Physiological and Biochemical Responses of Melilotus albus to Saline and Alkaline Stresses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. Methods
2.2.1. Seed Germination
2.2.2. Physiological Analysis
2.2.3. Physiological Analysis
2.2.4. Statistical Analysis
3. Results
3.1. Effect of Saline–Alkali Stress on Sweet Clover Germination
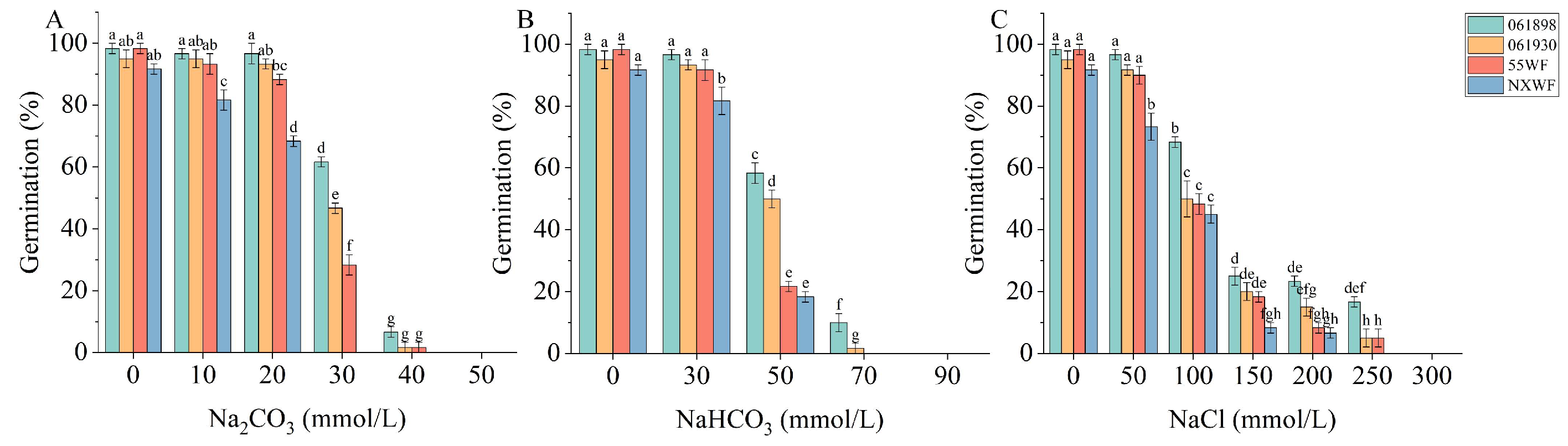
3.1.1. Effect of Saline–Alkali Stress on Sweet Clover Germination Rate
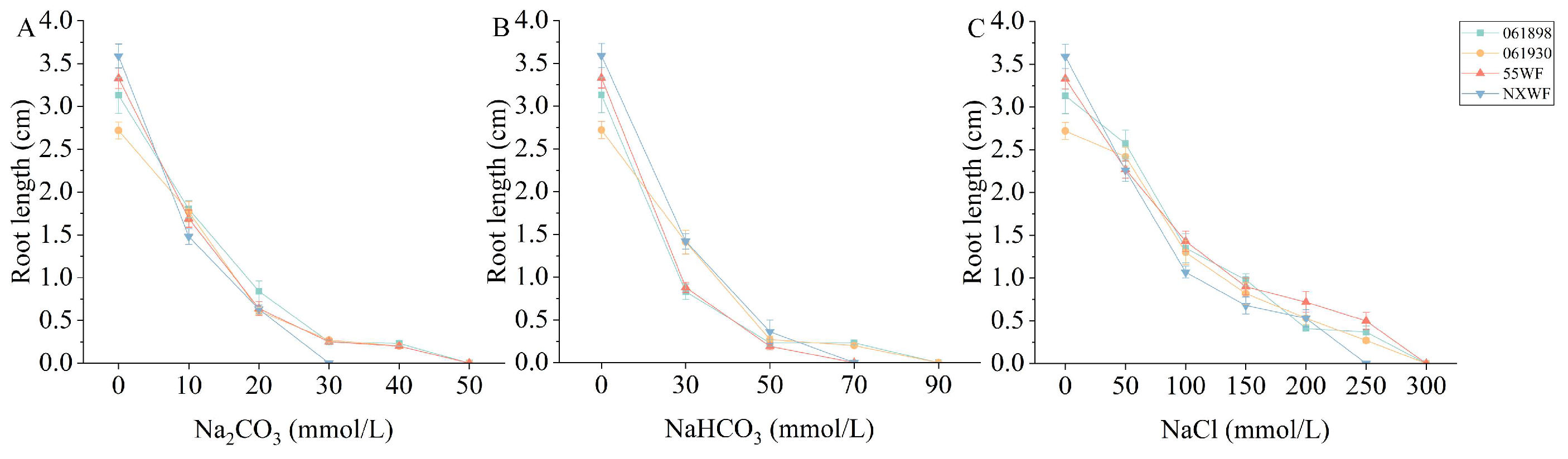
3.1.2. Effect of Saline–Alkali Stress on Sweet Clover Root Length
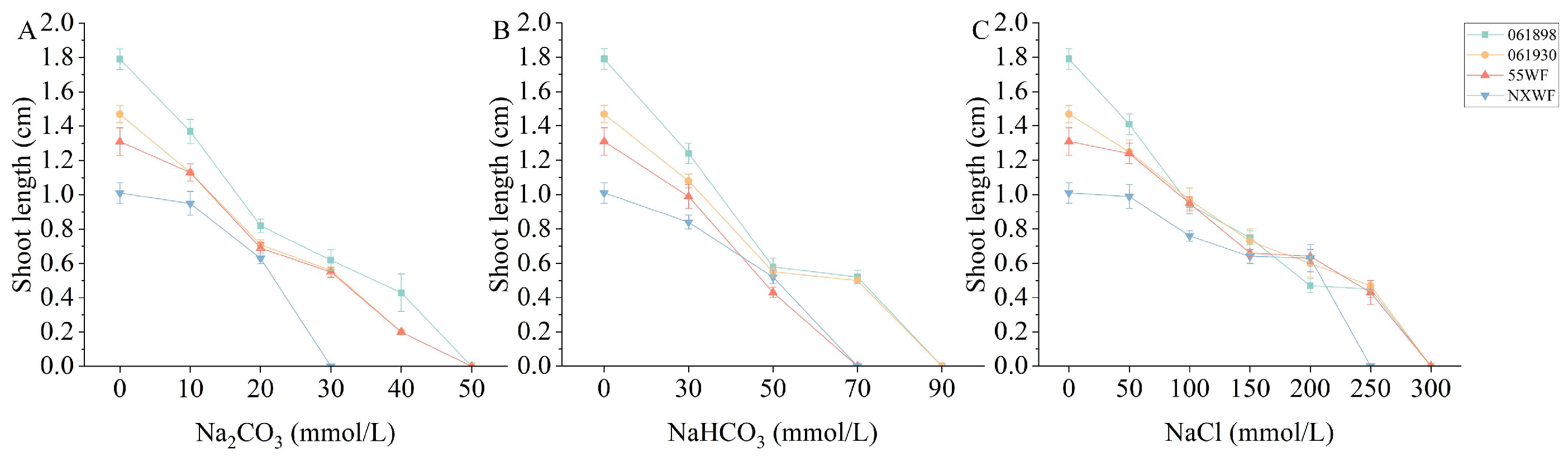
3.1.3. Effect of Saline–Alkali Stress on Sweet Clover Shoot Length
3.2. Effect of Saline–Alkali Stress on Sweet Clover Seedlings
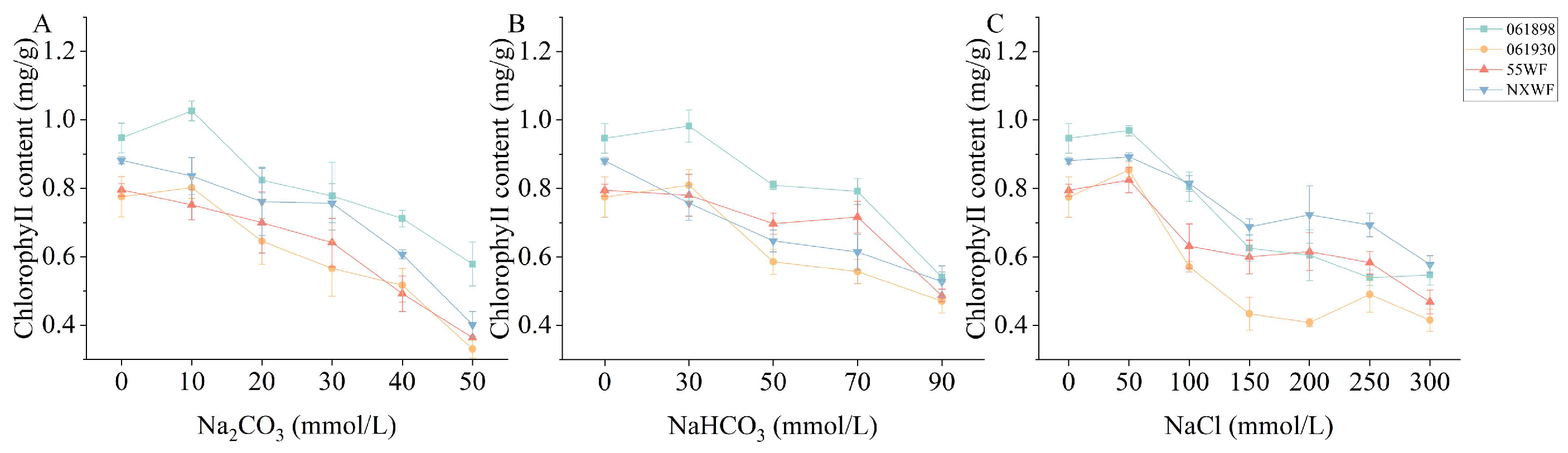
3.2.1. Effect of Saline–Alkali Stress on Sweet Clover Chlorophyll Content
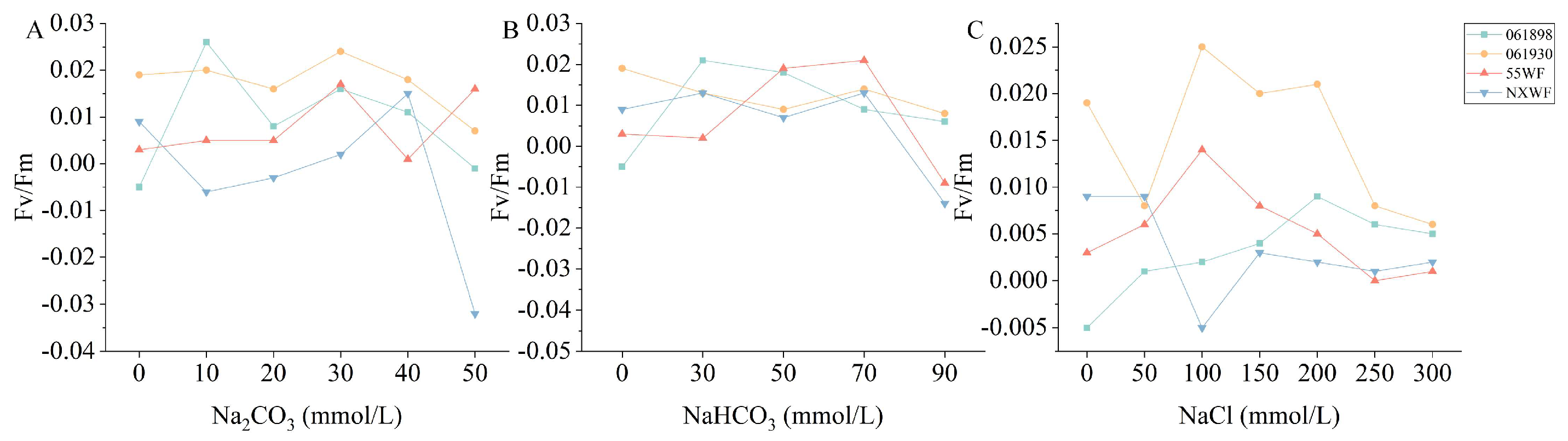
3.2.2. Effect of Saline–Alkali Stress on Sweet Clover Maximum Photochemical Quantum Yield of PSII
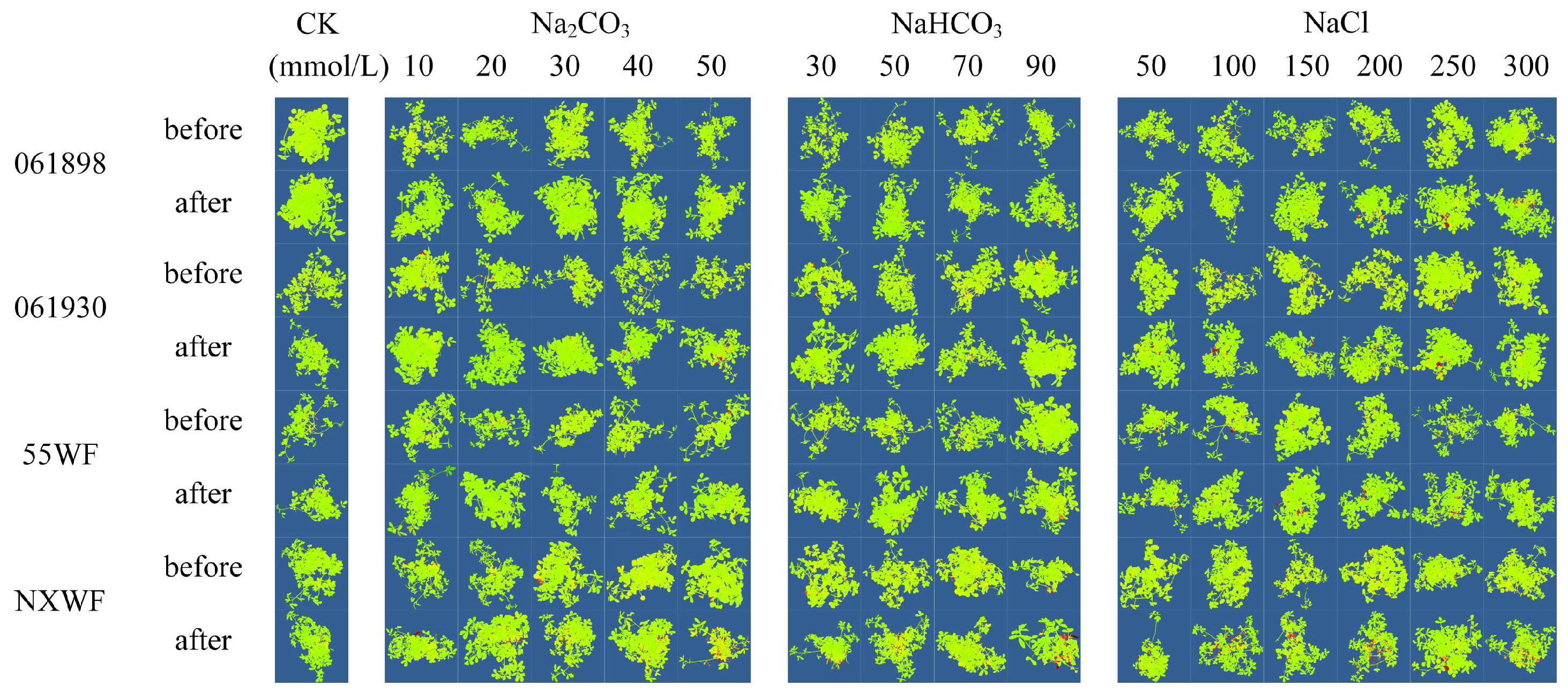
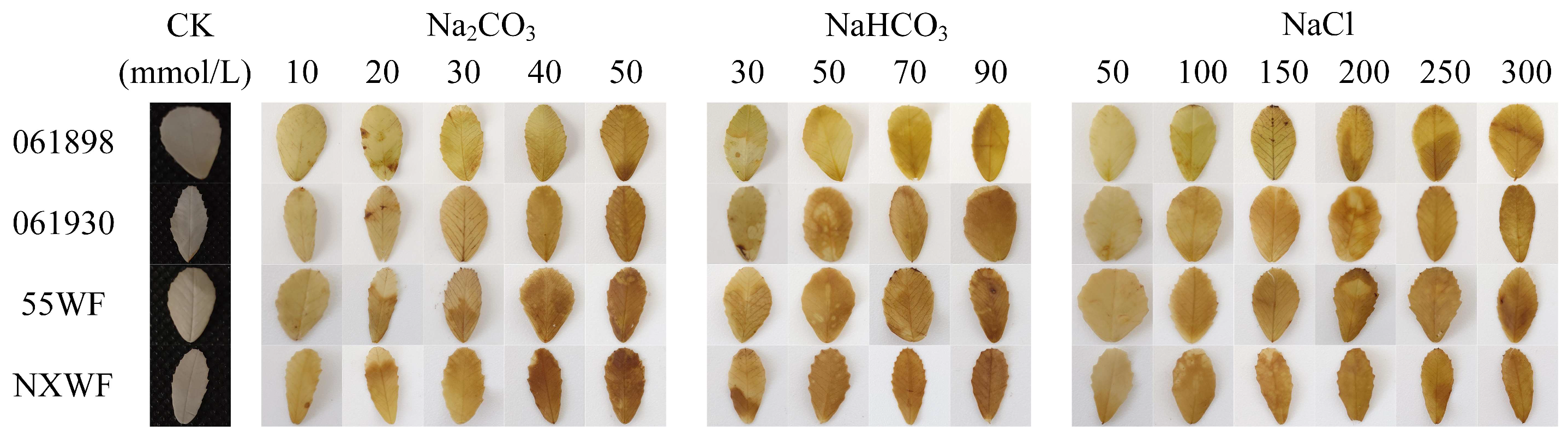
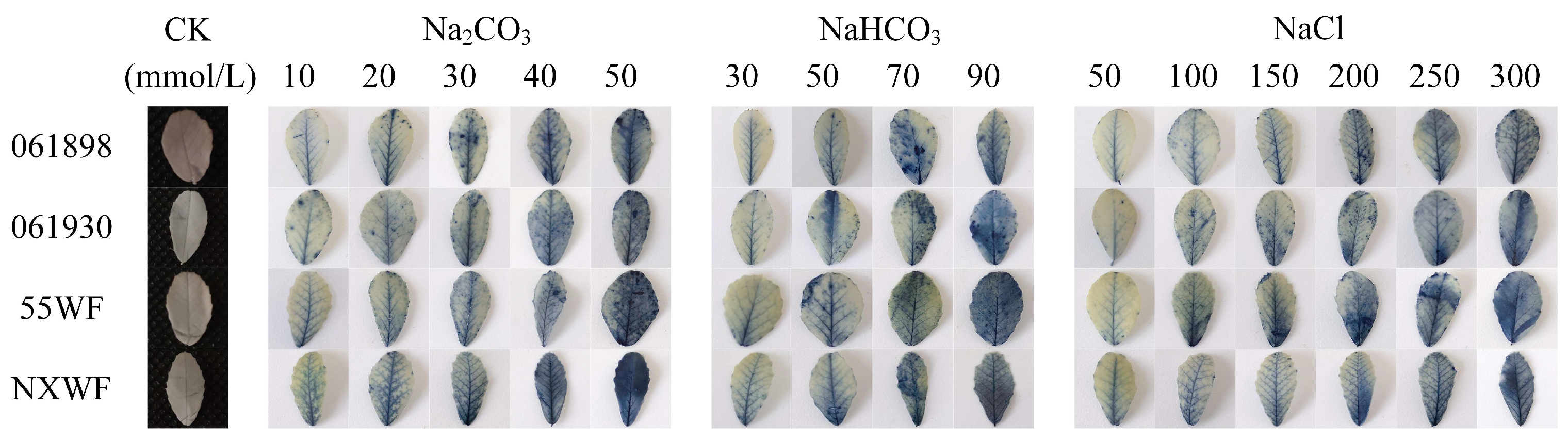
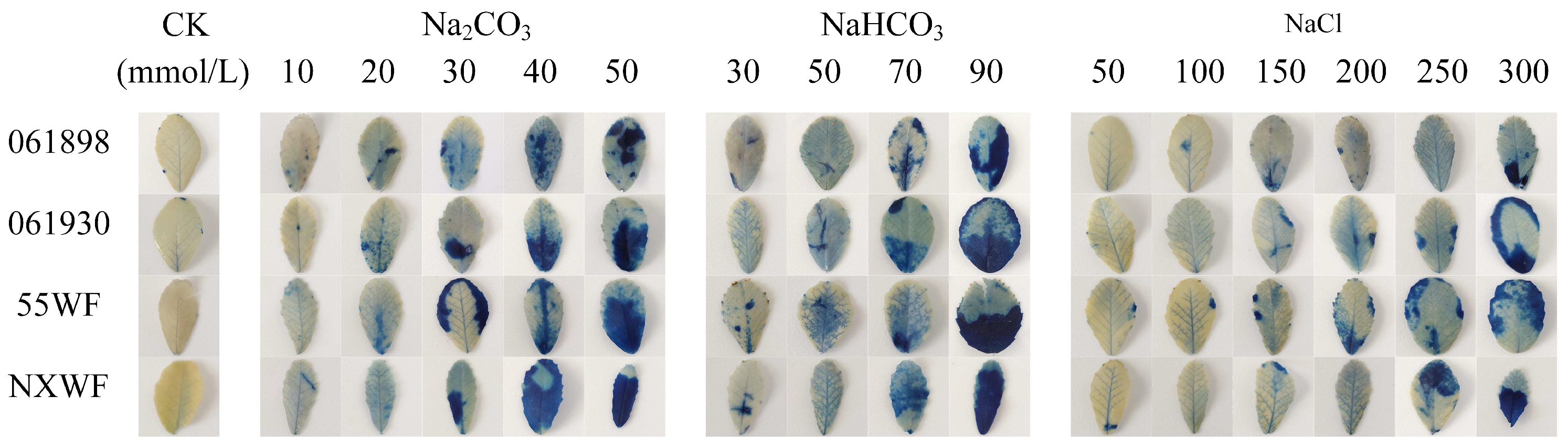
3.2.3. Effects of Saline–Alkali Stress on Sweet Clover ROS Accumulation and Cellular Damage in Leaves
3.2.4. Effect of Saline–Alkali Stress on Sweet Clover Leaf Cell Death
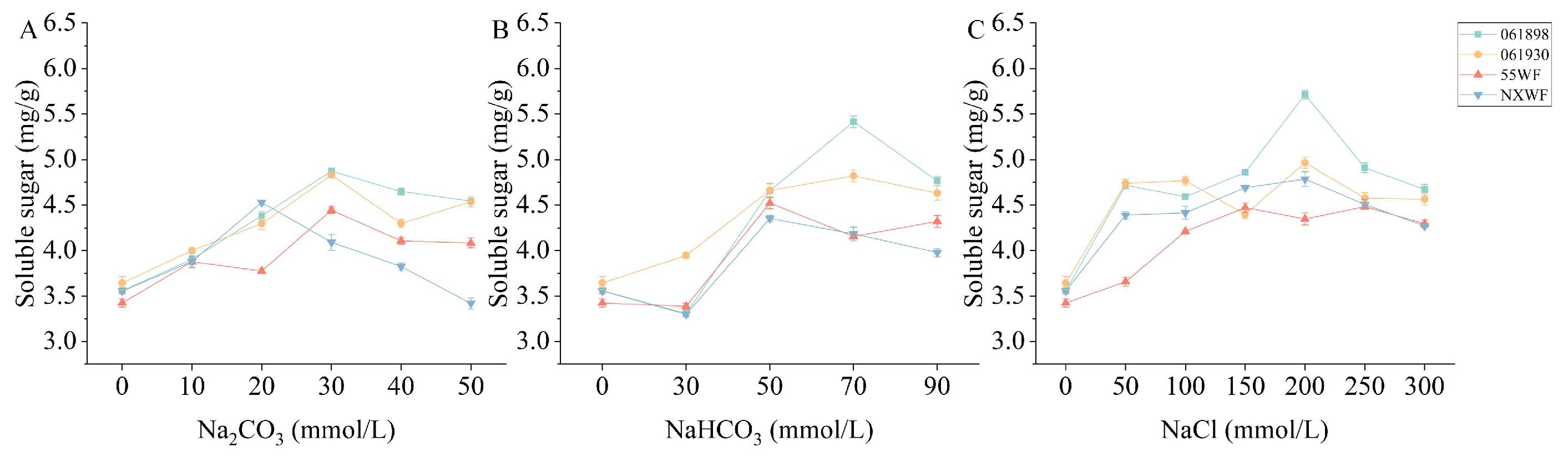
3.2.5. Effect of Saline–Alkali Stress on Sweet Clover Soluble Sugar Content
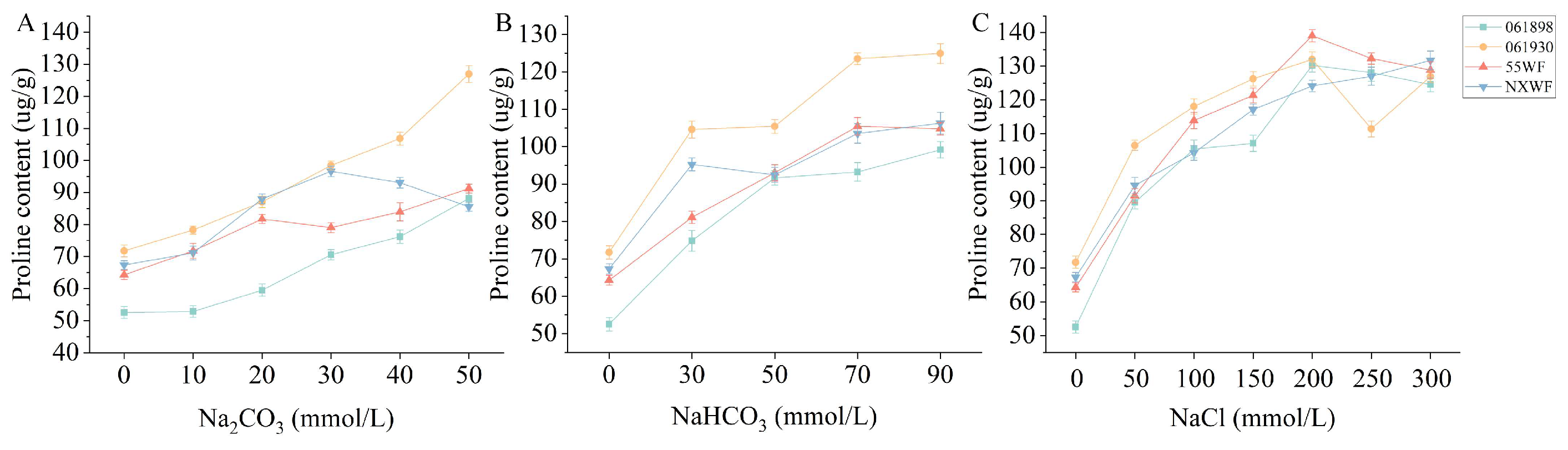
3.2.6. Effect of Saline–Alkali Stress on Sweet Clover Proline Content
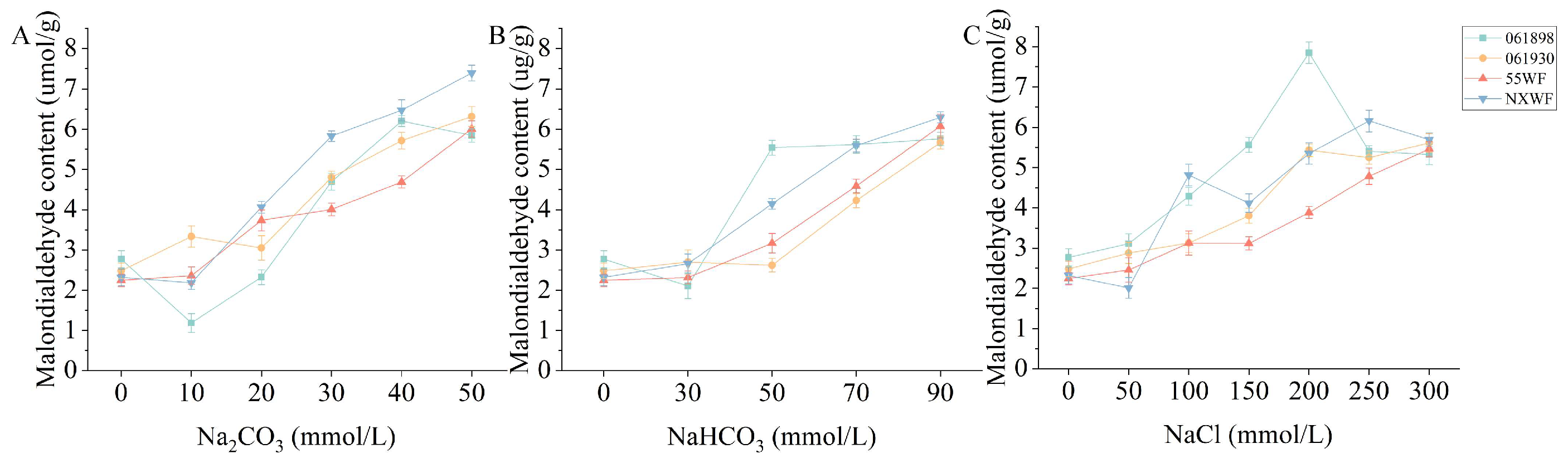
3.2.7. Effect of Saline–Alkali Stress on Sweet Clover Malondialdehyde Content
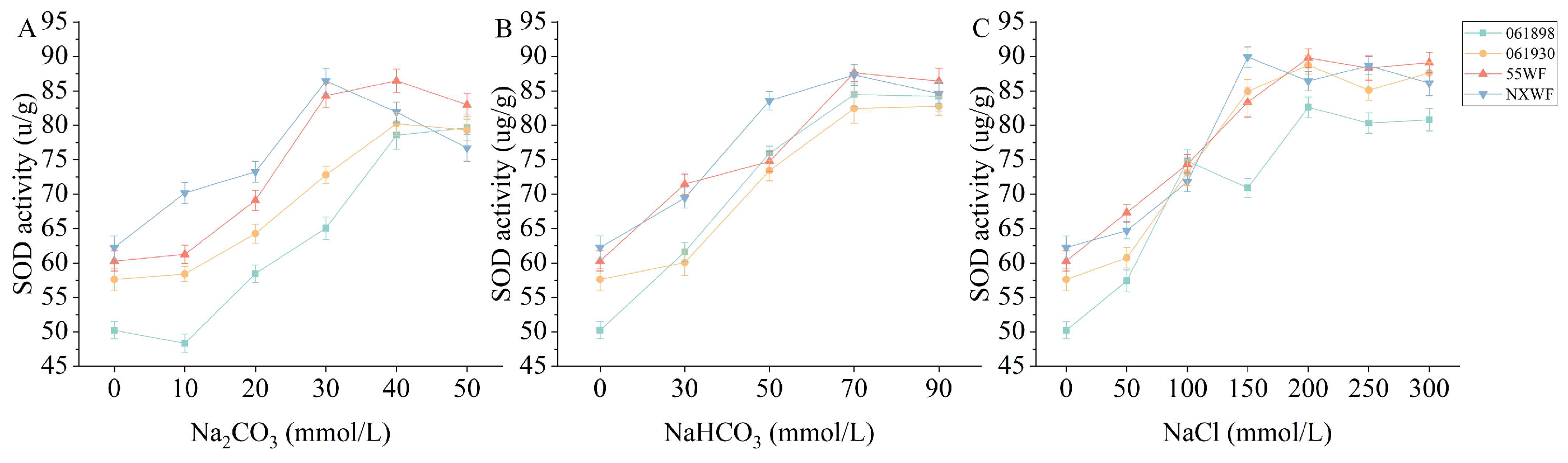
3.2.8. Effect of Saline–Alkali Stress on Sweet Clover SOD Activity
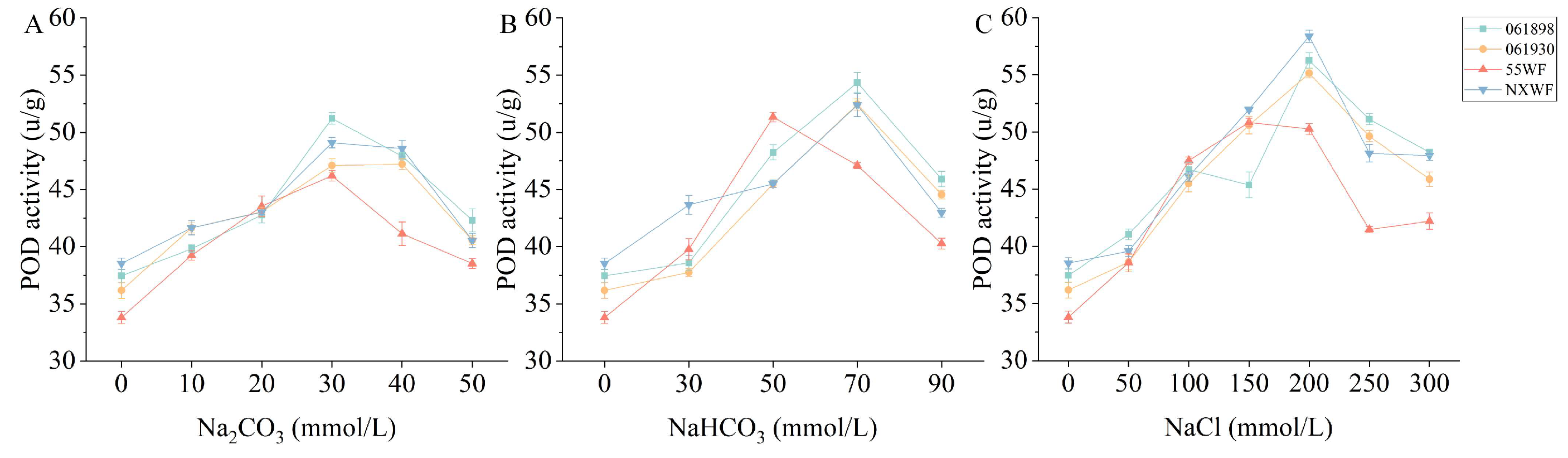
3.2.9. Effect of Saline–Alkali Stress on Sweet Clover POD Activity
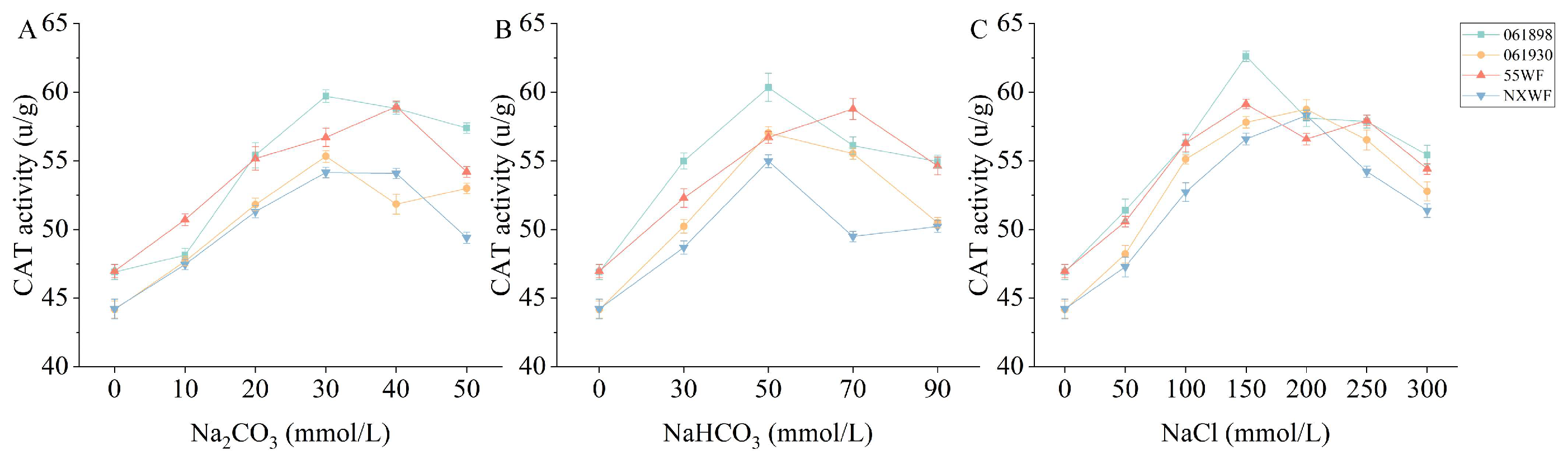
3.2.10. Effect of Saline–Alkali Stress on Sweet Clover CAT Activity
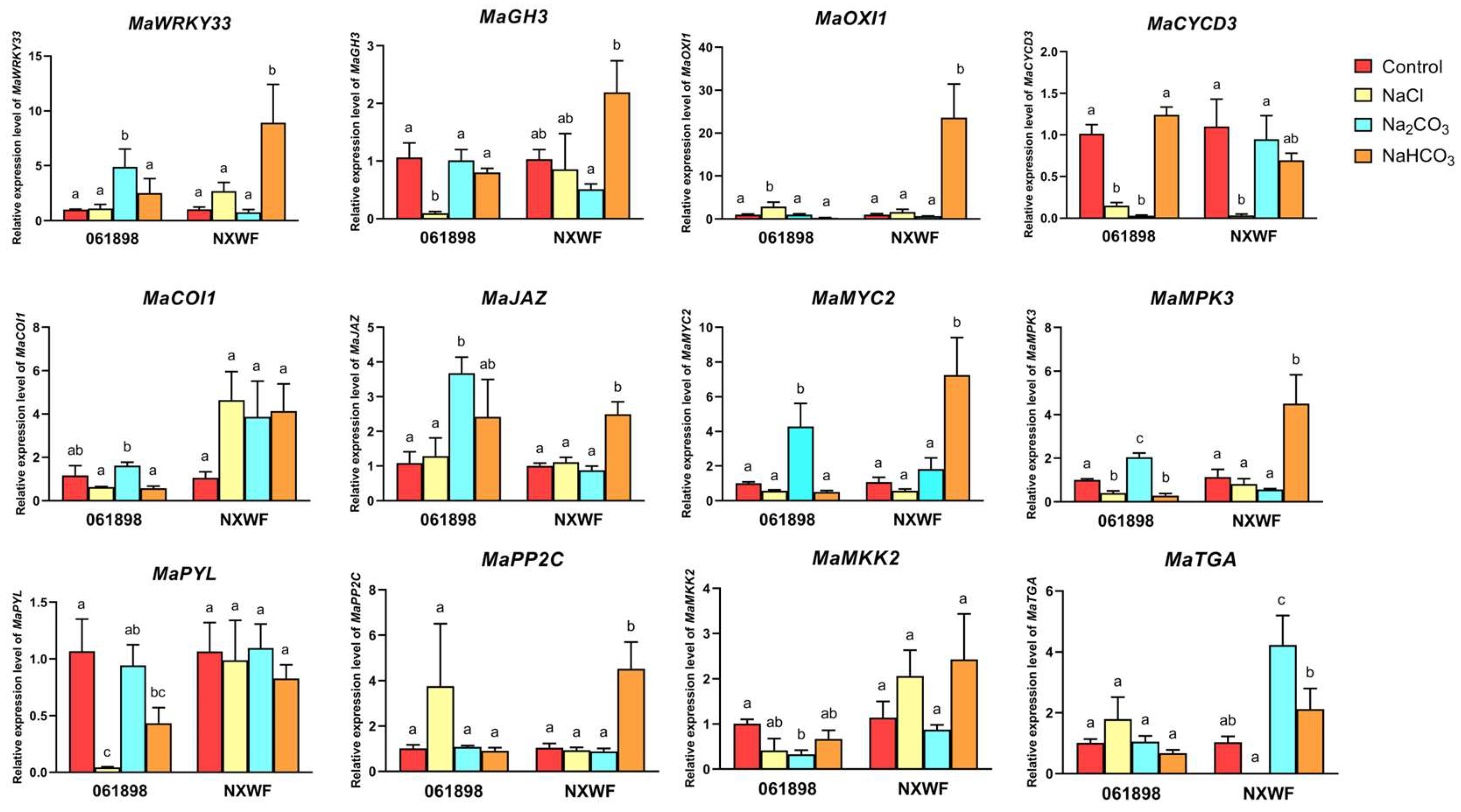
3.2.11. Gene Expression Level Analysis
4. Discussion
4.1. Effect of Saline–Alkali Stress on Sweet Clover Germination
4.2. Effect of Saline–Alkali Stress on Sweet Clover Seedlings
