Cryopreservation of Lavender Trumpet Tree (Handroanthus impetiginosus) Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
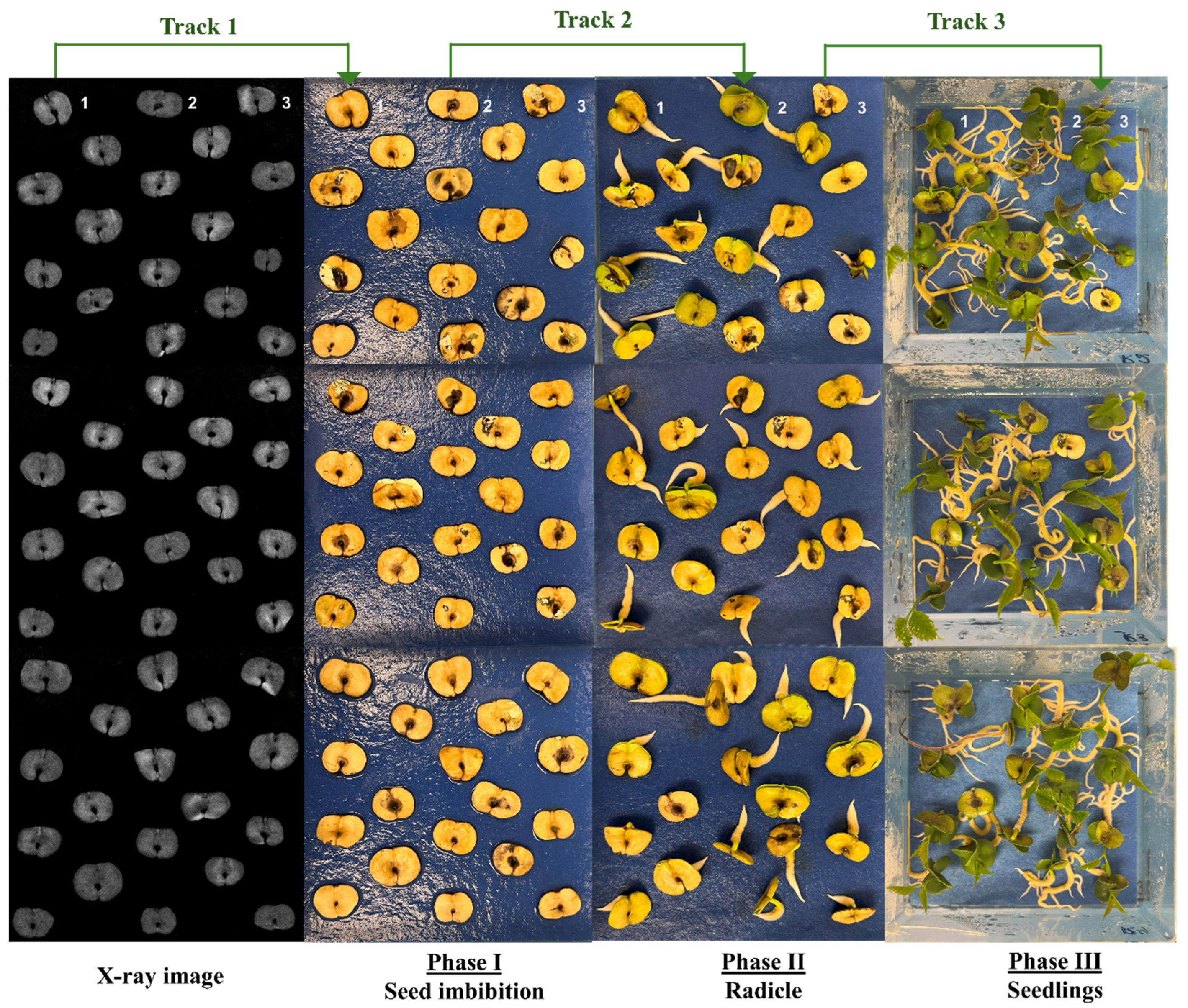
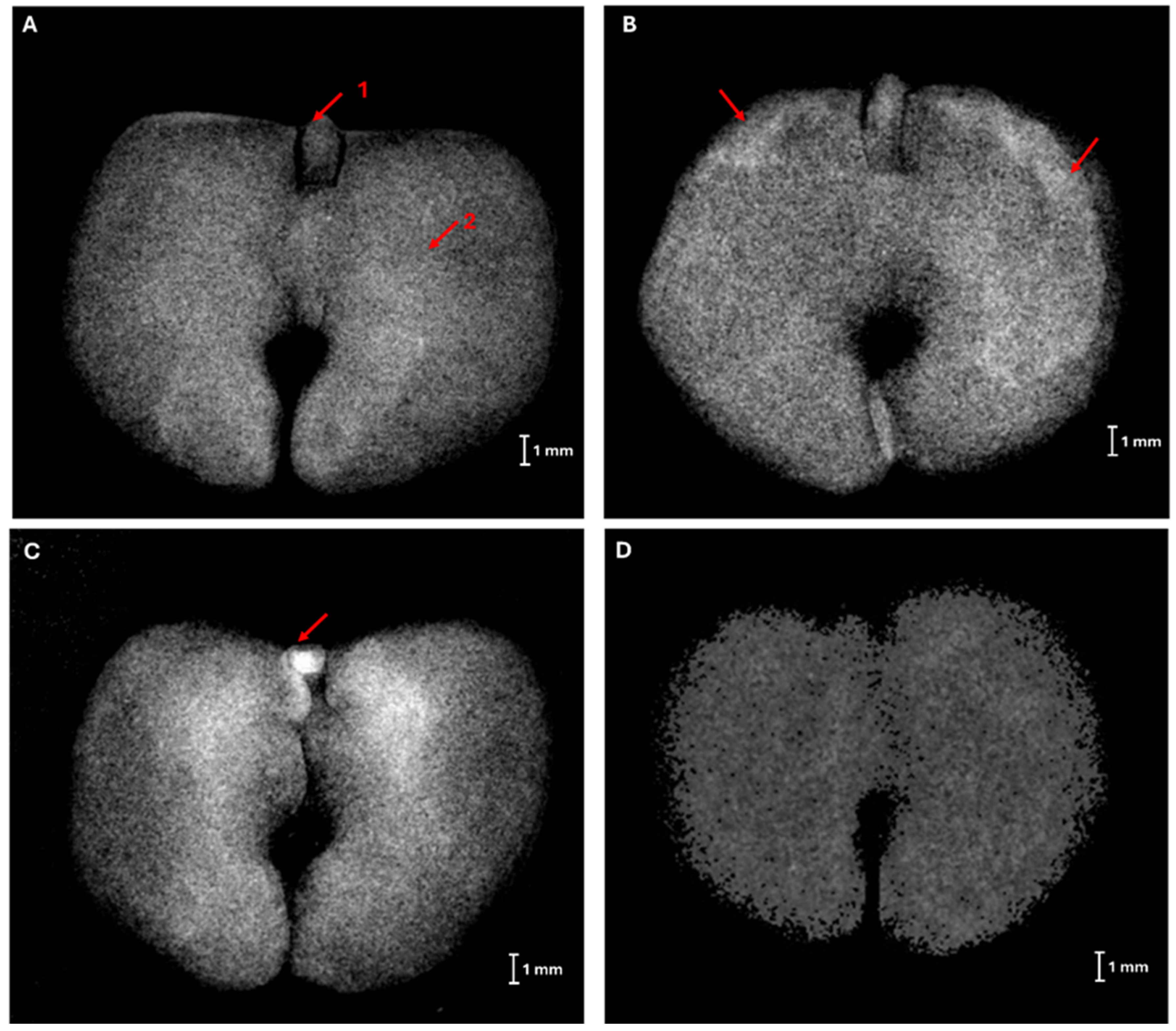
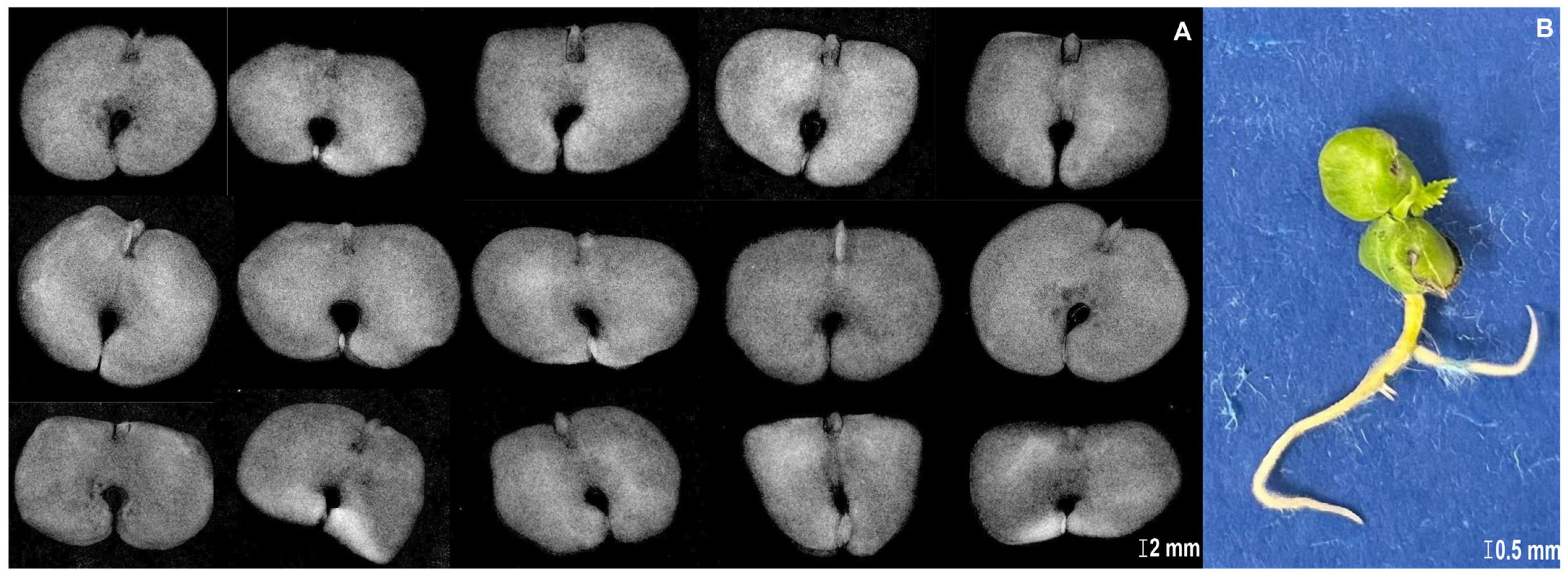
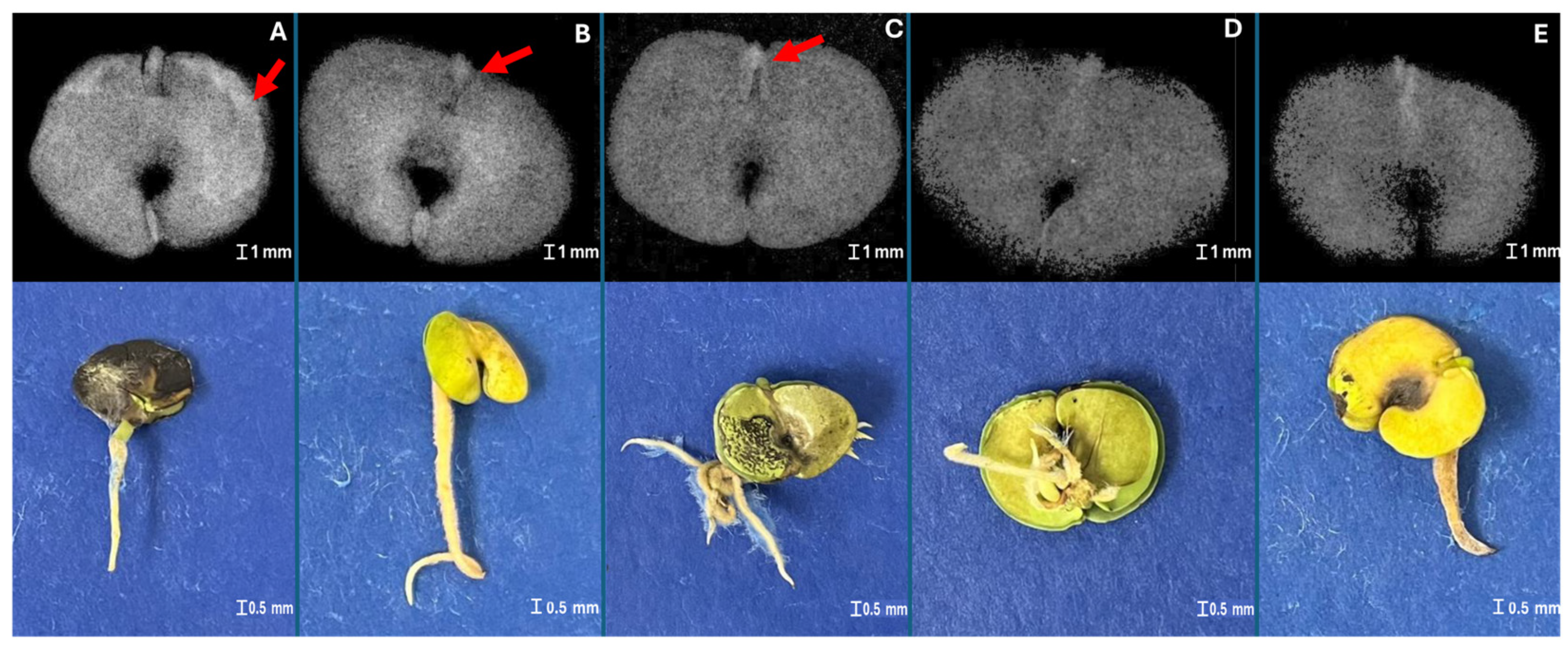
2.2. X-Ray Imaging in Seed Quality Assessment
2.3. Seeds Cryopreservation
- Control—seeds were not immersed in liquid nitrogen (LN) or vitrification solution; they were not placed in an ultra-freezer;
- UF—seeds stored in an ultra-freezer at −80 °C without vitrification solution;
- LN only—seeds immersed in LN without vitrification solution;
- PVS2—seeds immersed in PVS2 followed by LN;
- PVS2 + 1%PG—seeds immersed in PVS2 with 1.0% phloroglucinol (PG) followed by LN;
- PVS3—seeds immersed in PVS3 followed by LN;
- PVS3 + 1%PG—seeds immersed in PVS3 with 1.0% PG followed by LN.
