The Perfect Match: Testing the Effect of Increasing Red and Blue Ratio on Baby-Leaf Kale Growth, Yield and Physiology
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Settings and Design
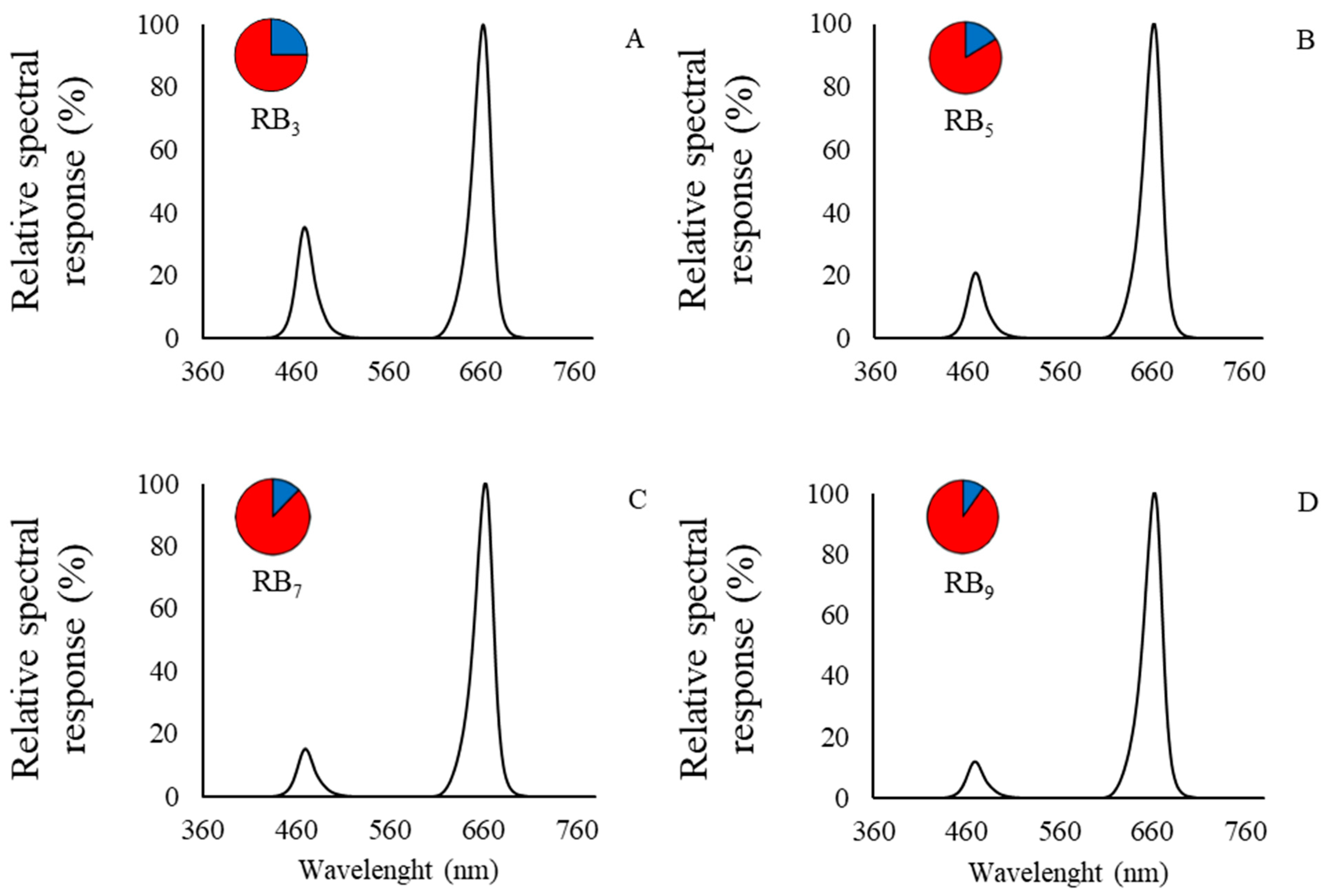
2.2. Light Treatments: Intensity, Quality and Photoperiod Properties
2.3. Morphological Measurements
2.4. Physiological Measurements
2.5. Resource Use Efficiency
2.6. Statistical Analysis
3. Results
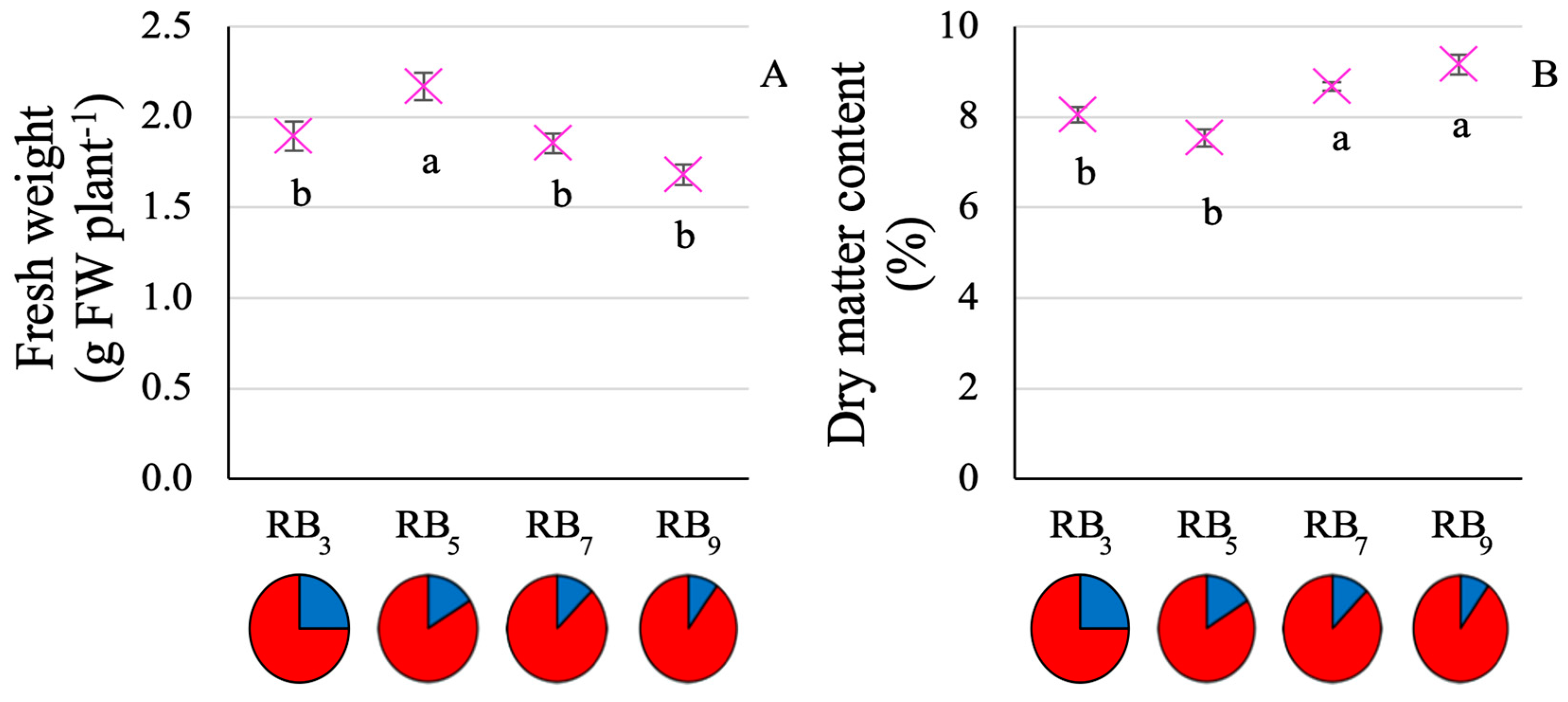
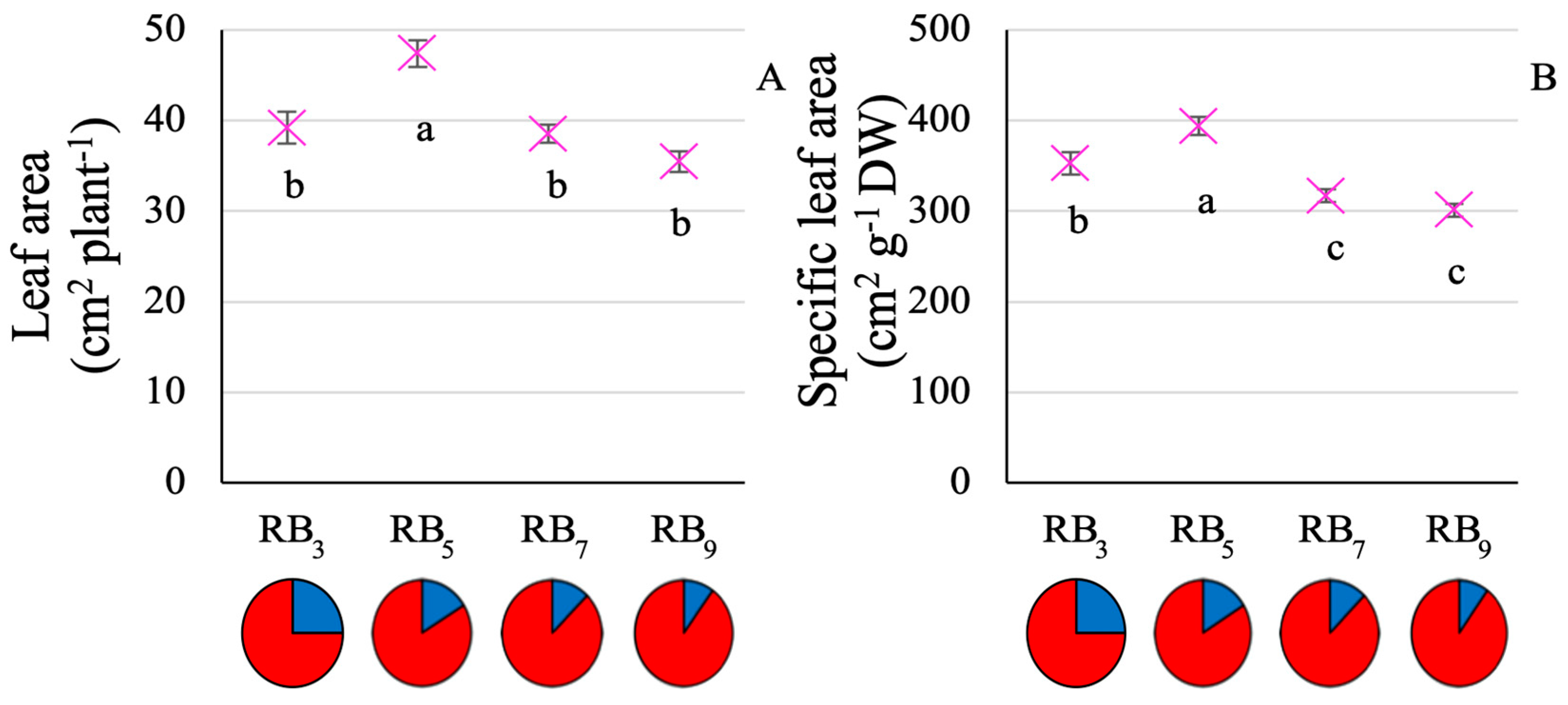
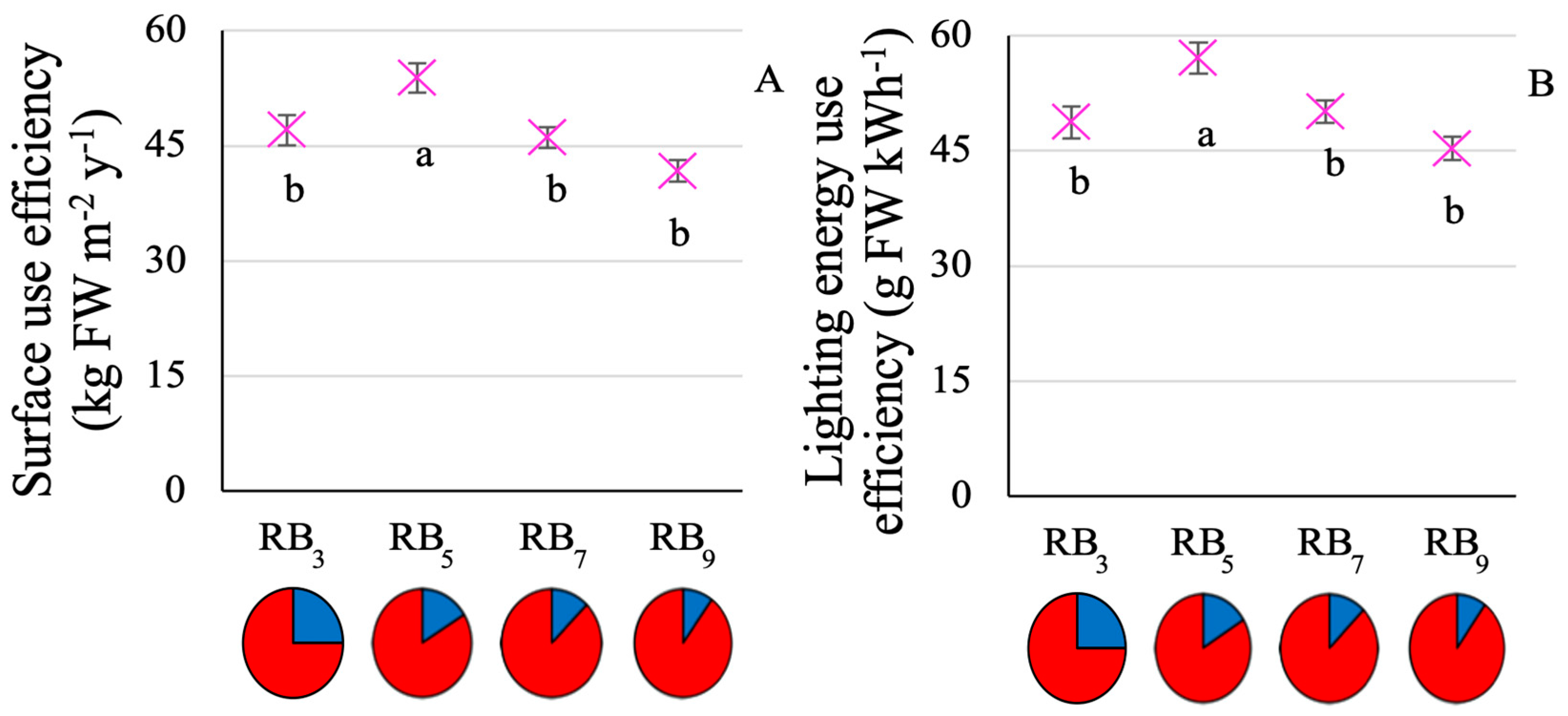
3.1. Plant Growth and Resource Use Efficiency
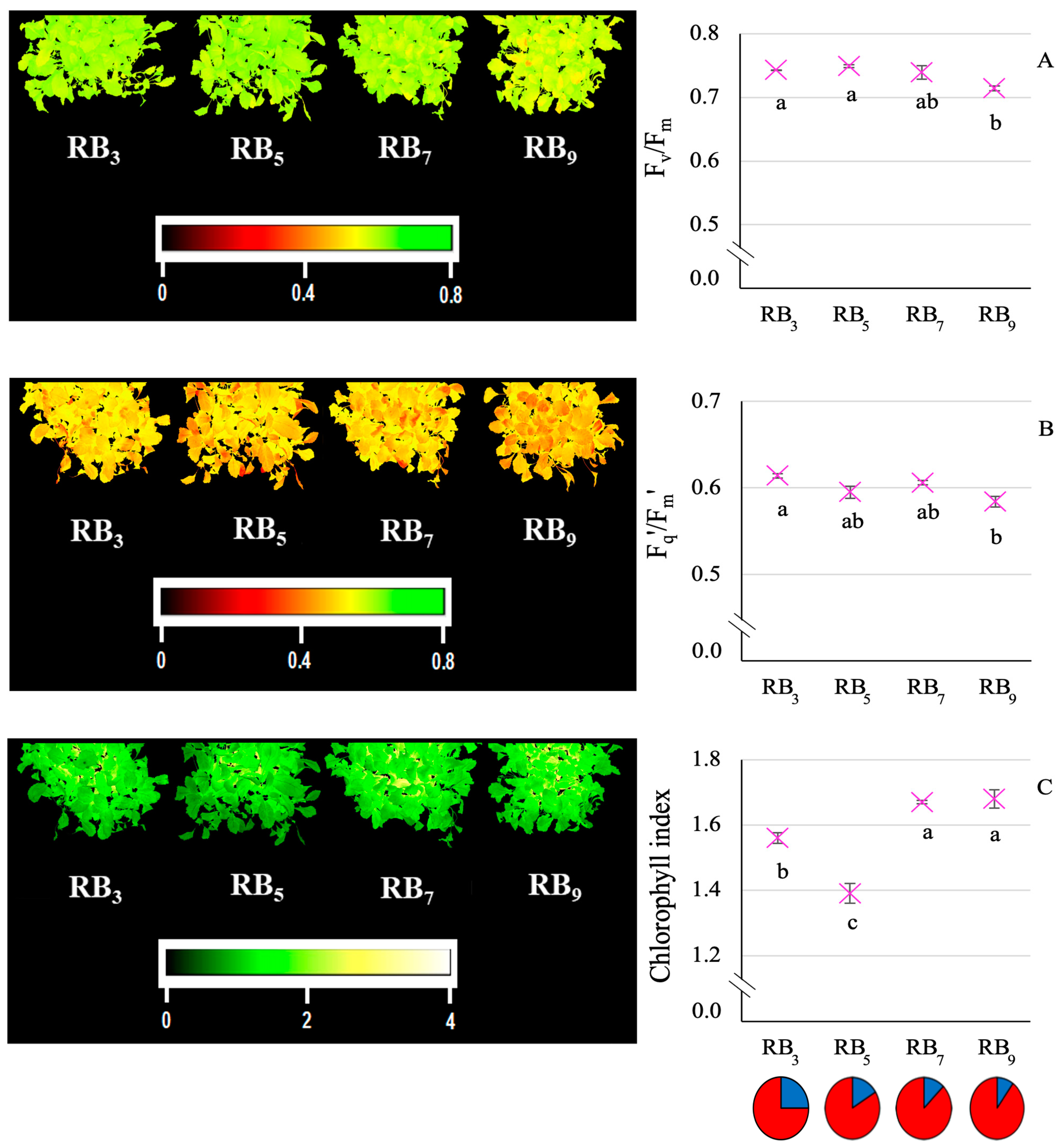
3.2. Physiological Results: Multispectral Data
4. Discussion
4.1. RB5 Allows for Optimized Baby-Leaf Kale Growth and Resource Use Efficiency
4.2. Photosynthetic Response to Rising RB Ratio in Kale
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Orsini, F.; Kahane, R.; Nono-Womdim, R.; Gianquinto, G. Urban Agriculture in the Developing World: A Review. Agron. Sustain. Dev. 2013, 33, 695–720. [Google Scholar] [CrossRef]
- FAO. Land Statistics 2001–2022-Global, Regional and Country Trends; FAOSTAT Analytical Briefs n.88; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Xi, L.; Zhang, M.; Zhang, L.; Lew, T.T.S.; Lam, Y.M. Novel Materials for Urban Farming. Adv. Mater. 2022, 34, 2105009. [Google Scholar] [CrossRef] [PubMed]
- Avgoustaki, D.D.; Xydis, G. How Energy Innovation in Indoor Vertical Farming Can Improve Food Security, Sustainability, and Food Safety? In Advances in Food Security and Sustainability; Cohen, M.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, pp. 1–51. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future Food-Production Systems: Vertical Farming and Controlled-Environment Agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Van Delden, S.H.; SharathKumar, M.; Butturini, M.; Graamans, L.J.A.; Heuvelink, E.; Kacira, M.; Kaiser, E.; Klamer, R.S.; Klerkx, L.; Kootstra, G.; et al. Current Status and Future Challenges in Implementing and Upscaling Vertical Farming Systems. Nat. Food 2021, 2, 944–956. [Google Scholar] [CrossRef]
- Bustamante, M.J. AgTech and the City: The Case of Vertical Farming and Shaping a Market for Urban-Produced Food. In Managing Digital Transformation; Andersson, P., Movin, S., Mähring, M., Teigland, R., Wennberg, K., Eds.; Stockholm School of Economics Institute for Research: Stockholm, Sweden, 2018; pp. 281–297. [Google Scholar]
- Martin, M.; Weidner, T.; Gullström, C. Estimating the Potential of Building Integration and Regional Synergies to Improve the Environmental Performance of Urban Vertical Farming. Front. Sustain. Food Syst. 2022, 6, 849304. [Google Scholar] [CrossRef]
- Thomaier, S.; Specht, K.; Henckel, D.; Dierich, A.; Siebert, R.; Freisinger, U.B.; Sawicka, M. Farming in and on Urban Buildings: Present Practice and Specific Novelties of Zero-Acreage Farming (ZFarming). Renew. Agric. Food Syst. 2015, 30, 43–54. [Google Scholar] [CrossRef]
- Zhang, H.; Asutosh, A.; Hu, W. Implementing Vertical Farming at University Scale to Promote Sustainable Communities: A Feasibility Analysis. Sustainability 2018, 10, 4429. [Google Scholar] [CrossRef]
- Paucek, I.; Appolloni, E.; Pennisi, G.; Quaini, S.; Gianquinto, G.; Orsini, F. LED Lighting Systems for Horticulture: Business Growth and Global Distribution. Sustainability 2020, 12, 7516. [Google Scholar] [CrossRef]
