Abstract
Pear decline (PD) phytoplasma populations were examined over one year in non-symptomatic pear trees with HW620 scions grafted onto three different rootstocks: OH×F87 (PD resistant), OH×F69 (PD susceptible), and Bartlett (PD susceptible). For all three rootstocks, populations were at a maximum during late summer for leaves and shoots, and reached their minimum in mid-winter for shoots and early spring for leaves. In contrast, roots exhibited maximum populations in mid-winter and minimum populations in mid-spring. For all tissue types, PD populations were consistently lowest in trees grafted onto OH×F87 rootstocks, intermediate in those on OH×F69, and highest on Bartlett rootstocks, demonstrating that the type of rootstock significantly impacts PD populations. While OH×F87 rootstocks had the lowest populations, they can still contain relatively high PD populations, particularly during periods with maximum populations. Future research could explore the development of even higher levels of PD resistance in pear rootstocks to reduce PD populations in both the rootstock and scion.
1. Introduction
Pear decline (PD) is a disease of the pear tree (Pyrus sp.) caused by the PD phytoplasma “Candidatus Phytoplasma pyri”, which belongs to the 16SrX-C phytoplasma subgroup based on 16S rRNA classification [1,2]. The symptoms of PD are influenced by environmental conditions, pear psylla infestation, the vigour of the tree, and rootstock tolerance [3]. Pear trees react to PD either with a quick decline, which often results in the sudden collapse of the tree, or a slow decline, which often occurs with leaf curl symptoms [2]. A quick decline has mostly been observed in pear trees grafted onto Asian pear rootstocks (P. serotina and P. ussuriensis), with foliar symptoms of sudden wilting of leaves over a two week period, leading to the death of the leaves and the scorched appearance of the whole tree [4,5]. A slow decline has been observed more in pear trees grafted onto more tolerant rootstocks, such as P. calleryana, P. betulaefolia and P. communis cvs. Bartlett, Old Home, and Hardy, with foliar symptoms observed primarily at the end of the summer and early fall [2,6].
The impact of the rootstock on scion symptoms can be partially explained by the annual fluctuation in PD phytoplasma populations [6,7]. Phytoplasmas are restricted to functional phloem sieve elements, and the populations of phytoplasma in the aerial part of plants depend on the active state of the phloem [8]. Under winter climatic conditions (below 0 °C), in trees, sieve tubes lose their function and become unsuitable for phytoplasmas, thus negatively affecting phytoplasma populations [6]. In pears, seasonal PD populations have been observed via DAPI staining and fluorescent microscopy, showing that PD becomes undetectable in the above-ground parts of the trees when temperatures drop below 0 °C during the winter months, but survives in the roots to recolonize the stem and branches in the following spring when a new functional phloem is produced [9]. Movement of PD phytoplasma from the roots to the newly developing sieve tubes in the stems and leaves occurs in May and June, at which time the concentration of PD phytoplasma is very low. From July until leaf fall, PD populations are systemic, reaching their highest numbers in September and October when the disease is well established [7,9]. Similar observations have been made for other tree species infected with phytoplasmas at temperatures below 0 °C, such as mulberry trees infected by mulberry dwarf phytoplasma [10,11], elm trees infected by elm yellows phytoplasma (EY) [8], paulownia trees infected with paulownia witches’ broom phytoplasma [12], lime trees infected by ”Ca. Phytoplasma aurantifolia” [13], and apple trees infected with AP phytoplasma [7,9], indicating that seasonal variation in phytoplasma populations is common.
Resistance to PD has been identified in different rootstocks. For example, P. communis cvs. grown on Bartlett rootstock show a moderate to low PD resistance [14]. Trees grown on several rootstocks of crosses of P. communis cv. Old Home with P. communis cv. Farmingdale (OH×F), such as OH×F87, 333, 18, and 267, show varying levels of resistance based on PD populations as detected via DAPI staining in young shoots and roots [9]. A high resistance was observed in OH×F87, while a high susceptibility was observed with OH×F217 and OH×69, with the latter being the most susceptible to PD [1].
Although it is known that PD phytoplasma populations vary over the year in pear tissues and that rootstock resistance to PD can affect PD populations throughout the trees, it is unknown if resistance affects the seasonal changes in PD populations. The goal of this study was to examine PD populations over a year in a 10-year-old orchard planted near Niagara-on-the-Lake in Ontario, Canada, with trees of P. communis cv. HW620 grafted onto Bartlett, OH×F87, and OH×F69 seedling rootstocks that had previously tested positive for PD phytoplasma via PCR.
The scion, HW620, was chosen for this study as it is a promising commercial variety producing good yields and large-sized fruit [15]. The rootstock Bartlett (syn. Williams), which was developed as a variety in England around 1796, was chosen for this study as pear trees in many parts of the world still use it as a rootstock [15,16]. OH×F69 and OH×F87 were both developed in the 1970s from crosses between the cultivars Old Home and Farmingdale [16], and were chosen for this study as they are popular commercial rootstocks primarily because they provide resistance to fire blight, woolly pear aphids, and Phytophthora root rot, and also provide tolerance to a high soil pH and drought [16,17]. In a study of six OH×F rootstocks infected by bud grafting, with PD infections confirmed via DAPI staining, which were compared with respect to foliar symptom severity with PD infections confirmed by DAPI staining, OH×F87 was the most resistant, while OH×F69 was the most susceptible [1]. Bartlett was not included in that study, and thus there is no direct comparison between Bartlett, OH×F69, and OH×F87 regarding their susceptibility to PD. However, Bartlett could be more susceptible than OH×F87, and possibly OH×F69, based on the description of the severity of foliar symptoms by Giunchedi et al. [14] and Seemüller et al. [1], but a direct comparison is difficult due to different locations with different climates, soil, and PD strains.
2. Materials and Methods
2.1. Sampling
In a commercial orchard planted in 1998 near Niagara-on-the-Lake (95 m above sea level) in Southern Ontario (Canada), fifteen asymptomatic pear trees of cv. HW620 grafted onto OH×F87, OH×F69, or Bartlett rootstocks (five trees per rootstock) which had tested positive for PD in 2008 using PD-specific nested PCR (P1/P7 followed by fPD/rPD primers) [18] were selected. From each tree, samples of approx. 0.5 g were obtained from roots 20–50 cm underground or leaves and shoots at heights of ~1–2 m or >2 m above ground level. Samples were collected at ~4–5 weeks intervals from June 2010 to May 2011. As a control, DNA was extracted from three asymptomatic pear trees of cv. HW620 grafted onto the same rootstocks in the same orchard testing negative for PD using the same PD-specific nested PCR assay. All tissue samples were immediately frozen and stored at −80 °C until further analysis.
