Abstract
Arbuscular mycorrhizal fungi (AMF) are known to enhance plant growth via stimulation of root system development. However, the extent of their effects and underlying mechanisms across different citrus genotypes remain to be fully elucidated. This study investigates the impact of Funneliformis mosseae (F. mosseae) inoculation on plant growth performance, root morphology, phosphorus (P), and indole-3-acetic acid (IAA) concentrations, as well as the expression of related synthesis and transporter genes in three citrus genotypes: red tangerine (Citrus tangerine ex. Tanaka), kumquat (Fortunella margarita L. Swingle), and fragrant citrus (Citrus junos Sieb. ex. Tanaka). Following 12 weeks of inoculation, significant improvements were observed in plant height, shoot and root biomass, total root length, average root diameter, second-order lateral root development, root hair density, and root hair length across all genotypes. Additionally, F. mosseae inoculation significantly increased root P and IAA concentrations in the three citrus genotypes. Notably, phosphatase activity was enhanced in F. margarita but reduced in C. tangerine and C. junos following inoculation. Gene expression analysis revealed a universal upregulation of the P transporter gene PT5, whereas expressions of the auxin synthesis gene YUC2, transporter gene LAX2, and phosphatase gene PAP1 were commonly downregulated. Specific to genotypes, expressions of YUC5, LAX5, PIN2, PIN3, PIN6, and expansin genes EXPA2 and EXPA4 were significantly upregulated in C. tangerine but downregulated in F. margarita and C. junos. Principal component analysis and correlation assessments highlighted a strong positive association between P concentration, P and auxin synthesis, and transporter gene expressions with most root morphology traits, except for root average diameter. Conversely, IAA content and phosphatase activities were negatively correlated with these root traits. These findings suggest that F. mosseae colonization notably enhances plant growth and root system architecture in citrus genotypes via modifications in P transport and IAA accumulation, indicating a complex interplay between mycorrhizal symbiosis and host plant physiology.
1. Introduction
Citrus fruits, renowned for their rich content of vitamins, carotenoids, sugars, and other beneficial compounds, are extensively cultivated across more than 140 countries, with China being a major producer [1]. However, the industry faces significant challenges due to the poor soil conditions—characterized by low fertility, particularly in phosphorus (P) levels, and reduced organic content—prevalent in the southern hills and mountainous regions where citrus is primarily grown [2]. These conditions not only constrain the extent and yield of citrus production but also impact the economic well-being and living standards of local farmers. Citrus plants typically exhibit a robust root system with limited fine roots and root hairs, which are crucial for nutrient absorption under natural cultivation conditions [3]. This limitation necessitates the adoption of innovative biotechnologies, such as arbuscular mycorrhizal (AM) technology, to enhance sustainable agriculture practices in these regions.
Arbuscular mycorrhizal fungi (AMF), beneficial soil microorganisms, form symbiotic relationships with approximately 80% of vascular plants, contributing to nutrient absorption, soil property regulation, and improved plant interactions within ecosystems [4]. Recognized for their multifaceted roles, these fungi are increasingly utilized as biological fertilizers and protectants in horticulture, particularly in fruit production in China [5]. Studies have demonstrated that AMF inoculation in fruit orchards can enhance fruit quality and soil health and increase antioxidant capacities depending on the fungal species involved [5,6]. Furthermore, AMF influence citrus fruit development by modulating gene expression related to sucrose synthesis and transport, thereby improving fruit size and sugar composition [7]. The interaction between AMF and citrus plants significantly affects root morphology, which is pivotal for nutrient uptake and overall plant health. Research indicates that AMF can optimize root architecture by adjusting parameters such as biomass, length, surface area, and lateral root number, with these effects varying among plant species and cultivars [8,9,10]. Specifically, mycorrhizal colonization has been shown to increase root hair density in host plant roots, enhancing water and nutrient absorption [11,12].
However, the influence of AMF on root system architecture (RSA) is subject to various factors, including soil P levels and hormonal balances. Studies on trifoliate orange (Poncirus trifoliata) and tea plants (Camellia sinensis L. O. Kuntze) have revealed that AMF infection promotes RSA, with improvements in root length, volume, surface area, and lateral root formation, which are further amplified by higher P concentrations [13,14]. In addition, AMF inoculation can also affect root hair growth, including their density and length under different soil P levels, and mycorrhiza-induced changes in root hair growth were closely related to upregulated expressions of expansin and simultaneous auxin accumulation in roots [15,16]. During the process, both changes in root morphology and root hairs induced by mycorrhizal are closely related to P metabolism and auxin signaling and transportation [17,18]. These findings underscore the critical role of AMF in facilitating nutrient acquisition and enhancing plant growth under varying soil conditions.
Despite the extensive research on citrus root systems, primarily focusing on trifoliate orange (P. trifoliata) rootstocks, kumquat (Fortunella margarita L. Swingle), red tangerine (Citrus tangerine ex. Tanaka), and fragrant citrus (Citrus junos Sieb. ex. Tanaka) are also commonly used rootstocks in citrus cultivation; there is a gap in understanding the symbiotic relationship between AMF and these different citrus genotypes. Thus, this study aims to address this gap by examining the effects of the AM fungus Funneliformis mosseae, which has been proven to establish a good symbiotic relationship with citrus fruits [11], on root development, P absorption, and indole-3-acetic acid (IAA) transportation in three widely cultivated citrus genotypes in China (as described below). The study aimed to link these physiological aspects to root mycorrhization, offering insights into the potential for optimizing citrus cultivation practices via targeted AMF inoculation.
2. Materials and Methods
2.1. Plant Culture
This study utilized genotypes of C. tangerine, F. margarita, and C. junos as plant materials. Seeds were initially soaked in 1 mol/L NaOH for 10 min and then disinfected with 70% ethanol. Following disinfestation, they were placed in sterilized river sand (autoclaved at 0.11 MPa and 121 °C for 2 h, particle size ≤ 2 mm) and maintained at a day/night temperature of 28/20 °C with a relative humidity of 80% to facilitate germination. Subsequently, uniform seedlings, approximately 4 cm in height and at the two-leaf stage, were transplanted into 2.5 L plastic pots containing river sand (particle size ≤ 4 mm). The sand, sourced from the Yangtze River, was pretreated with a 10% sulfuric acid–nitric acid solution and then autoclaved (121 °C, 0.11 MPa) for 2 h, following the protocol described by Liu et al. [16].
