Changes in Growth Parameters, C:N:P Stoichiometry and Non-Structural Carbohydrate Contents of Zanthoxylum armatum Seedling in Response to Five Soil Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Soil Basic Information
2.3. Seedling Culture
2.4. Sample Collection and Growth Parameter Measurement
2.5. Determination of Nutrient Element Content
2.6. Measurement of NSC Content
2.7. Statistical Analysis
3. Results
3.1. Influences of Soil Types on the Growth Parameters
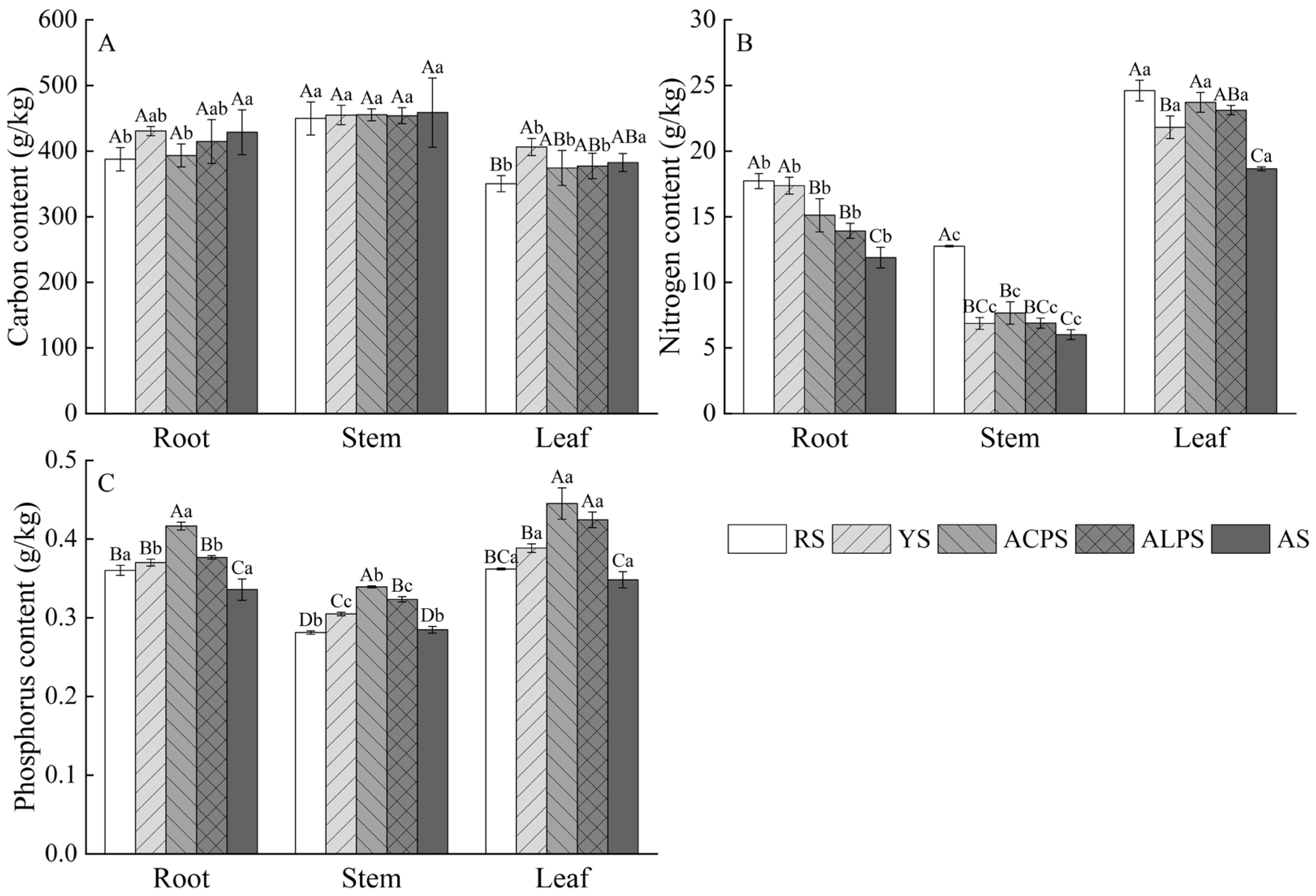
3.2. Influences of Soil Type on C, N, and P Contents
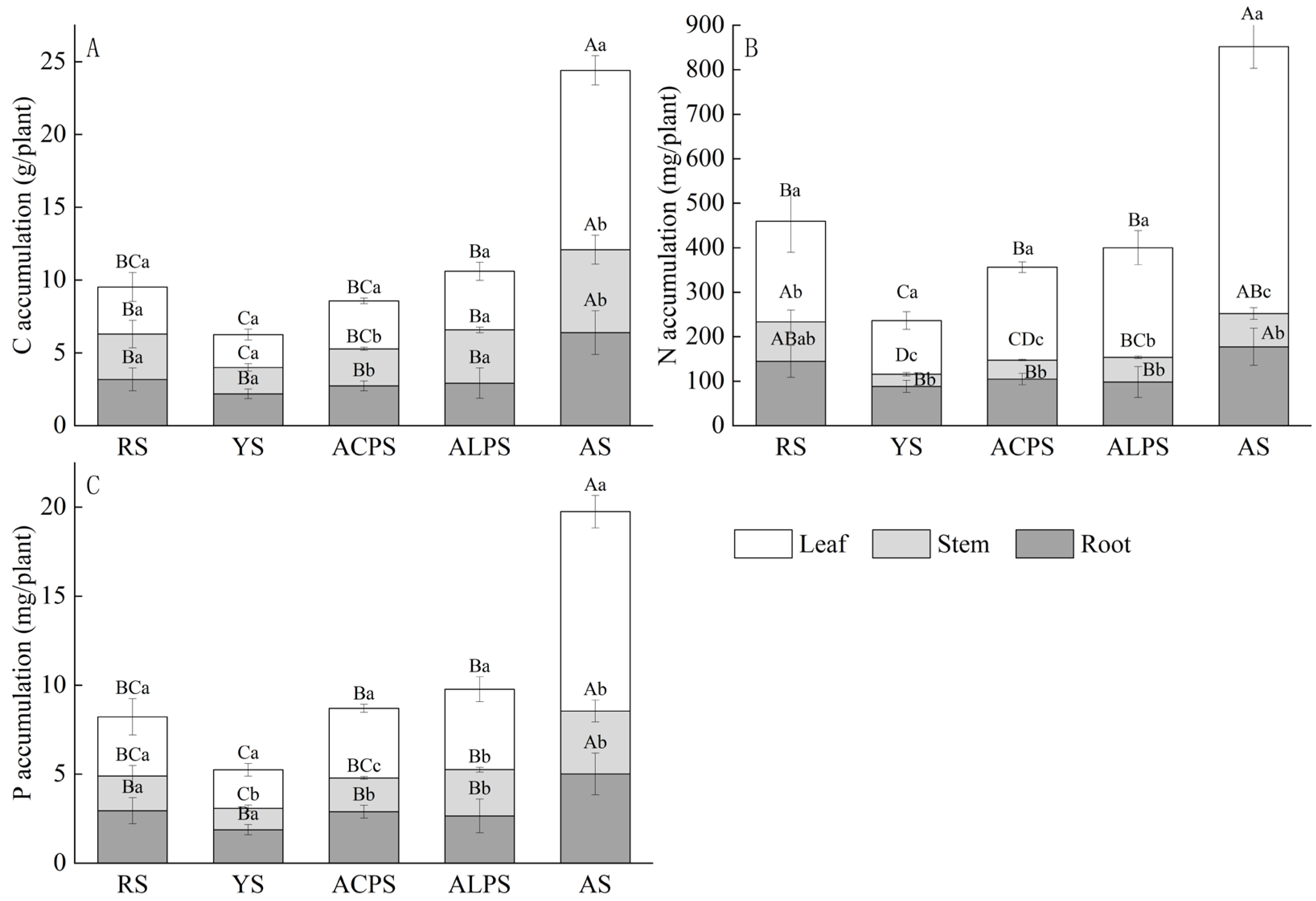
3.3. Infuences of Soil Types on the Accumulation of C, N, and P
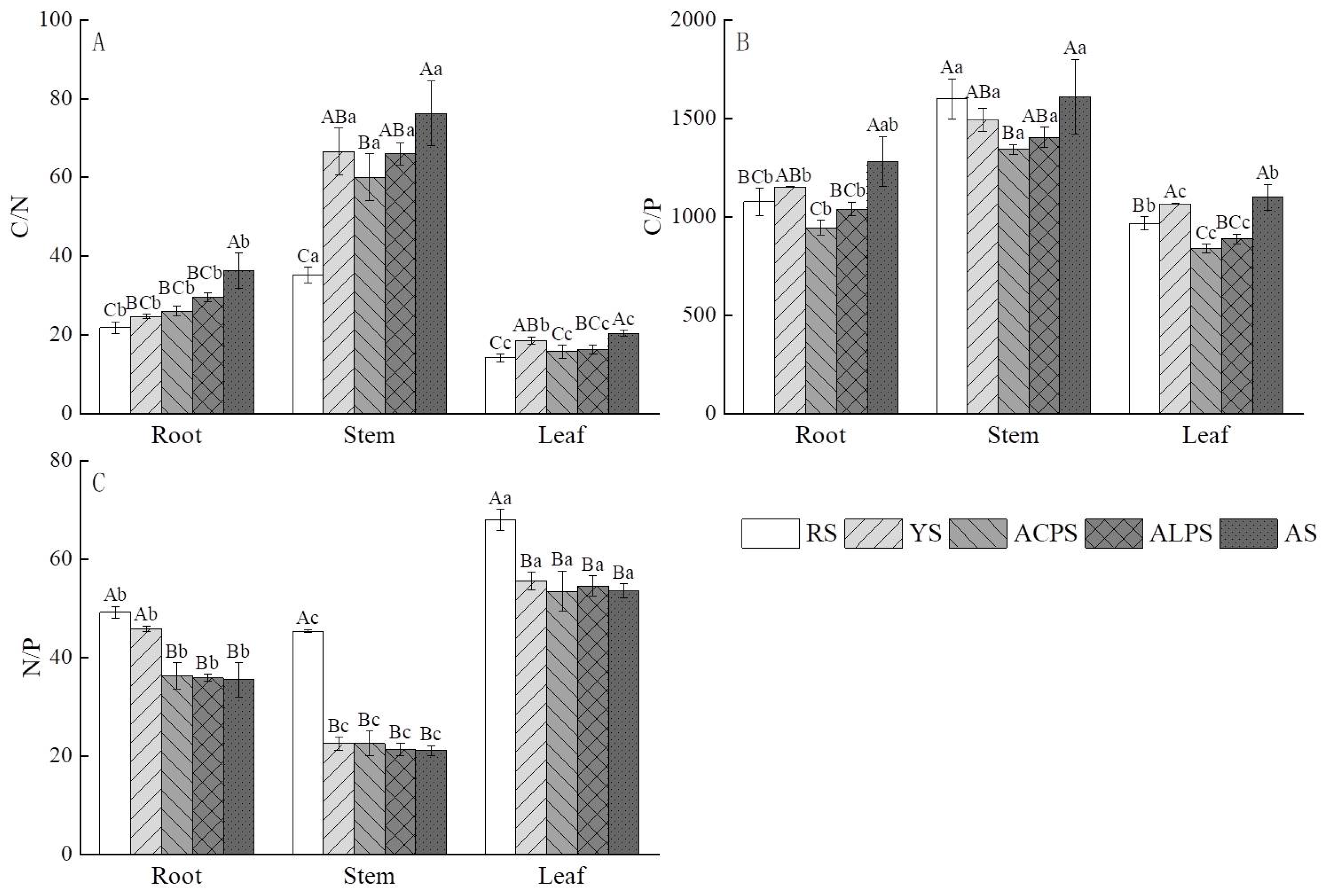
3.4. Influences of Five Soil Types on Stoichiometry
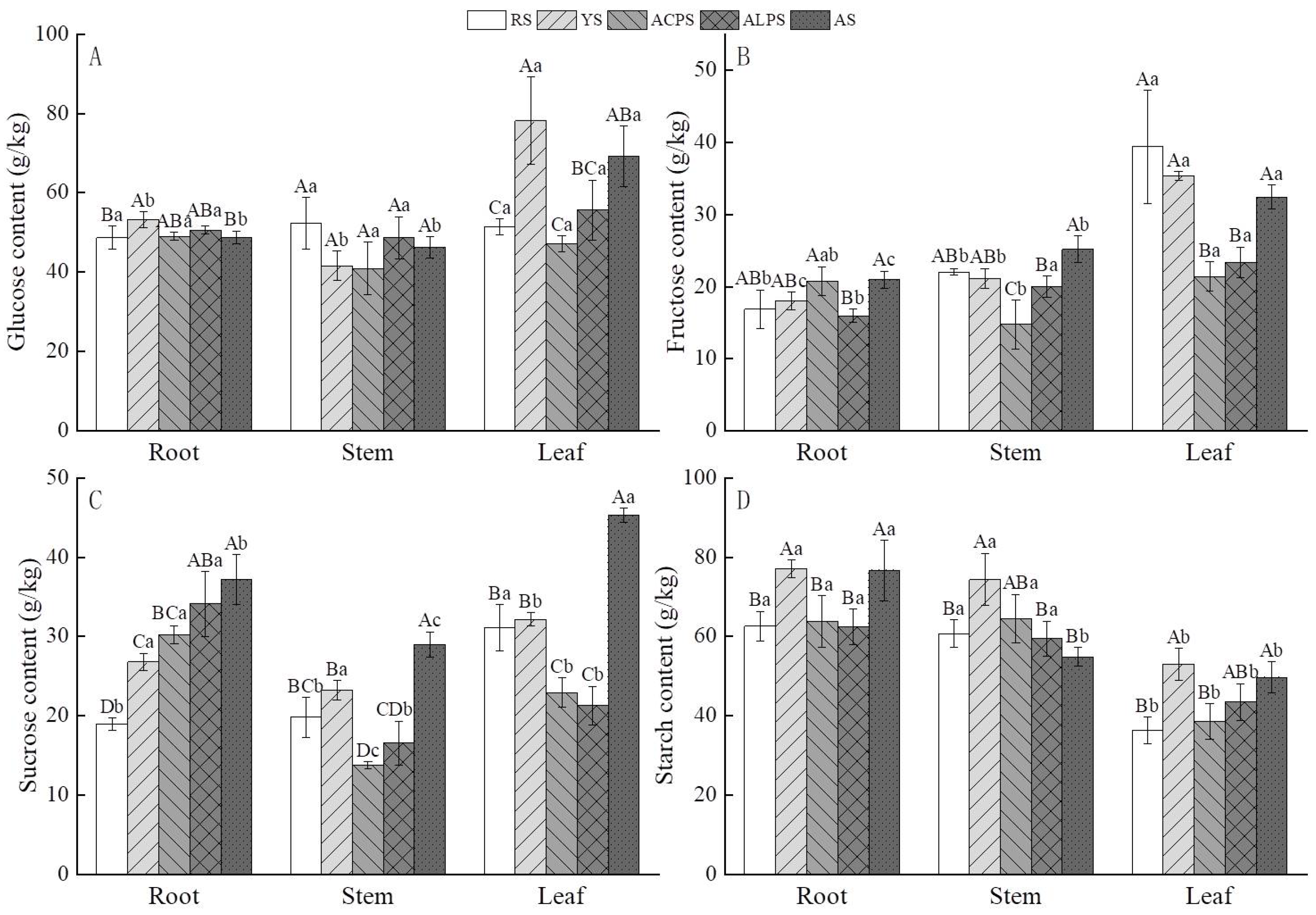
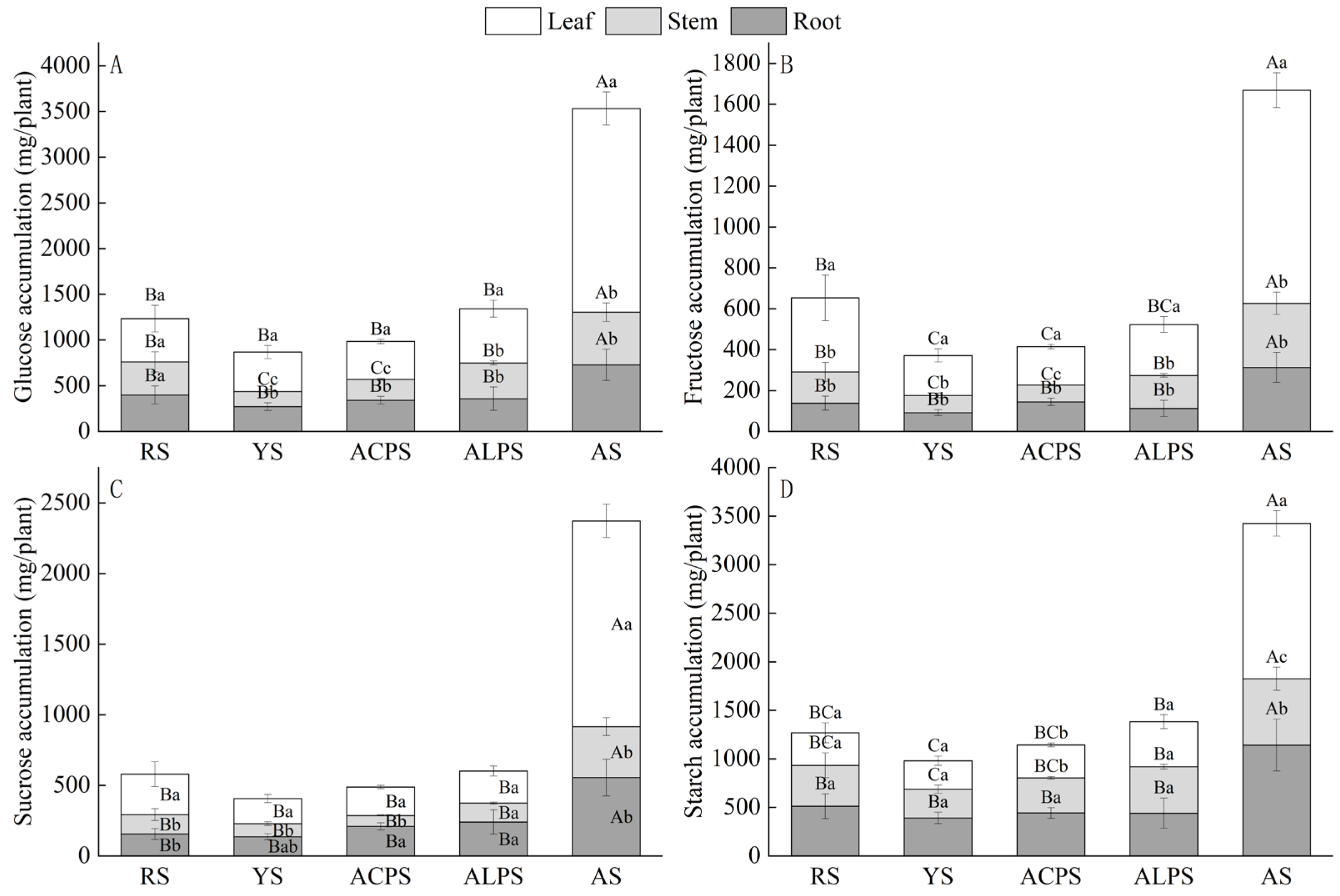
3.5. Effects of Five Soil Types on Non-Structural Carbohydrates (NSC)
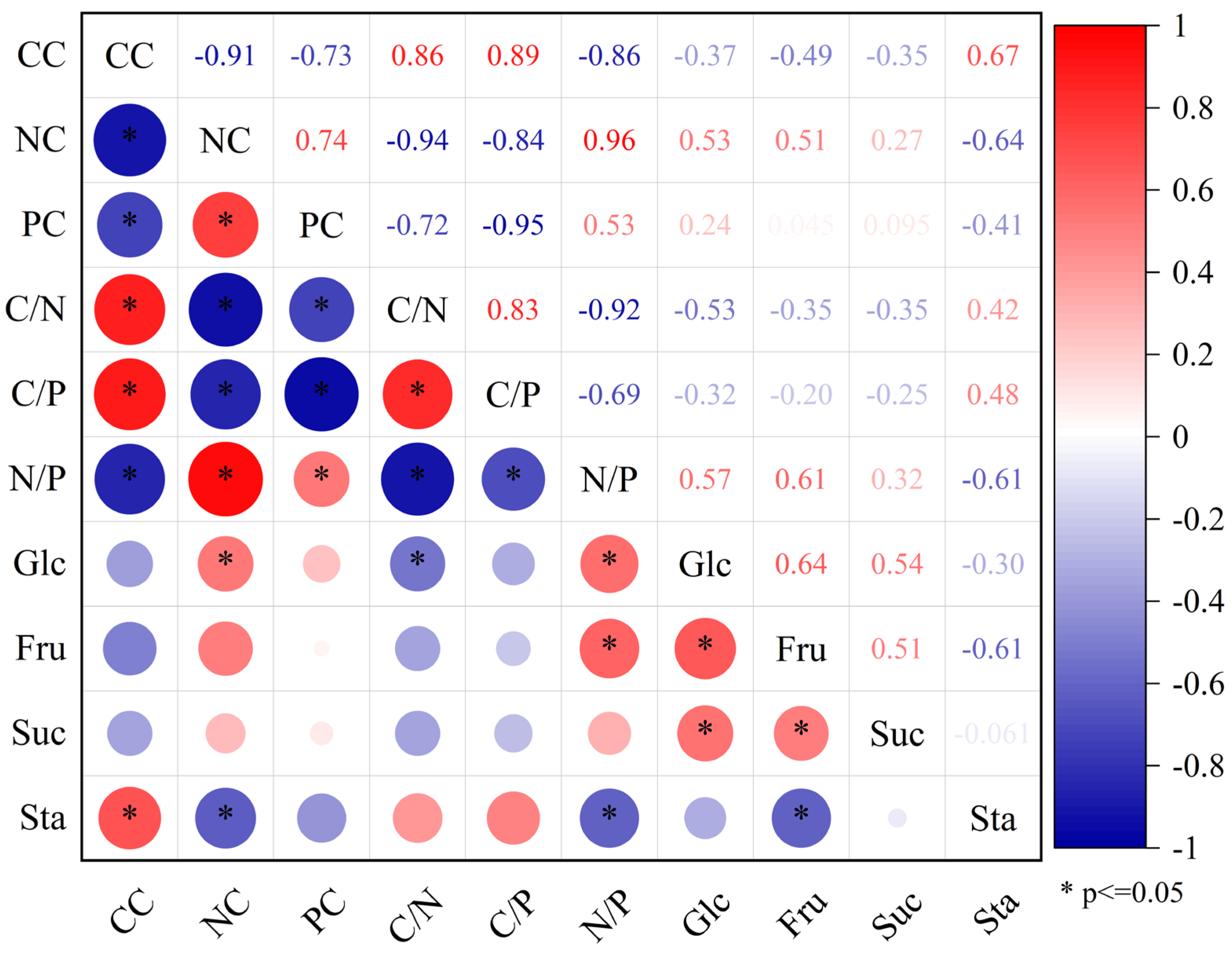
3.6. Correlation Analysis
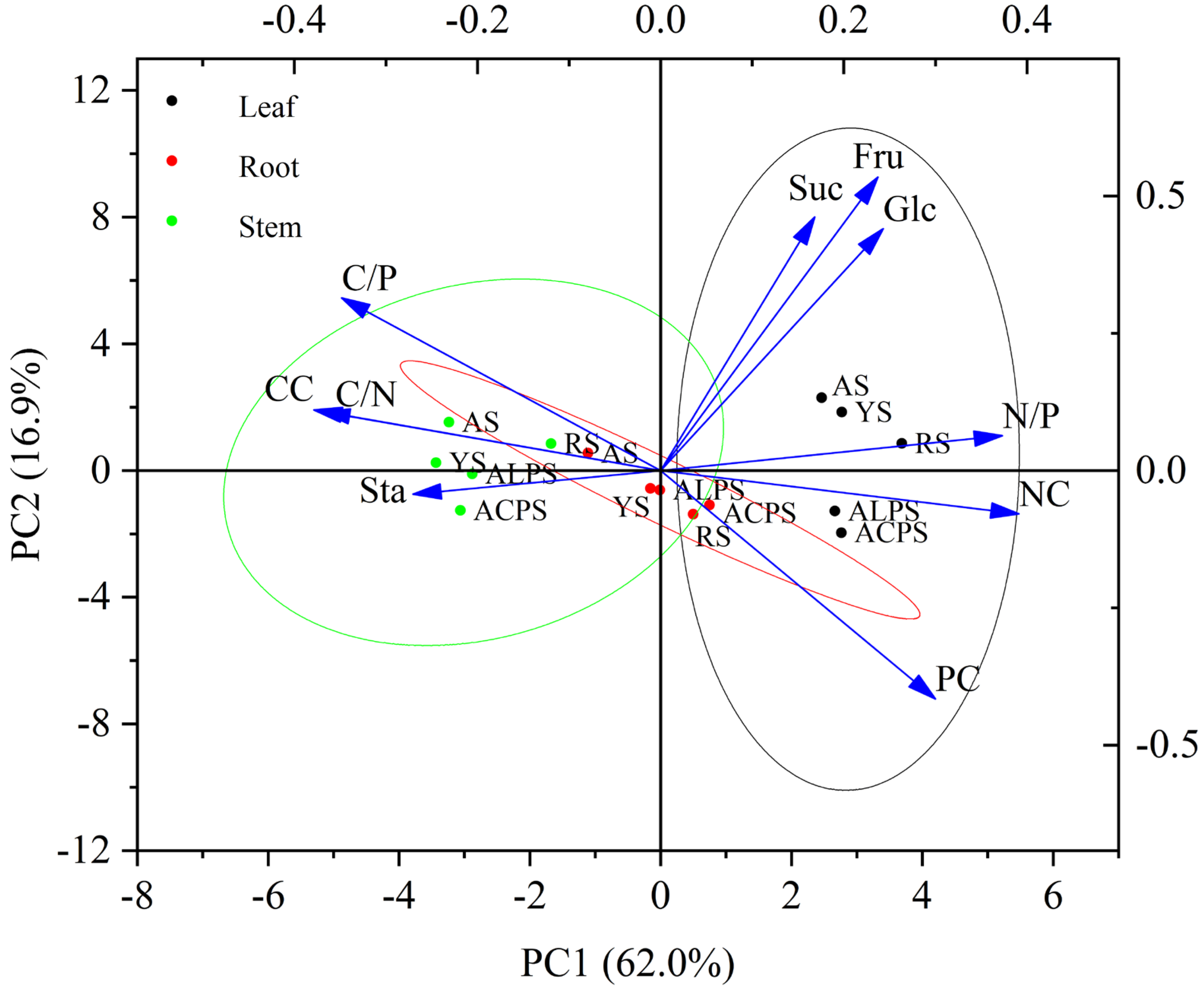
3.7. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dan, T.H.; Brix, H. Effects of soil type and water saturation on growth, nutrient and mineral content of the perennial forage shrub Sesbania sesban. Agroforest. Syst. 2017, 91, 173–184. [Google Scholar] [CrossRef]
- Lee, E.H.; Lee, B.E.; Kim, J.G. Effects of water levels and soil nutrients on the growth of Iris laevigata seedlings. J. Ecol. Environ. 2018, 42, 5–13. [Google Scholar] [CrossRef]
- Pei, J.; Li, H.; Li, S.; An, T.; Farmer, J.; Fu, S.; Wang, J. Dynamics of Maize carbon contribution to soil organic carbon in association with soil type and fertility level. PLoS ONE 2015, 10, e120825–e120840. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.; Liang, J.; Zhang, X. Effect of soil configuration on alfalfa growth under drought stress. Sustainability 2023, 15, 5400. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, T.; Liu, J.; Sun, J.; Zhang, P. Ecological stoichiometry, salt ions and homeostasis characteristics of different types of halophytes and soils. Front. Plant Sci. 2022, 13, 990246. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, Y.; Qian, W.; Wang, Y.J.; Ren, H.Y.; Gao, S.; Cao, G.X. Early growth characterization and antioxidant responses of Phellodendron chinense seedling in response to four soil types at three growth stages. Forests 2023, 14, 1746. [Google Scholar] [CrossRef]
- Kahkashan, P.; Najat, B.; Iram, S.; Iffat, S. Influence of soil type on the growth parameters, essential oil yield and biochemical contents of Mentha arvensis L. J. Essent. Oil-Bear. Plants 2016, 19, 76–81. [Google Scholar] [CrossRef]
- Ogundola, A.F.; Bvenura, C.; Ehigie, A.F.; Afolayan, A.J. Effects of soil types on phytochemical constituents and antioxidant properties of Solanum nigrum. S. Afr. J. Bot. 2022, 151, 325–333. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Penuelas, J. The elemental stoichiometry of aquatic and terrestrial ecosystems and its relationships with organismic lifestyle and ecosystem structure and function: A review and perspectives. Biogeochemistry 2012, 111, 1–39. [Google Scholar] [CrossRef]
- Ye, Y.; Liang, X.; Chen, Y.; Li, L.; Ji, Y.; Zhu, C. Carbon, nitrogen and phosphorus accumulation and partitioning, and C:N:P stoichiometry in late-season Rice under different water and nitrogen managements. PLoS ONE 2014, 9, e101776. [Google Scholar] [CrossRef]
- Sun, H.; Li, Q.; Lei, Z.; Zhang, J.; Song, X.; Song, X. Ecological stoichiometry of nitrogen and phosphorus in Moso bamboo (Phyllostachys Edulis) during the explosive growth period of new emergent shoots. J. Plant Res. 2019, 132, 107–115. [Google Scholar] [CrossRef]
- Niklas, K.J.; Owens, T.; Reich, P.B.; Cobb, E.D. Nitrogen/phosphorus leaf stoichiometry and the scaling of plant growth. Ecol. Lett. 2005, 8, 636–642. [Google Scholar] [CrossRef]
- Gusewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Jing, H.; Zhou, H.; Wang, G.; Xue, S.; Liu, G.; Duan, M. Nitrogen addition changes the stoichiometry and growth rate of different organs in Pinus Tabuliformis seedlings. Front. Plant Sci. 2017, 8, 1922–1932. [Google Scholar] [CrossRef]