- Fankhauser, C.; Chory, J. Light Control of Plant Development. Annu. Rev. Cell Dev. Biol. 1997, 13, 203–229. [Google Scholar] [CrossRef]
- Appolloni, E.; Pennisi, G.; Zauli, I.; Carotti, L.; Paucek, I.; Quaini, S.; Orsini, F.; Gianquinto, G. Beyond Vegetables: Effects of Indoor LED Light on Specialized Metabolite Biosynthesis in Medicinal and Aromatic Plants, Edible Flowers, and Microgreens. J. Sci. Food Agric. 2022, 102, 472–487. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Stamford, J.D.; Stevens, J.; Mullineaux, P.M.; Lawson, T. LED Lighting: A Grower’s Guide to Light Spectra. HortScience 2023, 58, 180–196. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Role of the Plant Factory With Artificial Lighting (PFAL) in Urban Areas. In Plant Factory. An Indoor Vertical Farming System for Efficient Quality Food Production; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: Amsterdam, The Netherlands, 2016; pp. 7–33. [Google Scholar] [CrossRef]
- Yokoyama, R. Energy Consumption and Heat Sources in Plant Factories. In Plant Factory Using Artificial Light. Adapting to Environmental Disruption and Clues to Agricultural Innovation; Anpo, M., Fukuda, H., Wada, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 177–184. [Google Scholar] [CrossRef]
- Penuela, J.; Ben, C.; Boldyrev, S.; Gentzbittel, L.; Ouerdane, H. The Indoor Agriculture Industry: A Promising Player in Demand Response Services. Appl. Energy 2024, 372, 123756. [Google Scholar] [CrossRef]
- Saini, R.K.; Ko, E.Y.; Keum, Y.-S. Minimally Processed Ready-to-Eat Baby-Leaf Vegetables: Production, Processing, Storage, Microbial Safety, and Nutritional Potential. Food Rev. Int. 2017, 33, 644–663. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Luna, M.C.; Selma, M.V.; Tudela, J.A.; Abad, J.; Gil, M.I. Baby-Leaf and Multi-Leaf of Green and Red Lettuces Are Suitable Raw Materials for the Fresh-Cut Industry. Postharvest Biol. Technol. 2012, 63, 1–10. [Google Scholar] [CrossRef]