The study employed Funneliformis mosseae (F. mosseae) (Nicol. & Gerd.) Schüßler & Walker [BGC XJ02] as the fungal material. This fungal strain, provided by the Bank of Glomeromycota in China (BGC), was propagated using white clover (Trifolium repens L.) as the host plant over a period of 12 weeks. During the transplantation of seedlings, each pot received an inoculation of 100 g (~3400 spores) of mycorrhizal inoculum, comprising fungal spores, extraradical mycelium, infected root segments, and growth substances (river sand and soil mixture), as the AMF treatment; the AMF strain is only inoculated once during seedling transplantation. The control (non-AMF) treatment consisted of 100 g of sterilized mycorrhizal inoculum (autoclaved at 0.11 MPa and 121 °C for 2 h) supplemented with 2.5 mL of a filtrate (through a 25 μm filter) from the inoculum to maintain similar microbial communities, excluding the AMF strain. Both the AMF-treated and control seedlings were cultivated in a controlled greenhouse environment where the photosynthetic photon flux density was 982 μmol/m2/s, with a day/night temperature of 27/20 °C, and the relative humidity ranged from 75 to 95%. Pots were replaced weekly to mitigate environmental variances. In the week following seedling transplantation, each pot was watered daily with 80 mL of distilled water. Thereafter, every three days, each pot was irrigated with 80 mL of a 1/10 P strength Hoagland solution [16] to support plant growth.
2.2. Experimental Design
The experiment was structured using a two-factor, completely randomized design, incorporating two main variables: inoculation status (with or without F. mosseae) and citrus genotype (comprising C. tangerine, F. margarita, and C. junos). Each treatment combination was replicated six times, resulting in a total of 36 pots. This design facilitated the assessment of the effects of fungal inoculation across different citrus genotypes, while the replication helped ensure the reliability of the results.
2.3. Variables Measurement
After 12 weeks of inoculation with AMF, seedlings—both AMF-inoculated and non-inoculated—were harvested. The shoots and roots were first exposed to a temperature of 105 °C for 30 min and then oven-dried at 70 °C for 48 h until a constant weight was achieved. The roots were cleaned under running tap water and scanned using an Epson Perfection V700 Photo Dual Lens System (J221A, Seiko Epson Corporation, Jakarta Selatan, Indonesia). The images obtained from this scanning process were analyzed for root morphological parameters (including total root length, projected area, surface area, volume, and average diameter) using WinRHIZO Pro 2007b software (Regent Instruments Inc., Quebec, QC, Canada). Then, the number of lateral roots was determined artificially on a test bed.
A selection of 1–2 cm segments from lateral roots, categorized by class, was randomly chosen for further analysis. These segments were cleared using 10% potassium hydroxide and stained with a 0.05% trypan blue solution [19]. The rate of mycorrhizal colonization in the lateral roots was determined following the method described by Shao et al. [14], using a Leica DME binocular light microscope (Leica Microsystem Inc., Wetzlar, Germany) for examination, and calculated as the percentage of AM infected root lengths against total root length.
Additionally, sixty 1 cm long root segments from the root hair zone, specifically 2–6 cm from the root tip, were randomly selected and fixed in 2.5% glutaraldehyde for 24 h. After dehydration via a graded ethanol series, the samples were critical-point dried [16]. Root hair growth was observed using a scanning electron microscope (JSM-6391LV, Japan Electronics Co., Ltd., Akishima, Japan), and measurements were conducted using Image J 1.43u software (National Institutes of Health, Bethesda, MD, USA, http://rsb.info.nih.gov/ij/, accessed on 1 April 2010.).
The P contents were analyzed using inductively coupled plasma–atomic emission spectrometry (ICP-AES, IRIS Advantage, Waltham, MA, USA) after digestion with nitric acid and perchloric acid. Acid phosphatase activity in the leaves and roots was quantified using the p-nitrophenyl phosphate substrate method [20].
IAA levels in the leaves and roots were extracted according to the method described by Dobrev and Kaminek [21] and measured by high-performance liquid chromatography (HPLC). The HPLC analysis utilized a mobile phase of 100% methanol and ultrapure water (2:3, v/v), with a 10 µL sample size, flow rate of 0.8 mL/min, column oven temperature of 30 °C, a total run time of 1 h, and detection performed at a wavelength of 254 nm.
2.4. Gene Expressions Analysis
Root samples were rapidly pulverized using liquid nitrogen, and RNA extraction was performed utilizing the EASY Spin Plus Plant Total RNA Extraction Kit (RN38, Aidlab Biotechnologies Co., Ltd., Beijing, China). Reverse transcription was carried out using the PrimeScript™ RT reagent Kit with gDNA Eraser (RR047A, Takara Bio Inc., Osaka Japan), strictly following the provided protocols. The study aimed to quantify the expression levels of auxin-related genes (YUC2, YUC5, PIN2, PIN3, PIN6, LAX2, LAX3, and LAX5), phosphate transporter genes (PT1, PT3, PT5, and PT6), phosphatase genes (PAP1 and PAP3), and expansins (EXPA2 and EXPA4) in the roots, using the 18S rRNA gene as an internal control for normalization purposes [22]. Specific primers for the target genes were designed utilizing Primer Premier 5.0 software (Palo Alto, CA, USA). The specificity of these primers was validated via the BLAST tool on the NCBI database. All primers were synthesized by Shanghai Sangon BioTech Co., Ltd. (https://www.sangon.com/, accessed on 8 July 2010), Wuhan, China, as outlined in Table 1.

Table 1.
Gene-specific primer sequences used in this work for qTR-PCR.
Quantitative PCR (qPCR) analyses were conducted on the CFX96 real-time PCR Detection System (BIO-RAD, California, CA, USA), with reaction mixtures and cycling conditions set in accordance with the guidelines established by Liu et al. [15]. The relative expression levels of the target genes were calculated using the comparative 2−ΔΔCT method, as described by Livak and Schmittgen [23], allowing for an accurate assessment of gene expression changes.
2.5. Statistical Analysis
The ANOVA procedure of SAS software (version 8.1, SAS Institute, Cary, NC, USA) was utilized to examine differences between treatments, and multiple comparison analysis was conducted using Duncan’s new complex polar difference method (p ≤ 0.05).
3. Results
3.1. Mycorrhizal Colonization
The study found that F. mosseae effectively colonized the root systems of all three citrus genotypes (C. tangerine, F. margarita, and C. junos). Characteristic mycorrhizal structures, including arbuscles (Figure 1a), vesicles (Figure 1b), and internal hyphae (Figure 1c), were seen within their roots, whereas an abundance of exterior hyphae (Figure 1d–f) was visible on the root surfaces. Among these citrus genotypes, mycorrhizal colonization rates varied from 21 to 44%, with a notable trend indicating higher colonization in C. tangerine, followed by C. junos and then F. margarita (Table 2, Figure 1).