2.4. Statistical Procedures
3. Results
3.1. X-Ray Imaging as Seed Quality Assessment
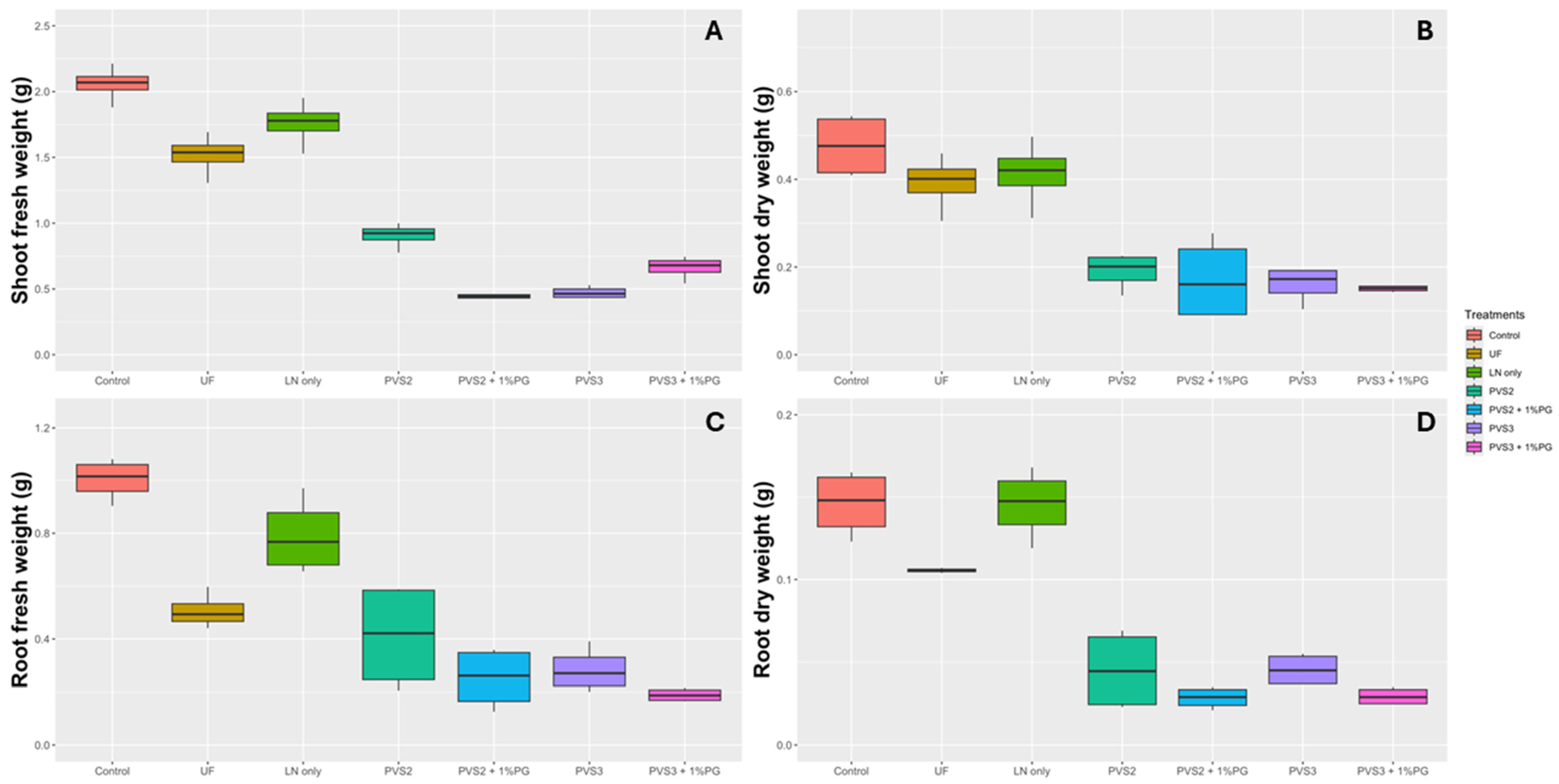
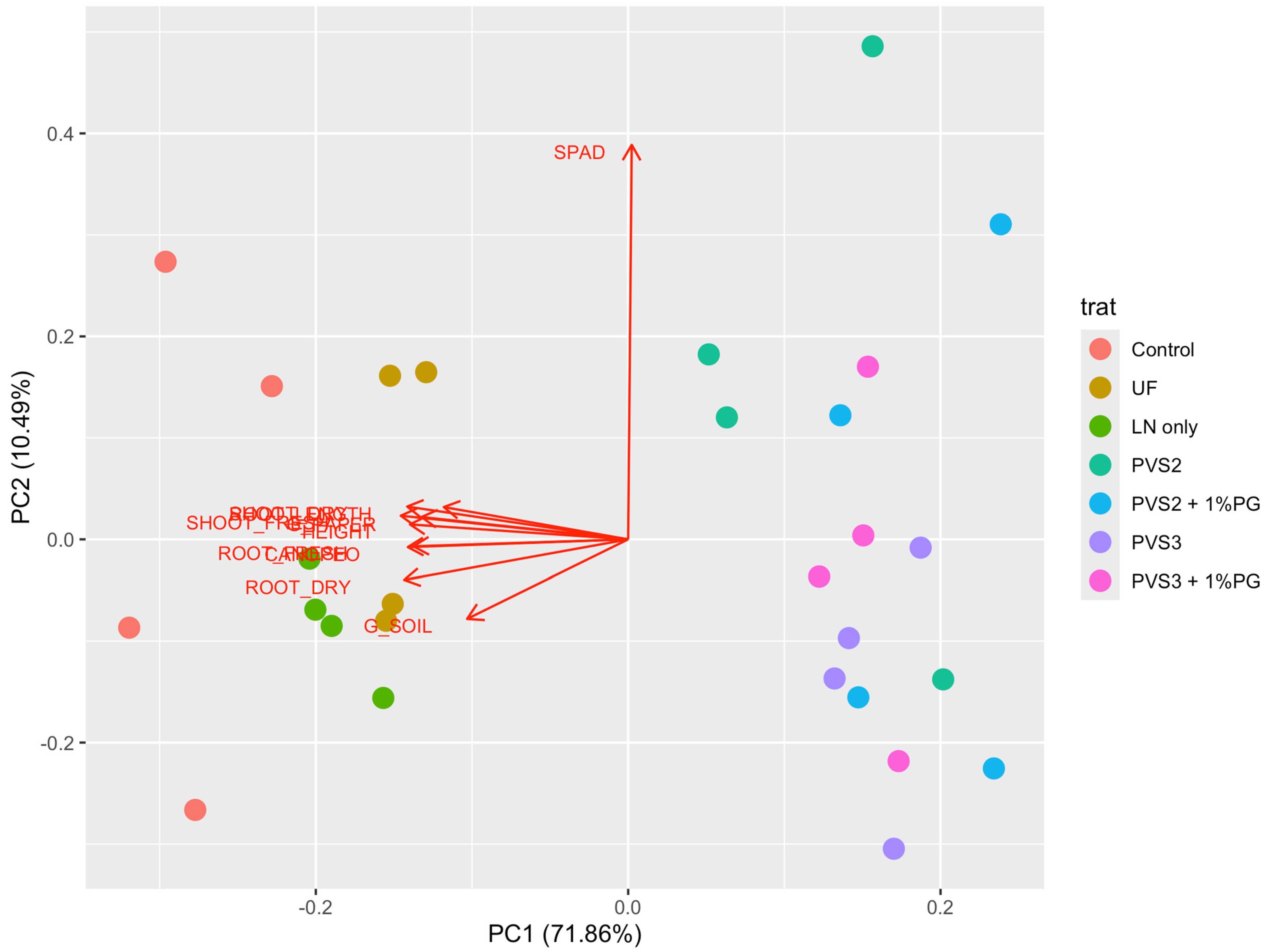
3.2. Seed Cryopreservation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas do Brasil, 8th ed.; Instituto Plantarum: Nova Odessa, Brazil, 2020; pp. 44–56. [Google Scholar]
- Silva-Junior, O.B.; Grattapaglia, D.; Novaes, E.; Collevatti, R.G. Genome Assembly of the Pink Ipê (Handroanthus impetiginosus, Bignoniaceae), a Highly Valued, Ecologically Keystone Neotropical Timber Forest Tree. Gigascience 2018, 7, gix125. [Google Scholar] [CrossRef] [PubMed]
- IUCN. The Red List of Threatened Species: BGCI, Tree Specialist Group Handroanthus impetiginosus. 2020. Available online: https://www.iucnredlist.org/species/144297143/173394208 (accessed on 20 January 2024).
