Conventional and Nano-Zinc Foliar Spray Strategies to Improve the Physico-Chemical Properties and Nutritional and Antioxidant Compounds of Timor Mango Fruits under Abiotic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Materials
2.2. Measurements and Determinations
2.2.1. Fruit Physical Measurements
2.2.2. Chemical Properties and Nutritional and Antioxidant Compound Measurements
2.3. Statistical Analysis
3. Results
3.1. Physical Characteristics
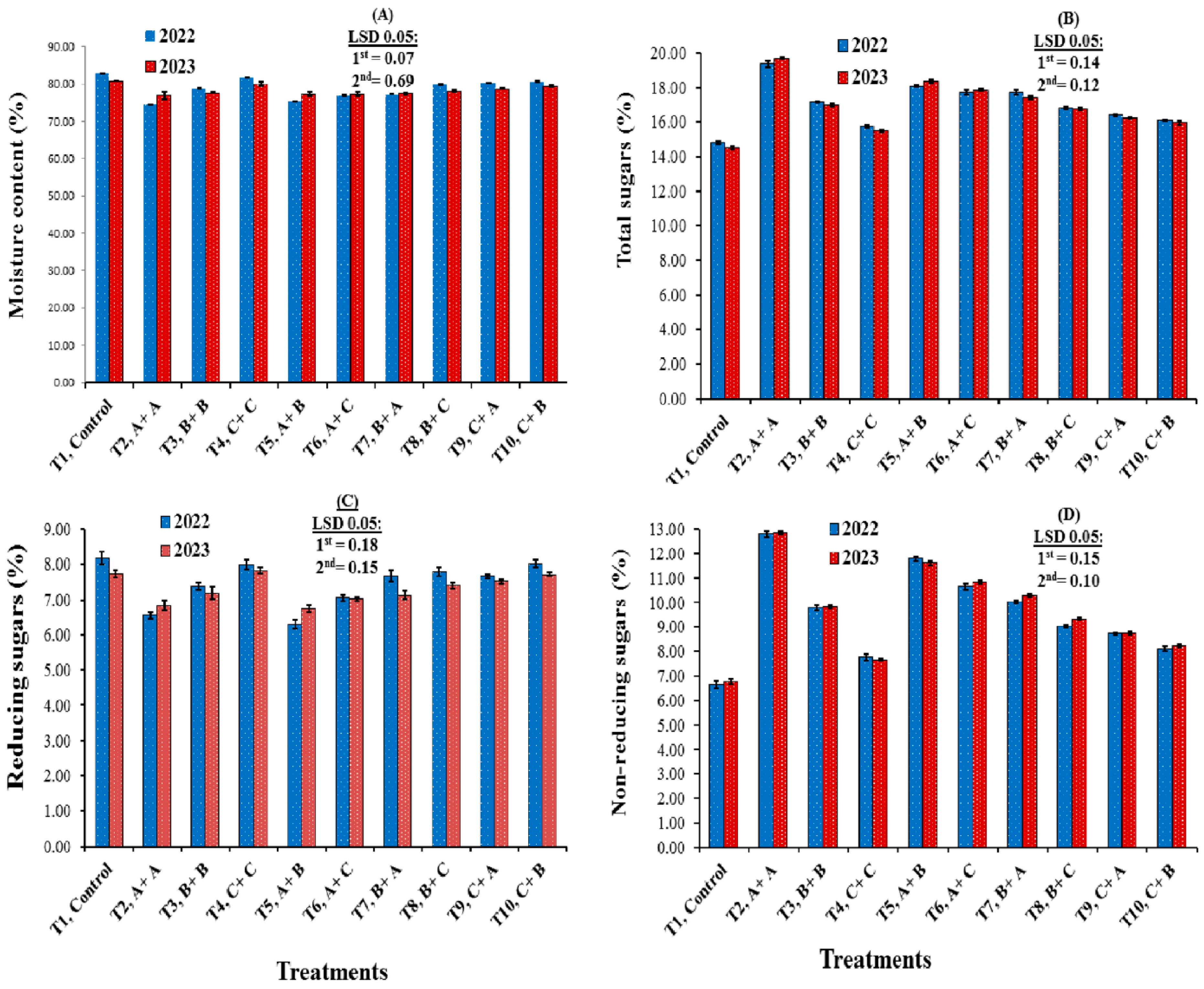
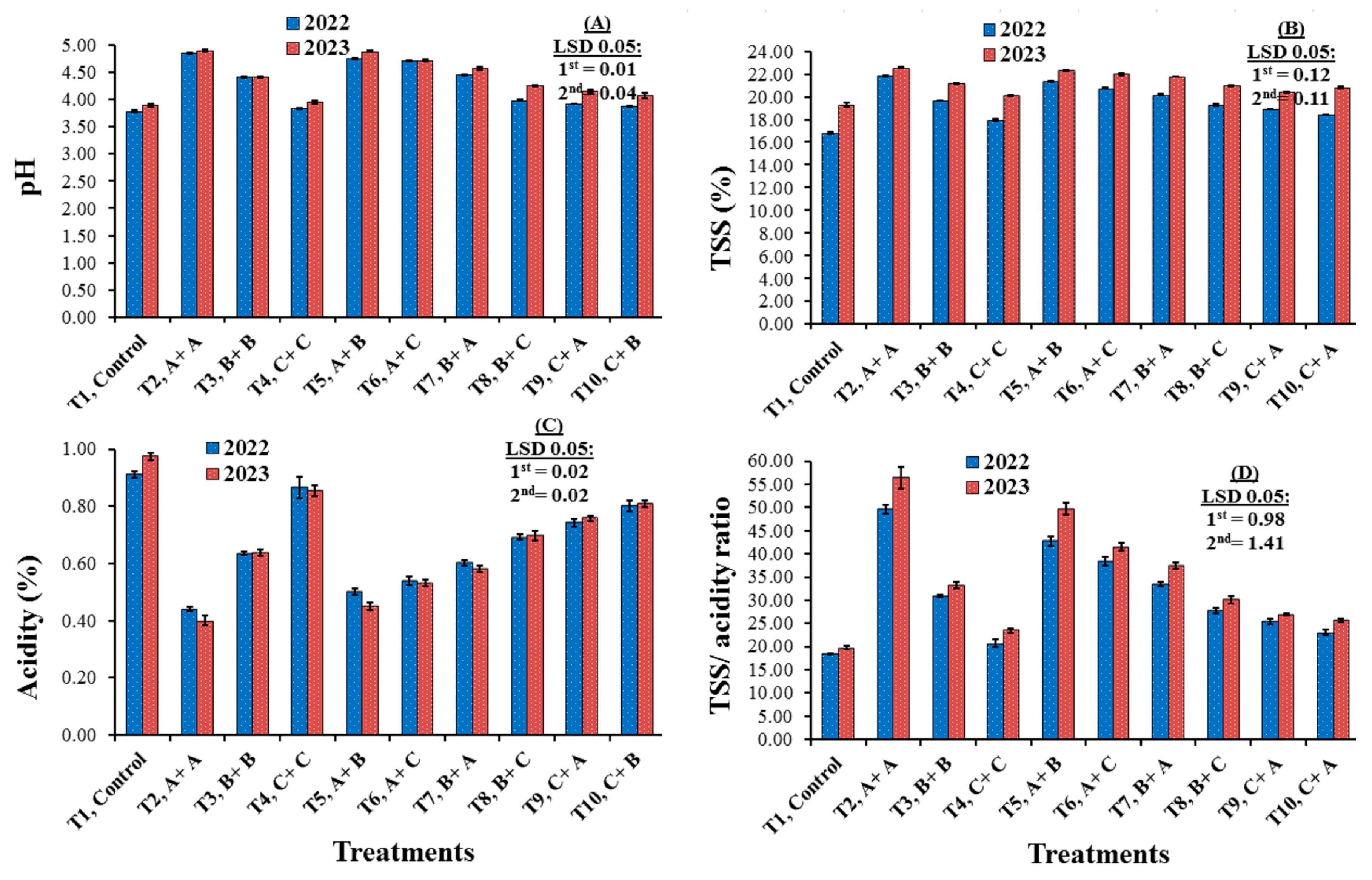
3.2. Chemical Characteristics
3.3. Mineral Contents
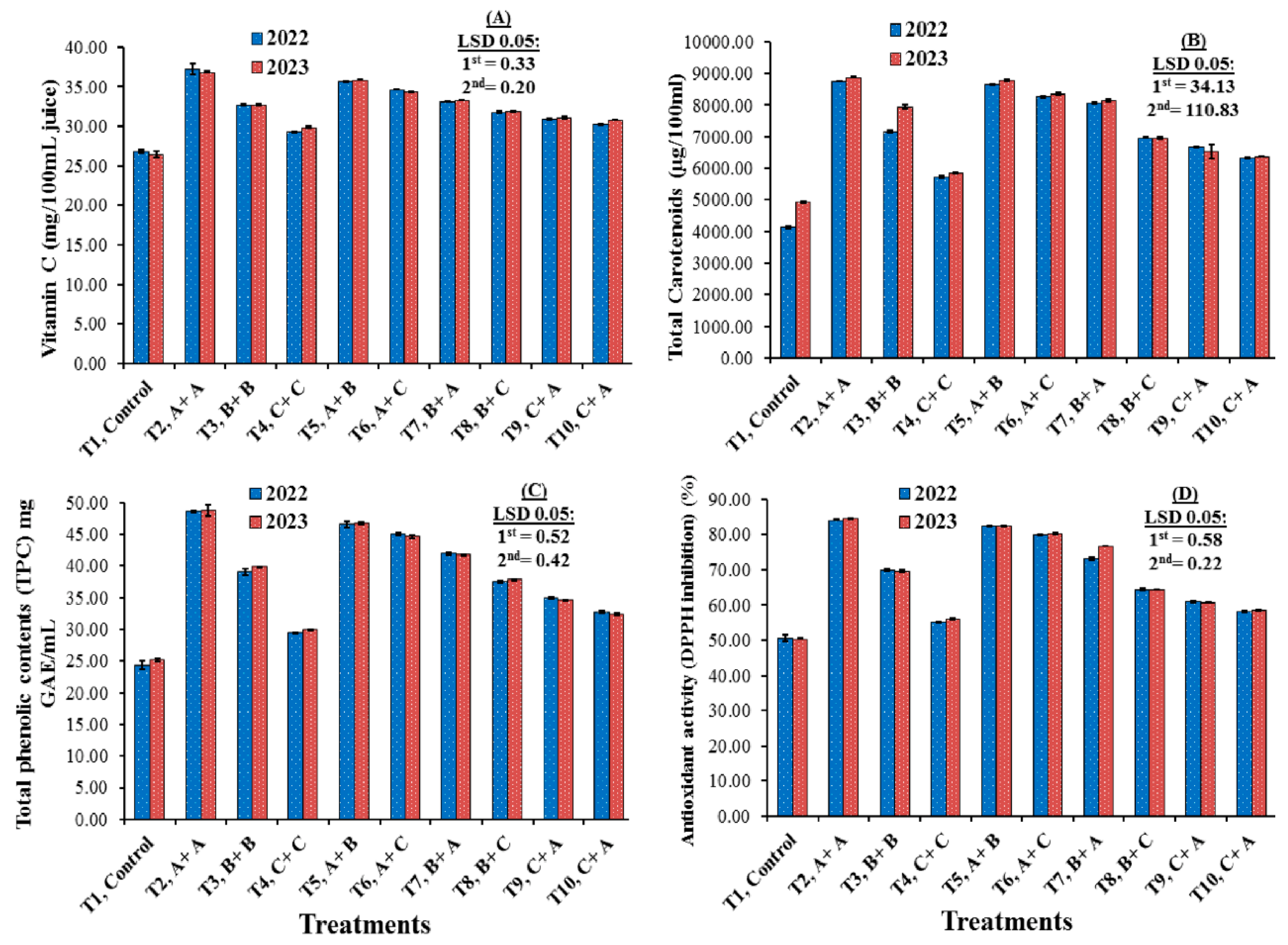
3.4. Antioxidant Compounds and Activities
4. Discussion
4.1. Physical Characteristics
4.2. Chemical Characteristics
4.3. Mineral Contents
4.4. Antioxidant Compounds and Activities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, T.; Luo, C.; Wan, W.; Liang, R.; Lu, T.; Li, Y.; Xie, F.; Chen, C.; Li, X.; Xie, X.; et al. Effects of thidiazuron on the quality and storage properties of mango fruit during postharvest. Food Qual. Saf. 2024, 8, fyad047. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, E.; Herrero-Lodares, C.; Sánchez-Prieto, M.; Olmedilla-Alonso, B.; Sánchez-Moreno, C.; De Ancos, B. Sustainable extraction methods of carotenoids from mango (Mangifera indica L. ‘Kent’) pulp: Ultrasound assisted extraction and green solvents. Food Chem. 2024, 450, 139253. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.A.; Kiloes, A.M.; Abdul Aziz, A.; Joyce, D.C. Impact of factors contributing to internal disorders of mango (Mangifera indica L.) fruit-A systematic literature review. Sci. Horticult. 2024, 331, 113150. [Google Scholar] [CrossRef]
- Alebidi, A.; Abdel-Sattar, M.; Mostafa, L.Y.; Hamad, A.S.A.; Rihan, H.Z. Synergistic effects of applying potassium nitrate spray with putrescine on productivity and fruit quality of mango Trees cv. Ewais. Agronomy 2023, 13, 2717. [Google Scholar] [CrossRef]
- Li, Z.; Bi, X.; Dai, Y.; Ren, R. Enhancing mango anthracnose control and quality maintenance through chitosan and iturin A coating. LWT-Food Sci. Technol. 2024, 198, 115955. [Google Scholar] [CrossRef]
- Sferrazzo, G.; Palmeri, R.; Restuccia, C.; Parafati, L.; Siracusa, L.; Spampinato, M.; Carota, G.; Distefano, A.; Di Rosa, M.; Tomasello, B.; et al. Mangifera indica L. Leaves as a Potential Food Source of Phenolic Compounds with Biological Activity. Antioxidants 2022, 11, 1313. [Google Scholar] [CrossRef]
- Abdel-Sattar, M.; Al-Obeed, R.S.; Makhasha, E.; Mostafa, L.Y.; Abdelzaher, R.A.E.; Rihan, H.Z. Improving mangoes’ productivity and crop water productivity by 24-epibrassinosteroids and hydrogen peroxide under deficit irrigation. Agric. Water Manag. 2024, 298, 108860. [Google Scholar] [CrossRef]
