Does the Physiological Age of Stock Plant Material Affect the Uptake of Indole-3-Butyric Acid (IBA) in Leafy Cuttings of Prunus subhirtella ‘Autumnalis’?
Abstract
1. Introduction
2. Material and Methods
2.1. Sample Preparation
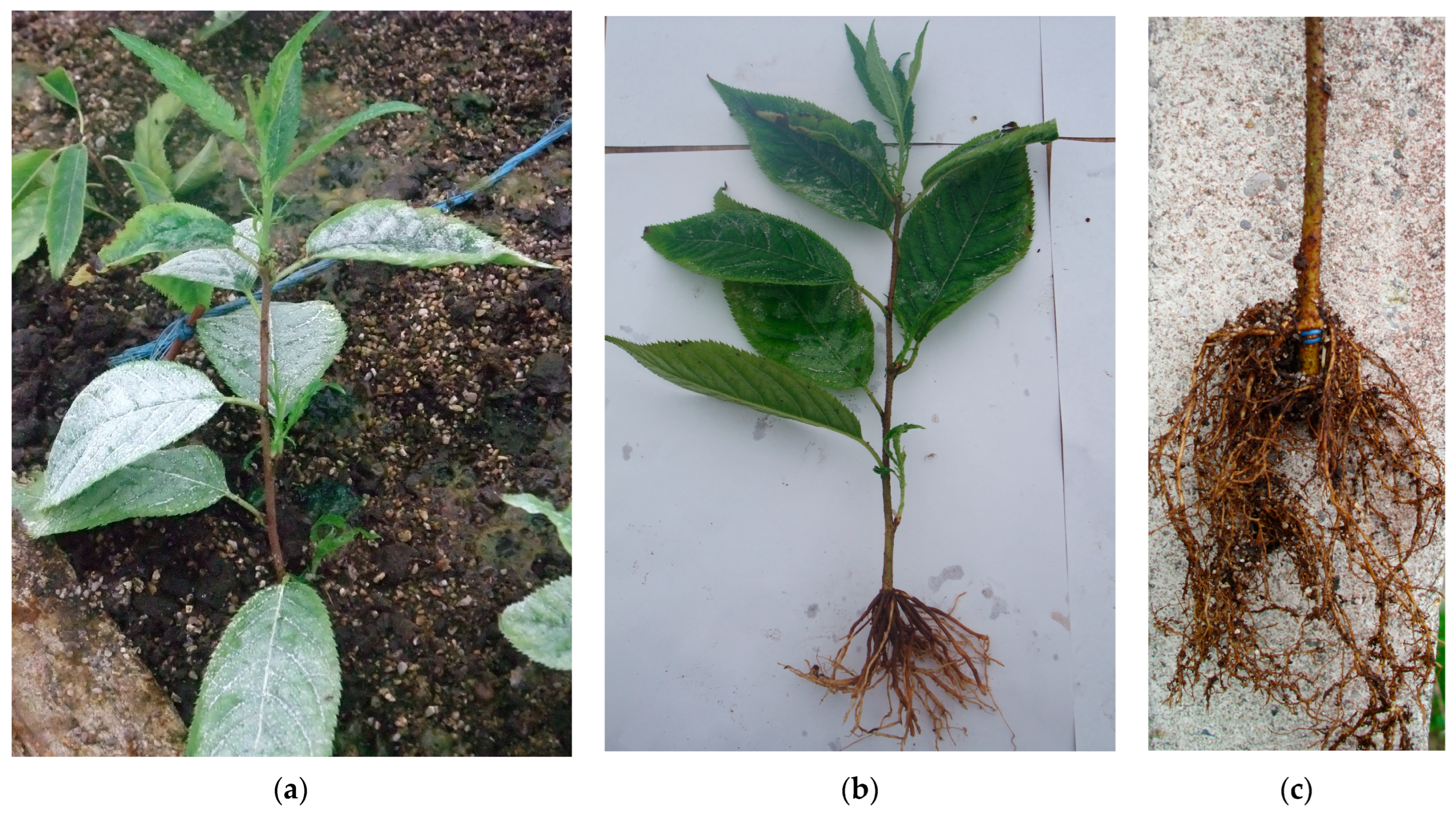
2.2. Evaluation of the Root Formation
2.3. Phytohormone Extraction and Analyses
2.4. Chemicals
2.5. Statistical Analysis
3. Results
3.1. Phytohormone Dynamics in Cuttings
3.2. Evaluation of Newly Formed Adventitious Root System
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vošnjak, M.; Osterc, G. Issues relating to the use of cold storage method in the production of herbaceous and woody cuttings of ornamental plants. Acta Agric. Slov. 2022, 118, 1–10. [Google Scholar]
- Legué, V.; Rigal, A.; Bhalerao, R.P. Adventitious root formation in tree species: Involvement of transcription factors. Physiol. Plant. 2014, 151, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Dong, Z.; Zheng, X.; Song, S.; Jiao, J.; Wang, M.; Song, C. Auxin and its interaction with ethylene control adventitious root formation and development in apple rootstock. Front. Plant Sci. 2020, 11, 574881. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Ruan, L.; Wang, L.; Cheng, H. Auxin-induced adventitious root formation in nodal cuttings of Camellia sinensis. Int. J. Mol. Sci. 2019, 20, 4817. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Hosseini, S.A.; Hajirezaei, M.R.; Druege, U.; Geelen, D. Adventitious rooting declines with the vegetative to reproductive switch and involves a changed auxin homeostasis. J. Exp. Bot. 2015, 66, 1437–1452. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B.; Rasmussen, A. The Physiology of Adventitious Roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Osterc, G.; Štampar, F. Differences in endo/exogenous auxin profile in cuttings of different physiological ages. J. Plant Physiol. 2011, 168, 2088–2092. [Google Scholar] [CrossRef]
- Pamfil, D.; Bellini, N. Auxin control in the formation of adventitious roots. Not. Bot. Horti Agrobot. Cluj Napoca 2011, 39, 307–316. [Google Scholar]
- Pacurar, D.I.; Perrone, I.; Bellini, C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol. Plant. 2014, 151, 83–96. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Vertocnik, A.; Town, C.D. Analysis of indole-3-butyric acid-induced adventitious root formation on Arabidopsis stem segments. J. Exp. Bot. 2005, 56, 2095–2105. [Google Scholar] [CrossRef]