4.2.1. Effect of Saline–Alkali Stress on Chlorophyll Content and Chlorophyll Fluorescence
4.2.2. Effects of Saline–Alkali Stress on H2O2 and O2− Accumulation and Cell Death
4.2.3. Effects of Saline–Alkali Stress on Osmoregulation MDA Content
4.2.4. Effect of Saline–Alkali Stress on Antioxidant Enzyme Activities (SOD, POD, CAT)
4.3. Sweet Clover Gene Expression under Saline–Alkali Treatment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Lian, P.; Zhou, G. Germination and Seeding Growth of Atriplex verrucif era Bieb·under NaCl Stress. Acta Bot. Boreali-Occident. Sin. 2008, 28, 2461–2466. [Google Scholar]
- Fang, S.; Hou, X.; Liang, X. Response Mechanisms of Plants under Saline-Alkali Stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Shi, L.X.; Yan, C.; Zhong, X.; Gu, F.X.; Liu, Q.; Xia, X.; Li, H. Ionomic and metabolic responses to neutral salt or alkaline salt stresses in maize (Zea mays L.) seedlings. BMC Plant Biol. 2017, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L. Studies on Physiological and Biochemical Characteristics Related to Salt Stress in Pistacia vera L.; Xinjiang Agricultural University: Xinjiang, China, 2004. [Google Scholar]
- Ye, T.; Wang, Y.; Feng, Y.Q.; Chan, Z. Physiological and metabolomic responses of bermudagrass (Cynodon dactylon) to alkali stress. Physiol. Plant. 2020, 171, 22–33. [Google Scholar] [CrossRef]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, W.Y.; Yun, D.J. A role for GIGANTEA. Plant Signal. Behav. 2013, 8, e24820. [Google Scholar] [CrossRef]
- Wang, H.; Takano, T.; Liu, S. Screening and Evaluation of Saline–Alkaline Tolerant Germplasm of Rice (Oryza sativa L.) in Soda Saline–Alkali Soil. Agronomy 2018, 8, 205. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Ma, L. Study on Effects and Assessments of NaCl Stress on Seed Germination and Physiological and Biochemical of Seedling of Herbages; Shandong Agricultural University: Taian, China, 2011. [Google Scholar]
- Wu, F.; Duan, Z.; Xu, P.; Yan, Q.; Meng, M.; Cao, M.; Jones, C.S.; Zong, X.; Zhou, P.; Wang, Y.; et al. Genome and systems biology of Melilotus albus provides insights into coumarins biosynthesis. Plant Biotechnol. J. 2022, 20, 592–609. [Google Scholar] [CrossRef]
