Abstract
In response to the near-threatened status of Handroanthus impetiginosus, primarily due to habitat loss and illegal logging, this study examines how X-ray imaging and cryopreservation impact the seed quality and viability essential for conservation. Seeds initially had a moisture content of 12.3%, reduced to 6.5% through desiccation. X-ray imaging allowed for detailed visualization of internal structures, identifying seeds as normal, abnormal, or dead based on damage and development. Normal seeds consistently germinated and produced healthy seedlings, while those with internal damage or excessive desiccation either resulted in abnormal seedlings or did not germinate. Various cryopreservation treatments were tested, including storage at −80 °C and liquid nitrogen immersion (LN), with and without vitrification solutions (PSV2; PVS3; PSV2 + 1% phloroglucinol; PSV3 + 1% phloroglucinol). Results indicated that immersion in LN without cryoprotectants achieved the highest germination and seedling viability, whereas vitrification solutions, such as PVS2 and PVS3, negatively affected germination. This study demonstrates that X-ray imaging is an effective tool for assessing seed quality and detecting internal damage, while cryopreservation without cryoprotectants is suitable for long-term seed storage. This work highlights the benefits of combining X-ray assessment with optimized cryopreservation techniques to support the conservation of threatened species.
1. Introduction
The tree species Handroanthus impetiginosus (Mart. ex DC.) Mattos, commonly known as lavender trumpet tree, can grow up to 30 m in height and have a trunk diameter of up to 80 cm. It is widely distributed in seasonally dry tropical forests, covering regions of South and Mesoamerica [1,2]. However, this species has been classified as near-threatened by the IUCN Red List [3] due to increased predatory human timber exploitation, and deforestation leading to reduced occurrence of natural habitats [4].
Forest restoration efforts involving this species face several challenges, including, but not limited to, competition with other species, slow growth and development time, and maintaining genetic diversity [1,5,6]. It is known that lavender trumpet tree is primarily propagated by seeds, and, therefore, understanding seed quality and conservation is crucial for preservation efforts in this species. Radiographic image analysis, herein X-ray, is a tool with significant potential for both primary and applied studies in seed analysis. This technique aims to clarify various aspects of seed structure and improve methodologies for assessing seed quality attributes [7,8].
The X-ray test is a non-destructive technique to assess seed quality, and it allows for subsequent physiological tests in the same seeds. These tests aim to correlate observations of the internal seed morphology with the characteristics of the resulting seedlings, and whether they display normal or abnormal development of each corresponding structure [9,10]. Additionally, the images obtained can be cataloged, archived, and later used to establish non-subjective and standardized evaluation criteria [11].
In addition to ensuring seed quality, species conservation efforts may require the long-term maintenance of a germplasm bank. Such a resource is important to protect and preserve plant species that face threats against their natural habitats. Additionally, it serves as a valuable repository for the study of these endangered species as well as their related taxa. In this context, cryopreservation is a key technique in seed conservation that enables the long-term storage and preservation of plant material [12] by preserving biological material in liquid nitrogen at extremely low temperatures, either in the liquid phase (−196 °C) or vapor phase (−150 °C). Cryopreservation should maintain the original characteristics of the plant material, ensuring its viability and normal development upon recovery after thawing [13].
However, due to the extremely low temperature of liquid nitrogen, seed moisture must be below 10% to prevent the formation of intracellular ice crystals during freezing, which can cause cell rupture and death [14,15]. Therefore, in some cases, the use of cryoprotective solutions is essential to ensure the successful recovery of organ tissues and the regeneration of plants after the cryopreservation process [16].
Among these methods, vitrification is the most widely used in cryopreservation protocols due to its simplicity and efficiency. It does not require sophisticated equipment and offers a high recovery rate and biological material survival rate [17]. The utilization of highly concentrated and pre-cooled cryoprotective solutions in vitrification prevents the formation of ice crystals and cellular damage and limits the diffusion of substances within the cell, such as water and other substrates, thereby inducing metabolic quiescence [18]. In this state, the cell metabolism is almost completely halted. The vitrification solution helps to prevent cell deterioration and create a stable environment that protects against structural and functional damage during cryopreservation. This protection is due to the water inside the cells forming a glass-like (vitreous) state, rather than ice crystals [19].
The goal of this study was to assess the use of X-ray imaging techniques in evaluating the quality of lavender trumpet seeds and to examine the germinability of seeds subjected to different cryopreservation treatments.
2. Materials and Methods
2.1. Plant Material
Handroanthus impetiginosus seeds were collected in Miami, southern Florida (25°46′31″ N, 80°12′32″ W). The collection took place in May 2023 from trees used for urban landscaping. This region has a humid subtropical climate and is classified as USDA plant hardiness zone 9a. The average annual precipitation is 1230 mm, with temperatures ranging from a minimum of 5.5 °C to a maximum of 32 °C.
The moisture content of the seeds was determined using a precise oven-drying method at 103 ± 3 °C for 17 ± 1 h, with two replicates of 10 g of seeds each [20]. For relevant comparisons, the moisture content was measured initially in the control group and again after one day of drying in silica gel to desiccate the seeds.
The moisture content as a percentage by fresh weight was calculated using the following formula:
where
Seed moisture content (%) = (M2 − M3/M2 − M1) × 100,
M1 = Weight of the weighing container with cover in grams.
M2 = Weight of the weighing container with cover and seeds before drying.
M3 = Weight of the weighing container with cover and seeds after drying.
2.2. X-Ray Imaging in Seed Quality Assessment
The seed quality X-ray experiment was conducted at the Seed Biology Laboratory of the University of Florida, located in Gainesville, FL, USA. For the internal morphology analysis of the seeds, 20 replications of 18 seeds each were used, distributed in transparent acrylic plates measuring 11 × 11 cm to facilitate image capture, totaling 360 seeds. All seeds were positioned similarly on the plate, and X-ray images were generated using the XPERT® 80 cabinet X-ray system (KUBTEC Scientific, Stratford, CT, USA). The equipment was set to a voltage of 90 kV, and the seeds were exposed to radiation for 20 s at a focal distance and resolution of 5 µm. The image contrast was calibrated to 49.5 μm. The voltage and exposure time settings were selected based on preliminary tests. The generated images were saved in JPEG format on a computer and subsequently analyzed.
Following this experiment, a germination test was conducted using the same experimental setup, and seeds were tracked over time by the same positions in the Gerbox as those used for X-ray imaging. For this step, seeds were distributed on filter paper that had been pre-moistened with distilled water at a ratio of 2.5 times the dry mass of the paper. All seeds were kept in a growth chamber at a constant temperature of 25 °C. Daily germination was evaluated until stabilization occurred 21 days after the test setup. The number of normal and abnormal seedlings and non-germinated seeds was recorded, photographed, and counted, with results expressed as percentages [21].