- Vanitha, K.; Kumar, M.; Sivakumar, V. Impact of Selected Plant Growth Regulators on Rooting Response of Stem Cuttings of Psidium guajava L. Int. J. Plant Soil Sci. 2023, 35, 320–325. [Google Scholar] [CrossRef]
- Bellini, C. A synthetic auxin for cloning mature trees. Nat. Biotechnol. 2024, 24, 13. [Google Scholar] [CrossRef]
- Bettoni, J.C.; van der Walt, K.; Aparecida Souza, J.; McLachlan, A.; Nadarajan, J. Sexual and asexual propagation of Syzygium maire, a critically endangered Myrtaceae species of New Zealand. N. Z. J. Bot. 2024, 62, 35–52. [Google Scholar] [CrossRef]
- Damodaran, S.; Strader, L.C. Indole 3-butyric acid metabolism and transport in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 851. [Google Scholar] [CrossRef]
- Frick, E.M.; Strader, L. Roles for IBA-derived auxin in plant development. J. Exp. Bot. 2018, 69, 169–177. [Google Scholar] [CrossRef]
- Kreiser, M.; Giblin, M.; Murphy, R.; Fiesel, P.; Braun, L.; Johnson, G.; Wyse, D.; Cohen, J.D. Conversion of indole-3-butyric acid to indole-3-acetic acid in shoot tissue of hazelnut (Corylus) and elm (Ulmus). J. Plant Growth Regul. 2016, 35, 710–721. [Google Scholar] [CrossRef]
