High Outcrossing Levels among Global Macadamia Cultivars: Implications for Nut Quality, Orchard Designs and Pollinator Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Sites and Processing
2.2. Kernel Genotyping
2.3. Data Analysis
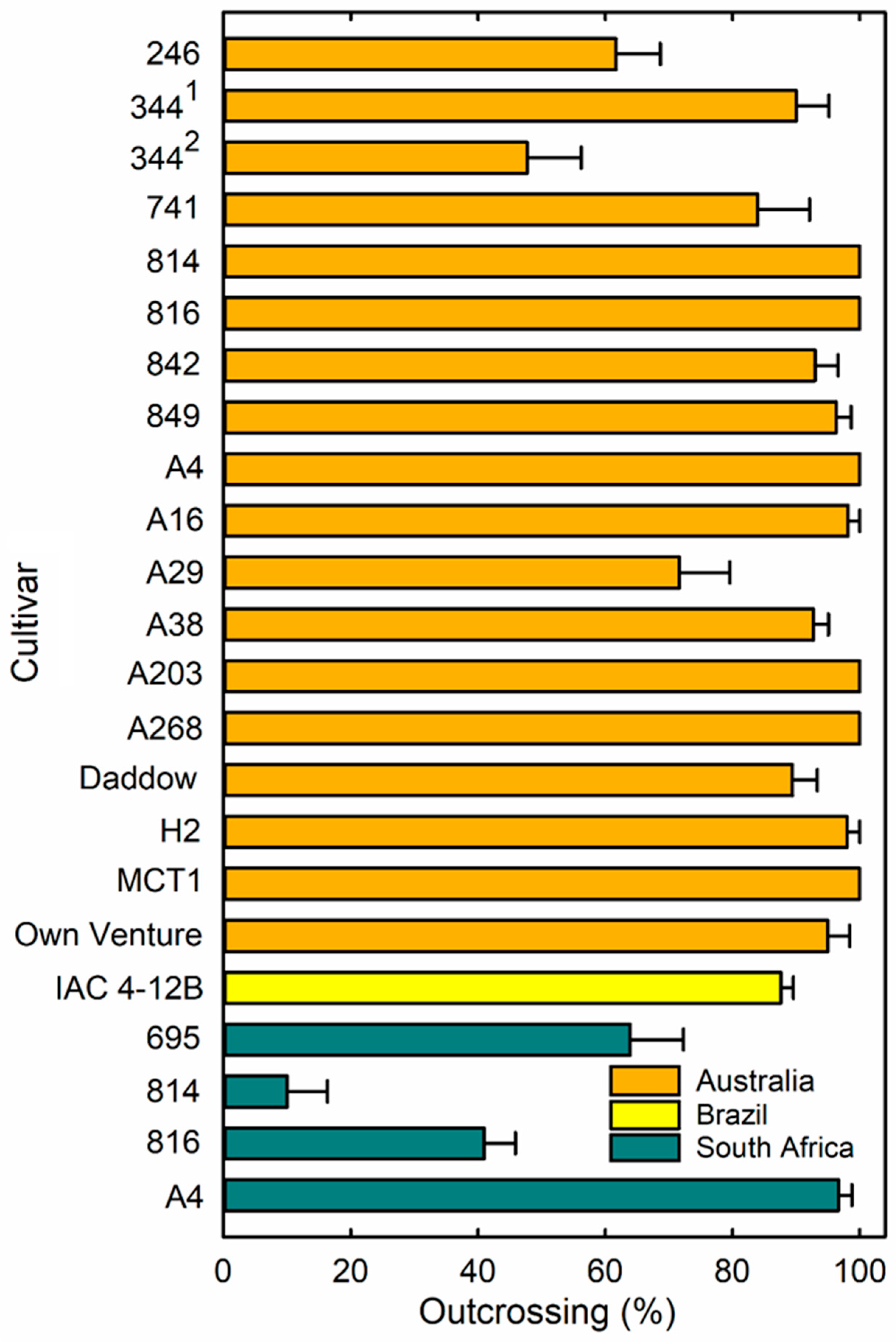
3. Results
3.1. Outcrossing Levels
3.2. Pollen-Parent Effects on Nut Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Cultivar | Country | Nut Size or Quality Parameter | |||
|---|---|---|---|---|---|
| Nut-in-Shell Mass (g) | Kernel Mass (g) | Kernel Recovery (%) | Oil Concentration (%) | ||
| 246 | Australia | 7.73 ± 0.18 | 2.56 ± 0.08 | 32.7 ± 0.6 | 75.8 ± 0.3 |
| 344 1 | Australia | 8.07 ± 0.18 | 2.58 ± 0.08 | 31.6 ± 0.7 | 77.8 ± 0.3 |
| 344 2 | Australia | 6.81 ± 0.28 | 1.90 ± 0.12 | 26.5 ± 1.0 | 74.7 ± 0.5 |
| 741 | Australia | 6.85 ± 0.18 | 2.58 ± 0.08 | 37.6 ± 0.5 | 77.8 ± 0.3 |
| 814 | Australia | 6.36 ± 0.15 | 2.34 ± 0.07 | 36.4 ± 0.8 | 77.3 ± 0.3 |
| 816 | Australia | 6.90 ± 0.14 | 2.97 ± 0.07 | 43.1 ± 0.5 | 77.4 ± 0.2 |
| 842 | Australia | 6.92 ± 0.18 | 2.51 ± 0.09 | 36.0 ± 0.8 | 77.9 ± 0.3 |
| 849 | Australia | 6.31 ± 0.15 | 2.61 ± 0.08 | 41.1 ± 0.8 | 76.7 ± 0.3 |
| A4 | Australia | 7.84 ± 0.17 | 3.58 ± 0.09 | 45.6 ± 0.5 | 76.7 ± 0.3 |
| A16 | Australia | 7.71 ± 0.15 | 3.20 ± 0.09 | 41.2 ± 0.6 | 78.8 ± 0.2 |
| A29 | Australia | 8.73 ± 0.19 | 3.26 ± 0.09 | 37.2 ± 0.5 | 77.0 ± 0.3 |
| A38 | Australia | 6.37 ± 0.20 | 2.32 ± 0.10 | 35.7 ± 0.8 | 77.6 ± 0.5 |
| A203 | Australia | 9.25 ± 0.26 | 3.21 ± 0.10 | 34.7 ± 0.4 | 79.3 ± 0.3 |
| A268 | Australia | 10.53 ± 0.32 | 3.74 ± 0.13 | 35.3 ± 0.7 | 78.1 ± 1.9 |
| Daddow | Australia | 8.36 ± 0.21 | 3.23 ± 0.08 | 38.6 ± 0.4 | 78.1 ± 0.2 |
| H2 | Australia | 8.74 ± 0.38 | 2.76 ± 0.13 | 31.7 ± 0.8 | 76.8 ± 0.3 |
| MCT1 | Australia | 6.94 ± 0.18 | 3.30 ± 0.10 | 47.8 ± 0.8 | 76.9 ± 0.4 |
| Own Venture | Australia | 8.22 ± 0.20 | 3.07 ± 0.09 | 37.4 ± 0.5 | 76.8 ± 0.3 |
| IAC 4-12B | Brazil | 5.42 ± 0.11 | 1.81 ± 0.05 | 33.5 ± 0.6 | — |
| 695 (Beaumont) | South Africa | 5.21 ± 0.10 | 2.15 ± 0.05 | 41.1 ± 0.5 | — |
| 814 | South Africa | 4.60 ± 0.11 | 1.76 ± 0.05 | 38.0 ± 0.6 | — |
| 816 | South Africa | 5.73 ± 0.17 | 2.91 ± 0.10 | 50.6 ± 0.7 | — |
| A4 | South Africa | 6.90 ± 0.17 | 3.53 ± 0.10 | 50.9 ± 0.6 | — |
References
- FAO. Statistical Yearbook. World Food and Agriculture 2021; FAO: Rome, Italy, 2021. [Google Scholar]
- Aizen, M.A.; Garibaldi, L.A.; Cunningham, S.A.; Klein, A.M. How much does agriculture depend on pollinators? Lessons from long-term trends in crop production. Ann. Bot. 2009, 103, 1579–1588. [Google Scholar] [CrossRef]
- Klein, A.-M.; Boreux, V.; Fornoff, F.; Mupepele, A.-C.; Pufal, G. Relevance of wild and managed bees for human well-being. Curr. Opin. Insect Sci. 2018, 26, 82–88. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.D.; Goodell, K. Pollen removal and deposition by honeybee and bumblebee visitors to apple and almond flowers. J. Appl. Ecol. 2001, 38, 1032–1044. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Aizen, M.A.; Harder, L.D. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 2009, 19, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Osterman, J.; Aizen, M.A.; Biesmeijer, J.C.; Bosch, J.; Howlett, B.G.; Inouye, D.W.; Jung, C.; Martins, D.J.; Medel, R.; Pauw, A.; et al. Global trends in the number and diversity of managed pollinator species. Agric. Ecosyst. Environ. 2021, 322, 107653. [Google Scholar] [CrossRef]