2.3. Seeds Cryopreservation
The cryopreservation experiment was conducted at the Environmental Horticulture Micropropagation Laboratory, Gainesville, FL, USA. The experimental design was completely randomized. There were seven treatments, with four replications and 50 seeds per plot, as follows:
- Control—seeds were not immersed in liquid nitrogen (LN) or vitrification solution; they were not placed in an ultra-freezer;
- UF—seeds stored in an ultra-freezer at −80 °C without vitrification solution;
- LN only—seeds immersed in LN without vitrification solution;
- PVS2—seeds immersed in PVS2 followed by LN;
- PVS2 + 1%PG—seeds immersed in PVS2 with 1.0% phloroglucinol (PG) followed by LN;
- PVS3—seeds immersed in PVS3 followed by LN;
- PVS3 + 1%PG—seeds immersed in PVS3 with 1.0% PG followed by LN.
The seeds were placed in 15 mL polypropylene centrifuge tubes for the cryopreservation treatments. When vitrification solutions were used (PVS2, PVS2 + 1%PG, PVS3, and PVS3 + 1%PG), the seeds were first placed in 15 mL of 2 mol L−1 glycerol solution and 0.4 mol L−1 sucrose for 60 min at 25 °C, as described by [22]. After 60 min, the loading solution was removed, and the seeds were immersed in vitrification solutions with or without adding 1% phloroglucinol. The compositions of the vitrification solutions were: PVS2—30% glycerol, 15% ethylene glycol, 15% dimethyl sulfoxide (DMSO), and 0.4 mol L−1 sucrose; and PVS3—30% glycerol, 15% propylene glycol, and 0.5 mol L−1 sucrose, as described by [23]. The samples were immersed for 120 min in each solution.
After 30 days in the ultra-freezer or liquid nitrogen, the tubes were removed and rapidly warmed in a water bath maintained at 39.7 °C until the vitrification solution was thawed. The seeds were then rinsed with distilled water and germination was assessed. Two germination tests were conducted: (1) Filter paper test: The paper was moistened with distilled water at 2.5 times its weight, the seeds were distributed over the paper, and the setup was placed in a transparent acrylic box (Gerbox) with dimensions of 11 cm × 11 cm × 3.5 cm and incubated for 21 days without water replacement [21]; (2) Soil test: the substrate consisted of coarse sand, peat, and pine bark. One seed was sown per cell in polypropylene trays with 72 cells, and the substrate was moistened with distilled water to 100% of its water-holding capacity. The acrylic boxes and trays were placed in a growth chamber set to 25 °C [21].
For the filter paper assay, only germination rate was assessed. In the soil test, the following parameters were evaluated. Seedling emergence was tested after 21 days, and after 60 days from the start of the test, the height (cm) and root length (cm) were measured, with the root length being the longest root measured using a millimeter ruler; fresh weight of the aerial part and roots, and dry weight of the aerial part and roots. To obtain the dry weight, the plant material was placed in an oven and dried at 80 °C for 48 h. The plant weights were recorded using a precision scale model AP50 (Denver Instrument Company, Denver, CO, USA) with a sensitivity of 0.0001 g, and the results were expressed as g per plant. Chlorophyll relative content was measured as SPAD values by evaluation of the second fully expanded leaf of each seedling, counted from the top downward, using a portable SPAD-502 chlorophyll meter (Minolta Co. Ltd., Osaka, Japan). The amount of green cover projected by the plants was captured in images using the advanced mobile application Canopeo® version 2.0. The mobile phone with the application was held at a height of 50 cm above the canopy, and the photos were taken under the same lighting conditions for all samples. A single-lens reflex camera with a focal length equivalence of approximately 26 mm was used.
2.4. Statistical Procedures
The data were subjected to analysis of variance, and for percentage values, a transformation using arcsin (x/100) was applied. Means were compared using Tukey’s test at a 5% significance level. Statistical analyses, principal component analysis, and Pearson correlation were performed using R software, version 4.4.1 [24].
3. Results
The average initial moisture content of the seeds was 12.3%. After one day in a silica gel desiccator, the moisture content decreased to 6.5%, and these seeds were used for both following studies: X-ray imaging and cryopreservation assay.
3.1. X-Ray Imaging as Seed Quality Assessment
The images corresponding to the X-rayed seeds, and all developmental stages from seed germination to seedling formation, are shown in Figure 1. The figure illustrates how each seed was individually tracked throughout the germination process.
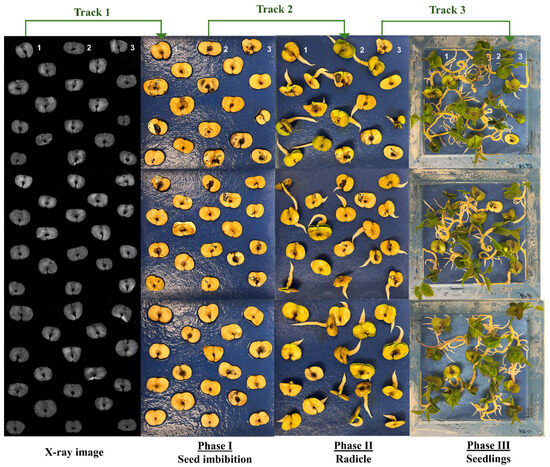
Figure 1.
X-ray images of seeds and the stages of germination development of Handroanthus impetiginosus (Mart. ex DC.) Mattos. Each seed was individually tracked throughout the germination process (e.g., track 1 corresponds to a single seed observation comparing X-ray image and seed imbibition; track 2 corresponds to a single seed observation comparing seed imbibition stage and radicle protrusion; track 3 corresponds to a single seed observation comparing radicle protrusion stage and seedling formation).
Through the X-ray images, it was possible to visualize the internal structures of the seed, where the hypocotyl-radicle axis and the thin bilobed leaf-like cotyledon were identified (Figure 2A). In addition, it was possible to observe internal damage overlapping with the seed structures, most observed in the cotyledons (Figure 2B). The hypocotyl-radicle axis appears straight and is easily visible in the X-ray image, as well as any damage present in this structure (Figure 2C). Figure 2D shows opaque blurriness associated with severe seed desiccation, which hinders the identification of any internal seed structures, as turgidity is not observed compared to other images.
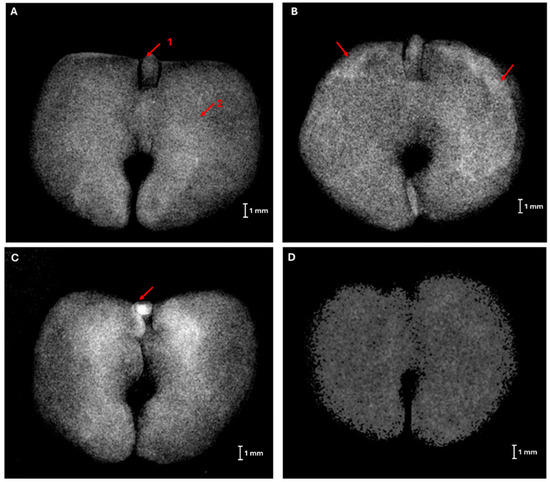
Figure 2.