- Reef, R.; Ball, M.C.; Feller, I.C.; Lovelock, C.E. Relationships among RNA: DNA ratio, growth and elemental stoichiometry in mangrove trees. Funct. Ecol. 2010, 24, 1064–1072. [Google Scholar] [CrossRef]
- Zhan, S.; Wang, Y.; Zhu, Z.; Li, W.; Bai, Y. Nitrogen enrichment alters plant N:P stoichiometry and intensifies phosphorus limitation in a steppe ecosystem. Environ. Exp. Bot. 2017, 134, 21–32. [Google Scholar] [CrossRef]
- Minden, V.; Kleyer, M. Internal and external regulation of plant organ stoichiometry. Plant Biol. 2014, 16, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Arif, M.; Liu, M.; Li, J.; Hu, X.; Geng, Q.; Yin, F.; Li, C. Plant-soil interactions and C:N:P stoichiometric homeostasis of plant organs in Riparian plantation. Front. Plant Sci. 2022, 13, 979023–979040. [Google Scholar] [CrossRef] [PubMed]
- Schreeg, L.A.; Santiago, L.S.; Wright, S.J.; Turner, B.L. Stem, root, and older leaf N:P ratios are more responsive indicators of soil nutrient availability than new foliage. Ecology 2014, 95, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huang, Z.; Wang, Z.; Chen, Y.; Wen, Z.; Liu, B.; Tigabu, M. Responses of leaf morphology, NSCs contents and C:N:P stoichiometry of Cunninghamia lanceolata and Schima superba to shading. BMC Plant Biol. 2020, 20, 354–364. [Google Scholar] [CrossRef]
- Xie, H.; Yu, M.; Cheng, X. Leaf non-structural carbohydrate allocation and C:N:P stoichiometry in response to light acclimation in seedlings of two subtropical shade-tolerant tree species. Plant Physiol. Bioch. 2018, 124, 146–154. [Google Scholar] [CrossRef]
- Long, R.W.; Adams, H.D. The osmotic balancing act: When sugars matter for more than metabolism in woody plants. Global Change Biol. 2023, 29, 1684–1687. [Google Scholar] [CrossRef]