- Carotti, L.; Pistillo, A.; Zauli, I.; Meneghello, D.; Martin, M.; Pennisi, G.; Gianquinto, G.; Orsini, F. Improving Water Use Efficiency in Vertical Farming: Effects of Growing Systems, Far-Red Radiation and Planting Density on Lettuce Cultivation. Agric. Water Manag. 2023, 285, 108365. [Google Scholar] [CrossRef]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated Digital Image Analysis for Rapid and Accurate Measurement of Leaf Area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between Leaf Chlorophyll Content and Spectral Reflectance and Algorithms for Non-Destructive Chlorophyll Assessment in Higher Plant Leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the Role of Red:Blue LED Lights on Resource Use Efficiency and Nutritional Properties of Indoor Grown Sweet Basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource Use Efficiency of Indoor Lettuce (Lactuca sativa L.) Cultivation as Affected by Red:Blue Ratio Provided by LED Lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef]
- Martínez-Moreno, A.; Frutos-Tortosa, A.; Diaz-Mula, H.; Mestre, T.C.; Martínez, V. Effect of the Intensity and Spectral Quality of LED Light on Growth and Quality of Spinach Indoors. Horticulturae 2024, 10, 411. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, J.; Song, S.; Su, W.; Liu, H. Growth, Nutritional Quality and Health-Promoting Compounds in Chinese Kale Grown under Different Ratios of Red:Blue LED Lights. Agronomy 2020, 10, 1248. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Laužikė, K.; Duchovskis, P.; Małek, S. Effect of Different Ratios of Blue and Red LED Light on Brassicaceae Microgreens under a Controlled Environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef]