- Jing, C. A comparison of Salt-Tolerance, Productivity and Nutritional Quality between Six Melilotus; Tarim University: Xinjiang, China, 2015. [Google Scholar]
- Zhang, J.; Di, H.; Luo, K.; Jahufer, Z.; Wu, F.; Duan, Z.; Stewart, A.; Yan, Z.; Wang, Y. Coumarin Content, Morphological Variation, and Molecular Phylogenetics of Melilotus. Molecules 2018, 23, 810. [Google Scholar] [CrossRef]
- McEwen, J.; Johnston, A.E. Yield and nitrogen-fixation of Melilotus alba. Field Crops Res. 1985, 12, 187–188. [Google Scholar] [CrossRef]
- Ge, G.; Liu, Y.; Jia, Y. Nutritional Value and Effect of Melilotus suaveolens Hay on Feeding Cashmere Goat. Acta Agrestia Sin. 2013, 21, 401–405. [Google Scholar] [CrossRef]
- Zhao, T.; Ma, C.; Wang, D.; Jing, Y.; Xi, L. Correlation between rhizobia distribution and the physical/chenical properties in soilof winter wheat intercropped with sweet clover. Acta Prataculturae Sin. 2018, 27, 45–55. [Google Scholar]
- Wu, F.; Luo, K.; Yan, Z.; Zhang, D.; Yan, Q.; Zhang, Y.; Yi, X.; Zhang, J. Analysis of miRNAs and their target genes in five Melilotus albus NILs with different coumarin content. Sci. Rep. 2018, 8, 14138. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Nian, L.; Ain, N.U.; Liu, X.; Yang, Y.; Zhu, X.; Haider, F.U.; Lv, Y.; Bai, P.; Zhang, X.; et al. Genome-Wide Identification and Expression Profiling of the SRS Gene Family in Melilotus albus Reveals Functions in Various Stress Conditions. Plants 2022, 11, 3101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, F.; Yan, Q.; Duan, Z.; Wang, S.; Ao, B.; Han, Y.; Zhang, J. Genome-Wide Analysis of the Rab Gene Family in Melilotus albus Reveals Their Role in Salt Tolerance. Int. J. Mol. Sci. 2022, 24, 126. [Google Scholar] [CrossRef]
- Liu, L.; Meng, S. Notification of the revision of the 2012 edition of the International Seed Testing Regulations. J. Nucl. Agric. Sci. 2012, 26, 762–763. [Google Scholar]
- Wang, A.; Zhang, C.; Wu, C.; Gao, M. Study on a Fast Method of Testing Chlorophyll Content in Maize. J. Maize Sci. 2008, 16, 97–100. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, J.; Ghosh, P.; Basu, D.; Das, S. Chickpea WRKY70 Regulates the Expression of a Homeodomain-Leucine Zipper (HD-Zip) I Transcription Factor CaHDZ12, which Confers Abiotic Stress Tolerance in Transgenic Tobacco and Chickpea. Plant Cell Physiol. 2017, 58, 1934–1952. [Google Scholar] [CrossRef]
