1. Introduction
In the Middle Ages, men presented bouquets of European cranberry bush (
Viburnum opulus L.) (
V. opulus) flowers to create positive feelings for women and manifest attention [
1]. Today,
V. opulus (family
Caprifoliaceae) is known as the snowball fruit, European cranberry bush, guelder rose, guilder rose, water elder, or cramp bark; it is widely cultivated around the world for ornamental or pharmaceutical purposes and less as edible fruit. The genus
Viburnum consists of over 200 species, among which the berries of
V. opulus have a bitter and astringent taste. The compounds responsible for bitterness and astringency make them unpleasant in terms of flavor and negatively affect the sensory quality of the final product [
2,
3,
4]. The use of alcohol tinctures made from the bark of the
V. opulus root, shrub, and its limbs for medicinal purposes dates back to 1882 by Purdy [
5], where it was used as a remedy to treat dysmenorrhea and uterine pain. According to the available data,
V. opulus fruits have been used by native inhabitants in northern parts of the United States, Canada, and Europe as uterine relaxant remedies and for eye disorders [
6]. More recent scientific findings imply that
V. opulus has medicinal properties for treating colds, coughs, tuberculosis, rheumatic aches, and ulcers, as well as stomach and kidney problems [
7,
8]. With the development of advanced technologies and analytical tools, a great deal of attention has been paid to the detailed analysis of
V. opulus morphological parts and to elucidating their components’ ability to treat various ailments within epidemiological and clinical trials with different therapeutic approaches. In some of these studies, affirmative effects on colon cancer [
9], testis and sperm damage [
10], inflammations [
11], endometriosis [
12], gastrointestinal mucosal damage [
13], and kidney stone formation [
14] are being reflected. Additionally, it has been well documented that the fruits of
V. opulus serve as ubiquitous reactive oxygen (ROS) and reactive nitrogen species (RNS) scavengers, and their apparent antioxidant abilities have also been well elucidated [
2,
13,
15]. Altun et al. [
16] emphasized the implication of the
V. opulus morphological parts, indicating the outstanding antioxidant activity (AOA) of branch and leaf extracts from it when prepared by maceration in cold distilled water. Furthermore, Dursun et al. [
17] underlined the significant impact of extraction methods—such as ultrasound-assisted, microwave-assisted, Soxhlet, and solvent-based—on the yield and quality of
V. opulus extracts. A recent study by Polka et al. [
18], who conducted a histochemical analysis of
V. opulus, revealed that the ethanol extracts of such morphological parts as bark, flowers, and fruit profusely contain various constituents, including flavan-3-ols (epicatechin and procyanidin B1), flavonols (quercetin), and hydroxycinnamates (chlorogenic and cryptochlorogenic acids). Among 13 of the phenolic compounds detected, the authors emphasized the relative abundance of flavan-3-ols in the 70% bark-derived ethanolic extracts that, among others, contributed to AOA the most. Contrary results have been reported by Karaçelik et al. [
19], who analyzed the antioxidant components of the
V. opulus juice, methanol, and acetonitrile extracts of seeds and skin. The exceptional composition of
V. opulus juice was underlined, showing the presence of 19 phenolic compounds, among which coumaroylquinic acid, chlorogenic acid, procyanidin B2 (dimer), and procyanidin trimer were dominant. Earlier, Velioglu et al. [
3] also revealed the presence of 13 phenolic compounds in the juice of
V. opulus, where chlorogenic acid was established as a prevalent compound that accounted for 54% or 2037 mg kg
−1 of the total phenolics. Kraujalyte et al. [
20] made a similar observation, indicating chlorogenic acid’s superiority over other phenolics (from 540 to 6939 mg kg
−1) among five of the
V. opulus genotypes tested. The most recent studies on antimicrobial activity have indicated the exceptional antimicrobial activity of
V. opulus juice [
21,
22,
23]. The relevance of this statement is becoming increasingly apparent as the problem associated with the spread of microorganisms that are resistant to topical antibiotics and therapies is turning from a local concern to a global one [
24].
The overuse of antibiotics is a primary cause leading to the development of antimicrobial resistance [
25]. As the issue of unwarranted and excessive use of antibiotics and the proliferation of microorganism resistance is exacerbated, scientists and medical microbiologists are sounding the alarm for a solution to be addressed. For preventive purposes, it is recommended to use herbal-derived formulations as an exceptional alternative to reduce the risk of morbidity from viral diseases. The recent findings in research into the antimicrobial properties of
V. opulus fruits indicate their effectiveness in combating pathogens. The abundance of chlorogenic acid and procyanidins in
V. opulus affects the rigidity and permeability of bacterial cell walls and membranes, disrupting their integrity and reducing ion exchange and ATF synthesis [
26]. This observation is reinforced by the study of Wu et al. [
27], who highlighted the interaction of chlorogenic acid with the membrane lipid and protein layers of
Staphylococcus aureus, thereby causing severe morphological changes that entailed the leakage of intracellular constituents and eventual cell apoptosis. A positive effect of chlorogenic acid was demonstrated by Ji et al. [
28], who highlighted the upregulation of interleukin-10 (IL-10) and interleukin-10 receptors (IL-10RA) in bovine mammary epithelial cells as a response to
S.
aureus-induced inflammation. The effectiveness of
V. opulus fruit extract in inhibiting the growth of
Aeromonas hydrophila ATCC 7965,
Bacillus cereus FMC 19,
Enterobacter aerogenes CCM 2531,
Escherichia coli DM,
Klebsiella pneumoniae FMC 5,
Proteus vulgaris FMC 1,
Pseudomonas aeruginosa ATCC 27853,
Salmonella typhimurium,
S. aureus Cowan 1, and
Yersinia enterocolitica EU has been proven [
29]. The antimicrobial activity of
V. opulus juice was also emphasized during analyses of the antimicrobial potential against
Salmonella Agona,
B. subtilis,
Listeria monocytogenes,
Enterococcus faecalis,
Micrococcus luteus, and
S. epidermidis [
21].