- Luo, G.; Li, J.; Guo, S.; Li, Y.; Jin, Z. Photosynthesis, nitrogen allocation, non-structural carbohydrate allocation, and C:N:P stoichiometry of Ulmus elongata seedlings exposed to different light intensities. Life 2022, 12, 1310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, J.; Li, M.; Zhang, G.; Yan, Q. Different light acclimation strategies of two coexisting tree species seedlings in a temperate secondary forest along five natural light levels. Forest Ecol. Manag. 2013, 306, 234–242. [Google Scholar] [CrossRef]
- Ai, Z.; Xue, S.; Wang, G.; Liu, G. Responses of non-structural carbohydrates and C:N:P stoichiometry of Bothriochloa ischaemum to nitrogen addition on the Loess Plateau, China. J. Plant Growth Regul. 2017, 36, 714–722. [Google Scholar] [CrossRef]
- Li, M.H.; Jiang, Y.; Wang, A.; Li, X.; Zhu, W.; Yan, C.F.; Du, Z.; Shi, Z.; Lei, J.; Schönbeck, L.; et al. Active summer carbon storage for winter persistence in trees at the cold Alpine treeline. Tree Physiol. 2018, 38, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; He, H.; Wu, Z.; Cong, Y.; Zong, S.; He, J.; Fu, Y.; Liu, K.; Sun, H.; Li, Y.; et al. Non-structural carbohydrate storage strategy explains the spatial distribution of treeline species. Plants 2020, 9, 384. [Google Scholar] [CrossRef]
- Smith, M.G.; Arndt, S.K.; Miller, R.E.; Kasel, S.; Bennett, L.T. Trees use more non-structural carbohydrate reserves during Epicormic than Basal resprouting. Tree Physiol. 2018, 38, 1779–1791. [Google Scholar] [CrossRef]
- Xu, D.; Zhuo, Z.; Wang, R.; Ye, M.; Pu, B. Modeling the distribution of Zanthoxylum armatum in China with Maxent modeling. Glob. Ecol. Conserv. 2019, 19, e691. [Google Scholar] [CrossRef]
- Devkota, K.P.; Wilson, J.; Henrich, C.J.; McMahon, J.B.; Reilly, K.M.; Beutler, J.A. Isobutylhydroxyamides from the pericarp of Nepalese Zanthoxylum Armatum inhibit Nf1-defective tumor cell line growth. J. Nat. Prod. 2013, 76, 59–63. [Google Scholar] [CrossRef]
- Agnihotri, S.; Wakode, S.; Ali, M. Chemical constituents isolated from Zanthoxylum armatum stem bark. Chem. Nat. Comp. 2017, 53, 880–882. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, S.; Singh, B. Quantitative and structural analysis of amides and lignans in Zanthoxylum armatum by UPLC-DAD-ESI-QTOF-MS/MS. J. Pharmaceut. Biomed. 2014, 94, 23–29. [Google Scholar] [CrossRef]