- Bennett, J.M.; Steets, J.A.; Burns, J.H.; Burkle, L.A.; Vamosi, J.C.; Wolowski, M.; Arceo-Gómez, G.; Burd, M.; Durka, W.; Ellis, A.G.; et al. Land use and pollinator dependency drives global patterns of pollen limitation in the Anthropocene. Nat. Commun. 2020, 11, 3999. [Google Scholar] [CrossRef]
- Larson, B.M.H.; Barrett, S.C.H. A comparative analysis of pollen limitation in flowering plants. Biol. J. Linn. Soc. 2000, 69, 503–520. [Google Scholar] [CrossRef]
- Knight, T.M.; Steets, J.A.; Vamosi, J.C.; Mazer, S.J.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mitchell, R.J.; Ashman, T.-L. Pollen limitation of plant reproduction: Pattern and process. Ann. Rev. Ecol. Evol. Syst. 2005, 36, 467–497. [Google Scholar] [CrossRef]
- Knight, T.M.; Steets, J.A.; Ashman, T.-L. A quantitative synthesis of pollen supplementation experiments highlights the contribution of resource reallocation to estimates of pollen limitation. Am. J. Bot. 2006, 93, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Wesselingh, R.A. Pollen limitation meets resource allocation: Towards a comprehensive methodology. New Phytol. 2007, 174, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Trueman, S.J.; Kämper, W.; Nichols, J.; Ogbourne, S.M.; Hawkes, D.; Peters, T.; Hosseini Bai, S.; Wallace, H.M. Pollen limitation and xenia effects in a cultivated mass-flowering tree, Macadamia integrifolia (Proteaceae). Ann. Bot. 2022, 129, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Trueman, S.J. The reproductive biology of macadamia. Sci. Hortic. 2013, 150, 354–359. [Google Scholar] [CrossRef]
- Heard, T.A. Behaviour and pollinator efficiency of stingless bees and honey bees on macadamia flowers. J. Apic. Res. 1994, 33, 191–198. [Google Scholar] [CrossRef]
- Grass, I.; Meyer, S.; Taylor, P.; Foord, S.; Hajek, P.; Tscharntke, T. Pollination limitation despite managed honeybees in South African macadamia orchards. Agric. Ecosyst. Environ. 2018, 260, 11–18. [Google Scholar] [CrossRef]
- Howlett, B.G.; Nelson, W.R.; Pattemore, D.E.; Gee, M. Pollination of macadamia: Review and opportunities for improving yields. Sci. Hortic. 2015, 197, 411–419. [Google Scholar] [CrossRef]
- Wallace, H.M.; Vithanage, V.; Exley, E.M. The effect of supplementary pollination on nut set of Macadamia (Proteaceae). Ann. Bot. 1996, 78, 765–773. [Google Scholar] [CrossRef]
- Willcox, B.K.; Howlett, B.G.; Robson, A.J.; Cutting, B.; Evans, L.; Jesson, L.; Kirkland, L.; Jean-Meyzonnier, M.; Potdevin, V.; Saunders, M.E.; et al. Evaluating the taxa that provide shared pollination services across multiple crops and regions. Sci. Rep. 2019, 9, 13538. [Google Scholar] [CrossRef] [PubMed]
- Sedgley, M. Pollen tube growth in macadamia. Sci. Hortic. 1983, 18, 333–341. [Google Scholar] [CrossRef]
- Sedgley, M.; Bell, F.D.H.; Bell, D.; Winks, C.W.; Pattison, S.J.; Hancock, T.W. Self- and cross-compatibility of macadamia cultivars. J. Hortic. Sci. 1990, 65, 205–213. [Google Scholar] [CrossRef]
- Meyers, N.; McConchie, C.; Turnbull, C.; Vithanage, V. Cross pollination and intervarietal compatibility in macadamia. Aust. Macadamia Soc. News Bull. 1995, 22, 5–8. [Google Scholar]
- Sacramento, C.K.; Pereira, F.M.; Perecin, D.; Sabino, J.C. Capacidade combinatória para fructificação em cultivares de nogueira macadâmia. Pesqui. Agropecu. Bras. 1999, 34, 2045–2049. [Google Scholar] [CrossRef]