2.2. DNA Extraction
DNA was extracted from petioles, leaf veins, or scrapings of vascular tissues below the bark of roots and shoots according to Green et al. (1999) [19]. Briefly, approx. 0.5 to 0.7 g of tissue was placed in a sample bag (Bioreba, Basel, Switzerland) with 5 mL of CTAB extraction buffer (100 mM Tris, pH 8.0, 1.4 M NaCl, 50 mM EDTA, pH 8.0, 2.5% (wt./vol) CTAB, 1% (wt./vol) polyvinylpyrrolidone (PVP-40)). The samples were ground inside the bag for 5 min in a Homex 6 Homogenizer (Bioreba, Basel, Switzerland), and a 0.5 mL aliquot of the homogenate was transferred to a 1.5 mL microcentrifuge tube and mixed (by inversion) with 4 μL of 100 mg/mL RNase A (Qiagen, Germantown, MD, USA). Following incubation at 65 °C with shaking (800 rpm) using a Thermo Mixer (VWR Canlab, Mississauga, ON, Canada) for 35 min, 1.8 mL of P3 buffer from a DNeasy Plant Mini Kit (Qiagen) was added and the DNA was extracted following the manufacturer’s protocol.
Concentrations of extracted DNA from PD-infected trees and healthy controls were measured by determining A260nm and A280nm using a NanoDrop (ND-1000) spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
2.3. Quantitative Real-Time PCR (qRT-PCR)
Real-time PCR assays were carried out in 96-well (0.2 mL) plates (VWR, Philadelphia, PA, USA) using a Stratagene MX3005P thermal cycler (Stratagene, La Jolla, CA, USA) with 25 μL reactions consisting of 3 μL of 10 ng/μL template DNA, 1.5 mM MgCl2, and 1X Taq PCR Master Mix (Qiagen, Hilden, Germany). The PHYTOF3 and PHYTOR3 primers targeting the 16S-23S rRNA intergenic spacer of the PD phytoplasma (Genbank accession GU565960 [5,20]) were used at 900 nM and 450 nM, respectively. A TaqMan probe (Integrated DNA Technologies Inc., Coralville, IA, USA) also targeting the 16S-23S rRNA intergenic spacer of the PD phytoplasma [20] was used at 200 mM and labelled at the 5′-end with the fluorescent dye 6-carboxyfluorescein (FAM) as a reporter and at the 3′-end with an Iowa Black® fluorescence quencher (FQ) (Table 1). Each sample was run in duplicate in the same plate. Reactions were conducted with one cycle of 5 min at 95 °C, followed by 45 cycles of 15 s at 95 °C and 30 s at 60 °C.

Table 1.
Primers and probe used for PD phytoplasma quantification.
2.4. Standard Curve Preparation for Quantitative Real-Time PCR (qRT-PCR) Analysis
For quantification of “Ca. Phytoplasma pyri” in the host plant, a standard curve was created by diluting plasmids containing a cloned partial “Ca. Phytoplasma pyri” 16S rRNA gene fragment (766 bp). To clone the 16S-23S rRNA intergenic spacer fragment, 40 ng of DNA isolated from mixed midribs tissues of several known PD-infected Bartlett seedlings was used. PCR amplification was performed in 25 μL containing 1X Taq PCR Master Mix (Qiagen), 1 μM each of primers FPDQ/RPDQ (designed in this study) (Table 1), and template [21]. The cycling conditions were one cycle of 94 °C for 10 min, followed by 40 cycles of 94 °C for 30 s, 54 °C for 30 s, 72 °C for 1 min 20 s, and a final extension cycle of 72 °C for 5 min. The resulting PCR product was purified from a 1% TAE agarose gel using the QIAquick Gel Extraction Kit (Qiagen) as recommended by the manufacturer. The extracted PCR product was cloned into the pGEM-T Easy plasmid vector (Promega, Madison, WI, USA), and plasmids containing the PCR product were detected by PCR with FPDQ/RPDQ primers. Plasmids were extracted according to the QIAGEN Plasmid Mini Kit protocol (Qiagen). The purified plasmid was quantified by a NanoDrop (ND-1000) spectrophotometer (NanoDrop Technologies). Standard curves were prepared with 10-fold serial dilutions of the cloned “Ca. Phytoplasma pyri” 16S rRNA gene fragment ranging in copy number from 102 to 107 (Figure S1). The following equation was used to calculate the corresponding copy number per microliter:
The molecular weight of plasmid + insert was calculated according to Baric et al. (2011) [22] as the sum of molecular weights of the total number of specific nucleotides and multiplied by the atomic mass unit (1.6605 × 10−27 g/u). The relative phytoplasma concentration was determined by performing the quantification principle of the 2−ΔΔCT method as described by Baric et al. (2011) [22].
2.5. Data Analysis
All quantitative data were subjected to mean comparisons using LSD at p ≤ 0.05 in SPSS statistical software v.21 (SPSS, Chicago, IL, USA). Environmental data for sampling location were obtained from Ontario Climate Centre, Environment Canada, Toronto.
3. Results
The monthly average daily temperature (Figure S2) and average daily precipitation (Figure S3) during the period of the study were generally similar to the 30-year averages at from the nearest weather station in St. Catharines (~5 km from the pear trees in this study). However, total summer temperatures were slightly higher and total winter temperatures were slightly lower than the 30-year average, and the total precipitation was slightly higher than the 30-year average for all months of the study, except for March and April of 2011. The months with an average daily temperature below 0 °C were December, January, and February, while the months above 20 °C were July and August.
In lower HW620 leaves on all rootstock genotypes, the pattern of the PD phytoplasma population was an increase from June to September, followed by a decline in October with no samples from November to March, and finally there was an increase from April to May the following year (Figure 1). During the summer, lower leaf PD populations were always significantly higher in Bartlett than OH×F87 rootstocks and significantly higher in Bartlett than in OH×F69 rootstocks, except in September when there was no significant difference between the rootstocks. PD populations in lower leaves of OH×F69 were significantly higher than those of OH×F87, except in June and August when there were no significant differences. The difference in PD populations between lower leaves on the rootstocks was most obvious in July (315.1, 171.8, and 37.9 PD cells/10 ng pear DNA with Bartlett, OH×F69, and OH×F87 rootstocks, respectively). During fall (October), PD populations in the lower leaves of HW620 were significantly different between all three rootstocks. No lower leaves were available from November to March, and therefore, no PD was detected. In the spring, PD populations significantly increased in Bartlett rootstock compared to OH×F87 rootstock in May, but they were significantly higher than those of OH×F69 in both May and April. There were no significant differences between OH×F69 and OH×F87 in the spring. In general, lower leaves on Bartlett rootstock had the highest PD populations compared to OH×F69 and OH×F87 rootstocks.