X-ray images of Handroanthus impetiginosus (A) Normal seed—arrow 1 highlights the isolated embryo showing the hypocotyl-radicle axis; arrow 2 indicates the thin bilobed leaf-like cotyledon; (B) mechanical damages to the cotyledons are highlighted; (C) damage to the hypocotyl-radicle axis indicated by the whitish coloration; (D) seeds showing signs of severe desiccation.
In the sample of 360 seeds, 77.5% of the seeds resulted in normal seedlings, 16% were classified as abnormal seedlings, and 6.5% were classified as dead seeds. Figure 3, Figure 4 and Figure 5 illustrate the practical implications of this research, showing the relationship between germination tests and X-ray images of H. impetiginosus seeds. Within the well-formed seeds (Figure 3A) subset, 100% of them germinated and consistently produced healthy seedlings, while seeds with physical damage, absence of structures, and/or severe desiccation may lead to abnormal seedlings (Figure 4) or resulted in dead seeds (Figure 5). This highlights the practical value of the X-ray test in producing clear X-ray images that reveal the seed’s internal structures and any physical damage that could hinder seedling formation.
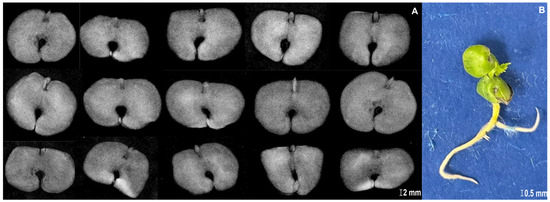
Figure 3.
X-ray images of Handroanthus impetiginosus seeds without internal damage (A), resulting in normal seedling (B).
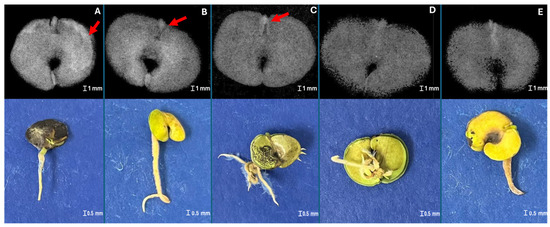
Figure 4.
X-ray images of Handroanthus impetiginosus seeds with deteriorated tissue (A), internal damage on the embryonic axis (B,C), and dehydrated seeds (D,E) resulting their respective abnormal seedlings.
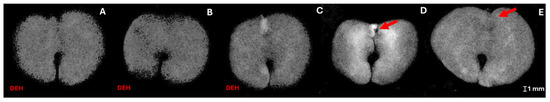
Figure 5.
X-ray images of Handroanthus impetiginosus dead seeds due to severe desiccation (A–C), deteriorated tissue on hypo-cotyl-radicle axis (D), and absence of critical structures (E). DEH—severe dehydration. arrow highlights the absence of the hypocotyl-radicle axis.
3.2. Seed Cryopreservation
There was no significant difference in germination on filter paper between treatments Control, UF, and LN only, with an average germination rate of 96%, 87.5%, and 86.7%, respectively. The lowest germination percentages were observed in treatments PVS2 + 1%PG (35%) and PVS3 (44%) (Figure 6A). In the soil assay, an increase in germination rate was noted for all treatments compared to the paper test. No significant differences were observed between treatments Control, UF, and LN only, and PVS3 + 1%PG, which had an average germination rate of 98.5%, 98%, 95%, and 92.5%, respectively (Figure 6B).
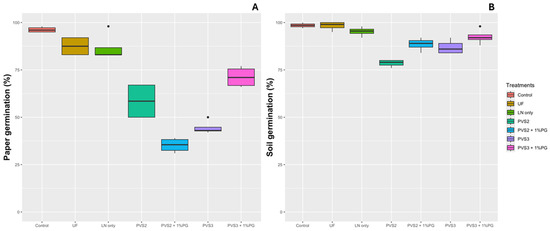
Figure 6.
Germination (%) of Handroanthus impetiginosus seeds under different cryopreservation treatments. (A) Germination in paper (%). (B) Germination in soil (%). Control—(no cryopreservation treatment applied); UF—seeds stored in an ultra-freezer at −80 °C without vitrification solution; LN only—seeds immersed in LN without vitrification solution; PVS2—seeds immersed in PVS2 followed by LN; PVS2 + 1%PG—seeds immersed in PVS2 with 1.0% phloroglucinol (PG) followed by LN; PVS3—seeds immersed in PVS3 followed by LN; PVS3 + 1%PG—seeds immersed in PVS3 with 1.0% PG followed by LN.
Seeds were sown in soil following the cryopreservation treatments. Seedling evaluation was carried out 60 days after the seeds were planted, and no differences were observed among treatments for the SPAD index (Figure 7A). For the green leaf area (Canopeo), UF and LN only were superior to the other cryopreservation treatments (Figure 7B). Regarding plant height, when the cryopreservation solution was used, a reduction in plant growth was observed, with an average reduction of 36% for the treatments PVS2, PVS2 + 1%PG, PVS3, and PVS3 + 1%PG compared to the other treatments (Control, UF, and LN only) (Figure 7C). For root length, treatments Control, UF, and LN only, PVS2, and PVS3 did not differ among themselves (Figure 7D).
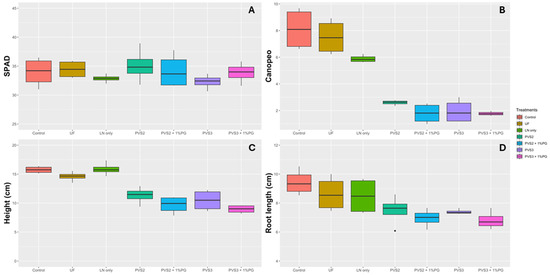
Figure 7.
Biometric and physiological parameters of Handroanthus impetiginosus seeds under different cryopreservation treatments. (A) SPAD index. (B) Canopeo. (C) Height (cm). (D) Root length (cm). Control—(no cryopreservation treatment applied); UF—seeds stored in an ultra-freezer at −80 °C without vitrification solution; LN only—seeds immersed in LN without vitrification solution; PVS2—seeds immersed in PVS2 followed by LN; PVS2 + 1%PG—seeds immersed in PVS2 with 1.0% phloroglucinol (PG) followed by LN; PVS3—seeds immersed in PVS3 followed by LN; PVS3 + 1%PG—seeds immersed in PVS3 with 1.0% PG followed by LN.
