions Committee: Gland, Switzerland, 2022. [Google Scholar]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for centromeric position on chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Stebbins, G.L. Chromosomal Evolution in Higher Plants, 1st ed.; Edward Arnold Publisher Ltd.: London, UK, 1971. [Google Scholar]
- Saenprom, K.; Saensouk, S.; Saensouk, P.; Senakun, C. Karyomorphological analysis of four species of Zingiberaceae from Thailand. Nucleus 2018, 61, 111–120. [Google Scholar] [CrossRef]
- Erdtman, G. Pollen Morphology and Plant Taxonomy: Angiosperms (An Introduction to Palynology. I); Corrected Reprint of the edition of 1952 with a new addendum; Hafner Publication Company: New York, NY, USA, 1972. [Google Scholar]
- Picheansoonthon, C.; Koonterm, S. Two new Kaempferia L. (Zingiberaceae) from southern Laos. Taiwania 2009, 54, 219–225. [Google Scholar]
- Saensouk, P.; Saensouk, S. Taxonomy, cytology, and palynology of Kaempferia pseudoparviflora (Zingiberaceae), a new and rare species from Northern Thailand. Asian J. Plant Sci. 2021, 20, 414–420. [Google Scholar] [CrossRef]
- Picheansoonthon, C.; Koonterm, S. A New Species of Kaempferia (Zingiberaceae) from Southern Laos. Taiwania 2008, 53, 406–409. [Google Scholar]
- Chakravorti, A.K. Multiplication of chromosome numbers in relation to speciation in Zingiberaceae. Sci. Cult. 1948, 14, 137–140. [Google Scholar]
- Saensouk, S.; Saensouk, P. Chromosome numbers of some Zingiberaceae in Thailand. Khon Kaen Univ. J. (Grad. Stud.) 2004, 9, 3–9. [Google Scholar]
- Omanakumari, N.; Matthew, P.M. Karyomorphological studies on three species of Kaempferia L. Cytologia 1984, 49, 709–715. [Google Scholar] [CrossRef]
- Omanakumari, N.; Matthew, P.M. Cytological studies on the genus Kaempferia L. from South India. Cell Chromosome Res. 1991, 14, 1–6. [Google Scholar]
- Sharma, A.K.; Bhattacharyya, N.K. Cytology of several members of Zingiberaceae. La Cellule 1959, 59, 297–346. [Google Scholar]
- Zou, P.; Newman, M.F.; Liao, J.P. Systematics of Zingiberaceae. Grana 2022, 61, 448–470. [Google Scholar] [CrossRef]

| Chro. Pair | Ls ± SD (µm) | Ll ± SD (µm) | LT ± SD (µm) | RL (%) | CI | Chromosome Type |
|---|---|---|---|---|---|---|
| 1 | 1.69 ± 0.08 | 2.77 ± 0.64 | 4.46 ± 0.73 | 11.96 | 0.62 | Submetacentric |
| 2 | 1.51 ± 0.09 | 2.48 ± 0.14 | 3.99 ± 0.23 | 10.69 | 0.62 | Submetacentric |
| 3 | 1.78 ± 0.09 | 2.08 ± 0.13 | 3.86 ± 0.22 | 10.35 | 0.54 | Metacentric |
| 4 | 1.49 ± 0.09 | 2.15 ± 0.13 | 3.65 ± 0.22 | 9.77 | 0.59 | Metacentric |
| 5 | 1.34 ± 0.08 | 2.27 ± 0.14 | 3.61 ± 0.21 | 9.67 | 0.63 | Submetacentric |
| 6 | 1.49 ± 0.09 | 1.93 ± 0.11 | 3.42 ± 0.20 | 9.17 | 0.56 | Metacentric |