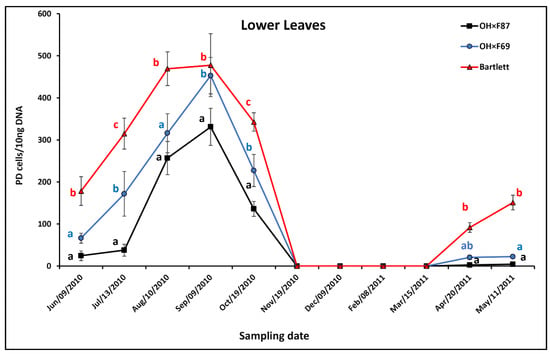
Figure 1.
qRT-PCR quantification of PD phytoplasma in lower leaves of cv. HW620 growing on rootstocks of Bartlett, OH×F69, and OH×F87. Each data point represents the average number of “Ca. Phytoplasma pyri” cells calculated from 10 ng of DNA samples from five (two biological reps) infected pear trees per rootstock (0.5 g of tissue per tree) collected monthly from June 2010 to May 2011. Vertical bars show standard errors. Means for the three rootstocks each month followed by the same letter are not significantly different according to Tukey’s statistical test at p ≤ 0.05. No leaves were available for collection from November to April.
In the upper leaves of HW620, the pattern of PD population was an increase from June to August on Bartlett and OH×F69 rootstocks versus a very limited increase on OH×F87 rootstock, which resulted in a peak for Bartlett in August, for OH×F69 in August and September, and for OH×F87 in September (Figure 2). This was followed by a decline in September and/or October with no upper leaf samples available from November to March. Finally, there was an increase in May the following year. In summer and fall, PD populations were significantly higher for HW620 on Bartlett than on OH×F87 rootstock at all months and significantly higher on Bartlett than OH×F69 rootstocks in August and September. Upper leaf PD populations were significantly higher on OH×F69 than OH×F87, except in October. The greatest difference between all three rootstocks was in August (715.9, 284.5, and 9.0 PD cells/10 ng pear DNA with Bartlett, OH×F69, and OH×F87 rootstocks, respectively). No PD was detected on the upper leaves in all rootstocks from November to March, as no upper leaves were available to sample. In April and May when the upper leaves first appeared, PD populations were not significantly different between any of the rootstocks. Compared to the lower leaves, PD populations on the upper leaves showed a similar pattern, with peak populations in late summer, but the difference between the three rootstocks was greater, with upper leaves having the highest PD populations on Bartlett followed by OH×F69 and then OH×F87 rootstocks.
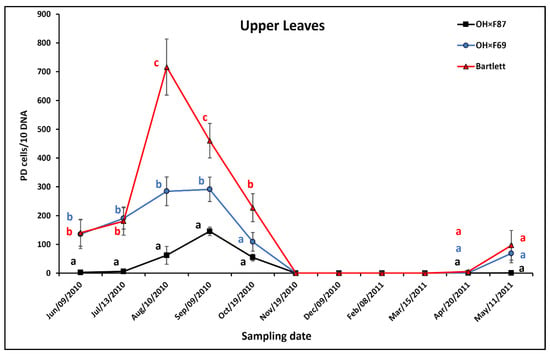
Figure 2.
qRT-PCR quantification of PD phytoplasma in upper leaves of cv. HW620 growing on rootstocks of Bartlett, OH×F69, and OH×F87. Each bar represents the average number of “Ca. Phytoplasma pyri” cells calculated from 10 ng of DNA samples from five (each two biological reps) infected pear trees per rootstock (0.5 g of tissue per tree) collected monthly from June 2010 to May 2011. Vertical bars show standard errors. Means for the three rootstocks each month followed by the same letter are not significantly different according to Tukey’s statistical test at p ≤ 0.05. No leaves were available for collection from November to April.
In lower shoots, the pattern of PD phytoplasma populations on Bartlett and OH×F69 rootstocks was an increase from June to August and/or September on all rootstocks, followed by a decline from September to December and then a gradual increase from December to May, except in the OH×F87 rootstock, where the populations remained relatively unchanged from December to May (Figure 3). During the increase in summer, PD populations were significantly higher in lower shoots in Bartlett rootstocks than OH×F87 rootstocks from June to September, but only significantly higher in Bartlett rootstocks than OH×F69 rootstocks in July and September. PD populations in lower shoots were always significantly higher in summer in OH×F69 than in OH×F87. The greatest difference between all three rootstocks was in July (271.6, 918.9, and 1455.5 PD cells/10 ng pear DNA, for OH×F87, OH×F69, and Bartlett, respectively). During the decline in late summer/fall, PD populations were significantly higher in lower shoots on Bartlett than OHF×87, except in November, but populations on Bartlett were not significantly different than on OH×F69. In winter and spring, PD populations did not show significant differences in lower shoots for HW620 on all rootstocks, except for a significantly higher PD population on Bartlett than on OH×F87 rootstock in May. Overall, lower shoots on Bartlett and OH×F69 rootstocks had significantly higher PD populations than those on OH×F87 rootstocks.
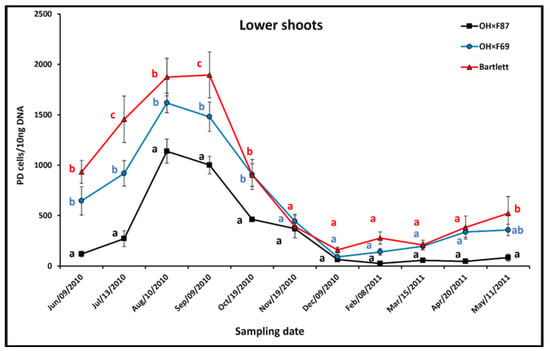
Figure 3.
qRT-PCR quantification of PD phytoplasma in lower shoots of cv. HW620 growing on rootstocks of Bartlett, OH×F69, and OH×F87. Each bar represents the average number of Ca. P. pyri cells calculated from 10 ng of DNA samples from five (each two biological reps) infected pear trees per rootstock (0.5 g of tissue per tree) collected monthly from June 2010 to May 2011. Vertical bars show standard errors. Means for the three rootstocks each month followed by the same letter are not significantly different according to Tukey’s statistical test at p ≤ 0.05.
In upper shoots, the pattern of PD phytoplasma populations was an increase from June to August on all rootstock genotypes, then a decline from August to December or February, followed by an increase from February to May (Figure 4). During the increase in summer, upper shoot PD populations were significantly higher in Bartlett than OH×F87 from June to September, but only significantly different in OH×F69 in August. The most obvious difference between the upper shoot PD populations on the three rootstocks was in August (1083.5, 676.3, and 452.9 PD cells/10 ng pear DNA, for Bartlett, OH×F69, and OH×F87, respectively). During the decline in fall, PD populations were only significantly higher in Bartlett than OH×F87 only in October, but were never significantly different between Bartlett and OH×F69 rootstocks. During winter, PD populations were never significantly different between upper shoots in the three rootstocks. In spring, significantly higher upper shoot PD populations were only observed in May in Bartlett compared to OH×F87 rootstocks. The pattern of PD population dynamics was similar between upper and lower shoots, with the same effect as rootstock genotype, except for a more pronounced peak in populations observed in upper shoots on the Bartlett rootstock in August.