- Liu, N.; Lin, Z. Use of Evans Blue for Testing Cell Viability of Intact Leaves of Plant. Plant Physiol. J. 2011, 47, 570–574. [Google Scholar]
- Wang, S.; Wan, C.; Wang, Y.; Chen, H.; Zhou, Z.; Fu, H.; Sosebee, R.E. The characteristics of Na+, K+ and free proline distribution in several drought-resistant plants of the Alxa Desert, China. J. Arid Environ. 2004, 56, 525–539. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Cui, C.; Wang, M.; Guo, J.; Wen, Z.; Li, A. Experiment improvement of the soluble sugar content determination by enthrone colorimetric method. Lab. Sci. 2013, 16, 19–20. [Google Scholar]
- Li, Z.; Wu, X. Experimental Design Scheme for the Effect of Drought Stress on Content of Malondialdehyde of Indoor Ornamental Plants. Tianjin Agric. Sci. 2016, 22, 49–51. [Google Scholar]
- Guo, F.X.; Zhang, M.X.; Chen, Y.; Zhang, W.H.; Xu, S.J.; Wang, J.H.; An, L.Z. Relation of several antioxidant enzymes to rapid freezing resistance in suspension cultured cells from alpine Chorispora bungeana. Cryobiology 2006, 52, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fu, X.Z.; Peng, T.; Huang, X.S.; Fan, Q.J.; Liu, J.H. Spermine pretreatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol. 2010, 30, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Gutterman, Y. Seed Germination in Desert Plants. Endeavour 2014, 19, 47. [Google Scholar]
- Xu, X.Y.; Fan, R.; Zheng, R.; Li, C.M.; Yu, D.Y. Proteomic analysis of seed germination under salt stress in soybeans. J. Zhejiang Univ. Sci. B 2011, 12, 507–517. [Google Scholar] [CrossRef]
- Wei, J.; Cai, Q.; Li, Y.; Shang, L.; Bu, X.; Yu, Z.; Ma, R. Research Progress on Response Mechanism of the Plant to Saline-Alkali Stress. Shandong Agric. Sci. 2022, 54, 156–164. [Google Scholar]
- Dai, M.; Wang, P.; Sun, J.; Zhou, T. Research Progress of Saline-alkali Stress Effect on Seeds’ Germination and Its Physiological and Biochemical Mechanism. North. Hortic. 2015, 10, 176–179. [Google Scholar]