- Pimenta, J.M.A.; Souza, W.M.A.T.D.; Ferrari, C.D.S.; Vieira, F.D.A.; Fajardo, C.G.; Pacheco, M.V. Selection of Handroanthus impetiginosus Mother Trees to Support Seed Collection Areas. Rev. Árvore 2023, 47, e4706. [Google Scholar] [CrossRef]
- Martins, J.R.; Edvaldo, A.A.S.; Alvarenga, A.A.; Rodrigues, A.C.; Ribeiro, D.E.; Toorop, P.E. Seedling Survival of Handroanthus impetiginosus (Mart. ex DC) Mattos in a Semi-Arid Environment Through Modified Germination Speed and Post-Germination Desiccation Tolerance. Braz. J. Biol. 2015, 75, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, J.M.A.; Felix, F.C.; Araújo, J.S.O.D.; Fajardo, C.G.; Pacheco, M.V. Selection of ISSR Molecular Primers for Studies of Genetic Diversity in Handroanthus impetiginosus (Mart. ex DC) Mattos. Rev. Caatinga 2022, 35, 231–238. [Google Scholar] [CrossRef]
- Piovesan, A.; Vancauwenberghe, V.; Van De Looverbosch, T.; Verboven, P.; Nicolaï, B. X-ray Computed Tomography for 3D Plant Imaging. Trends Plant Sci. 2021, 26, 1171–1185. [Google Scholar] [CrossRef]
- Pessoa, H.P.; Copati, M.G.F.; Azevedo, A.M.; Dariva, F.D.; Almeida, G.Q.D.; Gomes, C.N. Combining Deep Learning and X-ray Imaging Technology to Assess Tomato Seed Quality. Sci. Agric. 2023, 80, e20220121. [Google Scholar] [CrossRef]
- Silva, P.P.; Freitas, R.A.; Cícero, S.M.; Marcos Filho, J.; Nascimento, W.M. Image Analysis to Associate Morphological and Physiological Characteristics in Pumpkin Seeds. Hortic. Bras. 2014, 32, 210–214. [Google Scholar] [CrossRef]
- Medeiros, A.D.D.; Araújo, J.D.O.; León, M.J.Z.; Silva, L.J.D.; Dias, D.C.F.D.S. Parameters Based on X-Ray Images to Assess the Physical and Physiological Quality of Leucaena leucocephala Seeds. Ciência Agrotecnologia 2018, 42, 643–652. [Google Scholar] [CrossRef]
- Gomes Junior, F.G.; Duijn, B.V. Three-Dimensional (3-D) X-Ray Imaging for Seed Analysis. Seed Test. Int. 2017, 154, 48–52. Available online: https://www.cabdirect.org/cabdirect/abstract/20173322335 (accessed on 30 February 2024).
- Gantait, S.; Kundu, S.; Wani, S.H.; Das, P.K. Cryopreservation of Forest Tree Seeds: A Mini-Review. J. For. Environ. Sci. 2016, 32, 311–322. [Google Scholar] [CrossRef]
- Engelmann, F.; Dussert, S. Cryopreservation. In Conservation of Tropical Plant Species; Normah, M., Chin, H., Reed, B., Eds.; Springer: New York, NY, USA, 2012; pp. 107–119. [Google Scholar] [CrossRef]
- Engelmann, F. Use of Biotechnologies for the Conservation of Plant Biodiversity. Vitr. Cell. Dev. Biol.-Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Bozkurt, Y. Cryopreservation Biotechnology in Biomedical and Biological Sciences; IntechOpen: London, UK, 2018; pp. 129–154. [Google Scholar] [CrossRef]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Roque-Borda, C.A.; Kulus, D.; Souza, A.V.; Kaviani, B.; Vicente, E.F. Cryopreservation of agronomic plant germplasm using vitrification-based methods: An overview of selected case studies. Int. J. Mol. Sci. 2021, 22, 6157. [Google Scholar] [CrossRef] [PubMed]
- El Merzougui, S.; Benelli, C.; El Boullani, R.; Serghini, M.A. The cryopreservation of medicinal and ornamental geophytes: Application and challenges. Plants 2023, 12, 2143. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Kim, U.; Lee, S.G.; Ryu, B.; Kim, J.; Igor, A.; Kim, J.S.; Jung, C.R.; Park, J.H.; Kim, C.Y. Vitrification for cryopreservation of 2D and 3D stem cells culture using high concentration of cryoprotective agents. BMC Biotechnol. 2020, 20, 45. [Google Scholar] [CrossRef]
- International Seed Testing Association. Determination of moisture content. Int. Rules Seed Test. 2024, 2024, 1–22. [Google Scholar] [CrossRef]
- Brasil. Instruções para a Análise de Sementes de Espécies Florestais; Ministério da Agricultura, Pecuária e Abastecimento (MAPA); Secretaria de Defesa Agropecuária: Brasília, Brazil, 2013; pp. 54–79. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/sementes-e-mudas/publicacoes-sementes-e-mudas/instrucoes-para-analise-de-sementes-de-especies-florestais/view (accessed on 20 January 2024).
- Nishizawa, S.; Sakai, A.; Amano, Y.; Matsuzawa, T. Cryopreservation of Asparagus (Asparagus officinalis L.) Embryogenic Suspension Cells and Subsequent Plant Regeneration by Vitrification. Plant Sci. 1993, 91, 67–73. [Google Scholar] [CrossRef]
- Sakai, A.; Engelmann, F. Vitrification. In Cryopreservation of Plant Germplasm I: Theory and Practice; Bajaj, M.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 73–100. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 20 January 2024).