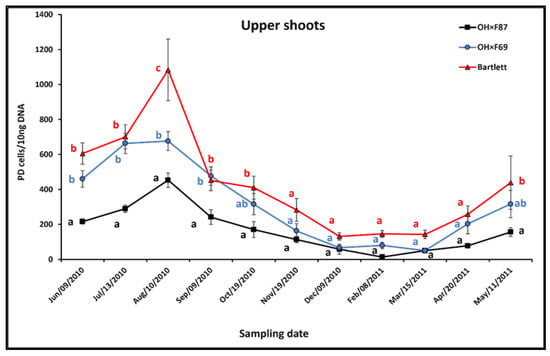
Figure 4.
qRT-PCR quantification of PD phytoplasma in upper shoots of cv. HW620 growing on rootstocks of Bartlett, OH×F69 and OH×F87. Each bar represents the average number of “Ca. Phytoplasma pyri” cells calculated from 10 ng of DNA samples from five (each two biological reps) infected pear trees per rootstock (0.5 g of tissue per tree) collected monthly from June 2010 to May 2011. Vertical bars show standard errors. Means for the three rootstocks each month followed by the same letter are not significantly different according to Tukey’s statistical test at p ≤ 0.05.
In roots, the pattern of PD phytoplasma populations was an increase from June to December for all rootstock genotypes, then a decrease from December to May (Figure 5). During summer and fall, root PD populations on the different rootstocks were not significantly different, except for Bartlett, in which they were significantly higher than OH×F69 and OH×F87 in August and November and significantly higher than OH×F87 in October. In winter, Bartlett had significantly higher root PD populations than both OH×F69 and OH×F87 in December; Bartlett had significantly higher PD populations compared than OH×F87 in February; and Bartlett had significantly higher PD populations than OH×F69 in March, which was also significantly higher than OH×F87. The most obvious difference was in December (9594.5, 4838.1, and 4679.5 PD cells/10 ng pear DNA for Bartlett, OH×F69, and OH×F87, respectively). In spring, there were no significant differences between PD populations in all three rootstocks.
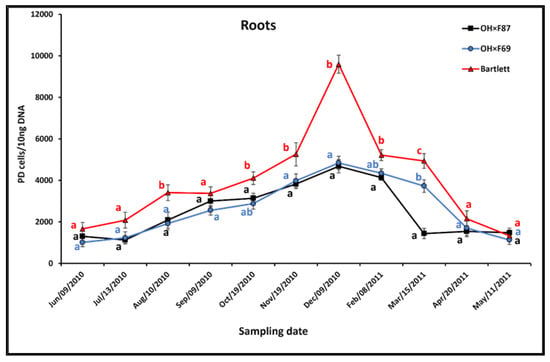
Figure 5.
qRT-PCR quantification of PD phytoplasma in roots of cv. HW620 growing on rootstocks of Bartlett, OH×F69 and OH×F87. Each bar represents the average number of “Ca. Phytoplasma pyri” cells calculated from 10 ng the DNA samples from five (each two biological reps) infected pear trees per rootstock (0.5 g of tissue per tree) collected monthly from June 2010 to May 2011. Vertical bars show standard errors. Means for the three rootstocks each month followed by the same letter are not significantly different according to Tukey’s statistical test at p ≤ 0.05.
A comparison of PD populations was conducted between various tissues on Bartlett rootstock revealed root tissue, consistently showing significantly higher PD populations than other tissues, except for June and May, when there were no significant differences between roots versus lower and upper shoots (Figure 6). For shoots, PD populations were generally higher in lower than upper shoots, with significant differences in August and September. For leaves, there were no significant differences in PD populations between upper and lower leaves, except for July and April, when the lower leaves had significantly higher PD populations than the upper leaves.
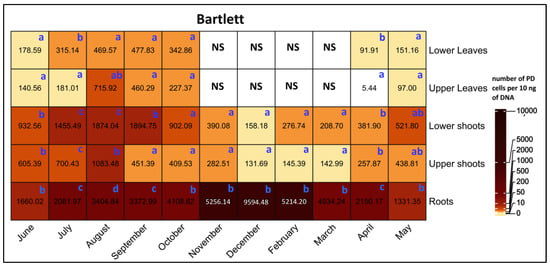
Figure 6.
Heat map of qRT-PCR quantification of PD phytoplasma in lower leaves, upper leaves, lower shoots, upper shoots, and roots of HW620 pear trees on Bartlett rootstock collected monthly between June 2010 and May 2011. Numbers represent the mean of the average number of PD phytoplasma cells determined from 10 ng of DNA samples from five infected pear trees of each rootstock (0.5 g of tissue per tree). Means followed by the same letter at each column (month) are not significantly different according to Tukey’s statistical test at p ≤ 0.05. No leaves were available during the months of November to April.
A comparison of PD populations between various tissues on OH×F69 rootstocks revealed that roots had significantly higher PD populations than other tissues, except for in June, July, and August, when there were no significant differences between lower shoots and roots (Figure 7). The PD populations in lower shoots were significantly higher than in the upper shoots in August. For leaves, PD populations were never significantly different between lower and upper leaves.
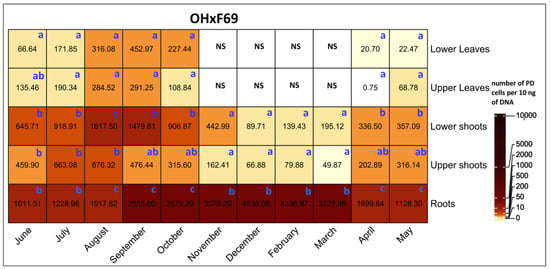
Figure 7.
Heat map of qRT-PCR quantification of PD phytoplasma in lower leaves, upper leaves, lower shoots, upper shoots, and roots of HW620 pear trees on OH×F69 rootstocks collected monthly between June 2010 and May 2011. Numbers represent the mean of the average number of PD phytoplasma cells determined from 10 ng of DNA samples from five infected pear trees of each rootstock (0.5 g of tissue per tree). Means followed by the same letter at each column (month) are not significantly different according to Tukey’s statistical test at p ≤ 0.05. No leaves were available during the months of November to April.
A comparison of PD populations between various tissues on OH×F87 rootstocks demonstrated that root tissues exhibited significantly higher PD populations than all other tissues across all sampling months (Figure 8). For shoots, PD populations were significantly higher in the lower shoots than the upper shoots in August and September. There were no significant differences in PD populations between the upper and lower leaves throughout the sampling period.
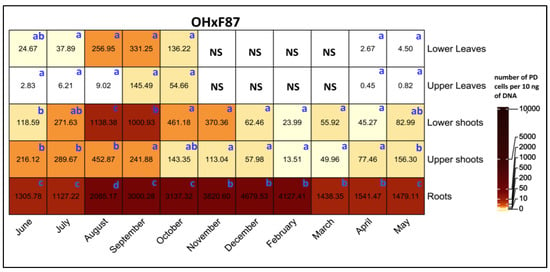
Figure 8.