- Nooreen, Z.; Kumar, A.; Bawankule, D.U.; Tandon, S.; Ali, M.; Xuan, T.D.; Ahmad, A. New chemical constituents from the fruits of Zanthoxylum armatum and its in vitro anti-inflammatory profile. Nat. Prod. Res. 2019, 33, 665–672. [Google Scholar] [CrossRef]
- Bhatt, V.; Sharma, S.; Kumar, N.; Singh, B. A new lignan from the leaves of Zanthoxylum armatum. Nat. Prod. Commun. 2017, 12, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Qian, W.; Yi, L.; Mao, Y.-D.; Ye, Y.-L.; Ren, H.-Y.; Gu, T.; Zhang, D.-J.; Cao, G.-X.; Gao, S. Chemical composition and antioxidant activity of Zanthoxylum armatum leaves in response to plant age, shoot type and leaf position. Forests 2023, 14, 1022. [Google Scholar] [CrossRef]
- Zhi, X.; Song, Y.; Yu, D.; Qian, W.; He, M.; Lin, X.; Zhang, D.; Gao, S. Early growth characterization and C:N:P stoichiometry in Firmiana simplex seedlings in response to shade and soil types. Forests 2023, 14, 1481. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, K.; Zhang, Q.; Wang, W.B. Ecophysiological responses of Jatropha curcas L. seedlings to simulated acid rain under different soil types. Ecotox. Environ. Safe 2019, 185, 109705–109717. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, X.; Sun, Y.; Liu, S.; Hu, H.; Xie, J.; Chen, G.; Xiao, Y.; Tang, Y.; Tu, L. C:N:P stoichiometry responses to 10 years of nitrogen addition differ across soil components and plant organs in a subtropical Pleioblastus amarus forest. Sci. Total Environ. 2021, 796, 148925–148937. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Yang, Y.; Zou, J.; Ma, N.; Liu, T.; Wang, H.; Hu, Y.; Gao, S. Responses of the growth and nutrient stoichiometry in Ricinus communis seedlings on four soil types. J. Elementol. 2022, 27, 223–238. [Google Scholar] [CrossRef]
- Tang, N.; Cao, Z.; Wu, P.; Liu, Y.; Lou, J.; Hu, Y.; Sun, X.; Si, S.; Chen, Z. Comparative transcriptome analysis reveals hormone, transcriptional and epigenetic regulation involved in prickle formation in Zanthoxylum armatum. Gene 2023, 871, 147434–147448. [Google Scholar] [CrossRef]