- Brancalion, P.H.; De Almeida, D.R.; Vidal, E.; Molin, P.G.; Sontag, V.E.; Souza, S.E.; Schulze, M.D. Fake legal logging in the Brazilian Amazon. Sci. Adv. 2018, 4, eaaUF192. [Google Scholar] [CrossRef]
- Franca, C.S.; Persson, U.M.; Carvalho, T.; Lentini, M. Quantifying timber illegality risk in the Brazilian forest frontier. Nat. Sustain. 2023, 6, 1485–1495. [Google Scholar] [CrossRef]
- Hay, F.R.; Probert, R.J. Advances in seed conservation of wild plant species: A review of recent research. Conserv. Physiol. 2013, 1, cot030. [Google Scholar] [CrossRef]
- Arkhipov, M.V.; Priyatkin, N.S.; Gusakova, L.P.; Karamysheva, A.V.; Trofimuk, L.P.; Potrakhov, N.N.; Shchukina, P.A. Microfocus X-ray method for detecting hidden defects in seeds of woody forest species and other types of vascular plants. Tech. Phys. 2020, 65, 324–332. [Google Scholar] [CrossRef]
- Kunishima, N.; Takeda, Y.; Hirose, R.; Kalasová, D.; Šalplachta, J.; Omote, K. Visualization of internal 3D structure of small live seed on germination by laboratory-based X-ray microscopy with phase contrast computed tomography. Plant Methods 2020, 16, 7. [Google Scholar] [CrossRef]
- Felix, F.C.; Medeiros, J.A.; Pacheco, M.V. Morfologia de sementes e plântulas de Handroanthus impetiginosus (Mart ex DC) Mattos. Rev. Ciências Agrárias 2018, 41, 1028–1035. [Google Scholar] [CrossRef]
- Carvalho, L.R.D.; Silva, E.A.A.D.; Davide, A.C. Classificação de sementes florestais quanto ao comportamento no armazenamento. Rev. Bras. Sementes 2006, 28, 15–25. [Google Scholar] [CrossRef]
- Guimarães, C.C.; Faria, J.M.R.; Oliveira, J.M.; Silva, E.A.A.D. Avaliação da perda da tolerância à dessecação e da quantidade de DNA nuclear em sementes de Peltophorum dubium (Spreng.) Taubert durante e após a germinação. Rev. Bras. Sementes 2011, 33, 207–215. [Google Scholar] [CrossRef]
- Pereira, W.V.S.; Faria, J.M.R.; Tonetti, O.A.O.; Silva, E.A.A. Perda da tolerância à dessecação em sementes de Copaifera langsdorffii durante a germinação. Braz. J. Biol. 2014, 74, 501–508. [Google Scholar] [CrossRef]
- Gomes, K.B.P.; Matos, J.M.M.; Martins, I.S.; Martins, R.C.C. X-ray test to evaluate the physiological potential of Platypodium elegans Vog seeds (Fabaceae). Sci. Agropecu. 2016, 7, 305–311. [Google Scholar] [CrossRef][Green Version]
- Pinheiro, D.T.; Medeiros, A.D.; Soares, T.F.N.S.; Capobiango, N.P.; Dias, D.C.T.F. Image analysis using X-ray to evaluate seed quality of Anadenanthera peregrina (L.) Speg. Ciência Florest. 2022, 32, 1309–1322. [Google Scholar] [CrossRef]
- Araújo, J.D.O.; Pinheiro, D.T.; Queiroz, G.B.; Soares, J.M.; Hoshide, A.K.; Morais Junior, V.T.M.D.; Soares da Rocha, S.J.S.; Dias, D.C.F.D. Selection of superior Senna macranthera seeds, carbon stock, and seedling survival, and costs for habitat restoration. Sustainability 2023, 15, 9875. [Google Scholar] [CrossRef]
- Liu, U.; Cossu, T.A.; Davies, R.M.; Forest, F.; Dickie, J.B.; Breman, E. Conserving orthodox seeds of globally threatened plants ex situ in the Millennium Seed Bank, Royal Botanic Gardens, Kew, UK: The status of seed collections. Biodivers. Conserv. 2020, 29, 2901–2949. [Google Scholar] [CrossRef]
- Salomão, A.N.; Santos, I.R.I.; José, S.C.B.R. Cryopreservation of Pyrostegia venusta (Ker Gawl) Miers seeds. Hoehnea 2020, 47, e1042019. [Google Scholar] [CrossRef]
- Castro, M.G.B.; Medeiros, A.D.; Silva, J.M.; Silva, L.J. Physical integrity of endive and chicory seeds determined by automated analysis of radiographic images. Rev. Bras. Ciências Agrárias 2021, 16, e9072. [Google Scholar] [CrossRef]
- Vendrame, W.; Takane, R.; Cardoso, L.; Alvarez, L.; Tadeu, R. Cryopreservation of seeds of Melocactus zehntneri Braun ex Ritter f. and Cereus gounellei Luetzelb ex Schum. by the vitrification method. Agron. Sci. Biotechnol. 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Silva, J.D.J.; Alencar, S.D.S.; Gomes, R.A.; Matias, J.R.; Pelacani, C.R.; Dantas, B.F. Conservation and physiological quality of Handroanthus spongiosus (Rizzini) S. Grose (Bignoniaceae) seeds. J. Seed Sci. 2022, 44, e202244007. [Google Scholar] [CrossRef]