In the assessment of the seedlings’ shoot (Figure 8A) and root fresh weight (Figure 8C), it was observed that the control treatment was superior to all the others, with average weights of 2.057 g and 1.004 g, respectively. However, interestingly, LN only also performed well compared to the other cryopreservation treatments, with average weights of 1.759 g and 0.7905 g for shoot and root fresh weight, respectively. Treatments PVS2, PVS2 + 1%PG, PVS3, and PVS3 + 1%PG showed reduction in their shoot dry mass accumulation by 48%, 74%, 73%, and 62%, respectively, compared to treatment LN only, which is cryopreservation treatment without the addition of any protective solution. For fresh root weight (Figure 8B), the reductions were 48%, 68%, 64%, and 76%, respectively, compared to LN only. Regarding root dry weight, treatments Control and LN only were superior to the others, and did not differ from each other (Figure 8D).
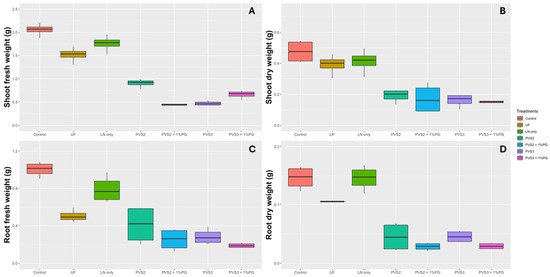
Figure 8.
Plant growth parameters of Handroanthus impetiginosus seeds under different cryopreservation treatments. (A) Shoot fresh weight (g). (B) Shoot dry weight (g). (C) Root fresh weight (g). (D) Root fresh weight (g). Control—(no cryopreservation treatment applied); UF—seeds stored in an ultra-freezer at −80 °C without vitrification solution; LN only—seeds immersed in LN without vitrification solution; PVS2—seeds immersed in PVS2 followed by LN; PVS2 + 1%PG—seeds immersed in PVS2 with 1.0% phloroglucinol (PG) followed by LN; PVS3—seeds immersed in PVS3 followed by LN; PVS3 + 1%PG—seeds immersed in PVS3 with 1.0% PG followed by LN.
When analyzing the Pearson correlation matrix (Figure 9), significant positive and negative correlations were found among the variables. The correlations between germination rate and seedling development parameters varied from −0.11 (between soil germination and SPAD) to 0.89 (between paper germination and shoot fresh weight). All the seedling biometric and physiological parameters showed good positive correlations among themselves, except for SPAD, which did not have a strong correlation with any variable. The correlation values for the biometric and physiological parameters range from 0.67 (between root length and root dry weight) to 0.93 (between shoot fresh weight and root dry weight).
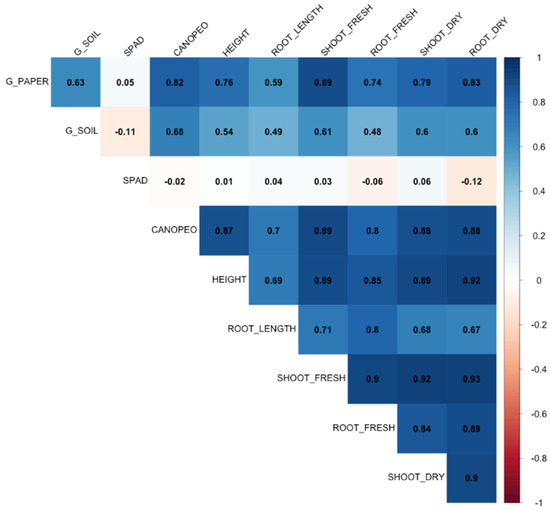
Figure 9.
Pearson correlation matrix between the analyzed variables of Handroanthus impetiginosus seeds under different cryopreservation treatments. Where G_PAPER = Germination in paper (%). G_SOIL = Germination in soil; SPAD = SPAD Index. CANOPEO. HEIGHT, ROOT_LENGHT = plant height and root length, respectively, in cm; SHOOT_FRESH, SHOOT_DRY, ROOT_FRESH, ROOT_DRY = shoot fresh and dry weight, root fresh and dry weight, respectively, in grams.
When considering the multivariate analysis of the cryopreservation treatments, the mean variation explained by canonical variables 1 and 2 was 82.35% of the total variance (Figure 10). The principal component analysis (PCA) played a key role in segregating the data into two clusters, with treatments UF and LN only showing behavior similar to the control, while treatments PVS2 to PVS3 + 1%PG formed a distinct group. It was observed that all growth variables analyzed, except for SPAD, distinguished a cluster containing the treatments control, UF, and LN only from the other treatments.
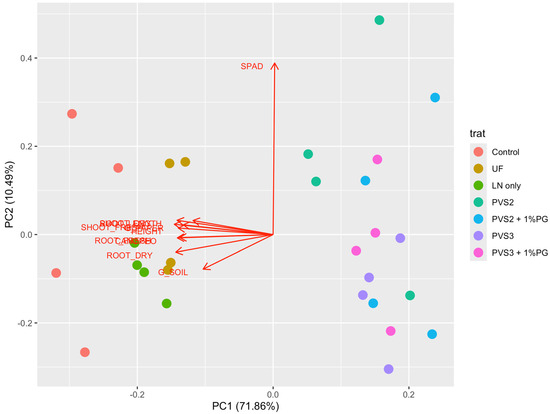
Figure 10.
Principal component analysis (PCA) by dispersion of cryopreservation treatments and the variables analyzed in Handroanthus impetiginosus seeds. Where G_PAPER = Germination in paper (%). G_SOIL = Germination in soil; SPAD = SPAD Index. CANOPEO. HEIGHT, ROOT_LENGHT = plant height and root length, respectively, in cm; SHOOT_FRESH, SHOOT_DRY, ROOT_FRESH, ROOT_DRY = shoot fresh and dry weight, root fresh and dry weight, respectively, in grams. Control—(no cryopreservation treatment applied); UF—seeds stored in an ultra-freezer at −80 °C without vitrification solution; LN only—seeds immersed in LN without vitrification solution; PVS2—seeds immersed in PVS2 followed by LN; PVS2 + 1%PG—seeds immersed in PVS2 with 1.0% phloroglucinol (PG) followed by LN; PVS3—seeds immersed in PVS3 followed by LN; PVS3 + 1%PG—seeds immersed in PVS3 with 1.0% PG followed by LN.
4. Discussion
The illegal logging of Handroanthus spp. timber in the Brazilian forests represents a significant conservation challenge. Handroanthus spp., known for its high economic value, is often targeted by illegal loggers who use fraudulent permits and documentation to disguise their activities as legal [25]. This issue is exacerbated by weak enforcement and regulatory loopholes, leading to substantial deforestation and ecological damage [26]. This pervasive problem of illegal logging threatens the genetic integrity and survival of these important trees [25,26]. Given these circumstances, preserving Handroanthus species through seed conservation techniques can be an alternative to ensure that this species will not go extinct.