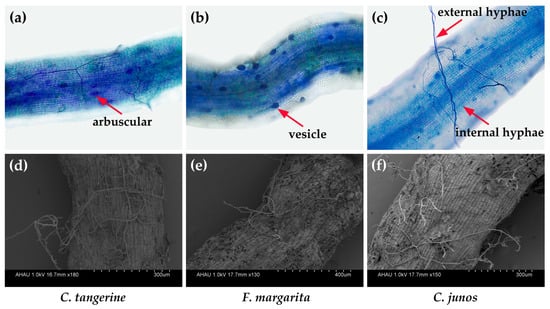
Figure 1.
Root mycorrhizal colonization of three citrus genotype plants by F. mosseae. Root AM colonization in C. tangerine (a), F. margarita (b) and C. junos (c), and external hyphae in C. tangerine (d), F. margarita (e) and C. junos (f).

Table 2.
Effect of F. mosseae on root AM colonization, plant height, and shoot and root biomass of three citrus genotype plants.
3.2. Plant Growth
Inoculation with F. mosseae significantly increased growth and biomass in all three citrus genotypes, as shown in Table 2. The degree of improvement in plant growth performance due to AMF was most significant in C. junos, with increases of 113, 227, and 185%, respectively. C. tangerine had the next largest growth response, increasing by 64, 243, and 154%, respectively. F. margarita responded the least to AMF inoculation, increasing by 38, 96, and 466%, respectively (Table 2).
3.3. Root System Architecture
In this study, inoculation with F. mosseae considerably improved root architectural characteristics in the investigated citrus genotypes when compared to controls without AMF treatment, as shown in Table 3. C. tangerine showed a 6% increase in total root length, 57% in average root diameter, and 267% in the number of 2nd-order lateral roots. F. margarita improved by 20% in total root length, 9% in average root diameter, and 31% in the number of 2nd-order lateral roots. For C. junos, the increases were 9% in total root length, 9% in average diameter, and 103% in the number of 2nd-order lateral roots.

Table 3.
Effect of F. mosseae on root system architecture and lateral root numbers of three citrus genotype plants.
In addition, F. mosseae inoculation increased the root surface area of F. margarita by 9%. Following F. mosseae inoculation, C. junos showed considerable increases in root surface area (9%), root volume (13%), average root diameter (5%), and the number of 1st-order lateral roots (28%). However, the project area of the plants in all three citrus genotypes remained constant after F. mosseae inoculation (Table 3).
3.4. Root Hair Growth
F. mosseae colonization had a considerable impact on root hair properties across the three citrus genotypes, as can be seen in Figure 2a–i. C. tangerine had the highest root hair density, whereas C. junos had the longest root hairs, independent of whether they were infected with F. mosseae. Notably, seedlings colonized by F. mosseae had significantly higher root hair density and length: 15 and 19% in C. tangerine, 58 and 50% in F. margarita, and 14 and 24% in C. junos, respectively. Furthermore, F. mosseae inoculation increased root hair diameter by 24% in C. tangerine, but no significant alterations were seen in the other two citrus genotypes.
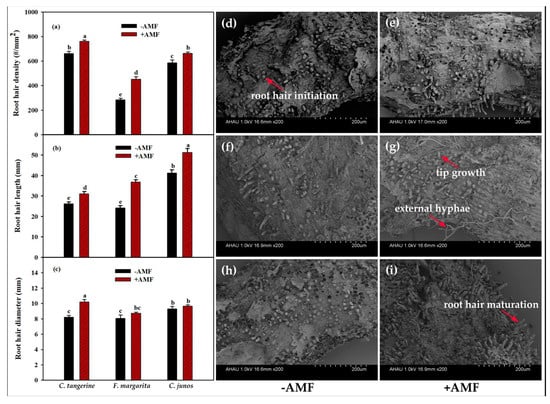
Figure 2.
Effect of F. mosseae on root hair development of three citrus genotypes. (a) Root hair density; (b) root hair length; (c) root hair diameter; and root hair morphology of non-AMF C. tangerine (d), AMF C. tangerine (e), non-AMF F. margarita (f), AMF F. margarita (g), non-AMF C. junos (h), and AMF C. junos (i). The different letters (a, b, c, d, and e) indicate significant differences (p ≤ 0.05) in the different AMF treatment treatments.
3.5. P Content and Phosphatase Activity
Inoculation with F. mosseae significantly increased P content in the roots of all three citrus genotypes. However, the degree of increase varied. The greatest significant increase was seen in C. tangerine, followed by C. junos, and the least in F. margarita, demonstrating a genotype-specific response to F. mosseae colonization (Figure 3A). Contrary to predictions, higher phosphatase activity following F. mosseae colonization was only seen in F. margarita, with an increase of 18%. In contrast, phosphatase activity in C. tangerine and C. junos seedlings was reduced by 45% and 55%, respectively, compared to non-colonized seedlings (Figure 3B).
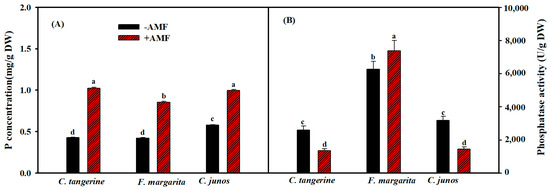
Figure 3.
Effect of F. mosseae on root P concentration (A) and root phosphatase activity (B) of three citrus genotype plants. The different letters (a, b, c, and d) indicate significant differences (p ≤ 0.05) in the different AMF treatment treatments.
3.6. IAA Content
Inoculation with F. mosseae resulted in a considerable increase in root IAA concentration across all three citrus genotypes investigated, while the amount of the increase varied significantly. The greatest increase was seen in F. margarita, where root IAA level increased by 32-fold, demonstrating a significant influence of F. mosseae colonization on auxin production in this genotype. Furthermore, the root IAA content of C. tangerine and C. junos rose by 164% and 64%, respectively (Figure 4).
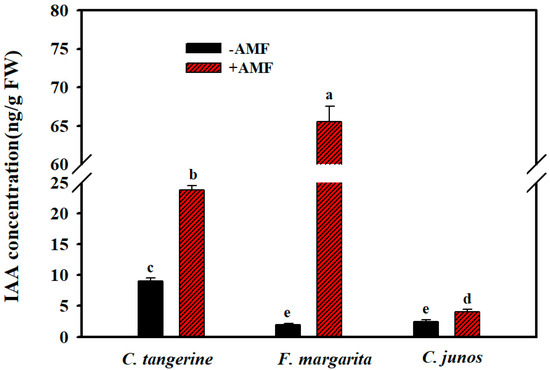
Figure 4.
Effect of AMF F. mosseae on root IAA concentration of three citrus genotype plants. The different letters (a, b, c, d and e) indicate significant differences (p ≤ 0.05) in the different AMF treatment treatments.