- Nag, S.; Saha, K.; Choudhuri, M.A. Role of Auxin and Polyamines in Adventitious Root Formation in Relation to Changes in Compounds Involved in Rooting. J. Plant Growth Regul. 2001, 20, 182–194. [Google Scholar] [CrossRef]
- De Klerk, G.-J.; van der Krieken, W.; de Jong, J.C. Review the formation of adventitious roots: New concepts, new possibilities. Vitr. Cell. Dev. Biol.-Plant 1999, 35, 189–199. [Google Scholar] [CrossRef]
- Ford, Y.Y.; Bonham, E.C.; Cameron, R.W.F.; Blake, P.S.; Judd, H.L.; Harrison-Murray, R.S. Adventitious rooting: Examining the role of auxin in an easy- and a difficult-to-root plant. Plant Growth Regul. 2002, 36, 149–159. [Google Scholar] [CrossRef]
- Osterc, G.; Mikulic, M.P.; Stampar, F. Quantification of IAA metabolites in the early stages of adventitious rooting might be predictive for subsequent differences in rooting response. J. Plant Growth Regul. 2016, 35, 534–542. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Cao, S.; Guo, Q.; Sun, Y.; Niu, D.; Long, C.; Fan, Y.; Li, Y. The RpTOE1-RpFT Module Is Involved in Rejuvenation during Root-Based Vegetative Propagation in Robinia pseudoacacia. Int. J. Mol. Sci. 2022, 23, 5079. [Google Scholar] [CrossRef]