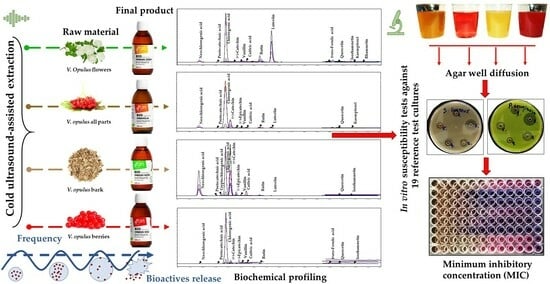
Currently, limited information is available on the effective concentrations of specific V. opulus extracts that can inhibit or delay the growth of pathogens. Most studies have focused on antimicrobial activity probing, which has been conducted using the Kirby–Bauer disk diffusion susceptibility method. Thus, this study’s primary goal was to examine four commercially available V. opulus extracts that were obtained from (1) flowers, (2) a combination of morphological parts (i.e., flowers, berries without seeds, leaves, buds, and bark), (3) bark, and (4) berries without seeds. To achieve this, a qualitative and quantitative analysis of the bioactive profiles and antimicrobial susceptibility test (MIC analysis) of reference test cultures were conducted, and the lowest concentrations that could inhibit the growth of microorganisms were determined.
4. Discussion
The study conducted by Mizzi et al. [
40] found that sugars can act as either synergists or antagonists to pathogenic microorganisms by either promoting or demoting their growth. Based on this finding, the first step in analyzing
V. opulus extracts was establishing their sugar profile and content. Perova et al. [
1] observed double to quadruple as much fructose in
V. opulus berries, which fluctuated from 2650 to 4040 mg 100 g
−1. The observed ratio of glucose to fructose in the extract of a
V. opulus berry was 1:1, which is consistent with the findings reported by Perova et al. [
1] for
V. opulus berries, who indicated a glucose content from 2860 to 4640 mg 100 g
−1. The presence of glucose in the fruits and flowers of
V. opulus was previously reported by Polka et al. [
18], who reported concentrations ranging from 2.0 to 15.3 g 100 mL
−1 on a dry weight basis (DW). However, those authors also reported an absence of this sugar in bark extract. The variation in fructose and glucose content can be attributed to the origin of the fruit, the type of morphological part, the extraction method used, and the expression of the results. This is the first report highlighting ribose’s presence in
V. opulus extracts. However, this sugar alcohol has already been documented in mulberry, apple, grape, apricot, and pear juice extracts [
41]. Xylose, a hemicellulose-derived sugar that is usually extracted after partial or complete hydrolysis of lignocellulose materials [
42], has also been observed as part of
V. opulus extracts derived from a mixture of morphological parts. However, the presence of xylose, as the structural component of opulosides and anthocyanidins, was also mentioned by Perova et al. [
1], who detected it as part of the non-target profiling of iridoid glucosides and anthocyanins. A similar observation was made by Kajszczak et al. [
7], who revealed the abundance of iridoids in green parts, mainly in the leaves and young stems, of the
V. opulus plant. This observation reinforces the funding of the present study since the extract was prepared using all the morphological parts of the
V. opulus shrub, i.e., flowers, whole berries, leaves, buds, and bark. Prolonged ultrasonic treatment promotes the release of structurally bound cell components, including xylose moieties, as was reported by Wang et al. [
43]. The release of bound components is conditioned by an ultrasound’s ability to increase cell wall permeability, and it will eventually disrupt it, thus leading to a more efficient mass transfer between the solvent and plant material. This statement can be reinforced by an early observation made by Sun et al. [
44]. It is worth noting that the total sugar content in
V. opulus extracts did not exceed 2.5%, which is far from the 20.0% concentration required to inhibit the growth of
S. aureus and
E. coli, as stated by Mizzi et al. [
40]. On the other hand, sugars in small quantities can be regarded as growth-promoting compounds since they serve as substrates for the microorganism, resulting in enhanced microbial growth instead of inhibition [
45].
Along with the undebatable physiological benefits inherent to amino acids (AAs) [
46], which were long considered simply building blocks for protein synthesis [
46], it has recently been shown that AAs act not only as growth-promoting substrates for pathogenic bacteria and opportunistic pathogens, but they also support the bacteria evasion that occurs in host immune defense [
47]. Therefore, as in the case of saccharides, the profile and concentrations of individual free amino acids in
V. opulus extracts were specified for the first time. The results make it possible to draw an interconnection between the AA content and the antimicrobial activity of
V. opulus extracts. The AAs analysis conducted by HPLC-ESI-TQ-MS/MS revealed relative content and distribution patterns over four
V. opulus extracts. The proline concentration in the
V. opulus extracts obtained from bark and berries was four and twenty-one times lower than in flower-derived extract. A high proline concentration in flower-derived extract has been associated with its role in plant metabolism and resistance to multiple abiotic and biotic stresses during flowering and plant growth and development [
48]. Hosseinifard et al. [
49] highlighted the dominance of proline in tomato flowers, revealing a 60 times higher concentration than that observed in other morphological parts such as leaves, roots, and fruit. Despite the contribution of dietary proline to collagen biosynthesis [
50], its direct involvement in the proliferation of disease-causing, biofilm-forming pathogenic microorganisms such as
S.