The use of seeds as plant material to be preserved requires research of several critical characteristics to ensure their successful long-term viability and quality. Some important factors are moisture content, seed viability, genetic stability, and species-specific requirements for storage conditions, including temperature and light sensitivity [27].
X-ray imaging is highly effective for identifying seed quality. It provides detailed, non-destructive views of both external and internal seed structures. This technology allows for the precise examination of features such as embryo development, endosperm completeness, and internal defects like cracks or voids, which are not visible from the outside [11,28]. In addition, high or low moisture content in seeds can impact X-ray imaging quality. Seeds with higher moisture levels may produce unclear images of internal structures and more opaque images, as X-rays are more likely to be absorbed. Conversely, extremely dry seeds could produce more distinct images, but the seeds may also be more fragile and susceptible to damage during the X-ray process [10,29].
In this study, internal seed structures were clearly illustrated in the obtained high-resolution X-ray images. The first group, represented by normal seedlings (Figure 3B), corresponds to seeds that were intact or had minor damage, and contained all well-developed morphological structures, including two cotyledons, epicotyl, hypocotyl, primary root, and secondary roots, as described by [30]. The second group (abnormal seedlings) shows intermediate damage to structures, compromising the physiological quality of the seeds (Figure 4). The third group of images (dead seeds) includes seeds that did not germinate due to the absence of and severe damage to the hypocotyl-radicle axis and desiccation (Figure 5).
Severe dehydration was one of the biggest causes of seedling abnormality or dead seeds. Although H. impetiginosus seeds exhibit orthodox behavior [31], they naturally possess a certain level of desiccation tolerance. When seeds are dehydrated beyond these limits, several consequences can be observed, including an increase in intracellular solute concentration, which can lead to intensified destructive chemical reactions. When crystallized, these solutes can cause protein denaturation and the rupture of cell membranes, damaging seed structures, and as a consequence, the imbibition process is affected, leading to irreversible damage or even seed death [32,33].
The results of this study are consistent with observations made in other plant species with flattened seeds. Studies demonstrated the effectiveness of using X-ray images to assess and identify the internal morphology of seeds from Platypodium elegans Vog. and Solanum lycopersicum, highlighting the direct association between the internal seed structures, presence of damage, and seedling formation [8,34]. The application of X-ray testing was also practical in evaluating the internal morphology of seeds from Leucaena leucocephala Lam., Cucumis sativus L., and Capsicum annuum L., showing a correlation with germination [11,28]. Additionally, these studies established the relationship between the physical and physiological quality of seeds from Anadenanthera peregrina (L) Speg and Senna macranthera (DC. ex Collad.) H.S. Irwin and Barneby [35,36]. Therefore, analyzing the internal aspects with X-ray imaging accurately detected potential issues and predicted germination success.
In terms of conservation, the identification of healthy and well-formed seeds is key to ensure the success of plant regeneration after a cryopreservation treatment. The use of X-ray imaging in terms of conservation is already employed for several species, including threatened species, as an initial quality assessment to ensure purity and seed quantity, as well as a non-destructive quality assessment over time during long-term storage [37]. In this context, well-formed H. impetiginosus seeds were selected for cryopreservation based on previous results of X-ray imaging. These selected seeds should achieve a high germination rate (the control treatment on Figure 6), and they ensured that seed quality did not impact the response to cryopreservation treatments. The moisture content of 6.5% has proven ideal for H. impetiginosus seeds, making it suitable for applying X-ray imaging and some conservation techniques.
For cryopreservation, high moisture content can lead to ice crystal formation within the seed structures, making lower moisture levels preferable [38,39]. In this case, the orthodox nature of H. impetiginosus seeds allowed them to tolerate drying to low moisture levels without compromising their metabolism, maintaining their integrity during prolonged cold storage [31,40].
Regarding the temperature used for cryopreservation, the results indicated that treatments at −80 °C and −196 °C (LN) were the most efficient in long-term seed conservation. Previous research has also compared the use of temperature ranges other than the ones provided by LN, and their potential in long-term seed storage. Interestingly, no difference was observed in the germination percentage of Handroanthus spongiosus (Rizzini) S. Grose seeds stored for 30 days in a freezer (−20°C) compared to those stored in liquid nitrogen, with both temperatures being recommended for the cryopreservation of this species [41].
Cryoprotectants are substances used to protect biological material from damage caused by freezing or extreme dehydration during cryopreservation processes, broadly classified into non-penetrating and penetrating types, each operating through distinct mechanisms [16]. Non-penetrating cryoprotectants, such as PVS3, function by dehydrating cells and drawing water out to minimize external ice formation. In contrast, penetrating cryoprotectants replace intracellular water, preventing ice formation within cells and stabilizing them in a vitrified, glass-like state [17,42]. Combined solutions, such as PVS2, integrate both strategies, offering dual protection by simultaneously removing water externally and internally.
Direct immersion of H. impetiginosus seeds in LN was efficient in seed recovery. The use of cryoprotectant solutions (PVS2, PVS3), with or without the addition of phloroglucinol, was ineffective for seed cryopreservation, as it also reduced seed germination and plant growth. A similar finding was reported in the study [43], where direct freezing of Cedrela odorata L. seeds without any vitrification solution (PVS2 and PVS3), proved to be the most effective protocol for preserving the seeds’ germinative capacity. Likewise, Astronium urundeuva M. Allemão Engl. and Sinningia leucotricha (Hoehne) seeds can also be cryopreserved in liquid nitrogen without cryoprotectants (PVS2; PVS2 + 1% phloroglucinol and PVS3) [44,45].
The use of PVS2 and PVS3 negatively impacted the height of Cedrela odorata L. seedlings [43]. Similarly, PVS1, PVS2, PVS3, and PVS2 combined with 1% phloroglucinol were found to adversely affect seedling height, root length, and total dry mass in Sinningia leucotricha (Hoehne) Moore [44]. In Genipa americana L. seed cryopreservation, liquid nitrogen preserved seed germination; however, the cold treatment reduced seedling height and root length [46].
However, the PVS2 solution was effective for cryopreserving Quercus variabilis seeds in liquid nitrogen [47]. Similarly, PVS2 and PVS2 combined with 1% phloroglucinol were effective for the cryopreservation of Miltonia flavescens seeds [48]. No differences were found in height, root length, root dry mass, or shoot dry mass between Astronium urundeuva M. Allemão Engl. seedlings immersed in cryoprotectant solutions (PVS2, PVS2 + 1% phloroglucinol, and PVS3) [45]. Additionally, it has been reported that the cryoprotectant PVS2 increases the dry biomass of Hylocereus costaricensis seedlings by mitigating oxidative stress associated with prolonged liquid nitrogen exposure [49].