- Maghuly, F.; da Camara Machadob, A.; Leopolda, S. Long-term stability of marker gene expression in Prunus subhirtella: A model fruit tree species. J. Biotechnol. 2007, 127, 310–321. [Google Scholar] [CrossRef]
- Trapp, M.A.; De Souza, G.D.; Rodrigues-Filho, E.; Boland, W.; Mithofer, A. Validated method for phytohormone quantification in plants. Front. Plant Sci. 2014, 5, 98911. [Google Scholar]
- López-Villamor, A.; Míguez-Soto, B.; Fernández-López, J. Adventitious root formation in Castanea sp. semi-hard cuttings is under moderate genetic control caused mainly by non-additive genetic variance. Can. J. For. Res. 2017, 47, 946–956. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, M.; Luo, X.; Kuang, R.; Yang, H.; Yao, J.; Huang, B.; Wei, Y. Transcriptomic analysis of papaya (Carica papaya L.) shoot explants obtained by leaf-and stem-inoculation methods for adventitious roots induction. Sci. Hortic. 2021, 276, 109762. [Google Scholar] [CrossRef]
- Babashpour, M.; Shakueefar, S.; Valipour, V. Effects of Indole-3-butyric Acid on the Rooting Ability of Semi-hardwood Bougainvillea sp. Cuttings. Mod. Appl. Sci. 2012, 6, 121–123. [Google Scholar] [CrossRef]
- Camellia, N.A.; Thohirah, L.A.; Abdullah, N.A.P.; Mohd Khidir, O. Improvement on Rooting Quality of Jatropha curcas Using Indole Butyric Acid (IBA). Res. J. Agric. Biol.Sci. 2009, 5, 338–343. [Google Scholar]