- Naznin, M.; Lefsrud, M.; Gravel, V.; Azad, M. Blue Light Added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Niu, G. Plant Responses to Light. In Plant Factory. An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 153–166. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, G.; Liang, W.; Wu, D.; Sun, Q.; Hao, Y. Effects of LED Light Quality on Broccoli Microgreens Plant Growth and Nutrient Accumulation. J. Plant Growth Regul. 2024, 43, 3481–3489. [Google Scholar] [CrossRef]
- Kang, W.H.; Park, J.S.; Park, K.S.; Son, J.E. Leaf Photosynthetic Rate, Growth, and Morphology of Lettuce under Different Fractions of Red, Blue, and Green Light from Light-Emitting Diodes (LEDs). Hortic. Environ. Biotechnol. 2016, 57, 573–579. [Google Scholar] [CrossRef]
- Franklin, K.A. Shade Avoidance. New Phytol. 2008, 179, 930–944. [Google Scholar] [CrossRef]
- Cope, K.R.; Snowden, M.C.; Bugbee, B. Photobiological Interactions of Blue Light and Photosynthetic Photon Flux: Effects of Monochromatic and Broad-Spectrum Light Sources. Photochem. Photobiol. 2014, 90, 574–584. [Google Scholar] [CrossRef]
- Clavijo-Herrera, J.; Van Santen, E.; Gómez, C. Growth, Water-Use Efficiency, Stomatal Conductance, and Nitrogen Uptake of Two Lettuce Cultivars Grown under Different Percentages of Blue and Red Light. Horticulturae 2018, 4, 16. [Google Scholar] [CrossRef]