| 7 | 1.15 ± 0.08 | 1.99 ± 0.11 | 3.15 ± 0.19 | 8.44 | 0.63 | Submetacentric |
| 8 | 1.53 ± 0.08 | 1.60 ± 0.11 | 3.13 ± 0.19 | 8.39 | 0.51 | Metacentric |
| 9 | 1.32 ± 0.08 | 1.74 ± 0.10 | 3.06 ± 0.18 | 8.20 | 0.57 | Metacentric |
| 10 | 1.13 ± 0.07 | 1.43 ± 0.09 | 2.55 ± 0.17 | 6.84 | 0.56 | Metacentric |
| 11 | 0.88 ± 0.06 | 1.56 ± 0.09 | 2.44 ± 0.15 | 6.53 | 0.64 | Submetacentric |
| Chro. Pair | Ls ± SD (µm) | Ll ± SD (µm) | LT ± SD (µm) | RL (%) | CI | Chromosome Type |
|---|---|---|---|---|---|---|
| 1 | 1.54 ± 0.08 | 2.53 ± 0.63 | 4.07 ± 0.71 | 10.5 | 0.62 | Submetacentric |
| 2 | 1.69 ± 0.09 | 2.29 ± 0.14 | 3.98 ± 0.23 | 10.26 | 0.58 | Metacentric |
| 3 | 1.65 ± 0.09 | 2.30 ± 0.13 | 3.95 ± 0.23 | 10.19 | 0.58 | Metacentric |
| 4 | 1.59 ± 0.09 | 2.20 ± 0.13 | 3.79 ± 0.22 | 9.77 | 0.58 | Metacentric |
| 5 | 1.22 ± 0.07 | 2.46 ± 0.14 | 3.68 ± 0.21 | 9.51 | 0.67 | Submetacentric |
| 6 | 1.71 ± 0.09 | 1.96 ± 0.11 | 3.67 ± 0.20 | 9.46 | 0.53 | Metacentric |
| 7 | 1.07 ± 0.08 | 2.54 ± 0.12 | 3.62 ± 0.20 | 9.33 | 0.70 | Subtelocentric |
| 8 | 1.39 ± 0.08 | 2.00 ± 0.12 | 3.39 ± 0.19 | 8.76 | 0.59 | Metacentric |
| 9 | 1.25 ± 0.07 | 1.87 ± 0.10 | 3.12 ± 0.18 | 8.04 | 0.60 | Submetacentric |
| 10 | 1.12 ± 0.07 | 1.77 ± 0.10 | 2.90 ± 0.17 | 7.47 | 0.61 | Submetacentric |
| 11 | 1.01 ± 0.06 | 1.59 ± 0.09 | 2.60 ± 0.16 | 6.70 | 0.61 | Submetacentric |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saensouk, P.; Saensouk, S.; Boonma, T.; Rakarcha, S.; Srisuk, P.; Imieje, V.O. Kaempferia sakolchaii sp. nov. and K. phuphanensis var. viridans var. nov. (Zingiberaceae), Two New Taxa from Northeastern Thailand. Horticulturae 2024, 10, 430. https://doi.org/10.3390/horticulturae10050430
Saensouk P, Saensouk S, Boonma T, Rakarcha S, Srisuk P, Imieje VO. Kaempferia sakolchaii sp. nov. and K. phuphanensis var. viridans var. nov. (Zingiberaceae), Two New Taxa from Northeastern Thailand. Horticulturae. 2024; 10(5):430. https://doi.org/10.3390/horticulturae10050430
Chicago/Turabian StyleSaensouk, Piyaporn, Surapon Saensouk, Thawatphong Boonma, Sarayut Rakarcha, Pathomthat Srisuk, and Vincent O. Imieje. 2024. "Kaempferia sakolchaii sp. nov. and K. phuphanensis var. viridans var. nov. (Zingiberaceae), Two New Taxa from Northeastern Thailand" Horticulturae 10, no. 5: 430. https://doi.org/10.3390/horticulturae10050430
APA StyleSaensouk, P., Saensouk, S., Boonma, T., Rakarcha, S., Srisuk, P., & Imieje, V. O. (2024). Kaempferia sakolchaii sp. nov. and K. phuphanensis var. viridans var. nov. (Zingiberaceae), Two New Taxa from Northeastern Thailand. Horticulturae, 10(5), 430. https://doi.org/10.3390/horticulturae10050430