- Howlett, B.G.; Read, S.F.J.; Alavi, M.; Cutting, B.T.; Nelson, W.R.; Goodwin, R.M.; Cross, S.; Thorp, T.G.; Pattemore, D.E. Cross-pollination enhances macadamia yields, even with branch-level resource limitation. HortScience 2019, 54, 609–615. [Google Scholar] [CrossRef]
- Hardner, C. Macadamia domestication in Hawai‘i. Genet. Resour. Crop Evol. 2016, 63, 1411–1430. [Google Scholar] [CrossRef]
- Alam, M.M.; Wilkie, J.; Topp, B.L. Early growth and graft success in macadamia seedling and cutting rootstocks. Acta Hortic. 2018, 1205, 637–643. [Google Scholar] [CrossRef]
- Vithanage, V.; Meyers, N.; McConchie, C. Maximising the Benefits from Cross-Pollination in Macadamia Orchards; Horticulture Australia Ltd.: Sydney, Australia, 2002. [Google Scholar]
- Kämper, W.; Trueman, S.J.; Ogbourne, S.M.; Wallace, H.M. Pollination services in a macadamia cultivar depend on across-orchard transport of cross pollen. J. Appl. Ecol. 2021, 58, 2529–2539. [Google Scholar] [CrossRef]
- Trueman, S.; De Silva, A.; Kämper, W.; Nichols, J.; Hosseini Bai, S.; Wallace, H.; Royle, J.; Peters, T.; Ogbourne, S. Pollen parentage of nuts during premature nut drop: Do self-pollinated nuts drop and cross-pollinated nuts remain? Aust. Macadamia Soc. News Bull. 2022, 50, 19–20. [Google Scholar]
- De Silva, A.L.; Kämper, W.; Wallace, H.M.; Ogbourne, S.M.; Hosseini Bai, S.; Nichols, J.; Trueman, S.J. Boron effects on fruit set, yield, quality and paternity of macadamia. Agronomy 2022, 12, 684. [Google Scholar] [CrossRef]
- Richards, T.E.; Kämper, W.; Trueman, S.J.; Wallace, H.M.; Ogbourne, S.M.; Brooks, P.R.; Nichols, J.; Hosseini Bai, S. Relationships between nut size, kernel quality, nutritional composition and levels of outcrossing in three macadamia cultivars. Plants 2020, 9, 228. [Google Scholar] [CrossRef]
- Langdon, K.S.; King, G.J.; Nock, C.J. DNA paternity testing indicates unexpectedly high levels of self-fertilisation in macadamia. Tree Genet. Genomes 2019, 15, 29. [Google Scholar] [CrossRef]
- Kodad, O.; Estopañán, G.; Juan, T.; Socias i Company, R. Xenia effects on oil content and fatty acid and tocopherol concentrations in autogamous almond cultivars. J. Agric. Food Chem. 2009, 57, 10809–10813. [Google Scholar] [CrossRef] [PubMed]
- Kämper, W.; Thorp, G.; Wirthensohn, M.; Brooks, P.; Trueman, S.J. Pollen paternity can affect kernel size and nutritional composition of self-incompatible and new self-compatible almond cultivars. Agronomy 2021, 11, 326. [Google Scholar] [CrossRef]
- Denney, J.O. Xenia includes metaxenia. HortScience 1992, 27, 722–728. [Google Scholar] [CrossRef]
- Fattahi, R.; Mohammadzedeh, M.; Khadivi-Khub, A. Influence of different pollen sources on nut and kernel characteristics of hazelnut. Sci. Hortic. 2014, 173, 15–19. [Google Scholar] [CrossRef]
- Meyers, N.M.; Morris, S.C.; McFadyen, L.M.; Huett, D.O.; McConchie, C.A. Investigation of sampling procedures to determine macadamia fruit quality in orchards. Aust. J. Exp. Agric. 1999, 39, 1007–1012. [Google Scholar] [CrossRef]
- Trueman, S.J.; Richards, S.; McConchie, C.A.; Turnbull, C.G.N. Relationships between kernel oil content, fruit removal force and abscission in macadamia. Aust. J. Exp. Agric. 2000, 40, 859–866. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Fazekas, A.J.; Herbert, P.D.N. Semi-automated, membrane-based protocol for DNA isolation from plants. Plant Mol. Biol. Rep. 2008, 26, 186. [Google Scholar] [CrossRef]