Heat map of qRT-PCR quantification of PD phytoplasma in lower leaves, upper leaves, lower shoots, upper shoots, and roots of HW620 pear trees of OH×F87 rootstocks collected monthly between June 2010 and May 2011. Numbers represent the mean of the average number of PD phytoplasma cells determined from 10 ng of DNA samples from five infected pear trees of each rootstock (0.5 g of tissue per tree). Means followed by the same letter at each column (month) are not significantly different according to Tukey’s statistical test at p ≤ 0.05. No leaves were available during the months of November to April.
4. Discussion
Seasonal changes in sieve tube elements can have a major impact on phytoplasmas in plants as they live exclusively in functional phloem sieve tube elements, and thus their survival directly depends on the nutrients available within the phloem [7,9,23]. For many woody plants, in fall and winter, sieve elements in the above-ground parts become inactive due to the formation of dormancy callose at the sieve plates (mediated by beta-1,3-glucan synthetase), which blocks the flow of phloem assimilates [9,24,25]. In contrast, most sieve elements in roots remain active over winter [7]. As a result, there is an accumulation of carbohydrates in the roots and a reduction in carbohydrates in the stem [26,27,28,29,30]. This negatively affects the main source of phytoplasma nutrition in stems [11,31,32]. In spring, dormancy callose is digested by beta-1,3-glucanase, allowing above-ground sieve elements to become fully functional [33,34]. Also, new functional sieve elements are differentiated from the vascular cambium in both above-ground parts and roots [25].
For apples and pears, stem sieve tubes degenerate in late fall and early winter, resulting in AP and PD phytoplasmas being eliminated from aerial tissues during winter [25]. Only a few small turgid sieve tubes adjacent to the cambial zone were observed in stems of apples and pears in January and February, but these presumably functional sieve elements did not result in the survival of AP and PD phytoplasmas over winter as detected via DAPI staining [6]. While AP and PD phytoplasmas were eliminated in stems each winter, they survived in the roots, as detected via graft transmission and DAPI staining [7,9]. With new phloem being produced in late winter and early spring, the phytoplasmas recolonized above-ground tissues by mass flow movement in the sieve tubes from the roots as sugars started to flow to sink organs like the stem meristems, thus spreading progressively upwards in the stems [22]. Therefore, phytoplasma populations in apples and pears can show strong seasonal colonisation patterns in locations where the temperatures drop below 0 °C.
In contrast, phytoplasma colonization of above-ground parts can persist over winter if temperatures remain above 0 °C. For example, AP phytoplasma was detected by real-time quantitative PCR in apple tree shoots over winter in northern Italy [22], where winter temperatures are approx. 0 °C, and PD phytoplasma was detected via nested non-quantitative PCR in the stems of pear trees during winter in northeastern Spain [35], where winter temperatures are approx. 8–9 °C. Also, PD in the branches of three pear cultivars was detected at higher levels during winter compared to summer months via nested non-quantitative PCR in the Czech Republic, where winter temperatures are approximately 2–4 °C [36,37].
Although studies have examined phytoplasma population dynamics in different plant tissues during the growing season, none have quantified these populations via quantitative real-time PCR, which is much more sensitive and precise than DAPI fluorescence staining or non-quantitative PCR [38]. Also, there are no previous reports on the impact of PD phytoplasma resistance of different rootstocks on the populations in the scion over a growing season, which could be important since the overwintering survival of the phytoplasmas is dependent upon the roots of apples and pears when winter temperatures are below 0 °C.
Using quantitative real-time PCR, this study was able to show that although PD populations differed between the above-ground tissues on Bartlett, OH×F69, and OH×F87 rootstocks, their seasonal patterns were generally not altered by the rootstock. In lower and upper leaves, PD populations increased in the spring, peaked in late summer, and then declined in autumn until leaf fall in November. The seasonal patterns in lower and upper shoots over the year were similar to those in lower and upper leaves, except that the shoot PD populations persisted over the winter and thus continued to decline until December or February before increasing in March or April before leaves emerged. Roots, however, almost had the exactly reverse pattern, with PD populations decreasing in the spring a minimum in mid-summer, and then increasing in autumn to maximum populations in December and February.
In comparison, PD phytoplasma populations, as determined via DAPI staining in the leaves and shoots of pear trees in southwestern Germany, were lowest in April and May and highest in August and September, with the peak correlating with the appearance of leaf reddening and leaf curling [1,39]. AP phytoplasma populations in apple shoots in Northern Italy increased from March to September, and then gradually declined until February, whereas populations in the roots increased from December to May and then gradually declined from June to October, as determined via real-time quantitative PCR [22]. Thus, the seasonal fluctuations found in this study in the Niagara region of Canada with winter temperatures of approx. −3 °C are similar to those of phytoplasmas in apples and pears growing in other cold winter locations.
For any given month, PD populations in this study were normally higher in lower than upper leaves, but those PD populations were always lower than those in shoots. PD populations each month were also typically higher in lower than upper shoots, but these were less than root PD populations. The higher PD population in lower than upper tissues (both leaves and shoots) could be due to re-colonisation of PD phytoplasma from roots to above-ground tissues, starting with spread of the pathogen first into the lower parts of the tree. Similarly, ESFY phytoplasma in several Prunus species could be detected via non-quantitative PCR in all lower shoots in March and February, but only in half of the upper shoots, which was speculated to be due to ESFY phytoplasma re-colonizing stems in spring from the roots, starting with the lower parts of the tree [23]. The higher PD populations in shoots compared to leaves could be due to the phytoplasmas multiplying more efficiently in non-photosynthetic compared to photosynthetic tissue. The AY phytoplasma population was higher in leaf tip cultures of photosynthetic mutants of Oenothera compared to wild-type plants, which was proposed to be due to the sensitivity of phytoplasmas to oxygen, which is normally abundant in photosynthetically active cells where it is released through photosystem II [40]. The higher PD populations in roots compared to above-ground parts could be due to roots having higher amount of phloem per mass of tissue compared to aerial plant organs, which was proposed to be the reason for the higher populations of AP phytoplasma in the roots compared to the shoots of apples determined via quantitative real-time PCR [22].
While the patterns of the seasonal variation in PD populations were similar between the three rootstock genotypes, the rootstock genotype did impact the size of the population. Regardless of tissue type, PD populations in the OH×F87 rootstock were usually the lowest, followed by populations in the OH×F69 rootstock, and populations on Bartlett rootstock were usually the highest. Some notable exceptions to this were PD populations that were not significantly different in upper leaves of scions on Bartlett and OH×F69 rootstocks in June and July and lower in the shoots and upper leaves of scions on Bartlett and OH×F69 rootstocks from November to May. Thus, scion tissues on Bartlett rootstocks were typically, but not always, more susceptible than those on OH×F69 rootstocks, but both were more susceptible than those on OH×F87 rootstocks. The most obvious impact of rootstocks on PD populations for upper above-ground tissues occurred in August, whereas for lower above-ground tissues it occurred in June and July and for roots, it occurred in December.