aureus,
E.
coli,
E.
chaffeensis,
Clostridium difficile,
Helicobacter spp., and
Cryptococcus neoformans was highlighted by Christgen et al. [
51] and Cleaver et al. [
52], who revealed its role as a respiratory substrate and osmolyte that ensures protection against environmental stress. Glutamic acid is the second most prevalent amino acid (AA) identified in the
V. opulus extracts. Its contribution to the total AA amount was 5.2 to 29.2% in the
V. opulus extracts. The highest glutamic acid content was found in the berry extract, while the lowest was in the flower extract. The presence of berries in the extract derived from a mixture of morphological parts can reinforce glutamic acid’s prevalence, as Asadpoor et al. [
53] reported for the nine fruit juices. The role of glutamic acid as a sole carbon and nitrogen source for microorganisms was revealed by Liu et al. [
54], who indicated a growth-promoting ability toward
S.
enterica and
Pseudomonas spp. A recent study showed disparities in the AA metabolism in
C.
albicans biofilms, with a prominent upregulation of arginine, proline, aspartate, and glutamate metabolism in high biofilm-forming isolates [
55]. The presence of alanine in the extracts of
V. opulus, specifically in the flower extract, makes it attainable and worthwhile to assume a possible contribution of this extract to the growth of selected opportunistic bacteria. This is relevant as the extensive proliferation of
E.
coli K-12 and EC-14 was observed by Muranaka et al. [
56] in infected model mice with an excess of alanine. This observation was supported by Díaz-Pascual et al. [
57], who noted that
E.
coli colonies in a controllable biofilm model system metabolized alanine as a carbon source in oxic conditions rather than in anoxic ones. The high demand for alanine in bacteria is conditioned by its direct involvement in the synthesis of peptidoglycan, a pivotal component of the cell wall of both Gram-negative and Gram-positive bacteria [
58]. The observed EAA and BCAA values in the
V. opulus extracts were much higher than those reported for the vinegar [
59] and fermented juice [
60] of
V. opulus. The content of the total individual AAs in the extracts varied significantly (
p < 0.05) between the investigated
V. opulus morphological parts. However, the highest total AA content was found in the extract obtained from flowers of
V. opulus. A similar observation was made by Kajszczak et al. [
7], who indicated that the flowers of
V. opulus are more abundant in protein than the fruit, stalk, and leaves. Overall, from a nutritional standpoint, the extracts obtained from flowers and a mix of
V. opulus morphological parts can be highlighted as exceptional, as the AA levels were found to be many times higher than those reported by others for plant extracts. However, recent findings have indicated that some AAs can induce the growth of biofilm-forming bacteria, such as
E.
coli,
P.
aeruginosa, and
S.
aureus. Therefore, additional studies are required to determine the influence of
V. opulus extracts on the growth of opportunistic bacteria.
Previous research has examined the chemical composition of different parts of
V. opulus, including its fruits, flowers, and bark, indicating a relative abundance of bioactives and their fluctuations over different morphological parts [
61]. However, most of these studies were based on investigating the dried morphological parts of the
V. opulus plant rather than extracts in their original state. Therefore, it is essential to comprehend better the profile and concentrations of the bioactives present in
V. opulus extracts. Preliminary spectrophotometric studies have shown that group compounds, particularly TPC, are present in ample amounts, as indicated in
Table 4. The values observed in the present study contradict the findings of Polka and Podsędek [
62], as well as Polka et al. [
18], for dried
V. opulus-obtained extracts and
V. opulus parts, who highlighted the superiority of the
V. opulus bark over both flowers and fruits. Dienaite et al. [
63], studying the yield of TPC from dried
V. opulus pomace as the function of extraction solvent utilized, observed relatively higher TPC values obtained by ethanol rather than water as a sole solvent. The concentrations of TPC in their study were four to fifteen times higher than in the present study. The choice of solvent should depend on the type of matrix and the nature of the substances being extracted. Plant materials contain hydrophilic compounds, i.e., compounds that are preferably soluble in water, but these do not appear in organic solvents and vice versa. In the research of Radenkovs et al. [
37], an augmented effect upon extractability of TPC from wild crab apple pomace and higher antioxidant activity (AOA) was obtained by utilizing 30% ethanol instead of other solvents or their aqueous solutions. On this occasion, water played the swelling agent role, increasing the plant cell contact surface [
64]. Since the studied
V. opulus extracts were intended as a homeopathic remedy for the preventive treatment of colds in children and adults, water was used as the only solvent to prepare the extracts industrially. The high TPC content in the
V. opulus extracts derived from berries and bark is expected to contribute to the inhibition of opportunistic microorganisms, as reported by Mahboubi et al. [
65] and Ispiryan et al. [
66] for
Punica granatum L. and
Rubus idaeus L. extracts, respectively.