- Khandaker, M.M.; Saidi, A.; Badaluddin, N.A.; Yusoff, N.; Majrashi, A.; Alenazi, M.M.; Saifuddin, M.; Alam, M.A.; Mohd, K.S. Effects of Indole-3-Butyric Acid (IBA) and rooting media on rooting and survival of air layered wax apple (Syzygium samarangense) CV Jambu Madu. Braz. J. Biol. 2022, 82, e256277. [Google Scholar] [CrossRef]
- Strader, L.C.; Culler, A.H.; Cohen, J.D.; Bartel, B. Conversion of endogenous indole-3-butyric acid to indole-3-acetic acid drives cell expansion in Arabidopsis seedlings. Plant Physiol. 2010, 153, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Osterc, G.; Stefancic, M.; Stampar, F. Juvenile stock plant material enhances root development through higher endogenous auxin level. Acta Physiol. Plant. 2009, 31, 899–903. [Google Scholar] [CrossRef]
- Awotedu, B.F.; Omolola, T.O.; Akala, A.O.; Awotedu, O.L.; Olaoti-Laaro, S.O. Vegetative Propagation: A Unique Technique of Improving Plants Growth. Int. Sci. J. World News Nat. Sci. 2021, 35, 83–101. [Google Scholar]
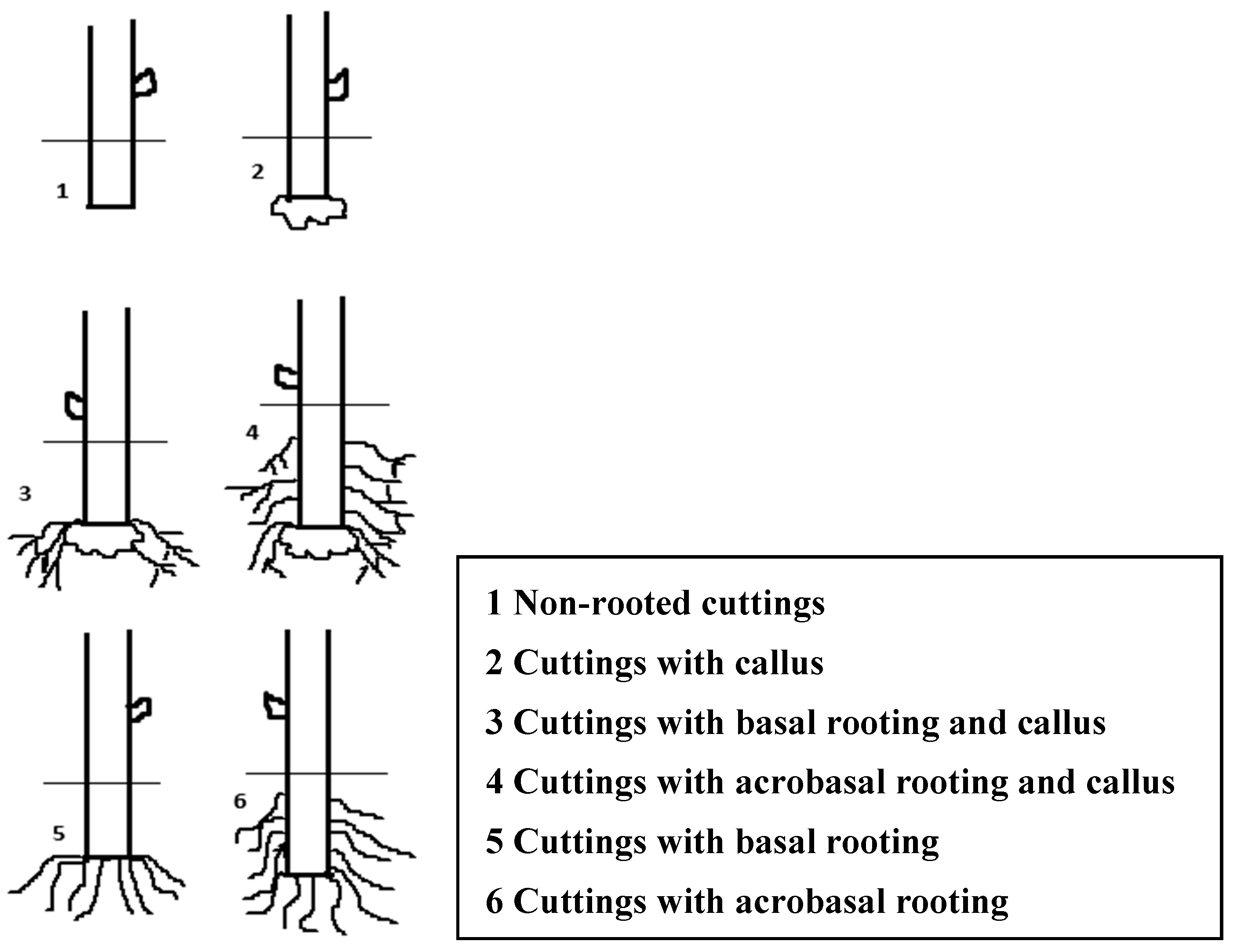
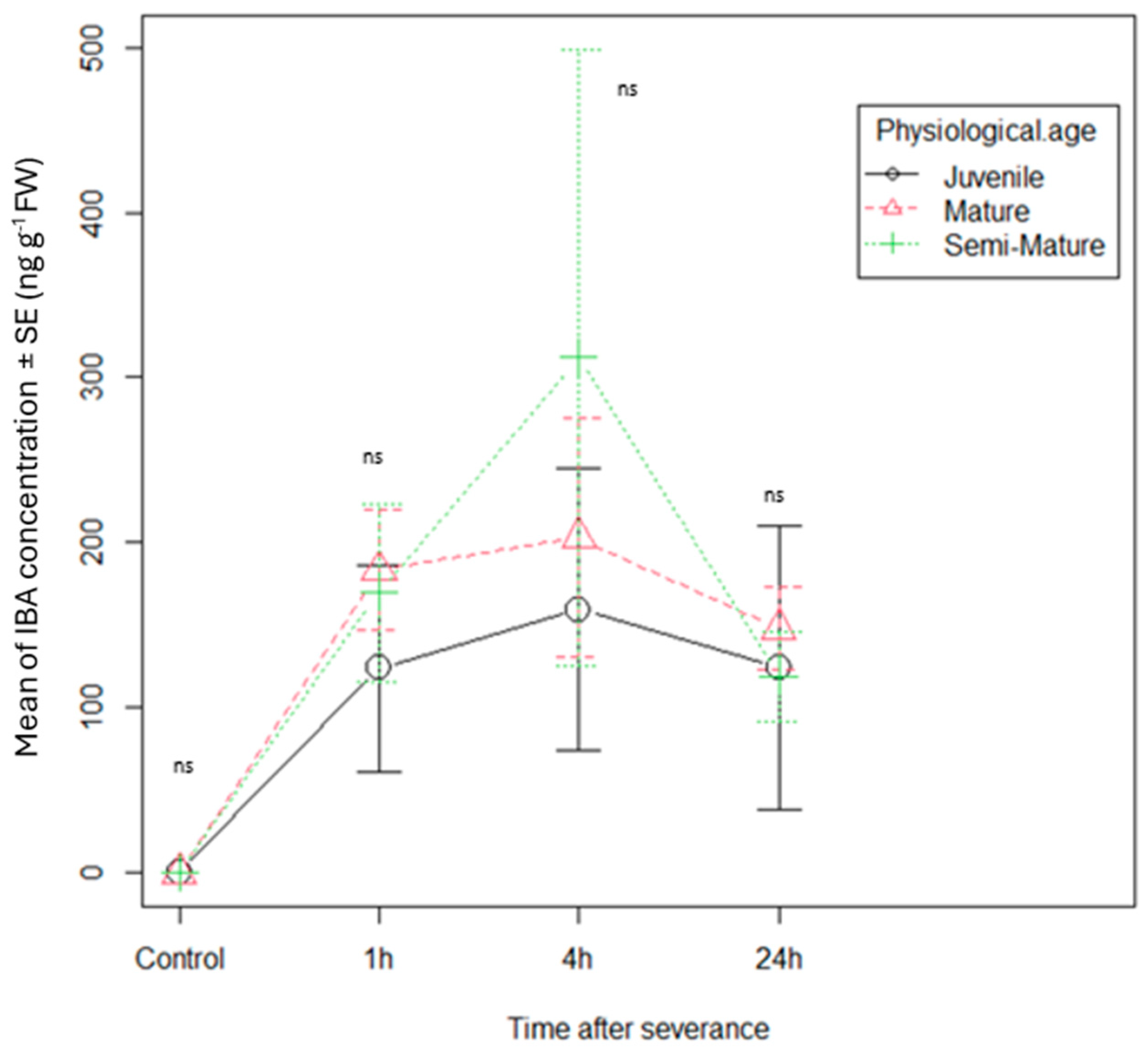
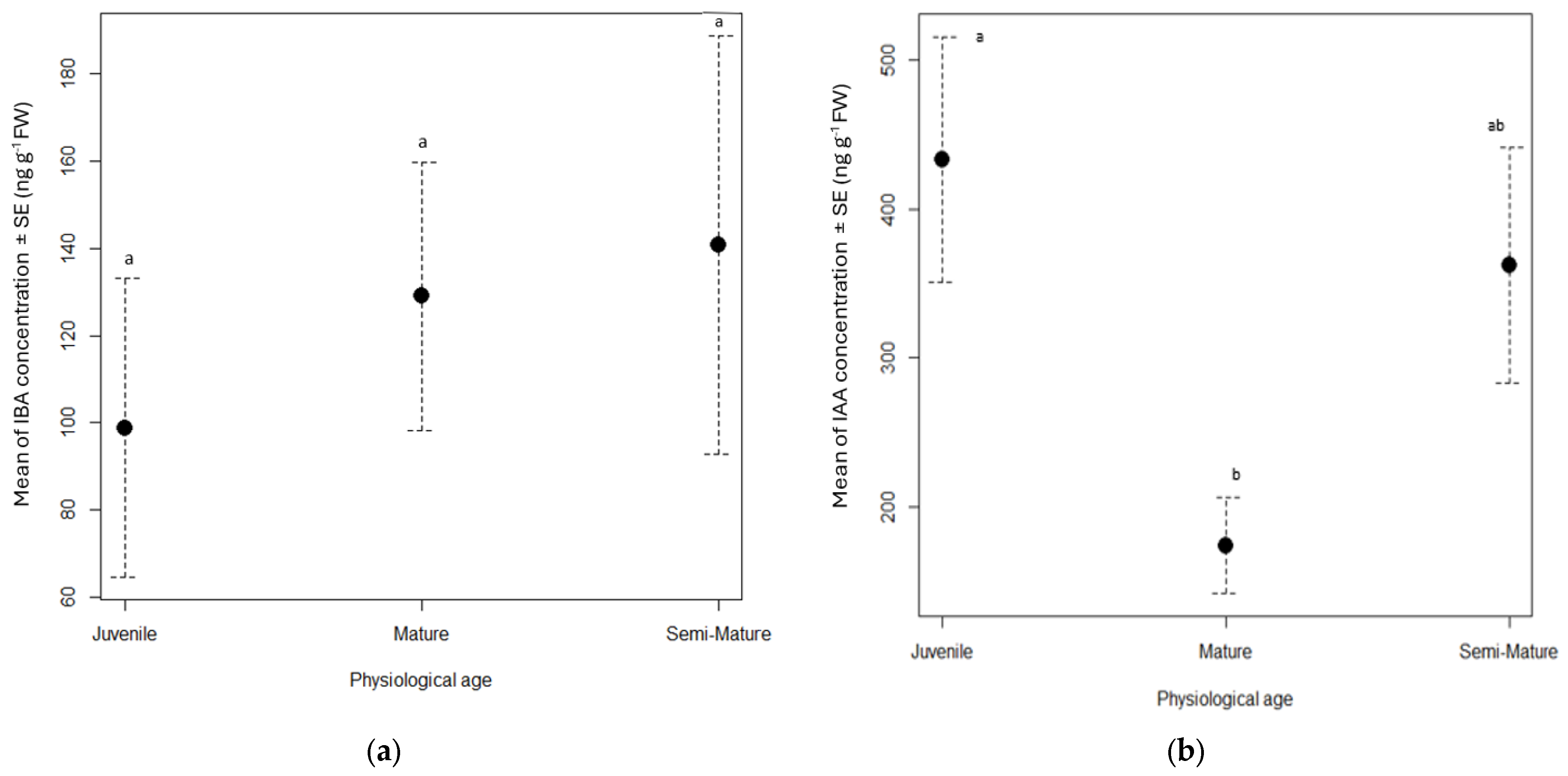
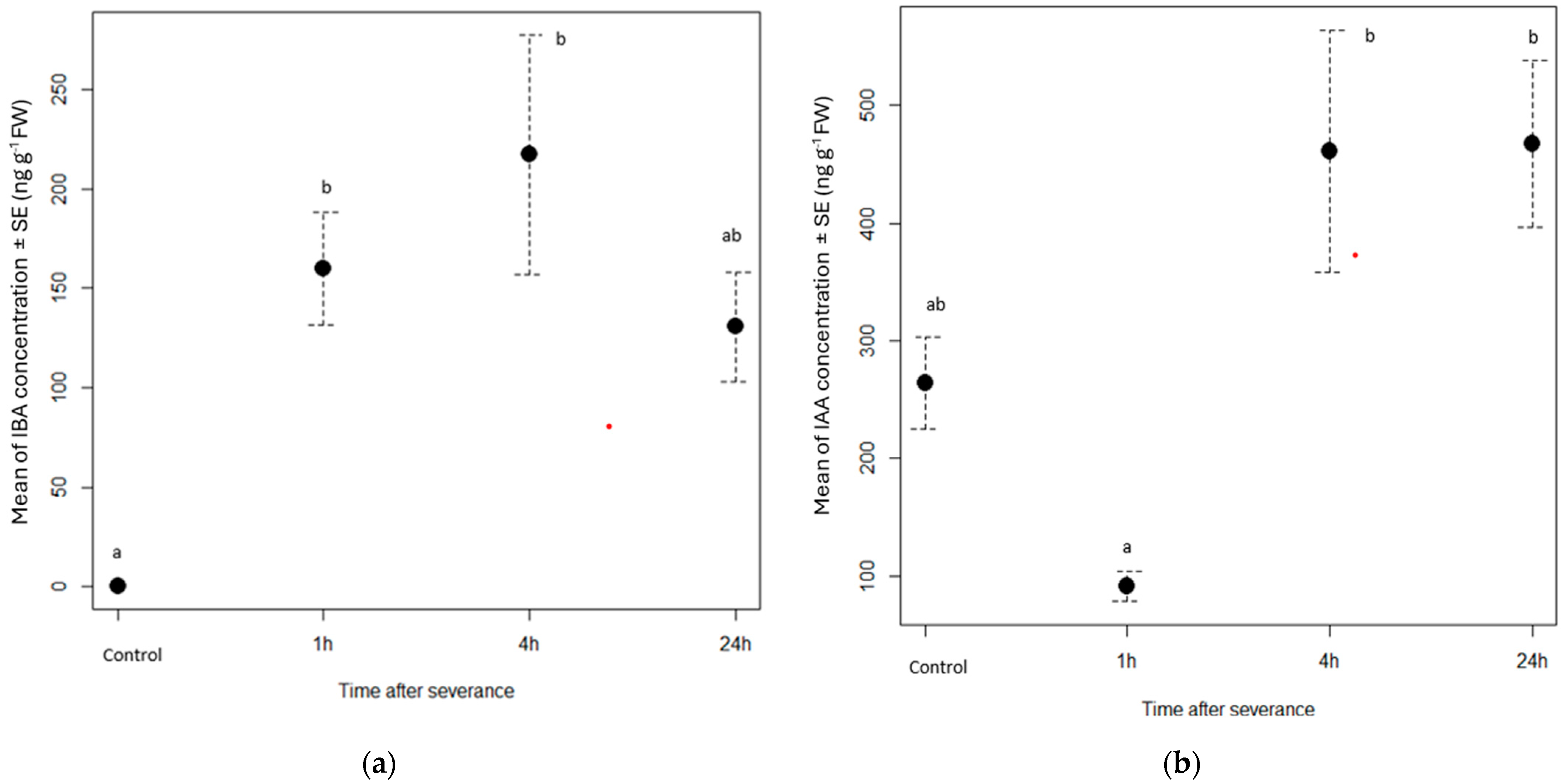