The findings of this study suggest that the application of PVS for the cryopreservation of large seeds, such as those of H. impetiginosus, may be unnecessary and potentially counterproductive. This conclusion aligns with the natural tolerance of these seeds to drying [31], as demonstrated by their ability to maintain viability at a water content of 6.5%. At such reduced water levels, the seeds are likely to vitrify at high cooling rates without the need for additional cryoprotectants. This inherent property simplifies their cryopreservation protocols and avoids potential complications associated with PVS.
In this study, the same seed moisture content (6.5%) was used across all treatments, which may align better without protocols involving the direct use of PVS solutions or without an initial pre-drying treatment. Standard vitrification protocols often include a loading solution to gradually dehydrate tissues before applying PVS [42]. However, since the seeds had a much lower water content compared to the loading solution, this step may have caused rehydration rather than dehydration, reducing the effectiveness of the treatment.
Moreover, large seeds present specific challenges in effectively absorbing vitrification solutions. The thick, impermeable seed coats characteristic of species like H. impetiginosus [30] hinder the penetration of cryoprotectants. Additionally, their low metabolic activity reduces osmotic uptake, limiting the distribution of vitrification solutions within the seed tissues. The internal structural complexity of large seeds further exacerbates this issue, leading to uneven absorption and potentially incomplete vitrification [50].
The variability in plant responses to cryoprotectants is due to a combination of species-specific characteristics, tissue types, and the nature of the cryoprotectants themselves. The PVS2 and PVS3 solutions are hypertonic, which can cause osmotic stress to cells, leading to dehydration and damage to cell membranes, affecting the ability to maintain homeostasis and potentially causing direct cell injury [51]. Although the toxic effects of these solutions are widely recognized, the alterations caused by cryoprotective solutions still lack a comprehensive explanation [42]. This underscores the urgent need for more research to bridge the gap between the literature that explores the biochemical effects of cryoprotectants and that which investigates their toxic effects in cryobiological applications [52].
Phloroglucinol (1,3,5-trihydroxybenzene) is frequently used in plant tissue cryopreservation because it helps mitigate the toxic effects of cryoprotectant solutions on plant cells. With properties similar to plant hormones like cytokinins and auxins, phloroglucinol promotes cell stability and growth even under the stress of freezing conditions. Its action reduces toxicity while enhancing cell recovery and viability, making it an effective additive for maintaining plant tissue health during cryopreservation [53,54]. Despite its other beneficial qualities, this study found phloroglucinol ineffective in reducing the toxic effects of vitrification solutions.
The combined evaluation of germination on paper and in soil allowed for the identification of toxicity levels during the plants’ recovery and growth after the application of different cryopreservation methodologies. All variables evaluated, except SPAD, demonstrated accuracy in characterizing the cryopreservation treatments, as demonstrated by the Person correlation and PCA.
Altogether, our findings highlight the importance of considering the biological and structural characteristics of seeds when designing cryopreservation protocols. Recognizing these inherent attributes can optimize genetic resource conservation while reducing procedural complexity and minimizing potential adverse effects.
In summary, the practical value of the X-ray test has been confirmed, producing clear radiographic images that reveal the seed’s internal structures and any physical damage that could hinder seedling formation. Thus, it is evident that visual inspection of X-rays is a highly effective tool that allows for the reliable evaluation of the physical quality of H. impetiginosus seeds. Cryopreserving H. impetiginosus seeds only using LN, without any vitrification solutions, proves to be the most effective treatment for long-term storage, as germination success after this cryopreservation treatment resulted in approximately 100% recovery rate in soil. This method highlights the simplicity and efficacy of cryopreservation when avoiding the complications associated with cryoprotectants, which can otherwise impede seed germination, emergence, and seedling development.
5. Conclusions
The use of radiographic images in seed analysis is a direct and reliable method. It allows for the immediate and accurate visualization of internal seed damage, establishing a clear correlation with parameters that define physiological quality.
For the cryopreservation of Handroanthus impetiginosus seeds, the use of vitrification solutions is not a prerequisite. The straightforward process of immersing seeds directly in liquid nitrogen allows for the recovery of seedlings without any hindrance to their development.
Author Contributions
Conceptualization, W.A.V. and K.F.L.P.; methodology, T.S.C., S.E.M. and W.A.V.; software, T.S.C. and V.M.P.; validation, K.F.L.P., W.A.V. and T.S.C.; formal analysis, T.S.C. and V.M.P.; investigation, T.S.C., S.E.M. and V.M.P.; resources, H.E.P. and W.A.V.; data curation, T.S.C.; writing—original draft preparation, T.S.C.; writing—review and editing, K.F.L.P., D.B., H.E.P. and W.A.V.; visualization, T.S.C.; supervision, D.B.; project administration, W.A.V.; funding acquisition, T.S.C. and W.A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thank the Coordination for the Improvement of Higher Education Personnel (CAPES—PrInt), Brazil for supporting this research under project number 88887.716805/2022-00 and The USDA National Institute of Food and Agriculture has provided support for this study, specifically under Hatch project 7001563. Special thanks are extended to M.S. Gabriel O. Matsumoto and M.S. Antonio Maricélio B. Souza for their invaluable assistance. Their support was instrumental in the successful completion of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas do Brasil, 8th ed.; Instituto Plantarum: Nova Odessa, Brazil, 2020; pp. 44–56. [Google Scholar]
- Silva-Junior, O.B.; Grattapaglia, D.; Novaes, E.; Collevatti, R.G. Genome Assembly of the Pink Ipê (Handroanthus impetiginosus, Bignoniaceae), a Highly Valued, Ecologically Keystone Neotropical Timber Forest Tree. Gigascience 2018, 7, gix125. [Google Scholar] [CrossRef] [PubMed]
- IUCN. The Red List of Threatened Species: BGCI, Tree Specialist Group Handroanthus impetiginosus. 2020. Available online: https://www.iucnredlist.org/species/144297143/173394208 (accessed on 20 January 2024).