The observed TFC values were partially similar to those reported by Düz et al. [
67] for
V. opulus extracts derived from berries, flowers, and branches when using four different solvents for extraction. The authors found the highest concentration of TFC in the ethyl acetate extracts of
V. opulus obtained from leaves, and the lowest was found in the water extracts. However, Mahboubi et al. [
65] highlighted a statistically better extractability of TFC from
Punica granatum L. flowers by methanol, whereas those extracted by water and ethanol were much worse. Relatively lower values of TFC were observed in the
V. opulus extracts of the current study, which were conditioned by the weak solubility of flavonoids in water compared with organic solvents such as acetonitrile, methanol, ethanol, or ethyl acetate [
64]. Like in the case of TPC, a strong and positive correlation between TFC and the growth of pathogenic bacteria was revealed by Sartini et al. [
68], who indicated their anti-staphylococcal activity toward suppressing the growth of
S. aureus. In a detailed study on the antimicrobial activity of flavonoids, Donadio et al. [
69] reported the possible action mechanisms of flavonoids on the growth of pathogenic bacteria, among which the inhibition of transporters such as efflux pumps responsible for the extrusion of noxious compounds, both in Gram-positive and Gram-negative bacteria was highlighted. The synergistic effect of plant material-derived flavonoids on the cell wall integrity has also been revealed, indicating the potential contribution toward disruptions of membrane ion exchange and reducing ATP synthesis by depleting cell membranes. The relative ampleness of molecules containing substituents such as -OH in the B-ring defines the antimicrobial activity of a particular compound [
70].
The observed TTC values partially agree with those reported by Polka and Podsędek [
62], who indicated that the TTC in
V. opulus extracts range from 327.0 to 5029 mg (cyanidin equivalents, CYE) 100 mL
−1 DW. Interestingly, the highest content of TFC was observed in bark-derived extracts rather than berry ones, as found in the current study. According to the comprehensive research by Ucella-Filho et al. [
71], tannin-rich water extracts obtained from the plants of families such as
Fabaceae,
Combretaceae,
Anacardiaceae,
Punicaceae, and
Curtisiaceae have been shown to have prominent antimicrobial activity against various types of bacteria, including
E. coli,
L. innocua,
S. aureus,
E. faecalis,
B. cereus,
Shigella dysenteriae,
P. aeruginosa,
S. enterica,
S. typhimurium,
S. flexneri,
Neisseria gonorrhoea,
K. pneumoniae,
A. baumannii,
E. aerogenes,
Neisseria meningitidis, and
C. albicans. The remarkable antimicrobial activity of tannins is conditioned by their ability to bind to cell membranes through hydrophobic interactions via hydrogen bonding, which decreases membrane potential and increases permeability [
72]. Due to the chelating of mineral macronutrients, such as iron or zinc, which are involved in bacterial metabolism and possess primarily electron transport function and catalytic or structural cofactors of many vital enzymes, tannins cause iron deprivation, thereby leading to irreversible cell death [
73]. It is worth noting that the antimicrobial potential of tannins depends primarily on their chemical nature rather than on concentrations. Farha et al. [
74] highlighted the superior antimicrobial activity of hydrolysable tannins, such as tannic acid, compared with monomeric catechin due to the abundance of the -OH groups in each of the ten galloyl groups. Given the results of the TTC content and the observations mentioned above, the prepared
V. opulus water extracts can be regarded as a potential alternative for preventing bacterial infections.
The TAC values observed in this study are consistent with those reported by Kajszczak et al. [
7] regarding the fruit of
V. opulus, which were ranged from 6.0 to 53.0 mg CGE 100 mL
−1 FW. However, the TAC content in fresh
V. opulus fruit was found to be three times higher, as reported by Česonienė et al. [
23]. The fluctuations in the TAC content observed in the present study and those reported by other researchers can be explained by the relative susceptibility of these compounds to degradation under a pH higher than 7 and by differences found in the extraction solvents applied [
75,
76]. Interestingly, Demirdöven et al. [
77] reported a similar TAC content observed in red cabbage extracts (
Brassica oleracea L.), which was approached through optimized ultrasound-assisted extraction utilizing 42% ethanol as a sole solvent. It has been highlighted that some Gram-positive bacteria are more susceptible to anthocyanin action than Gram-negative bacteria [
78]. The mechanisms underlying anthocyanin activity include the membrane and intracellular interactions of these compounds. The study by Lacombe et al. [
79] demonstrated the ability of anthocyanin fractions derived from
Vaccinium macrocarpon to contribute to the inhibition of
E. coli O157:H7 at a native pH. In contrast, the antimicrobial activity was markedly reduced after a pH adjustment to neutral. The authors observed cell damage causing the leakage of nucleotides and other cytoplasm macromolecules, along with cell aggregation and disintegration, leading to death. Anthocyanins extracted from the fruit of
Punica granatum L. and
Vaccinium vitis-idaea, among others, contribute significantly to the inhibition of
E. coli and
S. enterica, as outlined in the review article of Ma et al. [
80]. However, individual anthocyanin constituents that most exhibit antimicrobial activity cannot be identified since fruit extracts or juices are represented by a diversity of compounds such as organic acids, phenolic acids, flavonoids, anthocyanins, and saccharides that synergistically contribute to morphological changes in the bacterial cells, which leads to their irreversible death [
78].