| Phytohormone | Retention Time (min) | Pseudomolecular Ions (m/z) | Fragmentation Pattern (Relative Peak Intensity %) |
|---|---|---|---|
| IBA | 7.87 | 202 | 158 (100), 184 (9.67), 115 (0.34) |
| IAA | 5.50 | 174 | 130 (100) |
| Physiological Age of Stock Plant | Successfully Rooted Leafy Cuttings ± SE (%) | Rooted Cuttings but Failed ± SE (%) | Cuttings with Basal Root Development ± SE (%) | Cuttings with Acrobasal Root Development ± SE (%) | Cuttings with Callus Formation ± SE (%) |
|---|---|---|---|---|---|
| Juvenile | 78.33 ± 9.57 ab | 20.00 ± 8.16 a | 76.25 ± 1.25 a | 23.75 ± 1.5 ab | 23.13 ± 5.38 ab |
| Semi-Mature | 95.00 ± 5.00 b | 12.50 ± 7.98 a | 53.75 ± 15.70 a | 44.17 ± 13.70 b | 12.08 ± 2.99 a |
| Mature | 68.33 ± 4.09 a | 21.67 ± 3.19 a | 89.72 ± 4.09 a | 10.28 ± 4.09 a | 52.92 ± 12.14 b |
| Physiological age of stock plant | Number of main roots ± SE | Length of the root bush ± SE (cm) | Length of the newly formed shoots ± SE (cm) | Number of the newly formed shoots ± SE | |
| Juvenile | 5.9 ± 0.20 a | 25.26 ± 1.21 a | 96.67 ± 9.53 a | 4.50 ± 0.33 a | |
| Semi-Mature | 6.80 ± 0.43 a | 21.65 ± 0.89 a | 99.49 ± 12.78 a | 6.02 ± 0.68 a | |