- Pimenta, J.M.A.; Souza, W.M.A.T.D.; Ferrari, C.D.S.; Vieira, F.D.A.; Fajardo, C.G.; Pacheco, M.V. Selection of Handroanthus impetiginosus Mother Trees to Support Seed Collection Areas. Rev. Árvore 2023, 47, e4706. [Google Scholar] [CrossRef]
- Martins, J.R.; Edvaldo, A.A.S.; Alvarenga, A.A.; Rodrigues, A.C.; Ribeiro, D.E.; Toorop, P.E. Seedling Survival of Handroanthus impetiginosus (Mart. ex DC) Mattos in a Semi-Arid Environment Through Modified Germination Speed and Post-Germination Desiccation Tolerance. Braz. J. Biol. 2015, 75, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, J.M.A.; Felix, F.C.; Araújo, J.S.O.D.; Fajardo, C.G.; Pacheco, M.V. Selection of ISSR Molecular Primers for Studies of Genetic Diversity in Handroanthus impetiginosus (Mart. ex DC) Mattos. Rev. Caatinga 2022, 35, 231–238. [Google Scholar] [CrossRef]
- Piovesan, A.; Vancauwenberghe, V.; Van De Looverbosch, T.; Verboven, P.; Nicolaï, B. X-ray Computed Tomography for 3D Plant Imaging. Trends Plant Sci. 2021, 26, 1171–1185. [Google Scholar] [CrossRef]
- Pessoa, H.P.; Copati, M.G.F.; Azevedo, A.M.; Dariva, F.D.; Almeida, G.Q.D.; Gomes, C.N. Combining Deep Learning and X-ray Imaging Technology to Assess Tomato Seed Quality. Sci. Agric. 2023, 80, e20220121. [Google Scholar] [CrossRef]
- Silva, P.P.; Freitas, R.A.; Cícero, S.M.; Marcos Filho, J.; Nascimento, W.M. Image Analysis to Associate Morphological and Physiological Characteristics in Pumpkin Seeds. Hortic. Bras. 2014, 32, 210–214. [Google Scholar] [CrossRef]
- Medeiros, A.D.D.; Araújo, J.D.O.; León, M.J.Z.; Silva, L.J.D.; Dias, D.C.F.D.S. Parameters Based on X-Ray Images to Assess the Physical and Physiological Quality of Leucaena leucocephala Seeds. Ciência Agrotecnologia 2018, 42, 643–652. [Google Scholar] [CrossRef]
- Gomes Junior, F.G.; Duijn, B.V. Three-Dimensional (3-D) X-Ray Imaging for Seed Analysis. Seed Test. Int. 2017, 154, 48–52. Available online: https://www.cabdirect.org/cabdirect/abstract/20173322335 (accessed on 30 February 2024).
- Gantait, S.; Kundu, S.; Wani, S.H.; Das, P.K. Cryopreservation of Forest Tree Seeds: A Mini-Review. J. For. Environ. Sci. 2016, 32, 311–322. [Google Scholar] [CrossRef]
- Engelmann, F.; Dussert, S. Cryopreservation. In Conservation of Tropical Plant Species; Normah, M., Chin, H., Reed, B., Eds.; Springer: New York, NY, USA, 2012; pp. 107–119. [Google Scholar] [CrossRef]
- Engelmann, F. Use of Biotechnologies for the Conservation of Plant Biodiversity. Vitr. Cell. Dev. Biol.-Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Bozkurt, Y. Cryopreservation Biotechnology in Biomedical and Biological Sciences; IntechOpen: London, UK, 2018; pp. 129–154. [Google Scholar] [CrossRef]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Roque-Borda, C.A.; Kulus, D.; Souza, A.V.; Kaviani, B.; Vicente, E.F. Cryopreservation of agronomic plant germplasm using vitrification-based methods: An overview of selected case studies. Int. J. Mol. Sci. 2021, 22, 6157. [Google Scholar] [CrossRef] [PubMed]
- El Merzougui, S.; Benelli, C.; El Boullani, R.; Serghini, M.A. The cryopreservation of medicinal and ornamental geophytes: Application and challenges. Plants 2023, 12, 2143. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kim, U.; Lee, S.G.; Ryu, B.; Kim, J.; Igor, A.; Kim, J.S.; Jung, C.R.; Park, J.H.; Kim, C.Y. Vitrification for cryopreservation of 2D and 3D stem cells culture using high concentration of cryoprotective agents. BMC Biotechnol. 2020, 20, 45. [Google Scholar] [CrossRef]
- International Seed Testing Association. Determination of moisture content. Int. Rules Seed Test. 2024, 2024, 1–22. [Google Scholar] [CrossRef]
- Brasil. Instruções para a Análise de Sementes de Espécies Florestais; Ministério da Agricultura, Pecuária e Abastecimento (MAPA); Secretaria de Defesa Agropecuária: Brasília, Brazil, 2013; pp. 54–79. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/sementes-e-mudas/publicacoes-sementes-e-mudas/instrucoes-para-analise-de-sementes-de-especies-florestais/view (accessed on 20 January 2024).
- Nishizawa, S.; Sakai, A.; Amano, Y.; Matsuzawa, T. Cryopreservation of Asparagus (Asparagus officinalis L.) Embryogenic Suspension Cells and Subsequent Plant Regeneration by Vitrification. Plant Sci. 1993, 91, 67–73. [Google Scholar] [CrossRef]
- Sakai, A.; Engelmann, F. Vitrification. In Cryopreservation of Plant Germplasm I: Theory and Practice; Bajaj, M.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 73–100. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 20 January 2024).
- Brancalion, P.H.; De Almeida, D.R.; Vidal, E.; Molin, P.G.; Sontag, V.E.; Souza, S.E.; Schulze, M.D. Fake legal logging in the Brazilian Amazon. Sci. Adv. 2018, 4, eaaUF192. [Google Scholar] [CrossRef]
- Franca, C.S.; Persson, U.M.; Carvalho, T.; Lentini, M. Quantifying timber illegality risk in the Brazilian forest frontier. Nat. Sustain. 2023, 6, 1485–1495. [Google Scholar] [CrossRef]
- Hay, F.R.; Probert, R.J. Advances in seed conservation of wild plant species: A review of recent research. Conserv. Physiol. 2013, 1, cot030. [Google Scholar] [CrossRef]
- Arkhipov, M.V.; Priyatkin, N.S.; Gusakova, L.P.; Karamysheva, A.V.; Trofimuk, L.P.; Potrakhov, N.N.; Shchukina, P.A. Microfocus X-ray method for detecting hidden defects in seeds of woody forest species and other types of vascular plants. Tech. Phys. 2020, 65, 324–332. [Google Scholar] [CrossRef]
- Kunishima, N.; Takeda, Y.; Hirose, R.; Kalasová, D.; Šalplachta, J.; Omote, K. Visualization of internal 3D structure of small live seed on germination by laboratory-based X-ray microscopy with phase contrast computed tomography. Plant Methods 2020, 16, 7. [Google Scholar] [CrossRef]