- Izzo, L.G.; Mickens, M.A.; Aronne, G.; Gómez, C. Spectral Effects of Blue and Red Light on Growth, Anatomy, and Physiology of Lettuce. Physiol. Plant. 2021, 172, 2191–2202. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Physiological Responses of Cucumber Seedlings under Different Blue and Red Photon Flux Ratios Using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Pennisi, G.; Sanyé-Mengual, E.; Orsini, F.; Crepaldi, A.; Nicola, S.; Ochoa, J.; Fernandez, J.; Gianquinto, G. Modelling Environmental Burdens of Indoor-Grown Vegetables and Herbs as Affected by Red and Blue LED Lighting. Sustainability 2019, 11, 4063. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Peng, Y.; Arkebauer, T.J.; Suyker, A.E. Productivity, Absorbed Photosynthetically Active Radiation, and Light Use Efficiency in Crops: Implications for Remote Sensing of Crop Primary Production. J. Plant Physiol. 2015, 177, 100–109. [Google Scholar] [CrossRef]
- Whitelam, G.C.; Halliday, K.J. Light and Plant Development. Annual Plant Reviews Volume 30; Blackwell Publishing: Oxford, UK, 2007. [Google Scholar] [CrossRef][Green Version]
- Assmann, S.M.; Shimazaki, K. The Multisensory Guard Cell. Stomatal Responses to Blue Light and Abscisic Acid1. Plant Physiol. 1999, 119, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, D.N.; Klein, J.D. LED Pre-Exposure Shines a New Light on Drought Tolerance Complexity in Lettuce (Lactuca sativa) and Rocket (Eruca sativa). Environ. Exp. Bot. 2020, 180, 104240. [Google Scholar] [CrossRef]
- Chaves, M.M. How Plants Cope with Water Stress in the Field? Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.C.F. Experiments on the Effect of Different Intensities of Desiccation on Bryophyte Survival, Using Chlorophyll Fluorescence as an Index of Recovery. J. Bryol. 2003, 25, 201–210. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, J.; Zhang, Z.; Wan, X.; Hu, J. Analyzing the Effect of Light on Lettuce Fv/Fm and Growth by Machine Learning. Sci. Hortic. 2022, 306, 111444. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, X.; Gao, L.; Chen, Q.; Qu, M. Blue Light Is More Essential than Red Light for Maintaining the Activities of Photosystem II and I and Photosynthetic Electron Transport Capacity in Cucumber Leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef]
- Rajagopal, S.; Murthy, S.D.S.; Mohanty, P. Effect of Ultraviolet-B Radiation on Intact Cells of the Cyanobacterium Spirulina Platensis: Characterization of the Alterations in the Thylakoid Membranes. J. Photochem. Photobiol. B Biol. 2000, 54, 61–66. [Google Scholar] [CrossRef]
- Melis, A. Photosystem-II Damage and Repair Cycle in Chloroplasts: What Modulates the Rate of Photodamage in Vivo? Trends Plant Sci. 1999, 4, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Henriques, F.S. Leaf Chlorophyll Fluorescence: Background and Fundamentals for Plant Biologists. Bot. Rev. 2009, 75, 249–270. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, X.M.; Cui, J. The Importance of Blue Light for Leaf Area Expansion, Development of Photosynthetic Apparatus, and Chloroplast Ultrastructure of Cucumis Sativus Grown under Weak Light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and Physiological Properties of Indoor Cultivated Lettuce in Response to Additional Far-Red Light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Jayalath, T.C.; Van Iersel, M.W. Canopy Size and Light Use Efficiency Explain Growth Differences between Lettuce and Mizuna in Vertical Farms. Plants 2021, 10, 704. [Google Scholar] [CrossRef]