- Peterson, B.K.; Weber, J.N.; Kay, E.H.; Fisher, H.S.; Hoekstra, H.E. Double digest RADseq: An inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 2012, 7, e37135. [Google Scholar] [CrossRef]
- Evans, L.J.; Jesson, L.; Read, S.F.J.; Jochym, M.; Cutting, B.T.; Gayrard, T.; Jammes, M.A.S.; Roumier, R.; Howlett, B.G. Key factors influencing forager distribution across macadamia orchards differ among species of managed bees. Basic Appl. Ecol. 2021, 53, 74–85. [Google Scholar] [CrossRef]
- McFadyen, L.; Robertson, D.; Sedgley, M.; Kristiansen, P.; Olesen, T. Post-pruning shoot growth increases fruit abscission and reduces stem carbohydrates and yield in macadamia. Ann. Bot. 2011, 107, 993–1001. [Google Scholar] [CrossRef]
- Olesen, T.; Huett, D.; Smith, G. The production of flowers, fruit and leafy shoots in pruned macadamia trees. Funct. Plant Biol. 2011, 38, 327–336. [Google Scholar] [CrossRef]
- Moncur, M.W.; Stephenson, R.A.; Trochoulias, T. Floral development of Macadamia integrifolia Maiden & Betche under Australian conditions. Sci. Hortic. 1985, 27, 87–96. [Google Scholar]
- Trueman, S.J.; Turnbull, C.G.N. Effects of cross-pollination and flower removal on fruit set in macadamia. Ann. Bot. 1994, 73, 23–32. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Influence of physical distance between cultivars on yield, outcrossing rate and selective fruit drop in avocado (Persea americana, Lauraceae). Ann. Appl. Biol. 2011, 158, 354–361. [Google Scholar] [CrossRef]
- Degani, C.; El-Batsri, R.; Gazit, S. Outcrossing rate, yield, and selective fruit abscission in ‘Ettinger’ and ‘Ardith’ avocado plots. J. Am. Soc. Hortic. Sci. 1997, 122, 813–817. [Google Scholar] [CrossRef]
- Degani, C.; Goldring, A.; Gazit, S. Pollen parent effect on outcrossing rate in ‘Hass’ and ‘Fuerte’ avocado plots during fruit development. J. Am. Soc. Hortic. Sci. 1989, 114, 106–111. [Google Scholar] [CrossRef]
- Trueman, S.J.; Nichols, J.; Farrar, M.B.; Wallace, H.M.; Hosseini Bai, S. Outcrossing rate and fruit yield of Hass avocado trees decline at increasing distance from a polliniser cultivar. Agronomy 2024, 14, 122. [Google Scholar] [CrossRef]
- Degani, C.; Stern, R.A.; El-Batsri, R.; Gazit, S. Pollen parent effect on the selective abscission of ‘Mauritius’ and ‘Floridian’ lychee fruitlets. J. Am. Soc. Hortic. Sci. 1995, 120, 523–526. [Google Scholar] [CrossRef]
- Dag, A.; Gazit, S.; Eisenstein, D.; El-Batsri, R.; Degani, C. Effect of the male parent on pericarp and seed weights in several Floridian mango cultivars. Sci. Hortic. 1999, 82, 325–329. [Google Scholar] [CrossRef]
- McConchie, C.A.; Meyers, N.M.; Vithanage, V.; Turnbull, C.G.N. Pollen Parent Effects on Nut Quality and Yield in Macadamia; Horticultural Research & Development Corporation: Sydney, Australia, 1997. [Google Scholar]
- Penter, M.G.; Nkwana, E.; Nxundu, Y. Factors influencing kernel breakage in the South African macadamia industry. S. Afr. Macadamia Grow. Assoc. Yearb. 2008, 16, 6–10. [Google Scholar]
- Australian Macadamia Society. The Australian Macadamia Industry; Australian Macadamia Society: Lismore, Australia, 2017. [Google Scholar]
- Australian Macadamia Society. Kernel Quality Standard for Processors; Australian Macadamia Society: Lismore, Australia, 2018. [Google Scholar]
- State of Queensland. Macadamia Industry Benchmark Report. 2009 to 2021 Seasons; State of Queensland: Brisbane, Australia, 2022.