- Shen, Z.; Pan, D.; Wang, J.; Zhang, R.; Li, D.; Gao, C.; Di, G.; Zhong, P. Effects of Saline-Alkaloid Stress on Seed Germination and Seedling Growth of Grasses. Acta Agrestia Sin. 2012, 20, 914–920. [Google Scholar]
- Lu, Y.; Su, C.; Li, H. Effects of different salts on seed germination and seedling growth of Trifolium repens. Acta Prataculturae Sin. 2013, 22, 123–129. [Google Scholar]
- Zheng, Z. Physiological Mechanisms of Hydrogen Sulfide in Regulating Seed Germination and Seedlings Growth of Processing Tomato under NaCl Stress; Shihezi University: Xinjiang, China, 2017. [Google Scholar]
- Liu, Y.; Qi, J.; Liu, Q.; Zhang, X. Study on salt tolerance of three Elymus nutans seeds in germination period. Grassl. Turf 2014, 34, 61–65. [Google Scholar]
- Liu, B.; Zhong, C. The Changes of Morphologic Characteristics and Antioxidant Enzyme Activity of Leymus chinensis under Different Levels of Salt-alkali Stress. Sci. Technol. Eng. 2016, 16, 158–161. [Google Scholar]
- Wang, Y.; Quan, B.; Piao, J.; Piao, M. Comparison about drought resistance of 4 forages at seedling stage unde rwater stress. Agric. Sci. J. Yanbian 2007, 2, 101–106. [Google Scholar]
- Zhang, J.; Miu, N.; Bai, Y.; Wan, X.; Lv, W. Changes of Chlorophyll Content and Antioxidant Enzyme Activity of Diploid Potato under Salt Stress. Crops 2014, 5, 59–63. [Google Scholar]
- Guo, H.; Hu, T.; Fu, J. Effects of saline sodic stress on growth and physiological responses of Lolium perenne. Acta Prataculturae Sin. 2012, 21, 118–125. [Google Scholar]
- Lu, A.; Zhang, F.; Xu, X.; Wang, X.; Yao, S. Effects of salt stress on growth and physiological characteristics of Echinochloa frumentacea seedlings. Acta Prataculturae Sin. 2021, 30, 84–93. [Google Scholar]
- Fang, Y.; Xue, L. Application of Chlorophyll Fluorescence Dynamics to Plant Physiology in Adverse Circumstance. Ecol. Sci. 2019, 38, 225–234. [Google Scholar]
- Rao, L.; Li, S.; Cui, X. Leaf morphology and chlorophyll fluorescence characteristics of mulberry seedlings under waterlogging stress. Sci. Rep. 2021, 11, 13379. [Google Scholar] [CrossRef]
- Xng, Q.; Yu, S.; Niu, Y.; Yu, K.; Song, M. Effects of salt stress on photosynthetic physiology and chlorophyll fluorescence characteristics of grape (Red Globe) seedlings. Agric. Res. Arid Areas 2011, 29, 96–100. [Google Scholar]