As the scion was the same for all plants, the difference in PD phytoplasma populations in leaves and shoots would be expected to be due the impact of only the rootstock. One possibility is that more resistant rootstocks limited PD populations in roots over winter, resulting in slower phytoplasma re-colonization of the above-ground parts in the spring. Bisognin et al. [35] also speculated that the low titer of AP phytoplasma in the roots of AP-resistant M. sieboldii likely contributed to AP resistance. However, one might expect to eventually see similar PD populations in the scion tissues on all rootstocks if the growing season was sufficiently long. Except for upper leaves, PD phytoplasma peaked at about the same time in the aerial parts of the tree for all three rootstock genotypes just before temperatures declined in the fall without reaching similar levels. However, this may not have been long enough for populations to reach the same levels, with all three rootstocks prior to the onset of leaf senescence.
A second possibility is the typically lower PD phytoplasma populations in leaves and shoots on more PD-resistant rootstocks were due to the greater expression of different defense compounds in the roots in response to PD infection, and these compounds then spread throughout the plant, affecting the pathogen in stems, leaves, and roots. Extraction of total proteins from midrib tissues of healthy and Flavescence dorée (FD)-infected grapes, followed by separation via two-dimensional gel electrophoresis and identification of spots over-expressed two or more times in FD-infected compared to non-infected grapevines, revealed a higher percentage of differentially expressed proteins in the functional category of cell responses to stress for resistant cultivars compared to susceptible grape cultivars in the midrib phloem [41]. It was proposed that the different levels of these phloem-associated proteins, along with other phloem-associated responses in the more resistant cultivar, were responsible for the different levels of FD phytoplasma [42]. If the resistant pear rootstocks in this study were expressing and exporting higher levels of defense phloem-associated compounds, then one might expect to see the timing of PD phytoplasma populations to be similar in the leaves and shoots on different rootstocks, only with lower PD populations with higher levels of rootstock resistance, which was observed in this study, thus supporting this hypothesis.
The greater triggering of defense compounds in more PD-resistant rootstocks could be due to greater recognition of the pathogen, such as via PTI (PAMP-triggered immunity), which is a result of recognition of microbial- or pathogen-associated molecular patterns (MAMPs or PAMPs) or ETI (effector-triggered immunity), which is a result of recognition of pathogen effectors [43]. Examples of phytoplasma MAMPs are cold shock proteins and translation elongation factor Tu [44,45], and examples of phytoplasma effectors are SecA-secreted proteins, such as SAP11, which is secreted into the cytoplasm of phloem sieve cells targeting plant cell nuclei [46]. Pathogen recognition is followed by defense signaling, which can be transmitted systemically in plants, such as that related to systemic acquired resistance (SAR) and induced systemic resistance (ISR), via the phloem [47]. SAR and ISR against phytoplasmas have been demonstrated, such as in the chrysanthemum yellow phytoplasma, where SAR was induced by benzothiadiazole (BTH) in Chrysanthemum carinatum [48], and in the Grapevine Yellows phytoplasma, where ISR was induced by endophytic bacteria in Vitis vinifera [49]. Thus, recognition of PD phytoplasma MAMPs or effectors in the resistant rootstocks could not only trigger defense gene expression in the roots, but could also send signals via the phloem, thus triggering defense gene expression throughout the scion.
This study demonstrated that the rootstock genotype affects PD phytoplasma populations not only in the roots but also in the lower and upper leaves and shoots of the scion throughout the period when tissue was available. This difference was observed even though the seasonal pattern of PD populations in leaves and shoots was almost the opposite of that in roots. Thus, growers can choose scion varieties that they prefer based on characteristics such as fruit quality, and only need to change to more resistant rootstocks to achieve increased control of PD. However, each rootstock–scion combination would need to be examined, as there is a close relationship between rootstocks, yield, and quality of the scion, such as the growth and fruit yield of scions of the Beyrouti and Dargazi cultivars on PyroDwarf versus OH×F69 rootstocks [50]. This study also demonstrated that tissues of the Bartlett rootstock were more susceptible than those of OH×F69 and OH×F87 rootstocks based on typically higher PD populations. These differences were more obvious during periods of high PD populations, such as in roots during winter months and leaves and shoots during summer months. As this is much longer than the period of PD symptoms, such as foliar reddening, curling, and general decline, plant breeders could use quantitative real-time PCR of PD phytoplasma to compare selections over a much longer period compared to only when symptoms appear. Quantitative real-time PCR can be used to detect the presence of PD phytoplasma even in the middle of winter if tissues are available. Thus far, only a few studies have used quantitative real-time PCR to examine phytoplasma colonization patterns [22,38,51]. However, this study demonstrated that quantitative real-time PCR can accurately and reliably show even slight differences in phytoplasma populations, and it should more often be used for quantifying these pathogens.
5. Conclusions
PD phytoplasma populations in pear trees were found to vary seasonally, with low levels in aerial parts during winter, which peaked in late summer and then declined in autumn. Conversely, root populations decrease in spring, reach a minimum in mid-summer, and then increase in autumn, with consistently higher numbers than above-ground parts. The rootstock type influences PD populations in all tissues, with OH×F87 generally having the lowest, OH×F69 having intermediate, and Bartlett generally having the highest populations. This suggests that using PD-resistant rootstocks alone can be an effective strategy for managing PD throughout the tree. Thus, breeders can aim for even higher resistance levels in pear rootstock cultivars to reduce PD populations throughout the tree. The mechanism behind this resistance remains to be determined, perhaps by using a multi-omics approach.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10020129/s1, Figure S1: Standard curve of PD 16S-23S rRNA intergenic spacer DNA (log DNA dilution) and threshold cycle (Ct) measured using real-time qPCR; Figure S2: The average daily temperature (°C) vs. month of the year at the St. Catherines airport weather station, Ontario, Canada, in year of study (solid line) and 30-year period (dotted line) (June 2010–May 2011); Figure S3: The average daily precipitation (mm) vs. month of the year at the St. Catharines airport weather station, Ontario, Canada, in 30-year period (dotted line) and the year of study (solid line) (June 2010–May 2011).