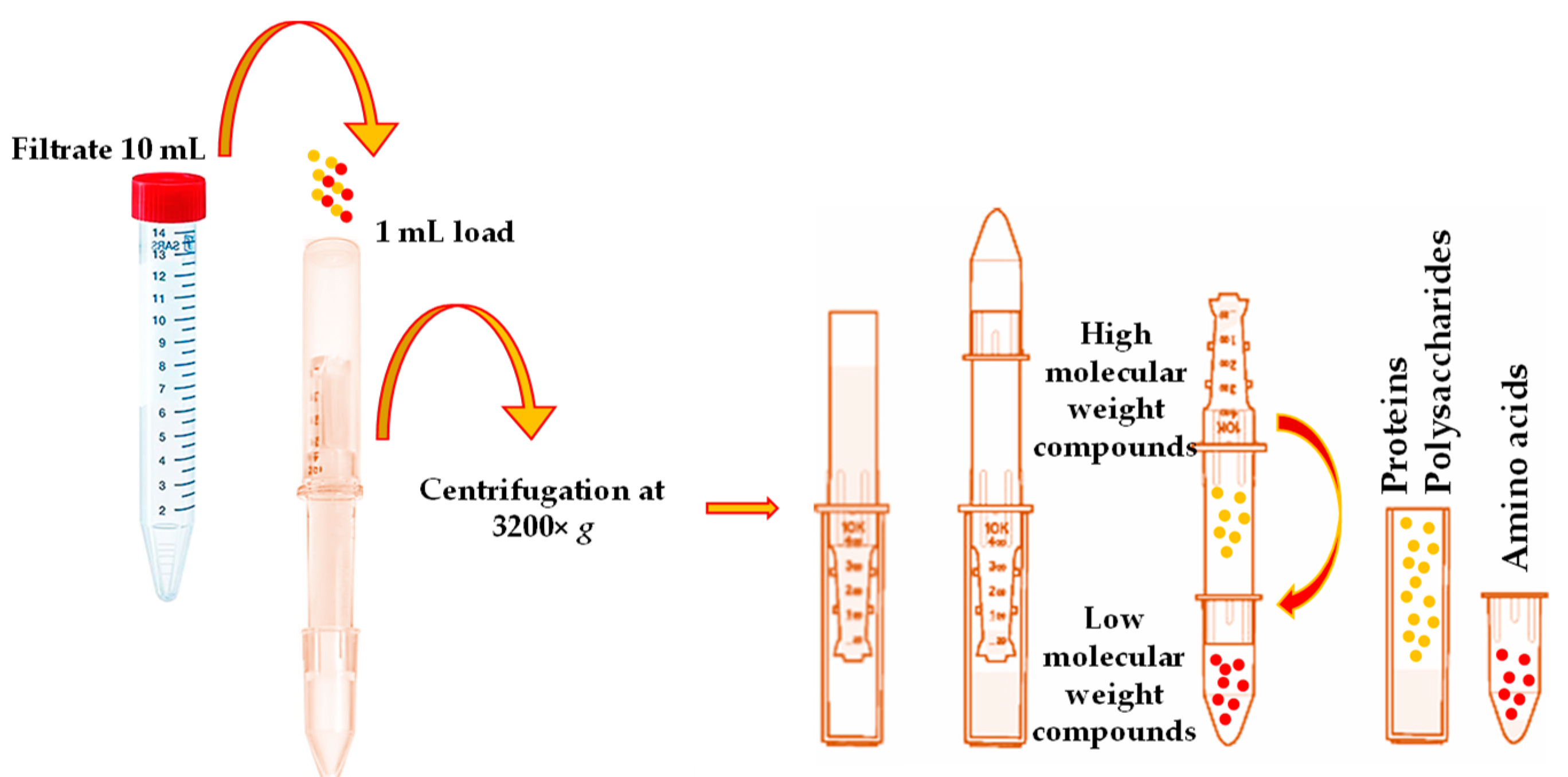
According to the obtained results, 18 individual, free phenolic compounds were qualitatively and quantitatively estimated after the purification of
V. opulus extracts by taking advantage of the SPE technique and using the SPE “Supel
™-Swift HLB” column. This study utilized the SPE as the high-molecular-weight compounds represented in plant matrices, such as proteins and polysaccharides, primarily affecting the correct quantification compounds of interest. The LC-ESI-TQ-MS/MS analysis of the
V. opulus extracts revealed the presence of flavonols, such as quercetin, rutin, luteolin and its glucoside, kaempferol, rhamnetin, and isorhamnetin; flavan-3-ols such as catechin and epicatechin; and hydroxycinnamic and hydroxybenzoic acid derivatives, such as vanillin, gallic, neochlorogenic, cryptochlorogenic, chlorogenic, protocatechuic, caffeic,
trans-ferulic, and
para-coumaric acids. Although the identification of the anthocyanin group representatives in this study was impossible due to technical limitations, the distribution pattern of the phenolics in the
V. opulus fruit was comparable to earlier studies by Kajszczak et al. [
7], who indicated the presence of all four (plus anthocyanins) phenolic groups in the
V. opulus fruit reported herein. Chlorogenic acid was found to be the most prevailing compound identified in the
V. opulus extracts, and the most substantial contribution of the compound to the total amount of individual phenolics investigated was observed for the
V. opulus extract derived from berries, which made up 90.3% of the total phenolics. Earlier, Velioglu et al. [
3] also established that chlorogenic acid was a prevalent compound among 13 phenolics identified in the juice of
V. opulus, accounting for 54% or 2037 mg kg
−1 of the total phenolics. This observation was further reinforced by Perova et al. [
1], who indicated that its contribution to the total amount of phenolics could be up to 96.2%. Altun and Yilmaz [
81] found an abundance of chlorogenic acid in the
V. opulus fruit, followed by leaves, which supports the findings of the current study. The abundance of chlorogenic acid in the fruit and juice of
V. opulus makes this object less attractive due to its remarkable bitterness and astringency [
82,
83]. However, this does not negate their use as active pharmaceutical ingredients [
8]. Recent studies examining the antimicrobial activity of chlorogenic acid have revealed its outstanding antimicrobial and antibiofilm effects against
Yersinia enterocolitica [
84]. This observation was also reinforced by Lou et al. [
85], who indicated its potential effectiveness in inhibiting three Gram-positive and three Gram-negative bacteria, including
S.