- Li, Q.; Ma, C.; Shang, Q. Effects of silicon on photosynthesis and antioxidative enzymes of maize under drought stress. Chin. J. Appl. Ecol. 2007, 18, 531–536. [Google Scholar]
- Su, L.; Bai, T.; Yu, H.; Wu, G.; Tan, L. Effects of Salt Stress on Seedlings Growth, Photosynthesis and Chlorophyll Fluorescence of Two Species of Artocarpus. Sci. Agric. Sin. 2019, 52, 2140–2150. [Google Scholar]
- Li, R.; Wang, Y.; Sun, Y.; Zhang, L.; Chen, A. Effects of salt stress on the growth, physiology, and biochemistry of five Bromus inermis varieties. Acta Prataculturae Sin. 2023, 32, 99–111. [Google Scholar] [CrossRef]
- An, Y.M.; Song, L.L.; Liu, Y.R.; Shu, Y.J.; Guo, C.H. De Novo Transcriptional Analysis of Alfalfa in Response to Saline-Alkaline Stress. Front. Plant Sci. 2016, 7, 931. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Guo, J.; Chen, Y.; Lu, P.; Liu, M.; Sun, P.; Zhang, Z. Roles of endophytic bacteria in Suaeda salsa grown in coastal wetlands: Plant growth characteristics and salt tolerance mechanisms. Environ. Pollut. 2021, 287, 117641. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Wang, T.; Wang, P. Mitigation Effect and Mechanism of Exogenous Melatonin on Maize Seedling under Salt Stress. Chin. J. Grassl. 2020, 42, 14–21. [Google Scholar]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–434. [Google Scholar] [CrossRef]
- Luo, D.; Liu, J.; Wu, Y.; Zhang, X.; Zhou, Q.; Fang, L.; Liu, Z. NUCLEAR TRANSPORT FACTOR 2-LIKE improves drought tolerance by modulating leaf water loss in alfalfa (Medicago sativa L.). Plant J. 2022, 112, 429–450. [Google Scholar] [CrossRef]
- Jia, X.; Deng, Y.; Sun, X.; Liang, L. Impacts of salt stress on the growth and physiological characteristics of Paspalum vaginatum. Acta Prataculturae Sin. 2015, 24, 204–212. [Google Scholar]
- Liu, A.; Zhang, Y.; Fang, Y.; Li, W.; Chen, Z. Effects of salt stress on the growth, antioxidant ability and salt stress protein of Calendula of ficinalis. Acta Prataculturae Sin. 2011, 20, 52–59. [Google Scholar]
- Mi, Y.; Wang, G.; Gong, C.; Cai, Z.; Wu, W. Effects of salt stress on growth and physiology of Isatis indigotica seedings. Acta Prataculturae Sin. 2018, 27, 43–51. [Google Scholar]