- Orford, R. Factors Influencing Total Kernel Recovery and Your Profitability. AusMac 2022 Conference; Australian Macadamia Society: Lismore, Australia. Available online: https://www.youtube.com/watch?v=O99Njr_I6oE (accessed on 11 October 2023).
- Ito, P.J.; Hamilton, R.A. Quality and yield of ‘Keauhou’ macadamia nuts from mixed and pure block plantings. HortScience 1980, 15, 307. [Google Scholar] [CrossRef]
- Mason, R.L.; Wills, R.B.H. Evaluation of the use of specific gravity as an objective index of the quality of Australian macadamia nuts. Food Technol. Aust. 1983, 35, 245–248. [Google Scholar]
- Stephenson, R.A.; Gallagher, E.C.; Doogan, V.J.; Mayer, D.G. Nitrogen and environmental factors influencing macadamia quality. Aust. J. Exp. Agric. 2000, 40, 1145–1150. [Google Scholar] [CrossRef]
- Trueman, S.J.; McConchie, C.A.; Turnbull, C.G.N. Ethephon promotion of crop abscission for unshaken and mechanically shaken macadamia. Aust. J. Exp. Agric. 2002, 42, 1001–1008. [Google Scholar] [CrossRef]
- Trueman, S.J. Yield responses to ethephon for unshaken and mechanically shaken macadamia. Aust. J. Exp. Agric. 2003, 43, 1143–1150. [Google Scholar] [CrossRef]
- Trueman, S.J. Preliminary evaluation of low ethephon doses for inducing fruit abscission of macadamia (Macadamia integrifolia) cv. A16. Trop. Agric. 2003, 80, 243–245. [Google Scholar]
- Herbert, S.W.; Walton, D.A.; Wallace, H.M. Pollen-parent affects fruit, nut and kernel development of Macadamia. Sci. Hortic. 2019, 244, 406–412. [Google Scholar] [CrossRef]
- Currey, A. Three years on shows the benefit of careful planning and flexibility. Aust. Macadamia Soc. News Bull. 2021, 49, 44–45. [Google Scholar]
- Kojetin, L. Colour sorting capacity builds capacity at Natara Macadamias. Aust. Macadamia Soc. News Bull. 2022, 50, 49–51. [Google Scholar]
- Ramírez, F.; Davenport, T.L. Apple pollination: A review. Sci. Hortic. 2013, 162, 188–203. [Google Scholar] [CrossRef]
- Connell, J.H.; Floyd, J.; Limberg, J.; Miller, B.; Boles, J. Influence of pollinizer arrangement, bloom timing, and almond cultivar ratios on orchard productivity and crop value. Acta Hortic. 2014, 1028, 77–82. [Google Scholar] [CrossRef]
- Kwon, J.H.; Jun, J.H.; Nam, E.Y.; Chung, K.H.; Yoon, I.K.; Yun, S.K.; Kim, S.J. Selection of a suitable pollinizer for ‘Summer Fantasia’ plum. HortScience 2017, 52, 1182–1187. [Google Scholar] [CrossRef]
- Australian Macadamia Society. Fact Sheet. Pollination; Australian Macadamia Society: Lismore, Australia, 2022. [Google Scholar]
- Kron, P.; Husband, B.C.; Kevan, P.G. Across- and along-row pollen dispersal in high-density apple orchards: Insights from allozyme markers. J. Hortic. Sci. Biotechnol. 2001, 76, 286–294. [Google Scholar] [CrossRef]
- Sáez, A.; Di Virgilio, A.; Tiribelli, F.; Geslin, B. Simulation models to predict pollination success in apple orchards: A useful tool to test management practices. Apidologie 2018, 49, 551–561. [Google Scholar] [CrossRef]
- Akinsanmi, O.A.; Wang, G.; Neal, J.; Russell, D.; Drenth, A.; Topp, B. Variation in susceptibility among macadamia genotypes and species to Phytophthora root decay caused by Phytophthora cinnamomi. Crop Prot. 2016, 87, 37–43. [Google Scholar] [CrossRef]