pneumonia,
S.
aureus,
B.
subtills,
E.
coli,
S.
dysenteriae, and
S.
typhimurium. It has been reported that chlorogenic acid interacts with the cell wall membrane, causing its permeabilization and partial leakage of nucleotides and other cytoplasm macromolecules, thus triggering cell inactivation [
86]. However, it has been proposed that the loss of cell components only partially affects the viability of microorganisms. Some intracellular processes in bacteria, apart from cell wall permeabilization, lie behind the antimicrobial activity of chlorogenic acid [
87]. The observations revealed the ability of chlorogenic acid to bind to shikimate pathway enzymes with high affinity and to inhibit their catalysis, thus causing irreversible cell death. In addition to chlorogenic acid, quinic acid derivatives, such as neochlorogenic and cryptochlorogenic acids, have also been found in the extracts of
V. opulus. The highest contribution of neochlorogenic acid to the total individual phenolics was observed for the
V. opulus extract derived from bark, which made up 11.1% of the phenolics investigated. Polka et al. [
18] highlighted the superiority of bark over fruit and flowers by considering the distribution of neochlorogenic and cryptochlorogenic acids, thus reinforcing the present study’s findings. Catechin, a phenolic compound, was found to be the second most abundant compound in the extracts of
V. opulus. The highest catechin concentration was observed in the bark extract, accounting for 57.7% of the total phenolic content. These results are consistent with the findings of Zakłos-Szyda et al. [
88], who reported that catechin is the main ingredient of
V. opulus fresh juice and the phenolic-rich fraction, which corresponded to 121 mg 100 g
−1 FW and 100.764 mg 100 g
−1 DW, respectively. It is worth noting that the results of the present study contradict those reported by Polka et al. [
18], who found no catechin in the
V. opulus fruit and an excess in the flowers. The differences in the fruit maturity stage, flower physiology stage, and extraction techniques may have influenced the extractability of phenolics. As with chlorogenic acid, monomeric catechin rather than galloylated forms possess promising antimicrobial activity against certain Gram-positive and Gram-negative bacteria [
89]. The ability of catechins to partition into the lipid bilayers of various components through hydrophobic interactions via hydrogen bonding between the -OH groups of catechins and the O atoms of lipophiles leads to a lateral expansion of membranes, and cell permeability increases [
89]. It has been experimentally established that antimicrobial activity, apart from reducing cell membrane potential, is conditioned by the formation of hydrogen peroxides through the oxidation of catechins, which leads to DNA damage and the oxidation of vital cell components such as organelles and membranes [
90]. Given the observations made by other researchers, it is worthwhile to assume that the presence of catechin, along with epicatechin, in the
V. opulus extracts that were obtained at high concentrations will substantially contribute to the inhibition of at least Gram-positive bacteria since the disability of negatively charged catechins to interact with model membranes of negative charge has been highlighted [
91]. The presence of luteolin as the third most prevailing phenolic was observed in the extracts of
V. opulus, and this is the first report revealing the existence of this type of flavonoid in
V. opulus flowers; however, their presence has already been evidenced in
V. opulus [
92] fruit and leaves [
61]. In a study on the antimicrobial activity of individual phenolics, the ability of flavonoids to inhibit the efflux pumps of
S.
aureus in a concentration-dependent manner has been established in the following order: myricetin > rhamnetin > kaempferol > apigenin > luteolin > quercetin [
69,
93]. Nevertheless, it should be stated that the other flavonoid representatives observed in the extracts of
V. opulus were detected at relatively low quantities, and their contribution to the antimicrobial activity will likely be negligible.