- Zamecnik, J.; Faltus, M.; Bilavcik, A. Vitrification solutions for plant cryopreservation: Modification and properties. Plants 2021, 10, 2623. [Google Scholar] [CrossRef]
- Velasco-García, M.V.; Hernández-Arroyo, D.G.; Muñoz-Gutiérrez, L.; Castillo-Martínez, C.R.; Vallejo-Reyna, M.Á.; García-Campusano, F. Seed cryopreservation of Cedrela odorata L: Germination and early nursery establishment. Rev. Mex. Cienc. For. 2022, 13, 31–55. [Google Scholar] [CrossRef]
- Stegani, V.; Alves, G.A.C.; Bertoncelli, D.J.; Faria, R.T.V. Criopreservação de sementes de rainha do abismo (Sinningia leucotricha). Ornam. Hortic. 2017, 23, 15–21. [Google Scholar] [CrossRef]
- Paula, J.C.B.; Guariz, H.R.; Ribeiro Júnior, W.A.; Shimizu, G.D.; Faria, R.T.; Oliveira, H.C. Cryopreservation of seeds of the Brazilian native species Aroeira-do-sertão (Astronium urundeuva M. Allemão Engl.). Rev. Caatinga 2022, 35, 915–924. [Google Scholar] [CrossRef]
- Souza, R.R.D.; Paiva, P.D.D.O.; Freitas, R.T.D.; Silva, D.P.C.D.; Reis, M.V.D.; Nery, F.C.; Paiva, R. Cryopreservation of Genipa americana seeds. Rev. Ciência Agronômica 2023, 54, e20228531. [Google Scholar] [CrossRef]
- Zhang, M.J.; Wang, Y.Z.; Xian, Y.; Cui, C.C.; Xie, X.M.; Tong, B.Q.; Han, B. Desiccation sensitivity characteristics and low-temperature storage of recalcitrant Quercus variabilis. Seed For. 2023, 14, 1837. [Google Scholar] [CrossRef]
- Paula, J.C.B.; Ribeiro Júnior, W.A.R.; Shimizu, G.D.; Men, G.B.; Faria, R.T. Cryopreservation in liquid nitrogen of Brazilian orchid seeds Miltonia flavescens Lindl. Rev. Agro@Mbiente On-Line 2020, 14, e20200016. [Google Scholar] [CrossRef]
- Santana, B.Í.D.; Paiva, R.; Reis, M.V.D.; Vilas-Boas, L.V.; Matos, E.M.; Campos, J.M.S.D. Seed cryopreservation without vitrification (PVS2) induces oxidative stimuli to promote endoreplication in red pitaya seedlings. Plant Cell Tissue Organ Cult. 2024, 156, 11. [Google Scholar] [CrossRef]
- Trusiak, M.; Plitta-Michalak, B.P.; Michalak, M. Choosing the right path for the successful storage of seeds. Plants 2022, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Best, B.P. Cryoprotectant toxicity: Facts, issues, and questions. Rejuvenation Res. 2015, 18, 422–436. [Google Scholar] [CrossRef]
- Fahy, G.M.; Wowk, B. Principles of Cryopreservation by Vitrification. In Cryopreservation and Freeze-Drying Protocols; Wolkers, W., Oldenhof, H., Eds.; Springer: New York, NY, USA, 2015; Volume 1257, pp. 21–82. [Google Scholar] [CrossRef]
- Silva, J.A.T.; Dobránszki, J.; Ross, S. Phloroglucinol in plant tissue culture. In Vitr. Cell. Dev. Biol.-Plant 2013, 49, 1–16. [Google Scholar] [CrossRef]
- Petti, C. Phloroglucinol Mediated Plant Regeneration of Ornithogalum dubium as the Sole “Hormone-Like Supplement” in Plant Tissue Culture Long-Term Experiments. Plants 2020, 9, 929. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, T.S.; Pereira, V.M.; El Merzougui, S.; Beleski, D.; Pérez, H.E.; Pivetta, K.F.L.; Vendrame, W.A. Cryopreservation of Lavender Trumpet Tree (Handroanthus impetiginosus) Seeds. Horticulturae 2024, 10, 1256. https://doi.org/10.3390/horticulturae10121256
Campos TS, Pereira VM, El Merzougui S, Beleski D, Pérez HE, Pivetta KFL, Vendrame WA. Cryopreservation of Lavender Trumpet Tree (Handroanthus impetiginosus) Seeds. Horticulturae. 2024; 10(12):1256. https://doi.org/10.3390/horticulturae10121256
Chicago/Turabian StyleCampos, Thiago Souza, Vania M. Pereira, Soumaya El Merzougui, David Beleski, Héctor E. Pérez, Kathia Fernandes Lopes Pivetta, and Wagner A. Vendrame. 2024. "Cryopreservation of Lavender Trumpet Tree (Handroanthus impetiginosus) Seeds" Horticulturae 10, no. 12: 1256. https://doi.org/10.3390/horticulturae10121256
APA StyleCampos, T. S., Pereira, V. M., El Merzougui, S., Beleski, D., Pérez, H. E., Pivetta, K. F. L., & Vendrame, W. A. (2024). Cryopreservation of Lavender Trumpet Tree (Handroanthus impetiginosus) Seeds. Horticulturae, 10(12), 1256. https://doi.org/10.3390/horticulturae10121256