3.7. Root EXP Expression
Compared to the non-mycorrhizal control, F. mosseae inoculation resulted in a considerable elevation of the expression levels of the expansin genes CtEXPA2 and CtEXPA4 in C. tangerine, with increases of 3.7-fold and 1.9-fold, respectively. However, in F. margarita, the presence of mycorrhizae resulted in a 1.3-fold and 4.8-fold decrease in FmEXPA2 and FmEXPA4 expressions, respectively, compared to the non-mycorrhizal treatment. Following F. mosseae colonization, the relative expression of CjEXPA2 was decreased by 7.1-fold, but CjEXPA4 expression was unaffected in C. junos (Figure 5).
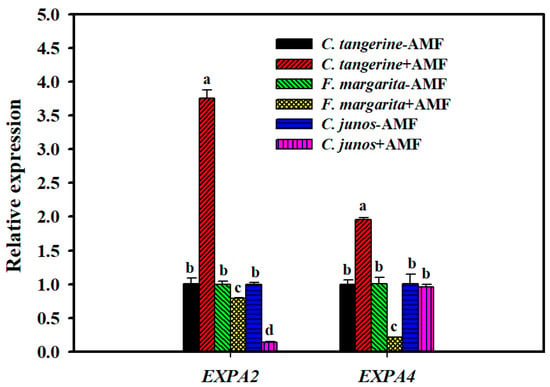
Figure 5.
Effect of F. mosseae on the relative expression of root expansin genes of three citrus genotype plants. The different letters (a, b, c, and d) indicate significant differences (p ≤ 0.05) in the different AMF treatment treatments.
3.8. P Transporter and Phosphatase Gene Expression
In this study, the phosphate transporter gene PT5 was significantly upregulated after F. mosseae inoculation, with relative expression increases of 28.2-fold in C. tangerine, 24.5-fold in F. margarita, and 71.6-fold in C. junos compared to the non-AMF control. Furthermore, F. mosseae inoculation increased CjPT1 expression by 2.9-fold in C. junos but had no significant effect on CjPT3 or CjPT6 expression.
In contrast, the expressions of PT1, PT3, and PT6 were significantly downregulated in C. tangerine by 1.6-fold, 1.4-fold, and 4.4-fold, respectively, and in F. margarita by 4.8-fold, 2.1-fold, and 14.3-fold (Figure 6). Furthermore, F. mosseae inoculation increased PAP3 expression by 1.2-fold in mycorrhizal red tangerine and 1.8-fold in C. junos. However, it reduced the relative expression of PAP3 by 2.5-fold in F. margarita. The study also found a significant downregulation of CtPAP1, FmPAP1, and CjPAP1 expressions by 4.8-fold, 50-fold, and 5-fold among the three citrus genotypes (Figure 6).
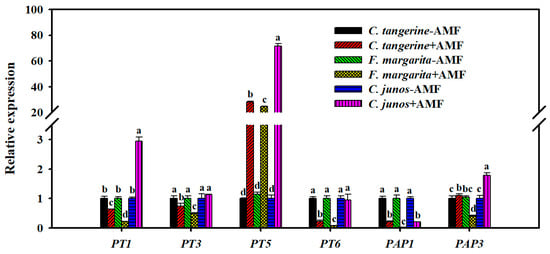
Figure 6.
Effect of F. mosseae on the relative expression of P transporter and phosphatase genes in roots of three citrus genotype plants. The different letters (a, b, c, and d) indicate significant differences (p ≤ 0.05) in the different AMF treatment treatments.
3.9. Root Auxin Related Gene Expression
The effect of F. mosseae colonization on the expression of auxin production and transport genes indicated substantial genotype-specific responses across the citrus genotypes investigated (Figure 7). F. mosseae substantially increased the expression of the auxin synthesis gene YUC5 in C. tangerine (CtYUC5) by 4.1-fold while decreasing expression in F. margarita (FmYUC5) and C. junos (CjYUC5) by 41.2- and 1.4-fold, respectively. Similarly, YUC2 expression was downregulated by 2.1-, 2.2-, and 1.2-fold in C. tangerine (CtYUC2), F. margarita (FmYUC2), and C. junos (CjYUC2), respectively.
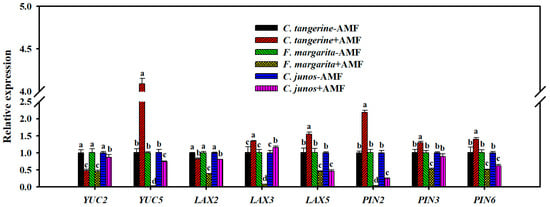
Figure 7.
Effect of F. mosseae on relative expression of root IAA synthesis and carrier genes of three citrus genotype plants. The different letters (a, b, c, and d) indicate significant differences (p ≤ 0.05) in the different AMF treatment treatments.
Regarding the auxin transporter genes LAX3 and LAX5, F. mosseae colonization resulted in an upregulation of 1.3-fold and 1.6-fold, respectively, in C. tangerine but caused a significant downregulation in F. margarita, with FmLAX2, FmLAX3, and FmLAX5 expressions decreasing by 2.6-fold, 12.5-fold, and 2.2-fold, respectively. In C. junos, F. mosseae upregulated CjLAX3 by 1.16-fold but downregulated CjLAX2 and CjLAX5 by 1.3-fold and 2.2-fold, respectively.
Additionally, the colonization significantly upregulated the expression of PIN-formulated genes CtPIN2, CtPIN3, and CtPIN6 in C. tangerine by 2.2-fold, 1.3-fold, and 1.4-fold, respectively. In contrast, in F. margarita, the expressions of FmPIN2, FmPIN3, and FmPIN6 were notably downregulated by 25-fold, 2-fold, and 2-fold, respectively. In C. junos, CjPIN2 and CjPIN6 expressions were downregulated by 4-fold and 1.6-fold, respectively.
3.10. Principal Component Analysis (PCA)
The PCA conducted in this study integrated variables related to AM colonization, root system architecture, lateral root development, P metabolism, and transport, as well as auxin synthesis and transport (Figure 8). The analysis revealed that the first two principal components accounted for 64.6% of the total variation observed (PC1: 38.8%, PC2: 25.8%). The PCA plot illustrated a distinct correlation among root AM colonization, root system architecture, and variables related to P and auxin metabolism and transport. Notably, the treatments involving AM colonization clustered together, regardless of the citrus genotype.