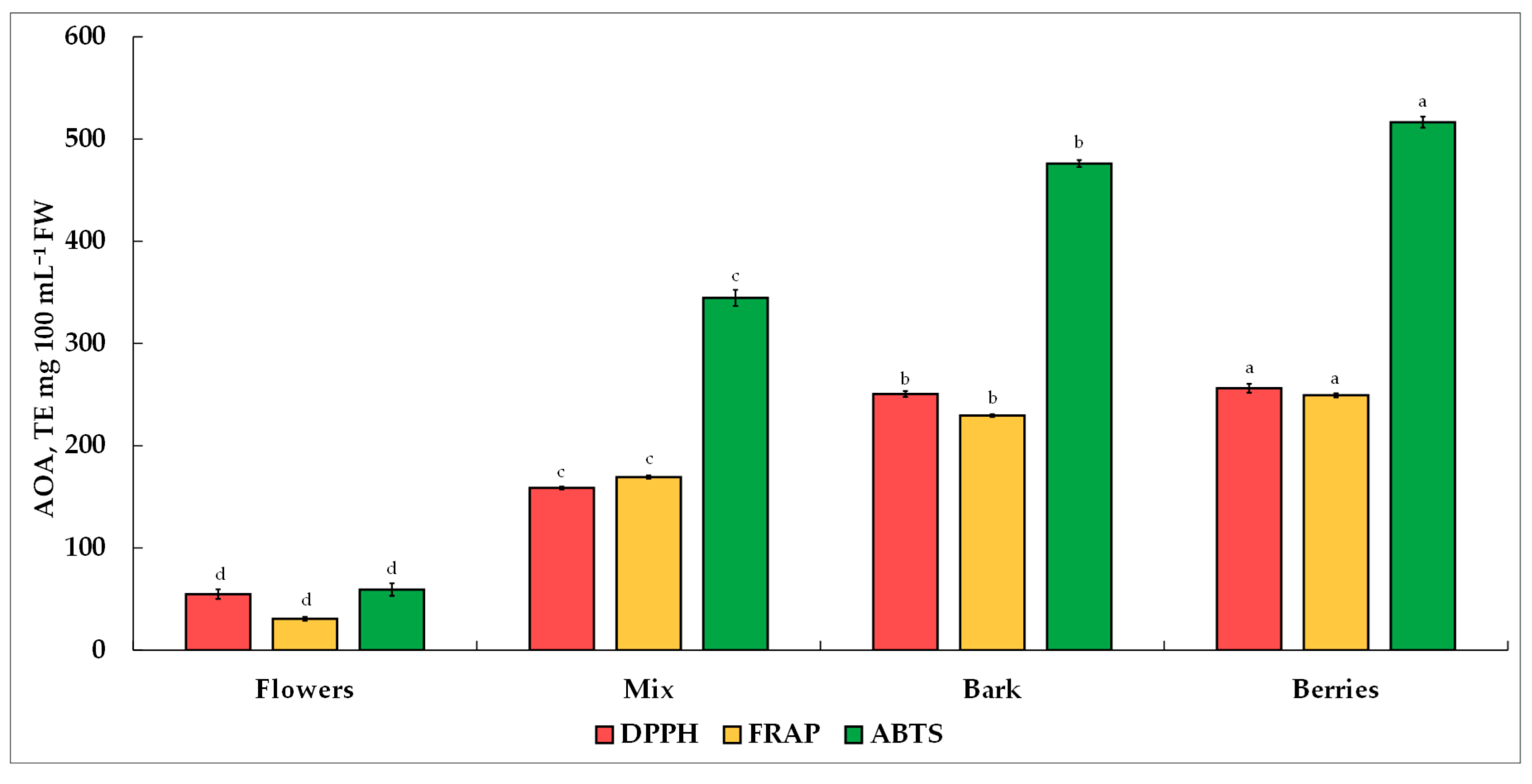
Antioxidants have become scientifically fascinating substances due to their tremendous clinically proven benefits, which include diminishing oxidative stress, supporting disease prevention and eye health, aiding brain function, contributing to mental health improvement, reducing inflammation, and stimulating probiotic development within gut microbiomes [
94,
95]. The mechanisms of antioxidants (AOAs) are primarily but not limited to scavenging free radicals such as superoxide anions and hydroxyl radicals [
96]. Given these statements, the ability of
V. opulus extracts to quench free radicals as part of a further study was established by three commonly used AOA methods, i.e., DPPH
•, FRAP, and ABTS
•+. The AOA of the different morphological parts of the
V. opulus extracts investigated was in the following order: berries > bark > mix > flowers. The results obtained by Polka and Podsędek [
62] indicated that the AOA of
V. opulus extracts is oriented toward hydroxyl radicals scavenging in the following effectiveness order: bark > flowers > fruits. Due to differences in expression units and extraction methods, the results of the present study are only in partial agreement with those reported by other researchers. Perova et al. [
1] reported a broader variation in the AOA pinpointed by DPPH
•, whereby they showed a range from 377.0 to 968.0 mg TE 100 g
−1 for dry
V. opulus fruits. Barak et al. [
97] observed a substantially higher AOA value for the water extract of
V. opulus dried fruit, which corresponded to 96.7 mg of butylated hydroxytoluene equivalent (BHTE) g
−1. The obtained data showed a direct relationship between the AOA of
V. opulus extracts and the TPC, TFC, and TTC values. However, a difference emerged between the AOA and individual phenolic compounds due to the distribution order and concentration of the individual phenolics observed in
V. opulus morphological parts, which was as follows: berries > mix > bark > flowers. A similar AOA pattern was obtained by the FRAP method, which showed the contribution of the
V. opulus obtained extracts to free radicals scavenging in the same order as was obtained by the DPPH
• method. This statement was additionally supported by an observation made in the earlier study by Muniyandi et al. [
98], who reported that tannins are better radical scavengers than flavonoids and anthocyanins. Some studies have reported a strong correlation between DPPH
• and ABTS
•+ and flavonoid values [
68,
99,
100]. However, according to the results obtained on the content of the investigated individual phenolic compounds and those of DPPH
•, FRAP, and ABTS
•+, it can be assumed a direct role of catechin toward AOA rather than flavonoids. The most substantial reactivity of catechins against free radicals that are determined by FRAP and ABTS
•+ was also reported by Grzesik et al. [
101], who revealed the superiority of monomeric catechin, epicatechin, and epigallocatechin gallates. Despite the extracts derived from
V. opulus berries and a mix containing the highest chlorogenic acid, their contribution to AOA was negligible. No substantial contribution of either total individual saccharides or AAs was observed toward AOA.