| Cultivar | Country | Location | Tree Separation within Row | Distance to Nearest Other Cultivars | Nearest Cultivar in Each Direction | Other Cultivars within 500 m |
|---|---|---|---|---|---|---|
| 246 | Australia | 28°43′39″ S 153°24′08″ E | 10 (50 m) | 1 and 1 row (10 m and 10 m) | 741, H2 | 333, 344, 508 |
| 344 1 | Australia | 24°58′36″ S 152°22′45″ E | 10 (40 m) | 6 and 6 rows (48 m and 48 m) | Daddow, A203 | 741, 842, 849, A4, A29, A268 |
| 344 2 | Australia | 28°44′29″ S 153°31′34″ E | 10 (35 m) | Mixed in the same row (3.5 m) | 246, 816, 849 | 333, 508, 741, 788, 791, A4 |
| 741 | Australia | 24°56′18″ S 152°21′38″ E | 10 (20 m) | 3 and 3 rows (30 m and 30 m) | A4, A203 | 814, 816, 842, 849, A16, A38, A268, Own Venture |
| 814 | Australia | 24°55′45″ S 152°21′51″ E | 5 (20 m) | 3 and 3 rows (24 m and 24 m) | 816, A16 | 741, 849, 842, A4, A38, A203, A268, Daddow, Own Venture |
| 816 | Australia | 24°58′50″ S 152°22′46″ E | 10 (40 m) | 4 and 4 rows (32 m and 32 m) | 842, A4 | 344, 741, 849, A29, A203, A268, Daddow |
| 842 | Australia | 24°58′46″ S 152°22′46″ E | 10 (40 m) | 10 and 11 rows (80 m and 88 m) | 741, 816 | 344, 849, A4, A29, A203, A268, Daddow |
| 849 | Australia | 24°58′55″ S 152°22′46″ E | 10 (40 m) | 7 and 8 rows (56 m and 64 m) 3 | A4, forest 3 | 344, 741, 816, 842, A29, A203, A268, Daddow |
| A4 | Australia | 24°45′44″ S 152°15′55″ E | 10 (40 m) | 4 and 5 rows (32 m and 40 m) | 849, A38 | 344, 842, A16, A268 |
| A16 | Australia | 24°55′47″ S 152°21′52″ E | 10 (40 m) | 4 and 4 rows (32 m and 32 m) | 814, A38 | 741, 816, 849, 842, A4, A203, A268, Daddow, Own Venture |
| A29 | Australia | 24°56′21″ S 152°21′38″ E | 10 (20 m) | 3 and 3 rows (30 m and 30 m) | A203, A203 | 741, 816, 842, 849, A4, A16, A38, A268, Daddow, Own Venture |
| A38 | Australia | 24°56′14″ S 152°21′58″ E | 10 (40 m) | 6 and 7 rows (48 m and 58 m) | A268, 842 | 741, 816, 849, A4, A16, A203, Daddow, Own Venture |
| A203 | Australia | 24°58′40″ S 152°22′46″ E | 10 (40 m) | 4 and 5 rows (32 m and 40 m) | 344, 741 | 816, 842, 849, A4, A29, A268, Daddow |
| A268 | Australia | 24°55′5″ S 152°21′55″ E | 10 (40 m) | 8 and 8 rows (56 m and 56 m) | 842, Daddow | 741, 816, 849, A4, A16, A38, A203, Own Venture |
| Daddow | Australia | 24°58′31″ S 152°22′45″ E | 10 (40 m) | 8 and 9 rows (64 m and 72 m) | 849, 344 | 741, 816, 842, A4, A29, A203, A268 |
| H2 | Australia | 28°43′47″ S 153°24′06″ E | 10 (50 m) | 1 and 1 row (10 m and 10 m) | 246, 508 | 333, 344, 660, 741 |
| MCT1 | Australia | 24°50′42″ S 152°17′40″ E | 10 (40 m) | Mixed in the same row (4 m) | See next column | 741, A4, A16, A38, Daddow, Heilscher |
| Own Venture | Australia | 24°55′51″ S 152°21′52″ E | 10 (40 m) | 4 and 4 rows (32 m and 32 m) | A38, 849 | 741, 816, 842, A4, A16, A203, A268, Daddow |
| IAC 4-12B | Brazil | 22°22′17″ S 48°26′38″W | 5 (20 m) | 4 and 5 rows (35 m and 50 m) | 246, 246 | 344, 420, 741, 816, 1014 |
| 695 (Beaumont) | South Africa | 25°48′36″ S 31°0′24″ E | 5 (30 m) | 17 trees 4 and 20 rows (102 m and 240 m) | 788, 814 | 741, 816, A4 |
| 814 | South Africa | 25°48′33″ S 31°0′35″ E | 5 (30 m) | 8 and 7 rows (100 m and 84 m) | 695, forest 3 | 788, 816 |
| 816 | South Africa | 25°48′21″ S 31°0′3″ E | 5 (30 m) | 26 trees 4 (130 m) | 741, 741 | 695, 788 |