- Ma, Y.; Tian, J.; Wang, X.; Huang, C.; Tian, M.; Wei, A. Fatty acid profiling and chemometric analyses for Zanthoxylum pericarps from different geographic origin and genotype. Foods 2020, 9, 1676. [Google Scholar] [CrossRef]
- Chen, X.; Wang, W.; Wang, C.; Liu, Z.; Sun, Q.; Wang, D. Quality evaluation and chemometric discrimination of Zanthoxylum bungeanum maxim leaves based on flavonoids profiles, bioactivity and HPLC-fingerprint in a common garden experiment. Ind. Crop. Prod. 2019, 134, 225–233. [Google Scholar] [CrossRef]
- Agnihotri, S.; Dobhal, P.; Ashfaqullah, S.; Chauhan, H.K.; Tamtal, S. Review of the botany, traditional uses, pharmacology, threats and conservation of Zanthoxylum armatum (Rutaceae). S. Afr. J. Bot. 2022, 150, 920–927. [Google Scholar] [CrossRef]
- Yousefi, M.; Hajabbasi, M.; Shariatmadari, H. Cropping system effects on carbohydrate content and water-stable aggregates in a calcareous soil of central Iran. Soil Till. Res. 2008, 101, 57–61. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H. Plant mixture balances terrestrial ecosystem C:N:P stoichiometry. Nat. Commun. 2021, 12, 4562–4571. [Google Scholar] [CrossRef] [PubMed]
- Kleyer, M.; Minden, V. Why Functional ecology should consider all plant organs: An allocation-based perspective. Basic Appl. Ecol. 2015, 16, 1–9. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; He, Y.; Li, S.; Liang, Z.; Peng, C.; Polle, A.; Luo, Z. Changes in carbon, nutrients and stoichiometric relations under different soil depths, plant tissues and ages in Black Locust plantations. Acta Physiol. Plant. 2013, 35, 2951–2964. [Google Scholar] [CrossRef]
- Maathuis, F.J. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Zhai, P.F.; Guan, J.X.; He, P.; Liu, H.Y.; Man, L.; Jiang, Y.; Ma, C.C. Changes of non-structural carbohydrates and nitrogen contents of needles and twigs in Pinus sylvestris var. mongolica plantations along an aridity gradient. Ying Yong Sheng Tai Xue Bao 2022, 33, 1518–1524. (In Chinese) [Google Scholar] [CrossRef]