| Mature | 6.01 ± 0.45 a | 23.37 ± 1.28 a | 72.86 ± 9.81 a | 3.92 ± 0.78 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunc, P.; Medic, A.; Veberic, R.; Osterc, G. Does the Physiological Age of Stock Plant Material Affect the Uptake of Indole-3-Butyric Acid (IBA) in Leafy Cuttings of Prunus subhirtella ‘Autumnalis’? Horticulturae 2024, 10, 296. https://doi.org/10.3390/horticulturae10030296
Kunc P, Medic A, Veberic R, Osterc G. Does the Physiological Age of Stock Plant Material Affect the Uptake of Indole-3-Butyric Acid (IBA) in Leafy Cuttings of Prunus subhirtella ‘Autumnalis’? Horticulturae. 2024; 10(3):296. https://doi.org/10.3390/horticulturae10030296
Chicago/Turabian StyleKunc, Petra, Aljaz Medic, Robert Veberic, and Gregor Osterc. 2024. "Does the Physiological Age of Stock Plant Material Affect the Uptake of Indole-3-Butyric Acid (IBA) in Leafy Cuttings of Prunus subhirtella ‘Autumnalis’?" Horticulturae 10, no. 3: 296. https://doi.org/10.3390/horticulturae10030296
APA StyleKunc, P., Medic, A., Veberic, R., & Osterc, G. (2024). Does the Physiological Age of Stock Plant Material Affect the Uptake of Indole-3-Butyric Acid (IBA) in Leafy Cuttings of Prunus subhirtella ‘Autumnalis’? Horticulturae, 10(3), 296. https://doi.org/10.3390/horticulturae10030296