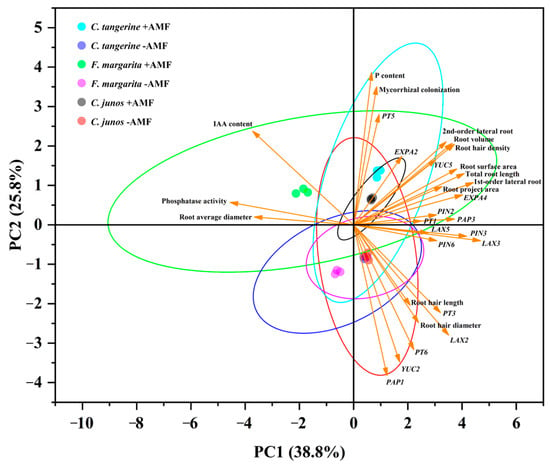
Figure 8.
Principal component analysis (PCA) from all six treatments and 30 variables, including RSA, P, and auxin transportation, assessed in different citrus genotypes inoculated with or without F. mosseae. PC1: variation between varieties; PC2: variation between inoculation and non-inoculation with F. mosseae.
Contrastingly, the non-AMF treatment appeared inversely related to vectors such as lateral root development and associated gene expressions. It also clustered near the vectors representing IAA content, phosphatase activity, and average root diameter (Figure 8). Non-AMF colonized C. junos and C. tangerine samples were associated with root hair diameter and length, along with some expressions of auxin synthesis and transport genes. However, these were positioned further away from AM-treated plants and variables associated with root system architecture and expansin expressions.
Both AM and non-AMF treatments in C. junos and C. tangerine were closely grouped with variables indicative of root architecture, P content, and expansin expressions, yet they were located at an intermediate distance from vectors related to root hair length, diameter, and auxin influx gene expressions.
3.11. Pearson Correlations
The Pearson correlation analysis conducted on the dataset, segregating non-AMF and AMF-colonized plants, aimed to identify correlations unique to each treatment group concerning root system architecture parameters and variables related to P and auxin (Figure 9). Six significant correlations were found to be common between both non-AMF and AMF treatments. These included positive correlations between P content and 2nd-order lateral roots, PIN3 and LAX3, YUC2 and PT5, and total root length with various parameters such as root surface area, project area, volume, lateral root numbers, and root hair density. Additionally, there were correlations between 2nd-order lateral roots and root hair variables, alongside a negative correlation between phosphatase activity and parameters such as total root length, root surface area, volume, 1st-order lateral roots, and root hair density.
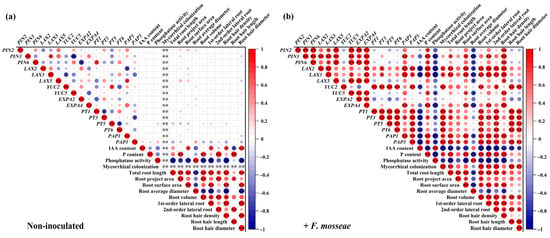
Figure 9.
Pearson correlations between root system architecture parameters and P and auxin transport in different citrus genotypes for non-inoculated treatments (a) or inoculated with the AMF F. mosseae (b). Red and blue colors indicate positive and negative relationships, respectively. Circle sizes indicate the correlation strength. *, p < 0.05, means significant; **, p < 0.01, means extremely significant.
Unique positive correlations in non-AMF plants included relationships between IAA content and root surface, 1st-order lateral roots, root hair density, and root hair diameter; root P content and root project area and root hair length; PIN6 and PT1; LAX5 and PT5; 1st-order lateral roots with root hair density and diameter; and 2nd-order lateral roots with root hair length (Figure 9a).
Conversely, AMF-colonized plants displayed a wider array of unique positive correlations. These encompassed relationships between root P and PIN2, PIN3, PIN5, LAX3, YUC5, AM colonization and most gene expressions with root system parameters; root hair density with most gene expressions, P content, and root system parameters; and root hair length and diameter with P transporter gene expressions (Figure 9b). Additionally, a negative correlation was observed between IAA content, phosphatase activity, root average diameter, and most gene expressions with root system parameters.
4. Discussion
Previous studies have demonstrated that inoculation with various AMF affects citrus plants differently, with F. mosseae showing the highest mycorrhizal colonization, suggesting a particularly beneficial symbiotic relationship with citrus plants [11]. This study was consistent with these findings, revealing significant variances in mycorrhizal dependency across three citrus genotypes (C. tangerine, C. junos, and F. margarita), further emphasizing the role of host genotype in influencing mycorrhizal colonization, which is consistent with previous research [24,25]. Moreover, the affinity between various AM fungi and host plants also contributes to differences in colonization rates, as evidenced by the higher infection rate of F. margarita compared to C. tangerine when inoculated with Diversispora spurca [26]. Furthermore, the data presented indicate that AMF inoculation, particularly with F. mosseae, greatly increases plant growth, with the degree of growth being genotype-dependent. This is consistent with Youpensuk et al. [27], who found varying growth responses in various citrus genotypes after mixed AMF inoculation. Li et al. [26] also reinforced this by demonstrating genotype-specific growth enhancement effects in citrus.
The RSA is critical for water and nutrient absorption, and AMF inoculation dramatically improves RSA characteristics such as root length, surface area, volume, and lateral root number, thus expanding the root coverage absorption area [28]. This increase in RSA was particularly evident in the present study of C. junos when infected with F. mosseae, which is similar to studies on lemon seedlings [14]. Variations in RSA enhancement between genotypes point to genotype-specific responses to AMF inoculation [25]. Additionally, AMF inoculation has been demonstrated to greatly improve root hair density and length, which were essential for nutrition and water absorption, across all tested citrus genotypes. This conclusion was consistent with previous studies revealing AMF’s favorable impacted on root hair formation under a variety of stress circumstances [16,29]. This study also showed a favorable link between mycorrhizal colonization and root hair development, indicating a synergistic interaction between citrus plant water and nutrient intake [11]. Inoculation with AMF could stimulate root hair development by changing the destiny of epidermal cells, impacting root hair cell morphogenesis and gene expression involved in their initiation and growth [30]. AMF infection also increases the production of expansins in the host plant, which are enzymes that allow cell wall loosening and play an important role in root hair formation, as well as the creation of symbiosis between AMF and the host plant [16,31]. In this work, AMF inoculation dramatically increased the expression of EXPA2 and EXPA4 genes in C. tangerine, which corresponded to the observed root hair development pattern. However, in C. junos and F. margarita, the expression of these genes was downregulated, suggesting that root hair growth in these species may be regulated by other expansin sub-families or genes [15]. A positive correlation was found between root hair density and the expression of EXPA4, indicating its involvement in root hair initiation [16].