- Sun, W.; Han, B.; Hu, X.; Hu, T. Malonyl Dialdehyde Changes of Alfalfa under Salt Stress. J. Anhui Agric. Sci. 2009, 37, 1905–1906+1911. [Google Scholar]
- Chandra, A.; Dubey, A. Effect of ploidy levels on the activities of delta(1)-pyrroline-5-carboxylate synthetase, superoxide dismutase and peroxidase in Cenchrus species grown under water stress. Plant Physiol. Biochem. 2010, 48, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Srivastava, S.; Penna, S.; D’Souza, S.F. Thiourea orchestrates regulation of redox state and antioxidant responses to reduce the NaCl-induced oxidative damage in Indian mustard (Brassica juncea (L.) Czern.). Plant Physiol. Biochem. 2011, 49, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Yan, L.; Mi, F.; Yu, J.; Jia, Z.; Yang, Z.; Yang, W. Salt-Tolerance of Seed Germination and Seedling Growth for Five Grass Species. Seed 2016, 35, 27–31. [Google Scholar]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Ishihama, N.; Yoshioka, H. Post-translational regulation of WRKY transcription factors in plant immunity. Curr. Opin. Plant Biol. 2012, 15, 431–437. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Ren, H.; Zhang, C.; Xiao, D.; Li, Y.; Hou, X.; Liu, T. Regulatory interaction of BcWRKY33A and BcHSFA4A promotes salt tolerance in non-heading Chinese cabbage [Brassica campestris (syn. Brassica rapa) ssp. chinensis]. Hortic. Res. 2022, 9, uhac113. [Google Scholar] [CrossRef]
- Sugimoto, M.; Oono, Y.; Gusev, O.; Matsumoto, T.; Yazawa, T.; Levinskikh, M.A.; Sychev, V.N.; Bingham, G.E.; Wheeler, R.; Hummerick, M. Genome-wide expression analysis of reactive oxygen species gene network in Mizuna plants grown in long-term spaceflight. BMC Plant Biol. 2014, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Rentel, M.C.; Lecourieux, D.; Ouaked, F.; Usher, S.L.; Petersen, L.; Okamoto, H.; Knight, H.; Peck, S.C.; Grierson, C.S.; Hirt, H. OXI1 kinase is necessary for oxidative burst-mediated signalling in Arabidopsis. Nature 2004, 427, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Lee, B.; Choi, G.; Shin, S.; Prasad, D.T.; Lee, O.; Kwak, S.S.; Kim, D.N.J.; Bahk, J.; Hong, J.C. NDP