- Wang, K.; Shen, C.; Cao, P.; Song, L.N.; Yu, G.Q. Changes of non-structural carbohydrates of Pinus sylvestris var. mongolica seedlings in the process of drought-induced mortality. Ying Yong Sheng Tai Xue Bao 2018, 29, 3513–3520. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Ai, W.; Zhan, H.; Ma, S.; Lu, X. Non-structural carbohydrates and growth adaptation strategies of Quercus mongolica Fisch. ex Ledeb. seedlings under drought stress. Forests 2023, 14, 404. [Google Scholar] [CrossRef]
| Soil Type | pH | Organic Matter (g·kg−1) | Total Nitrogen (g·kg−1) | Total Phosphorus (g·kg−1) |
|---|---|---|---|---|
| Red soil (RS) | 4.9 | 16.64 | 1.14 | 0.067 |
| Yellow soil (YS) | 4.8 | 33.50 | 1.89 | 0.080 |
| Acidic purple soil (ACPS) | 4.3 | 42.53 | 2.33 | 0.235 |
| Alkaline purple soil (ALPS) | 8.7 | 20.99 | 1.65 | 0.301 |
| Alluvial soil (AS) | 8.1 | 38.38 | 2.35 | 0.300 |
| Soil Type | RS | YS | ACPS | ALPS | AS | |
|---|---|---|---|---|---|---|
| Plant height (cm) | 56.1 ± 10.78 c | 44.72 ± 7.3 d | 54.14 ± 5.01 cd | 66.46 ± 4.56 b | 77.22 ± 9 a | |
| Ground diameter (mm) | 8.56 ± 0.64 b | 6.82 ± 0.53 d | 7.76 ± 0.19 c | 9.09 ± 0.69 b | 10.62 ± 0.7 a | |
| Fresh weight (g) | Leaf | 28.47 ± 8.64 b | 16.25 ± 2.35 c | 26.91 ± 1.22 b | 31.25 ± 5.08 b | 48.25 ± 3.93 a |
| Stem | 16.26 ± 5.01 b | 8.92 ± 0.95 c | 12.94 ± 0.66 bc | 18.09 ± 1.25 b | 27.64 ± 4.15 a | |
| Root | 36.3 ± 9.04 ab | 22.42 ± 0.74 b | 26.25 ± 6.76 b | 24.63 ± 10.19 b | 54.28 ± 19.21 a | |
| Dry weight (g) | Leaf | 9.21 ± 2.83 b | 5.53 ± 0.92 c | 8.79 ± 0.52 bc | 10.66 ± 1.65 b | 32.17 ± 2.62 a |
| Stem | 6.94 ± 2.1 b | 3.98 ± 0.56 c | 5.59 ± 0.22 bc | 8.05 ± 0.43 b | 12.44 ± 2.18 a | |
| Root | 8.17 ± 2.03 b | 5.08 ± 0.78 b | 6.93 ± 0.86 b | 7.05 ± 2.52 b | 14.91 ± 3.5 a | |
| RWC (%) | Leaf | 67.69 ± 0.27 | 66.06 ± 0.8 | 67.35 ± 0.43 | 65.86 ± 0.46 | 66.66 ± 2.2 |
| Stem | 57.28 ± 0.55 | 55.42 ± 2.4 | 56.73 ± 2.42 | 55.44 ± 1.09 | 55.11 ± 1.13 | |