AMF are known to improve plant growth and RSA by promoting nutrient absorption, such as P. The extraradical hyphae of AMF enhance the root–soil surface area, which improves P interception and delivery to the host [32]. The data revealed that AMF inoculation significantly enhanced root P concentrations across various citrus genotypes, consistent with findings reported in studies on other plants [33,34,35]. This increase in P content was attributed to mycorrhizal absorption and the transfer of phosphate from soil to host roots [36]. However, previous studies also revealed that the expression of P transport genes (PTs) determines the rate of P transport in plants during its uptake and transport process. Interestingly, AMF colonization was found to induce the downregulation of PTs in the roots of host plants, suggesting a reduced necessity for P mobilization from the soil due to efficient mycorrhizal absorption [37]. The investigation revealed differences in the expression levels of PT genes among citrus genotypes, with PT5 showing increased expression across all genotypes. This is consistent with previous study on citrus plants by Liu et al. [14], highlighting their significant role in P absorption and transportation. The association between P concentration, PT expression, and RSA parameters indicated that PTs enhance P absorption and transport, thereby facilitating RSA development via increased P availability. Furthermore, PCA and correlation analysis revealed significant positive associations between P content, PT expression, mycorrhizal colonization, and various RSA and root hair parameters, highlighting the interconnectedness of these factors in promoting plant health and nutrient uptake. This suggested the need for further research to elucidate the specific roles and mechanisms of different PTs in various citrus genotypes.
Phosphatases are pivotal in the uptake, assimilation, and metabolism of P, where microorganisms, enhanced by AMF, secrete phosphatase to catalyze the hydrolysis of organic P, thereby facilitating its availability to plants [38]. The present study found that AMF inoculation significantly increased phosphatase activity in F. margarita seedlings, indicating an enhanced secretion and activity of phosphatase by the extraradical hyphae of F. mosseae. In contrast, phosphatase activities in C. tangerine and C. junos seedlings decreased after AMF inoculation, which is consistent with observations that AMF may reduce root acid phosphatase activity under certain conditions [14], possibly due to the utilization of secreted phosphatases in activating P nutrients. The expression of acid phosphatase genes (PAPs), known to enhance phosphatase activity and induced under P deficiency [39], exhibited variation among the citrus genotypes after AMF inoculation. Significantly, PAP3 was upregulated in C. tangerine and C. junos but downregulated in F. margarita, deviating from the observed changes in phosphatase activity. This suggested that the effect of AMF on phosphatase secretion might be indirectly mediated via alterations in the host’s nutrient status [14]. Moreover, the consistent downregulation of PAP1 across all genotypes indicated a genotype-specific regulation of phosphatase activity by different PAPs, underscoring the necessity for further research on the expression of fungal acid phosphatase genes across various plant genotypes. Correlation analyses have confirmed a positive association between mycorrhizal colonization and PAP expression, linking PAP expression to the RSA and root hair variables, highlighting the complex interactions between mycorrhizal symbiosis and plant nutrient uptake mechanisms.
Auxin, a pivotal phytohormone involved in promoting plant growth and RSA development, experiences significant modulation following AMF inoculation [40]. The research revealed that AMF inoculation markedly increased the concentration of root IAA across all three citrus genotypes; although the extent of this increase varied, this is consistent with the previous studies on litchi colonized by Glomus intraradices and Gigaspora margarita [41] and on citrus colonized by F. mosseae [41] indicating AMF’s potential role in enhancing the expression of auxin synthesis genes. Flavin monooxygenases (YUC) as the key gene families for auxin synthesis [42], which was reported to have higher over-expression in AMF-colonized seedlings roots [16]. Nonetheless, the observed relationship between auxin synthesis genes, such as YUC, and IAA concentration underscores the complexity of auxin synthesis regulation, suggesting it was not exclusively governed by the levels of gene expression. This complexity pointed to a multifaceted interaction between AMF presence and plant hormonal dynamics, necessitating further investigation to fully elucidate the mechanisms underlying AMF-induced changes in auxin synthesis and its implications for plant growth and development.
Polar transport is a unique mode of auxin transport in plants, and IAA located in the root hair zone needs to rely on the auxin influx carrier auxin resistant 1 (AUX1)/like-AUX1 (AUX/LAX), the auxin efflux carrier PIN (PINFORMED) for transport between cells [43,44]. The study further investigated the impact of AMF on auxin transport, emphasizing the significance of auxin transporters such as AUX1, LAX, and PIN in the regulation of IAA distribution, which in turn affects RSA and root hair development. The result obtained from the present study on C. tangerine was in agreement with Yao et al.’s study [41] on litchi (Litchi chinensis Sonn.) seedlings. Nevertheless, the expressions of LAX2, LAX5, PIN2, and PIN6 were dramatically decreased in both F. margarita and C. junos. It is probable that AMF inoculation slows down the differentiation of quiescent center cells [45] and accumulates more auxin in the root cap for root elongation. The differential expression of these transporters among genotypes following AMF inoculation suggested a sophisticated interplay between AMF colonization and auxin transport mechanisms, which contributed to modifications in root architecture via changes in auxin distribution patterns. PCA and correlation analysis highlighted the comprehensive influence of AMF on auxin synthesis, transport, and RSA development, demonstrating positive correlations between auxin-related gene expressions and root system parameters, indicating that AM fungi can achieve more coordinated auxin transmembrane transport [46]. These insights underscore the complex role of AMF in influencing plant growth and development via intricate interactions with the host’s physiological processes. Specifically, the findings illuminate how AMF intervention could affect phosphatase activity and auxin dynamics, thereby facilitating the establishment and optimization of root architecture. This underlined the importance of AMF in modulating plant physiological processes, highlighting its potential utility in agricultural practices aimed at enhancing plant growth and development.
5. Conclusions
Inoculation with F. mosseae has been demonstrated to significantly enhance growth and RSA, including root hair development, across various citrus genotypes. This enhancement was associated with increased P and IAA concentrations, which were attributable to the upregulation of P and auxin synthesis and transporter genes mediated by AMF. The study found that F. mosseae inoculation specifically influenced the synthesis and transport of P and IAA, resulting in distinct response patterns among the citrus genotypes. This variation underscores the need for further research to elucidate the specific mechanisms behind these genotype-dependent responses. Additionally, F. mosseae inoculation was found to promote root hair growth by upregulating the expression of expansin genes, which are crucial for RSA development. In conclusion, the interplay of mycorrhizal symbiosis with enhanced P acquisition and IAA synthesis and transport significantly improves RSA in citrus genotypes, underscoring the multifaceted role of AMF in plant growth and development. This study provided new insights into the complex interplay between mycorrhizal symbiosis and host plant physiology, highlighting the potential of AMF as a valuable tool for sustainable citrus production.