- Felix, F.C.; Medeiros, J.A.; Pacheco, M.V. Morfologia de sementes e plântulas de Handroanthus impetiginosus (Mart ex DC) Mattos. Rev. Ciências Agrárias 2018, 41, 1028–1035. [Google Scholar] [CrossRef]
- Carvalho, L.R.D.; Silva, E.A.A.D.; Davide, A.C. Classificação de sementes florestais quanto ao comportamento no armazenamento. Rev. Bras. Sementes 2006, 28, 15–25. [Google Scholar] [CrossRef]
- Guimarães, C.C.; Faria, J.M.R.; Oliveira, J.M.; Silva, E.A.A.D. Avaliação da perda da tolerância à dessecação e da quantidade de DNA nuclear em sementes de Peltophorum dubium (Spreng.) Taubert durante e após a germinação. Rev. Bras. Sementes 2011, 33, 207–215. [Google Scholar] [CrossRef]
- Pereira, W.V.S.; Faria, J.M.R.; Tonetti, O.A.O.; Silva, E.A.A. Perda da tolerância à dessecação em sementes de Copaifera langsdorffii durante a germinação. Braz. J. Biol. 2014, 74, 501–508. [Google Scholar] [CrossRef]
- Gomes, K.B.P.; Matos, J.M.M.; Martins, I.S.; Martins, R.C.C. X-ray test to evaluate the physiological potential of Platypodium elegans Vog seeds (Fabaceae). Sci. Agropecu. 2016, 7, 305–311. [Google Scholar] [CrossRef][Green Version]
- Pinheiro, D.T.; Medeiros, A.D.; Soares, T.F.N.S.; Capobiango, N.P.; Dias, D.C.T.F. Image analysis using X-ray to evaluate seed quality of Anadenanthera peregrina (L.) Speg. Ciência Florest. 2022, 32, 1309–1322. [Google Scholar] [CrossRef]
- Araújo, J.D.O.; Pinheiro, D.T.; Queiroz, G.B.; Soares, J.M.; Hoshide, A.K.; Morais Junior, V.T.M.D.; Soares da Rocha, S.J.S.; Dias, D.C.F.D. Selection of superior Senna macranthera seeds, carbon stock, and seedling survival, and costs for habitat restoration. Sustainability 2023, 15, 9875. [Google Scholar] [CrossRef]
- Liu, U.; Cossu, T.A.; Davies, R.M.; Forest, F.; Dickie, J.B.; Breman, E. Conserving orthodox seeds of globally threatened plants ex situ in the Millennium Seed Bank, Royal Botanic Gardens, Kew, UK: The status of seed collections. Biodivers. Conserv. 2020, 29, 2901–2949. [Google Scholar] [CrossRef]
- Salomão, A.N.; Santos, I.R.I.; José, S.C.B.R. Cryopreservation of Pyrostegia venusta (Ker Gawl) Miers seeds. Hoehnea 2020, 47, e1042019. [Google Scholar] [CrossRef]
- Castro, M.G.B.; Medeiros, A.D.; Silva, J.M.; Silva, L.J. Physical integrity of endive and chicory seeds determined by automated analysis of radiographic images. Rev. Bras. Ciências Agrárias 2021, 16, e9072. [Google Scholar] [CrossRef]
- Vendrame, W.; Takane, R.; Cardoso, L.; Alvarez, L.; Tadeu, R. Cryopreservation of seeds of Melocactus zehntneri Braun ex Ritter f. and Cereus gounellei Luetzelb ex Schum. by the vitrification method. Agron. Sci. Biotechnol. 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Silva, J.D.J.; Alencar, S.D.S.; Gomes, R.A.; Matias, J.R.; Pelacani, C.R.; Dantas, B.F. Conservation and physiological quality of Handroanthus spongiosus (Rizzini) S. Grose (Bignoniaceae) seeds. J. Seed Sci. 2022, 44, e202244007. [Google Scholar] [CrossRef]
- Zamecnik, J.; Faltus, M.; Bilavcik, A. Vitrification solutions for plant cryopreservation: Modification and properties. Plants 2021, 10, 2623. [Google Scholar] [CrossRef]
- Velasco-García, M.V.; Hernández-Arroyo, D.G.; Muñoz-Gutiérrez, L.; Castillo-Martínez, C.R.; Vallejo-Reyna, M.Á.; García-Campusano, F. Seed cryopreservation of Cedrela odorata L: Germination and early nursery establishment. Rev. Mex. Cienc. For. 2022, 13, 31–55. [Google Scholar] [CrossRef]
- Stegani, V.; Alves, G.A.C.; Bertoncelli, D.J.; Faria, R.T.V. Criopreservação de sementes de rainha do abismo (Sinningia leucotricha). Ornam. Hortic. 2017, 23, 15–21. [Google Scholar] [CrossRef]
- Paula, J.C.B.; Guariz, H.R.; Ribeiro Júnior, W.A.; Shimizu, G.D.; Faria, R.T.; Oliveira, H.C. Cryopreservation of seeds of the Brazilian native species Aroeira-do-sertão (Astronium urundeuva M. Allemão Engl.). Rev. Caatinga 2022, 35, 915–924. [Google Scholar] [CrossRef]
- Souza, R.R.D.; Paiva, P.D.D.O.; Freitas, R.T.D.; Silva, D.P.C.D.; Reis, M.V.D.; Nery, F.C.; Paiva, R. Cryopreservation of Genipa americana seeds. Rev. Ciência Agronômica 2023, 54, e20228531. [Google Scholar] [CrossRef]
- Zhang, M.J.; Wang, Y.Z.; Xian, Y.; Cui, C.C.; Xie, X.M.; Tong, B.Q.; Han, B. Desiccation sensitivity characteristics and low-temperature storage of recalcitrant Quercus variabilis. Seed For. 2023, 14, 1837. [Google Scholar] [CrossRef]
- Paula, J.C.B.; Ribeiro Júnior, W.A.R.; Shimizu, G.D.; Men, G.B.; Faria, R.T. Cryopreservation in liquid nitrogen of Brazilian orchid seeds Miltonia flavescens Lindl. Rev. Agro@Mbiente On-Line 2020, 14, e20200016. [Google Scholar] [CrossRef]
- Santana, B.Í.D.; Paiva, R.; Reis, M.V.D.; Vilas-Boas, L.V.; Matos, E.M.; Campos, J.M.S.D. Seed cryopreservation without vitrification (PVS2) induces oxidative stimuli to promote endoreplication in red pitaya seedlings. Plant Cell Tissue Organ Cult. 2024, 156, 11. [Google Scholar] [CrossRef]
- Trusiak, M.; Plitta-Michalak, B.P.; Michalak, M. Choosing the right path for the successful storage of seeds. Plants 2022, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Best, B.P. Cryoprotectant toxicity: Facts, issues, and questions. Rejuvenation Res. 2015, 18, 422–436. [Google Scholar] [CrossRef]
- Fahy, G.M.; Wowk, B. Principles of Cryopreservation by Vitrification. In Cryopreservation and Freeze-Drying Protocols; Wolkers, W., Oldenhof, H., Eds.; Springer: New York, NY, USA, 2015; Volume 1257, pp. 21–82. [Google Scholar] [CrossRef]
- Silva, J.A.T.; Dobránszki, J.; Ross, S. Phloroglucinol in plant tissue culture. In Vitr. Cell. Dev. Biol.-Plant 2013, 49, 1–16. [Google Scholar] [CrossRef]
- Petti, C. Phloroglucinol Mediated Plant Regeneration of Ornithogalum dubium as the Sole “Hormone-Like Supplement” in Plant Tissue Culture Long-Term Experiments. Plants 2020, 9, 929. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).