Author Contributions
M.K.: Material preparation, Data collection, Data analysis, Visualization, Investigation; M.K., P.H.G. and D.M.H.: Conceptualization, Methodology; P.H.G. and D.M.H.: Supervision; M.K., P.H.G. and D.M.H.: Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Seemüller, E.; Lorenz, K.-H.; Lauer, U. Pear decline resistance in Pyrus communis rootstocks and progenies of wild and ornamental pyrus taxa. Acta Hortic. 1998, 472, 681–692. [Google Scholar] [CrossRef]
- Seemüller, E.; Schneider, B. ‘Candidatus Phytoplasma Mali’, ‘Candidatus Phytoplasma Pyri’ and ‘Candidatus Phytoplasma Prunorum’, the causal agents of apple proliferation, pear decline and European stone fruit yellows, respectively. Int. J. Syst. Evol. Microbiol. 2004, 54, 1217–1226. [Google Scholar] [CrossRef]
- Brewer, L.; Volz, R. Genetics and breeding of pear. In The Pear Genome; Korban, S.S., Ed.; Compendium of Plant Genomes; Springer International Publishing: Cham, Switzerland, 2019; pp. 63–101. ISBN 978-3-030-11048-2. [Google Scholar]
- Ogawa, J.M. Diseases of Temperate Zone Tree Fruit and Nut Crops; Publication/University of California; Division of Agriculture and Natural Resources; University of California, Division of Agriculture and Natural Resources: Oakland, CA, USA, 1991; ISBN 978-0-931876-97-4. [Google Scholar]
- Kaviani, M.; Goodwin, P.H.; Hunter, D.M. Differences in gene expression of pear selections showing leaf curling or leaf reddening symptoms due to pear decline phytoplasma. Plants 2022, 11, 427. [Google Scholar] [CrossRef]
- Schaper, U. Condition of the phloem and the persistence of mycoplasma-like organisms associated with apple proliferation and pear decline. Phytopathology 1982, 72, 736. [Google Scholar] [CrossRef]
- Seemüller, E.; Kunze, L.; Schaper, U. Colonization behavior of MLO, and symptom expression of proliferation-diseased apple trees and decline-diseased pear trees over a period of several years/Besiedlungsverhalten von MLO Und Symptomausbildung Bei Triebsuchtkranken Apfelbäumen Und Verfallskranken Birnbäumen Im Verlauf Mehrerer Jahre. Z. Für Pflanzenkrankh. Und Pflanzenschutz/J. Plant Dis. Prot. 1984, 91, 525–532. [Google Scholar]
- Braun, E.J. Histopathology of phloem necrosis in ulmus Americana. Phytopathology 1976, 66, 598. [Google Scholar] [CrossRef]
- Seemüller, E.; Schaper, U.; Zimbelmann, F. Seasonal Variation in the colonization patterns of mycoplasmalike organisms associated with apple proliferation and pear decline/Jahreszeitliche Veränderungen in Der Besiedlung Triebsuchtkranker Apfelbäume Und Verfallskranker Birnbäume Durch Mykoplasmaähnliche Organismen. Z. Für Pflanzenkrankh. Und Pflanzenschutz/J. Plant Dis. Prot. 1984, 91, 371–382. [Google Scholar]
- Jiang, H.; Wei, W.; Saiki, T.; Kawakita, H.; Watanabe, K.; Sato, M. Distribution patterns of mulberry dwarf phytoplasma in reproductive organs, winter buds, and roots of mulberry trees. J. Gen. Plant Pathol. 2004, 70, 168–173. [Google Scholar] [CrossRef]
- Primrose, S.B. Phytoplasmas: Bacteria that manipulate plants and insects. In Microbiology of Infectious Disease: Integrating Genomics with Natural History; Primrose, S.R., Ed.; Oxford University Press: Oxford, UK, 2022; ISBN 978-0-19-286384-3. [Google Scholar]
- Nakamura, H.; Ohgake, S.; Sahashi, N.; Yoshikawa, N.; Kubono, T.; Takahashi, T. Seasonal Variation of paulownia witches’-broom phytoplasma in paulownia trees and distribution of the disease in the Tohoku district of Japan. J. Res. 1998, 3, 39–42. [Google Scholar] [CrossRef]
- Mazraie, M.A.; Izadpanah, K.; Hamzehzarghani, H.; Salehi, M.; Faghihi, M.M. Spread and colonization pattern of ‘Candidatus Phytoplasma aurantifolia’ in lime plants [Citrus aurantifolia (christm.) swingle] as revealed by real-time pcr assay. J. Plant Pathol. 2019, 101, 629–637. [Google Scholar] [CrossRef]
- Giunchedi, L.; Poggi Pollini, C.; Bissani, R.; Babini, A.R.; Vicchi, V. Etiology of a pear decline disease in italy and susceptibility of pear variety and rootstock to phytoplasma-associated pear decline. Acta Hortic. 1995, 386, 489–495. [Google Scholar] [CrossRef]
- Hunter, D.M.; Slingerland, K.C. Evaluation of four fire blight-tolerant pear cultivars and selections for commercial pear production in Canada. Acta Hortic. 2008, 800, 541–546. [Google Scholar] [CrossRef]
- Brooks, L. History of the Old Home×Farmingdale pear rootstocks. Fruit Var. J. 1984, 38, 126–128. [Google Scholar]
- Hancock, J.F.; Lobos, G.A. Pears. In Temperate Fruit Crop Breeding: Germplasm to Genomics; Hancock, J.F., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 299–336. ISBN 978-1-4020-6907-9. [Google Scholar]
- Smart, C.D.; Schneider, B.; Blomquist, C.L.; Guerra, L.J.; Harrison, N.A.; Ahrens, U.; Lorenz, K.H.; Seemüller, E.; Kirkpatrick, B.C. Phytoplasma-specific PCR primers based on sequences of the 16S-23S rRNA spacer region. Appl. Environ. Microbiol. 1996, 62, 2988–2993. [Google Scholar] [CrossRef]
- Green, M.J.; Thompson, D.A.; MacKenzie, D.J. Easy and efficient DNA extraction from woody plants for the detection of phytoplasmas by polymerase chain reaction. Plant Dis. 1999, 83, 482–485. [Google Scholar] [CrossRef]
- Hunter, D.M.; Svircev, A.M.; Kaviani, M.; Michelutti, R.; Wang, L.; Thompson, D. First report of pear decline caused by ‘Candidatus Phytoplasma pyri’ in Ontario, Canada. Plant Dis. 2010, 94, 634. [Google Scholar] [CrossRef]
- Kaviani, M. Resistance to Pear Decline Phytoplasma and Its Relationship to Pathogen Overwintering, Host Response and Foliar Symptoms. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2014. [Google Scholar]
- Baric, S.; Berger, J.; Cainelli, C.; Kerschbamer, C.; Letschka, T.; Dalla Via, J. Seasonal colonisation of apple trees by ‘Candidatus Phytoplasma mali’ revealed by a new quantitative TaqMan Real-Time PCR approach. Eur. J. Plant Pathol. 2011, 129, 455–467. [Google Scholar] [CrossRef]