Author Contributions
Conceptualization, Conceptualization, C.-Y.L. and Q.-S.W.; methodology, C.-Y.L., Q.-S.W., X.-N.G. and F.-J.D.; data curation and statistical analysis, C.-Y.L., X.-N.G., F.-J.D. and Q.-S.W.; writing—original draft preparation, C.-Y.L. and Q.-S.W.; writing—review and editing, C.-Y.L. and Q.-S.W.; supervision, Q.-S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 32102315).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data supporting the findings of this study are included in this article.
Acknowledgments
The authors would like to extend their sincere appreciation to the National Natural Science Foundation of China (No. 32102315).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zayed, A.; Badawy, M.T.; Farag, M.A. Valorization and extraction optimization of citrus seeds for food and functional food applications. Food Chem. 2021, 355, 129609. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Wang, S.; Cao, M.-Q.; Zou, Y.-N.; Yao, Y.-X. Tempo-spatial distribution and related functionings of root glomalin and glomalin-related soil protein in a citrus rhizosphere. J. Anim. Plant Sci. 2014, 24, 245–251. [Google Scholar] [CrossRef]
- Florian, K.; Florian, V.; Li, X.; Hinrich, B.; Günter, N.; Benjamin, N.; Frank, h.; Uwe, L. Estimating the importance of maize root hairs in low phosphorus conditions and under drought. Ann. Bot. 2019, 124, 961–968. [Google Scholar] [CrossRef]
- Berger, F.; Gutjahr, C. Factors affecting plant responsiveness to arbuscular mycorrhiza. Curr. Opin. Plant Biol. 2021, 59, 101994. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chang, H.; Feng, Z.; Liu, X.; Zhu, H.; Yao, Q. Growth and photosynthetic responses of litchi seedlings to arbuscular mycorrhizal fungal inoculation: Differences between two genotypes. Not. Bot. Horti Agrobo. 2018, 46, 466–473. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Wang, Y.; Alqahtani, M.-D.; Wu, Q.-S. Positive changes in fruit quality, leaf antioxidant defense system, and soil fertility of beni-madonna tangor citrus (Citrus nanko × C. amakusa) after field AMF inoculation. Horticulturae 2023, 9, 1324. [Google Scholar] [CrossRef]
- Li, Q.-S.; Srivastava, A.-K.; Zou, Y.-N.; Wu, Q.-S. Field inoculation responses of arbuscular mycorrhizal fungi versus endophytic fungi on sugar metabolism associated changes in fruit quality of lane late navel orange. Sci. Hortic. 2023, 308, 111587. [Google Scholar] [CrossRef]
- Chen, W.; Li, J.; Zhu, H.; Xu, P.; Chen, J.; Yao, Q. The differential and interactive effects of arbuscular mycorrhizal fungus and phosphorus on the lateral root formation in Poncirus trifoliata (L.). Sci. Horti. 2017, 217, 258–265. [Google Scholar] [CrossRef]
- Caruso, T.; Mafrica, R.; Bruno, M.; Vescio, R.; Sorgonàb, A. Root architectural traits of rooted cuttings of two fig cultivars: Treatments with arbuscular mycorrhizal fungi formulation. Sci. Hortic. 2021, 283, 110083. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, H.; Twagirayezu, G.; Zhang, F.; Shi, Y.; Luo, C.; Yan, F.; Wang, Z.; Xing, D. Arbuscular mycorrhizal fungi adjusts root architecture to promote leaf nitrogen accumulation and reduce leaf carbon–nitrogen ratio of mulberry seedlings. Forests 2023, 14, 2448. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Liu, C.-Y.; Zhang, D.-J.; Zou, Y.-N.; He, X.-H.; Wu, Q.-H. Mycorrhiza alters the profile of root hairs in trifoliate orange. Mycorrhiza 2016, 26, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Hao, Y.; Wu, X.-L.; Dai, F.-J.; Abd-Allah, E.-F.; Wu, Q.-S.; Liu, S.-R. Arbuscular mycorrhizal fungi improve drought tolerance of tea plants via modulating root architecture and hormones. Plant Growth Regul. 2024, 102, 13–22. [Google Scholar] [CrossRef]
- Zhang, D.-J.; Xia, R.-X.; Cao, X. Ethylene modulates root hair development in trifoliate orange through auxin-signaling pathway. Sci. Horti. 2016, 213, 252–259. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Guo, X.-N.; Wu, X.-L.; Dai, F.-J.; Wu, Q.-S. The comprehensive effects of Rhizophagus intraradices and P on root system architecture and P transportation in Citrus limon L. Agriculture 2022, 12, 317. [Google Scholar] [CrossRef]
- Mohanty, S.-K.; Arthikala, M.-K.; Nanjareddy, K.; Lara, M. Plant-symbiont interactions: The functional role of expansins. Symbiosis 2018, 74, 1–10. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Wang, P.; Zhang, D.-J.; Zou, Y.-N.; Kuca, K.; Wu, Q.-S. Mycorrhiza-induced change in root hair growth is associated with IAA accumulation and expression of EXPs in trifoliate orange under two P levels. Sci. Hortic. 2018, 234, 227–235. [Google Scholar] [CrossRef]
- Chen, W.; Li, J.; Zhu, H.; Xu, P.; Chen, J.; Yao, Q. Arbuscular mycorrhizal fungus enhances lateral root formation in Poncirus trifoliata (L.) as revealed by RNA-Seq analysis. Front. Plant Sci. 2017, 8, 2039. [Google Scholar] [CrossRef]
- Cao, X.; Chen, C.-L.; Zhang, D.-J.; Shu, B.; Xiao, J.; Xia, R.-X. Influence of nutrient deficiency on root architecture and root hair morphology of trifoliate orange (Poncirus trifoliate L. Raf.) seedlings under sand culture. Sci. Hortic. 2013, 162, 100–105. [Google Scholar] [CrossRef]
- Phillips, J.-M.; Hayman, D.-S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McLachlan, K.-D.; Elliot, D.-E.; Marco, D.; Garran, J.-H.; De, M.-D. Leaf acid phosphatase isozymes in the diagnosis of phosphorus status in field-grown wheat. Aust. J. Agric. Res. 1987, 38, 1–13. [Google Scholar] [CrossRef]