kinase 2 interacts with two oxidative stress-activated MAPKs to regulate cellular redox state and enhances multiple stress tolerance in transgenic plants. Proc. Natl. Acad. Sci. USA 2003, 100, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Pitzschke, A.; Djamei, A.; Bitton, F.; Hirt, H. A major role of the MEKK1-MKK1/2-MPK4 pathway in ROS signalling. Mol. Plant 2009, 2, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Teige, M.; Scheikl, E.; Eulgem, T.; Dóczi, R.; Hirt, H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell 2004, 15, 141–152. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Depuydt, S.; Hardtke, C.S. Hormone signalling crosstalk in plant growth regulation. (Special Issue: Plant signalling pathways: A comparative evolutionary overview.). Curr. Biol. CB 2011, 21, R365–R373. [Google Scholar] [CrossRef]
- Goossens, J.; Fernández-Calvo, P.; Schweizer, F.; Goossens, A. Jasmonates: Signal transduction components and their roles in environmental stress responses. Plant Mol. Biol. 2016, 91, 673–689. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; You, J.; Xu, X.; Yang, Y.; Wang, J.; Zhang, D.; Mu, L.; Zhuang, X.; Shen, Z.; Guo, C. Physiological and Biochemical Responses of Melilotus albus to Saline and Alkaline Stresses. Horticulturae 2024, 10, 297. https://doi.org/10.3390/horticulturae10030297
Wang Z, You J, Xu X, Yang Y, Wang J, Zhang D, Mu L, Zhuang X, Shen Z, Guo C. Physiological and Biochemical Responses of Melilotus albus to Saline and Alkaline Stresses. Horticulturae. 2024; 10(3):297. https://doi.org/10.3390/horticulturae10030297
Chicago/Turabian StyleWang, Zhao, Jia You, Xiaoyue Xu, Ying Yang, Jianli Wang, Dongmei Zhang, Linlin Mu, Xu Zhuang, Zhongbao Shen, and Changhong Guo. 2024. "Physiological and Biochemical Responses of Melilotus albus to Saline and Alkaline Stresses" Horticulturae 10, no. 3: 297. https://doi.org/10.3390/horticulturae10030297
APA StyleWang, Z., You, J., Xu, X., Yang, Y., Wang, J., Zhang, D., Mu, L., Zhuang, X., Shen, Z., & Guo, C. (2024). Physiological and Biochemical Responses of Melilotus albus to Saline and Alkaline Stresses. Horticulturae, 10(3), 297. https://doi.org/10.3390/horticulturae10030297