- Jarausch, W.; Lansac, M.; Dosba, F. Seasonal colonization pattern of European stone fruit yellows phytoplasmas in different Prunus species detected by specific PCR. J. Phytopathol. 1999, 147, 47–54. [Google Scholar] [CrossRef]
- Mullendore, D.L.; Windt, C.W.; Van As, H.; Knoblauch, M. Sieve tube geometry in relation to phloem flow. Plant Cell 2010, 22, 579–593. [Google Scholar] [CrossRef]
- Evert, R.F. The Cambium and seasonal development of the phloem in Pyrus malus. Am. J. Bot. 1963, 50, 149–159. [Google Scholar] [CrossRef]
- Fadón, E.; Fernandez, E.; Behn, H.; Luedeling, E. A Conceptual framework for winter dormancy in deciduous trees. Agronomy 2020, 10, 241. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Carbohydrate sources and sinks in woody plants. Bot. Rev. 1992, 58, 107–222. [Google Scholar] [CrossRef]
- Tixier, A.; Gambetta, G.A.; Godfrey, J.; Orozco, J.; Zwieniecki, M.A. Non-Structural Carbohydrates in Dormant Woody Perennials; The tale of winter survival and spring arrival. Front. For. Glob. Chang. 2019, 2, 18. [Google Scholar] [CrossRef]
- Sivaci, A. Seasonal changes of total carbohydrate contents in three varieties of apple (Malus sylvestris miller) stem cuttings. Sci. Hortic. 2006, 109, 234–237. [Google Scholar] [CrossRef]
- Tromp, J. Nutrient reserves in roots of fruit trees, in particular carbohydrates and nitrogen. Plant Soil 1983, 71, 401–413. [Google Scholar] [CrossRef]
- Gallinger, J.; Gross, J. Phloem metabolites of Prunus Sp. rather than infection with “Candidatus Phytoplasma prunorum” influence feeding behavior of Cacopsylla pruni nymphs. J. Chem. Ecol. 2020, 46, 756–770. [Google Scholar] [CrossRef]
- Oshima, K.; Kakizawa, S.; Arashida, R.; Ishii, Y.; Hoshi, A.; Hayashi, Y.; Kagiwada, S.; Namba, S. Presence of two glycolytic gene clusters in a severe pathogenic line of “Candidatus Phytoplasma asteris”. Mol. Plant Pathol. 2007, 8, 481–489. [Google Scholar] [CrossRef]
- Savage, J.A. It’s all about timing—Or is it? Exploring the potential connection between phloem physiology and whole plant phenology. Am. J. Bot. 2020, 107, 848–851. [Google Scholar] [CrossRef]
- Aloni, R.; Raviv, A.; Peterson, C.A. The role of auxin in the removal of dormancy callose and resumption of phloem activity in Vitis vinifera. Can. J. Bot. 1991, 69, 1825–1832. [Google Scholar] [CrossRef]
- Errea, P.; Aguelo, V.; Hormaza, J.I. Seasonal variations in detection and transmission of pear decline phytoplasma. J. Phytopathol. 2002, 150, 439–443. [Google Scholar] [CrossRef]
- Kučerová, J.; Karešová, R.; Navrátil, M.; Válová, P. Seasonal occurrence of ‘Candidatus Phytoplasma pyri’ in pear trees in the Czech Republic. Bull. Insectology 2007, 60, 263–264. [Google Scholar]
- Križanac, I.; Mikect, I.; Musić, M.Š.; Škorić, D. Diversity of phytoplasmas infecting fruit trees and their vectors in Croatia/Diversität von Obstbaum Infizierenden Phytoplasmen Und Ihren Vektoren in Kroatien. J. Plant Dis. Prot. 2010, 117, 206–213. [Google Scholar] [CrossRef]
- Bisognin, C.; Schneider, B.; Salm, H.; Grando, M.S.; Jarausch, W.; Moll, E.; Seemüller, E. Apple proliferation resistance in apomictic rootstocks and its relationship to phytoplasma concentration and simple sequence repeat genotypes. Phytopathology 2008, 98, 153–158. [Google Scholar] [CrossRef]
- Seemüller, E.; Moll, E.; Schneider, B. Pear Decline resistance in progenies of Pyrus taxa used as rootstocks. Eur. J. Plant Pathol. 2009, 123, 217–223. [Google Scholar] [CrossRef]
- Sears, B.B.; Klomparens, K.L.; Wood, J.I.; Schewe, G. Effect of altered levels of oxygen and carbon dioxide on phytoplasma abundance in oenotheraleaftip cultures. Physiol. Mol. Plant Pathol. 1997, 50, 275–287. [Google Scholar] [CrossRef]
- Margaria, P.; Palmano, S. Response of the Vitis vinifera L. cv. ‘Nebbiolo’ proteome to flavescence dorée phytoplasma infection. Proteomics 2011, 11, 212–224. [Google Scholar] [CrossRef]
- Roggia, C.; Caciagli, P.; Galetto, L.; Pacifico, D.; Veratti, F.; Bosco, D.; Marzachì, C. Flavescence Dorée Phytoplasma titre in field-infected Barbera and Nebbiolo grapevines. Plant Pathol. 2014, 63, 31–41. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant Immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Sugio, A.; Hogenhout, S.A. The genome biology of phytoplasma: Modulators of plants and insects. Curr. Opin. Microbiol. 2012, 15, 247–254. [Google Scholar] [CrossRef]
- Sugio, A.; MacLean, A.M.; Kingdom, H.N.; Grieve, V.M.; Manimekalai, R.; Hogenhout, S.A. Diverse targets of phytoplasma effectors: From plant development to defense against insects. Annu. Rev. Phytopathol. 2011, 49, 175–195. [Google Scholar] [CrossRef]
- Bai, X.; Correa, V.R.; Toruño, T.Y.; Ammar, E.-D.; Kamoun, S.; Hogenhout, S.A. AY-WB phytoplasma secretes a protein that targets plant cell nuclei. Mol. Plant Microbe Interact. 2009, 22, 18–30. [Google Scholar] [CrossRef]
- Ryals, J.; Uknes, S.; Ward, E. Systemic acquired resistance. Plant Phys. 1994, 104, 1109–1112. [Google Scholar] [CrossRef]
- D’Amelio, R.; Marzachì, C.; Bosco, D. Activity of benzothiadiazole on chrysanthemum yellows phytoplasma (‘Candidatus Phytoplasma asteris’) infection in daisy plants. Crop Prot. 2010, 29, 1094–1099. [Google Scholar] [CrossRef]
- Bulgari, D.; Casati, P.; Crepaldi, P.; Daffonchio, D.; Quaglino, F.; Brusetti, L.; Bianco, P.A. Restructuring of endophytic bacterial communities in grapevine yellows-diseased and recovered Vitis vinifera L. Plants. Appl. Environ. Microbiol. 2011, 77, 5018–5022. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Yadegari, M.; Davarynejad, G.H.; Nemati, S.H. The rootstock and scion interaction effects on growth and bearing characteristics of young pear trees. J. Hortic. Sci. 2022, 36, 519–531. [Google Scholar] [CrossRef]
- Rekab, D.; Pirajno, G.; Cettul, E.; De Salvador, F.R.; Firrao, G. On the apple proliferation symptom display and the canopy colonization pattern of “Candidatus Phytoplasma mali” in Apple Trees. Eur. J. Plant Pathol. 2010, 127, 7–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).