| A4 | South Africa | 25°38′55″ S 31°18′33″ E | 4 (16 m) | 3 and 4 rows (24 m and 32 m) | 695, A16 | 344, 741, 788, 816, 842, 849, A38, A203, A268, Daddow, Nelmak 2 |
| Mother Cultivar × Pollen Parent | Country | Nut Size or Quality Parameter | |||
|---|---|---|---|---|---|
| Nut-in-Shell Mass (g) | Kernel Mass (g) | Kernel Recovery (%) | Oil Concentration (%) | ||
| 246 × 246 | Australia | 7.21 ± 0.25 a | 2.23 ± 0.11 a | 30.4 ± 0.9 a | 74.6 ± 0.6 a |
| 246 × 344 | Australia | 7.63 ± 0.33 a | 2.56 ± 0.13 ab | 33.6 ± 1.1 ab | 74.8 ± 0.7 a |
| 246 × 508 | Australia | 8.12 ± 0.19 a | 2.86 ± 0.12 b | 35.2 ± 1.3 b | 76.2 ± 1.1 a |
| 246 × 741 * | Australia | 6.80 ± 0.29 a | 2.31 ± 0.10 ab | 34.3 ± 1.0 b | 76.8 ± 0.4 a |
| 344 1 × 344 | Australia | 6.79 ± 0.74 a | 1.87 ± 0.33 a | 26.4 ± 2.6 a | 75.4 ± 1.5 a |
| 344 1 × 842 | Australia | 8.58 ± 0.31 b | 2.70 ± 0.14 b | 31.4 ± 0.7 ab | 77.9 ± 0.6 ab |
| 344 1 × 849 | Australia | 8.90 ± 0.24 b | 2.72 ± 0.11 b | 30.6 ± 0.8 ab | 77.0 ± 0.8 ab |
| 344 1 × Daddow * | Australia | 8.40 ± 0.26 b | 2.77 ± 0.12 b | 32.8 ± 0.9 b | 78.5 ± 0.5 b |
| 344 2 × 344 | Australia | 6.31 ± 0.34 a | 1.69 ± 0.15 a | 25.4 ± 1.3 a | 73.5 ± 0.5 a |
| 344 2 × 246 * | Australia | 8.35 ± 0.64 b | 2.61 ± 0.23 b | 31.2 ± 1.3 b | 77.0 ± 0.9 b |
| 741 × 741 * | Australia | 5.36 ± 0.32 a | 1.91 ± 0.10 a | 36.0 ± 1.3 a | 75.7 ± 0.8 a |
| 741 × 842 * | Australia | 7.12 ± 0.24 b | 2.63 ± 0.12 b | 36.9 ± 0.8 a | 77.7 ± 0.4 ab |
| 741 × A16 | Australia | 8.75 ± 0.32 c | 3.46 ± 0.09 c | 39.7 ± 1.4 a | 79.2 ± 0.3 b |
| 814 × 816 * | Australia | 5.75 ± 0.25 a | 1.96 ± 0.11 a | 33.5 ± 1.1 a | 77.6 ± 0.3 a |
| 814 × 849 * | Australia | 6.47 ± 0.26 ab | 2.47 ± 0.10 b | 38.3 ± 1.3 b | 78.2 ± 0.4 a |
| 814 × A16 | Australia | 7.57 ± 0.54 b | 3.01 ± 0.18 c | 38.8 ± 1.6 b | 80.0 ± 0.6 b |
| 816 × 842 * | Australia | 6.91 ± 0.25 a | 2.94 ± 0.11 a | 42.7 ± 0.9 a | 77.0 ± 0.4 a |
| 816 × 849 | Australia | 6.97 ± 0.24 a | 3.01 ± 0.10 a | 43.3 ± 1.0 a | 77.3 ± 0.4 a |
| 842 × 816 | Australia | 6.91 ± 0.26 a | 2.63 ± 0.12 a | 38.2 ± 1.3 a | 78.9 ± 0.4 a |
| 842 × Daddow * | Australia | 8.10 ± 0.32 b | 3.28 ± 0.11 b | 40.8 ± 1.2 a | 79.2 ± 0.3 a |
| 849 × 816 * | Australia | 6.02 ± 0.23 a | 2.54 ± 0.14 a | 41.6 ± 1.4 a | 76.9 ± 0.6 a |
| A4 × 344 | Australia | 6.95 ± 0.40 a | 3.23 ± 0.17 a | 46.6 ± 1.1 a | 79.7 ± 0.5 ab |
| A4 × A16 | Australia | 8.03 ± 0.36 ab | 3.69 ± 0.19 ab | 45.9 ± 0.8 a | 79.0 ± 0.6 a |
| A4 × A38 * | Australia | 8.40 ± 0.29 b | 4.01 ± 0.14 b | 47.9 ± 1.1 a | 80.7 ± 0.4 b |
| A16 × 814 * | Australia | 7.85 ± 0.43 a | 3.16 ± 0.20 a | 40.2 ± 1.1 a | 79.5 ± 0.8 a |
| A16 × A38 * | Australia | 7.74 ± 0.40 a | 3.32 ± 0.21 a | 42.6 ± 0.9 a | 78.8 ± 0.4 a |
| A29 × A29 | Australia | 8.26 ± 0.40 a | 2.95 ± 0.19 a | 35.4 ± 1.0 a | 75.5 ± 0.8 a |
| A29 × (H2 × 741) * | Australia | 8.32 ± 0.44 a | 3.14 ± 0.23 a | 37.7 ± 1.5 a | 78.5 ± 0.9 b |
| A203 × 344 * | Australia | 8.74 ± 0.19 a | 2.98 ± 0.11 a | 34.1 ± 1.0 a | 78.9 ± 0.6 a |
| A203 × Daddow * | Australia | 10.26 ± 0.46 b | 3.72 ± 0.28 b | 36.0 ± 1.5 a | 80.8 ± 1.0 a |
| A268 × 816 | Australia | 11.13 ± 0.78 a | 3.95 ± 0.32 a | 35.4 ± 0.7 a | 77.6 ± 1.1 a |
| A268 × 842 * | Australia | 10.12 ± 0.35 a | 3.48 ± 0.17 a | 34.1 ± 0.8 a | 78.1 ± 0.3 a |
| A268 × 849 | Australia | 10.01 ± 0.61 a | 3.56 ± 0.33 a | 35.1 ± 1.4 a | 76.8 ± 0.5 a |