- Gianquinto, G.; Fecondini, M.; Mezzetti, M.; Orsini, F. Steering Nitrogen Fertilisation by Means of Portable Chlorophyll Meter Reduces Nitrogen Input and Improves Quality of Fertigated Cantaloupe (Cucumis melo L. Var. Cantalupensis Naud.). J. Sci. Food Agric. 2010, 90, 482–493. [Google Scholar] [CrossRef]
| Lighting Treatments | Total Lighting Energy Use (kWh m−2 cycle−1) |
|---|---|
| RB3 | 56.8 |
| RB5 | 55.5 |
| RB7 | 54.2 |
| RB9 | 54.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zauli, I.; Rossini, E.; Pennisi, G.; Martin, M.; Crepaldi, A.; Gianquinto, G.; Orsini, F. The Perfect Match: Testing the Effect of Increasing Red and Blue Ratio on Baby-Leaf Kale Growth, Yield and Physiology. Horticulturae 2024, 10, 1134. https://doi.org/10.3390/horticulturae10111134
Zauli I, Rossini E, Pennisi G, Martin M, Crepaldi A, Gianquinto G, Orsini F. The Perfect Match: Testing the Effect of Increasing Red and Blue Ratio on Baby-Leaf Kale Growth, Yield and Physiology. Horticulturae. 2024; 10(11):1134. https://doi.org/10.3390/horticulturae10111134
Chicago/Turabian StyleZauli, Ilaria, Ernesto Rossini, Giuseppina Pennisi, Michael Martin, Andrea Crepaldi, Giorgio Gianquinto, and Francesco Orsini. 2024. "The Perfect Match: Testing the Effect of Increasing Red and Blue Ratio on Baby-Leaf Kale Growth, Yield and Physiology" Horticulturae 10, no. 11: 1134. https://doi.org/10.3390/horticulturae10111134
APA StyleZauli, I., Rossini, E., Pennisi, G., Martin, M., Crepaldi, A., Gianquinto, G., & Orsini, F. (2024). The Perfect Match: Testing the Effect of Increasing Red and Blue Ratio on Baby-Leaf Kale Growth, Yield and Physiology. Horticulturae, 10(11), 1134. https://doi.org/10.3390/horticulturae10111134