| Root | 77.39 ± 2.15 | 77.33 ± 3.6 | 72.86 ± 4.48 | 70.96 ± 1.73 | 71.47 ± 4.8 | |
| Target | Source of Variation | ||
|---|---|---|---|
| Soil Type | Organ | Soil Type × Organ | |
| C content | 1.947 | 26.113 ** | 0.452 |
| N content | 66.641 ** | 1172.280 ** | 9.021 ** |
| P content | 92.034 ** | 321.649 ** | 4.104 ** |
| C/N | 27.251 ** | 425.359 ** | 8.883 ** |
| C/P | 13.862 ** | 133.170 ** | 0.796 |
| N/P | 79.003 ** | 562.228 ** | 6.687 ** |
| Glucose content | 4.829 ** | 21.706 ** | 5.362 ** |
| Fructose content | 10.346 ** | 55.789 ** | 5.927 ** |
| Sucrose content | 49.574 ** | 68.128 ** | 14.443 ** |
| Starch content | 9.454 ** | 70.579 ** | 2.376 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, T.; Ren, H.; Wang, M.; Qian, W.; Hu, Y.; Yang, Y.; Yu, T.; Zhao, K.; Gao, S. Changes in Growth Parameters, C:N:P Stoichiometry and Non-Structural Carbohydrate Contents of Zanthoxylum armatum Seedling in Response to Five Soil Types. Horticulturae 2024, 10, 261. https://doi.org/10.3390/horticulturae10030261
Gu T, Ren H, Wang M, Qian W, Hu Y, Yang Y, Yu T, Zhao K, Gao S. Changes in Growth Parameters, C:N:P Stoichiometry and Non-Structural Carbohydrate Contents of Zanthoxylum armatum Seedling in Response to Five Soil Types. Horticulturae. 2024; 10(3):261. https://doi.org/10.3390/horticulturae10030261
Chicago/Turabian StyleGu, Tao, Hongyu Ren, Mengying Wang, Wenzhang Qian, Yunyi Hu, Yao Yang, Ting Yu, Kuangji Zhao, and Shun Gao. 2024. "Changes in Growth Parameters, C:N:P Stoichiometry and Non-Structural Carbohydrate Contents of Zanthoxylum armatum Seedling in Response to Five Soil Types" Horticulturae 10, no. 3: 261. https://doi.org/10.3390/horticulturae10030261
APA StyleGu, T., Ren, H., Wang, M., Qian, W., Hu, Y., Yang, Y., Yu, T., Zhao, K., & Gao, S. (2024). Changes in Growth Parameters, C:N:P Stoichiometry and Non-Structural Carbohydrate Contents of Zanthoxylum armatum Seedling in Response to Five Soil Types. Horticulturae, 10(3), 261. https://doi.org/10.3390/horticulturae10030261