The antimicrobial activity of the obtained
V. opulus extracts against 19 test microorganisms was probed using the agar well diffusion method. The selection of this screening method to test antimicrobial activity was based on evidence regarding the lack of direct interconnection between the complex mixtures represented by high-molecular-weight compounds of a hydrophilic and lipophilic nature deposited on a disc with bacteria distributed over media. Due to the compounds’ relatively low diffusivity magnitude, the Kirby–Bauer method only vaguely represents the antimicrobial activity. The initial screening showed that
V. opulus extracts had no antimicrobial activity against certain microorganisms, including
L.
monocytogenes,
B.
cereus,
C.
sakazakii,
E.
cloacae,
C.
perfringens,
E.
coli,
C.
freundii,
C.
albicans, and
A.
brasiliensis. Sangma et al. [
102] made a similar observation, thereby revealing less antimicrobial activity of the methanol extracts from the fruit and leaves of
V.
simonsii against Gram-negative
E.
coli and
S.
enterica and the microscopic fungus C.
ablicans. The lack of antimicrobial activity of the
V. opulus extracts that are rich in the compounds of negative surface charge can partially explain the resistance of Gram-negative bacteria, which is represented by a double membrane composed of phospholipids and lipopolysaccharides outside a thin peptidoglycan layer that is inside of a negative charge [
91]. This statement reinforces the observation regarding the antimicrobial activity of the
V. opulus extracts in inhibiting the Gram-negative bacteria selected in the current study. Fan et al. [
103] indicated that catechins, for instance, have a stronger affinity to the peptidoglycan of Gram-positive bacteria than the negatively charged lipopolysaccharides in the membrane of Gram-negative bacteria. A remarkable activity of the
V. opulus extract derived from berries against Gram-negative
C.
muytjensii and
P.
aeruginosa bacteria was revealed, which can be attributed to the abundance of the chlorogenic acid in this type of
V. opulus extract and its ability to act on the intracellular membrane of
P.
aeruginosa, thereby causing a permeability increase, as well as detachment and loss of ATP [
104]. A positive effect of catechin-rich
V. opulus extracts derived from bark was also observed inhibiting
C.
muytjensii, thus supporting the lateral expansion of the membrane and increasing cell permeability. Among the
V. opulus extracts investigated, the extract derived from berries exhibited the most remarkable antimicrobial activity against Gram-positive bacteria
S.
aureus. Düz et al. [
67] reported a substantially lower activity of the ethanol and water extracts of
V. opulus fruit against
S.
aureus despite the presence of a higher concentration of bioactive compounds than that was observed in the present study, the values of which corresponded to 18.6 and 14.0 mm. It is worth noting that the extracts obtained from
V. opulus, except from flowers and berries, did not contribute to the suppression of
L. innocua and
L. ivanovii growth. These results are consistent with the findings of Puupponen-Pimiä et al. [
105], who indicated the resistance of
L.
monocytogenes and
L. innocua to all eight berry extracts. The lack of inhibition is due to
Listeria spp. stress resistance and this genera’s ability to survive under harsh environmental conditions such as high acidity; the presence of osmolytes, oxidants, and bacteriocins; low and high pressure; and UV light [
106]. The presence of conjugative plasmids and transposons carrying antibiotic resistance makes up most of the
Listeria spp. isolates from clinical, food-borne, and environmental sources resistant to topical antibiotics [
107]. Overall, the extracts derived from
V. opulus effectively inhibited the Gram-positive and Gram-negative bacteria selected in the present study. The produced inhibition zones were similar to those reported by Česonienė et al. [
21] for
V. opulus ethanol extracts, and it was several times higher than those reported by Adebayo et al. [
108] for
V. opulus water extracts.
The MIC values for the
V. opulus extracts of different morphological parts were defined for the first time, generally reinforcing the data approached by the agar well diffusion method. This study indicates the remarkable inhibitory activity of the obtained
V. opulus extracts, wherein they acted in a homeopathic mode of action in a dose-dependent manner, thereby revealing that lower extract doses are more effective than higher ones. It is worth noting that the extract obtained from the flowers of
V. opulus did not show any significant inhibitory activity against the selected bacteria, except for
L. innocua, at either high or low extract doses. This suggests that
L. innocua is highly susceptible to the constituents present in flowers rather than in the bark or berries of
V. opulus. Additionally, the high concentration of AAs in the
V. opulus extract derived from flowers may have encouraged bacterial growth rather than inhibiting it. At higher concentrations, the effects of obtained
V. opulus extracts are less pronounced due to the presence of high-molecular-weight compounds such as pectic polysaccharides, proteins, and fats [
18]. These compounds limit the access of
V. opulus bioactives to the cell membranes, thus weakening the interaction between the bioactive substances and vital organs of the bacteria. This statement can be reinforced by an early observation made by Ildiz et al. [
109], who indicated a lack of antimicrobial activity of free
V. opulus fruit extracts at a concentration range of 2000–125 µL mL
−1 against four Gram-positive and four Gram-negative bacteria, which included two microscopic fungi. However, the same group of researchers reported that nano-scaled metallic objects engineered by green synthesis utilizing
V. opulus fruit extracts deliver remarkable antimicrobial activity, which is achieved by the ability of additional negatively charged regions of nanoparticles to interact with the cell membranes more effectively, thus leading to cell membrane damage. Overall, the obtained agar well diffusion and MIC values indicate the homeopathic potential and effectiveness of
V. opulus extracts in their original state in inhibiting Gram-positive and Gram-negative bacteria at relatively low concentrations. However, further studies are needed to pinpoint the molecular mechanisms of action.