| Daddow × Daddow | Australia | 8.40 ± 0.70 a | 3.01 ± 0.28 a | 35.7 ± 0.9 a | 78.6 ± 0.2 ab |
| Daddow × 344 | Australia | 7.94 ± 0.47 a | 3.30 ± 0.20 a | 41.7 ± 1.5 b | 79.7 ± 0.4 a |
| Daddow × 849 * | Australia | 8.91 ± 0.48 a | 3.45 ± 0.15 a | 39.0 ± 0.6 b | 77.7 ± 0.5 b |
| H2 × 508 * | Australia | 9.84 ± 0.49 a | 2.98 ± 0.13 a | 30.6 ± 1.1 a | 77.4 ± 0.3 a |
| MCT1 × A4 * | Australia | 7.32 ± 0.24 a | 3.33 ± 0.12 a | 45.8 ± 1.1 a | 77.0 ± 0.5 a |
| MCT1 × A16 | Australia | 6.62 ± 0.35 a | 3.33 ± 0.20 a | 50.3 ± 1.6 a | 75.8 ± 0.8 a |
| Own Venture × 849 * | Australia | 8.71 ± 0.46 a | 3.50 ± 0.22 a | 40.1 ± 1.2 a | 76.0 ± 0.4 a |
| IAC 4-12 B × IAC 4-12 B | Brazil | 5.41 ± 0.55 a | 1.69 ± 0.14 a | 31.7 ± 1.4 a | — |
| IAC 4-12 B × 246 * | Brazil | 5.35 ± 0.16 a | 1.82 ± 0.06 a | 34.1 ± 0.8 a | — |
| 695 × 695 | South Africa | 5.05 ± 0.16 a | 1.96 ± 0.07 a | 38.8 ± 0.8 a | — |
| 695 × 788 | South Africa | 5.63 ± 0.26 a | 2.49 ± 0.15 b | 44.0 ± 1.1 b | — |
| 695 × 814 | South Africa | 5.67 ± 0.39 a | 2.39 ± 0.17 ab | 42.2 ± 1.1 b | — |
| 695 × 816 * | South Africa | 4.99 ± 0.17 a | 2.10 ± 0.09 ab | 42.1 ± 0.9 b | — |
| 814 × 814 | South Africa | 4.45 ± 0.10 a | 1.67 ± 0.05 a | 37.4 ± 0.6 a | — |
| 816 × 816 | South Africa | 5.52 ± 0.23 a | 2.74 ± 0.13 a | 49.6 ± 0.9 a | — |
| 816 × 695 * | South Africa | 6.33 ± 0.45 a | 3.35 ± 0.30 a | 52.4 ± 1.7 a | — |
| 816 × 741 * | South Africa | 5.57 ± 0.29 a | 2.95 ± 0.19 a | 52.8 ± 1.5 a | — |
| A4 × 695 * | South Africa | 6.91 ± 0.19 a | 3.58 ± 0.12 a | 51.5 ± 0.8 a | — |
| A4 × A268 | South Africa | 7.44 ± 0.64 a | 3.69 ± 0.34 a | 49.7 ± 1.5 a | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trueman, S.J.; Penter, M.G.; Malagodi-Braga, K.S.; Nichols, J.; De Silva, A.L.; Ramos, A.T.M.; Moriya, L.M.; Ogbourne, S.M.; Hawkes, D.; Peters, T.; et al. High Outcrossing Levels among Global Macadamia Cultivars: Implications for Nut Quality, Orchard Designs and Pollinator Management. Horticulturae 2024, 10, 203. https://doi.org/10.3390/horticulturae10030203
Trueman SJ, Penter MG, Malagodi-Braga KS, Nichols J, De Silva AL, Ramos ATM, Moriya LM, Ogbourne SM, Hawkes D, Peters T, et al. High Outcrossing Levels among Global Macadamia Cultivars: Implications for Nut Quality, Orchard Designs and Pollinator Management. Horticulturae. 2024; 10(3):203. https://doi.org/10.3390/horticulturae10030203
Chicago/Turabian StyleTrueman, Stephen J., Mark G. Penter, Kátia Sampaio Malagodi-Braga, Joel Nichols, Anushika L. De Silva, Adalgisa Thayne Munhoz Ramos, Leonardo Massaharu Moriya, Steven M. Ogbourne, David Hawkes, Trent Peters, and et al. 2024. "High Outcrossing Levels among Global Macadamia Cultivars: Implications for Nut Quality, Orchard Designs and Pollinator Management" Horticulturae 10, no. 3: 203. https://doi.org/10.3390/horticulturae10030203
APA StyleTrueman, S. J., Penter, M. G., Malagodi-Braga, K. S., Nichols, J., De Silva, A. L., Ramos, A. T. M., Moriya, L. M., Ogbourne, S. M., Hawkes, D., Peters, T., Kasinadhuni, N., Hosseini Bai, S., Wallace, H. M., & Kämper, W. (2024). High Outcrossing Levels among Global Macadamia Cultivars: Implications for Nut Quality, Orchard Designs and Pollinator Management. Horticulturae, 10(3), 203. https://doi.org/10.3390/horticulturae10030203