- Sivankalyani, V.; Feygenberg, O.; Diskin, S.; Wright, B.; Alkan, N. Increased anthocyanin and flavonoids in mango fruit peel are associated with cold and pathogen resistance. Postharv. Biol. Technol. 2016, 111, 132–139. [Google Scholar] [CrossRef]
- Makhasha, E.; Al-Obeed, R.S.; Abdel-Sattar, M. Responses of Nutritional Status and Productivity of Timor Mango Trees to Foliar Spray of Conventional and/or Nano Zinc. Sustainability 2024, 16, 6060. [Google Scholar] [CrossRef]
- Abd El-Hamid, H.T.; Hafiz, M.A.; Wenlong, W.; Qiaomin, L. Detection of Environmental Degradation in Jazan Region on the Red Sea, KSA, Using Mathematical Treatments of Remote Sensing Data. Remote Sens. Earth Syst. Sci. 2019, 2, 183–196. [Google Scholar] [CrossRef]
- Sattar, A.; Wang, X.; Ul-Allah, S.; Sher, A.; Ijaz, M.; Irfan, M.; Abbas, T.; Hussain, S.; Nawaz, F.; Al-Hashimi, A.; et al. Foliar application of zinc improves morpho-physiological and antioxidant defense mechanisms, and agronomic grain biofortification of wheat (Triticum aestivum L.) under water stress. Saudi J. Biol. Sci. 2022, 29, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.S.; Husna, M.T.; Uddin, M.N.; Hossain, M.A.; Sarwar, A.K.M.G.; Ali, O.M.; Abdel