- Dobrev, P.-I.; Kaminek, M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J. Chromatogr. A 2002, 950, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-W.; Yuan, F.-R.; Long, G.-Y.; Qin, L.; Deng, Z.-N. Selection of reference genes for quantitative real-time RT-PCR analysis in citrus. Mol. Biol. Rep. 2012, 39, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Livak, L.-J.; Schmittgen, T.-D. Analysis of relative gene expression data using real-time quantitative PCR and 2−∆∆Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Erdinc, C.; Durak, E.-D.; Ekincialp, A.; Şensoy, S.; Demir, S. Variations in response of determinate common bean (Phaseolus vulgaris L.) genotypes to arbuscular mycorrhizal fungi (AMF) inoculation. Turk. J. Agric. For. 2017, 41, 1–9. [Google Scholar] [CrossRef]
- Felföldi, Z.; Vidican, R.; Stoian, V.; Roman, I.-A.; Sestras, A.-F.; Rusu, T.; Sestras, R.-E. Arbuscular mycorrhizal fungi and fertilization influence yield, growth and root colonization of different tomato genotype. Plants 2022, 11, 1743. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, Y.-N.; Wu, Q.-S. Effects of Diversispora spurca inoculation on growth, root system architecture and chlorophyll contents of four citrus genotypes. Int. J. Agric. Biol. 2013, 15, 342–346. [Google Scholar]
- Youpensuk, S.; Lordkaew, S.; Rerkasem, B. Genotypic variation in responses of Citrus spp. to arbuscular mycorrhizal fungi. J. Agric. Sci. 2009, 1, 59. [Google Scholar] [CrossRef][Green Version]
- Satria, B.; Fadli, M.; Herawati, N. Utilization of arbuskular mikoriza fungi [AMF] for growth and ready to release of three genotype gaharu [Aquilaria spp.]. IOP Conf. Ser. Earth Environ. Sci. 2021, 741, 012049. [Google Scholar] [CrossRef]
- Pang, J.; Bansal, R.; Zhao, H.; Bohuon, E.; Lambers, H.; Ryan, M.-H.; Ranathunge, K.; Siddique, K.-H.-M. The carboxylate-releasing phosphorus-mobilizing strategy can be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytol. 2018, 219, 518–529. [Google Scholar] [CrossRef]
- Rigas, S.; Ditengou, F.-A.; Ljung, K.; Daras, G.; Tietz, O.; Palme, K.; Hatzopoulos, P. Root gravitropism and root hair development constitute coupled developmental responses regulated by auxin homeostasis in the Arabidopsis root apex. New Phytol. 2013, 197, 1130–1141. [Google Scholar] [CrossRef]
- Balestrini, R.; Cosgrove, D.-J.; Bonfante, P. Differential location of α-expansin proteins during the accommodation of root cells to an arbuscular mycorrhizal fungus. Planta 2005, 220, 889–899. [Google Scholar] [CrossRef]
- Plassard, C.; Becquer, A.; Garcia, K. Phosphorus transport in mycorrhiza: How far are we? Trends Plant Sci. 2019, 24, 794–801. [Google Scholar] [CrossRef]
- De Oliveira, V.-H.; Mazzafera, P.; de Andrade, S.-A.-L. Alleviation of low phosphorus stress in Eucalyptus grandis by arbuscular mycorrhizal symbiosis and excess Mn. Plant Stress 2022, 5, 100104. [Google Scholar] [CrossRef]
- Sané, A.-K.; Diallo, B.; Kane, A.; Ngom, M.; Cissoko, M.; Sy, M.-O. Response to inoculation with arbuscular mycorrhizal fungi of two tomato (Solanum lycopersicum L.) varieties subjected to water stress under semi-controlled conditions. Agric. Sci. 2022, 13, 790–819. [Google Scholar] [CrossRef]
- El-Sherbeny, T.-M.-S.; Mousa, A.-M.; El-Sayed, E.-S.-R. Use of mycorrhizal fungi and phosphorus fertilization to improve the yield of onion (Allium cepa L.) plant. Saudi J. Biol. Sci. 2022, 29, 331–338. [Google Scholar] [CrossRef]
- Golubkina, N.; Amagova, Z.; Matsadze, V.; Zamana, S.; Tallarita, A.; Caruso, G. Effects of arbuscular mycorrhizal fungi on yield, biochemical characteristics, and elemental composition of garlic and onion under selenium supply. Plants 2020, 9, 84. [Google Scholar] [CrossRef]
- Shu, B.; Xia, R.-X.; Wang, P. Differential regulation of Pht1 phosphate transporters from trifoliate orange (Poncirus trifoliata L. Raf) seedlings. Sci. Hortic. 2012, 146, 115–123. [Google Scholar] [CrossRef]
- Chen, J.; van Groenigen, K.-J.; Hungate, B.-A.; Terrer, C.; van Groenigen, J.-W.; Maestre, F.-T.; Ying, S.-C.; Luo, Y.; Jorgensen, U.; Sinsabaugh, R.-L.; et al. Long-term nitrogen loading alleviates phosphorus limitation in terrestrial ecosystems. Glob. Chang. Biol. 2020, 26, 5077–5086. [Google Scholar] [CrossRef]
- Li, C.-C.; Gui, S.-H.; Yang, T.; Walk, T.; Wang, X.-R.; Liao, H. Identification of soybean purple acid phosphatase genes and their expression responses to phosphorus availability and symbiosis. Ann. Bot. 2012, 109, 275–285. [Google Scholar] [CrossRef]
- Gutjahr, C.; Sawers, R.-J.-H.; Marti, G.; Andrés-Hernández, L.; Yang, S.-Y.; Casieri, L. Transcriptome diversity among rice root types during asymbiosis and interaction with arbuscular mycorrhizal fungi. Proc. Natl. Acad. Sci. USA 2015, 112, 6754–6759. [Google Scholar] [CrossRef]
- Yao, Q.; Zhu, H.-H.; Chen, J.-Z. Growth responses and endogenous IAA and iPAs changes of litchi (Litchi chinensis Sonn.) seedlings induced by arbuscular mycorrhizal fungal inoculation. Sci. Hortic. 2005, 105, 145–151. [Google Scholar] [CrossRef]
- Pacheco-Villalobos, D.; Sankar, M.; Ljung, K.; Hardtke, C.-S. Disturbed local auxin homeostasis enhances cellular anisotropy and reveals alternative wiring of auxin-ethylene crosstalk in Brachypodium distachyon seminal roots. PLoS Genet. 2013, 9, e1003564. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.; Aryal, B.; Donato, M.-D.; Hao, P.-C. A critical view on ABC transporters and their interacting partners in auxin transport. Plant Cell Physiol. 2017, 58, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-J.; Luo, J. The PIN-FORMED auxin efflux carriers in plants. Int. J. Mol. Sci. 2018, 19, 2759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Swarup, R.; Bennett, M.; Schaller, G.-E.; Kieber, J.-J. Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical merstem. Curr. Biol. 2013, 23, 1979–1989. [Google Scholar] [CrossRef]
- Petrášek, J.; Friml, J. Auxin transport routes in plant development. Development 2009, 136, 2675–2688. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).