Latef, A.A.H.; Hossain, A. Heat Stress at Early Reproductive Stage Differentially Alters Several Physiological and Biochemical Traits of Three Tomato Cultivars. Horticulturae 2021, 7, 330. [Google Scholar] [CrossRef]
- Camejo, D.; Rodriguez, P.; Morales, M.A.; Dell’amico, J.M.; Torrecillas, A.; Alarcon, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.; Sami Ullah Qadir, S.U.; Alyemeni, M.N.; Ahmad, P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gen expression in Solanum lycopersicum. 3Biotech 2020, 10, 208. [Google Scholar] [CrossRef]
- Thounaojam, T.C.; Thounaojam, T.M.; Upadhyaya, H. Role of zinc oxide nanoparticles in mediating abiotic stress responses in plant. Nanobiotechnol. Plant Prot. 2021, 323–337. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.; Sun, J.; Guo, S. Exogenous salicylic acid increases the heat tolerance in tomato (Solanum lycopersicum L.) by enhancing photosynthetic efficiency and improving antioxidant defense through scavenging of reactive oxygen species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar] [CrossRef]
- Zhou, R.; Kong, L.; Yu, X.; Ottosen, C.-O.; Zhao, T.; Jiang, F.; Wu, Z. Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol. Plant. 2019, 41, 20. [Google Scholar] [CrossRef]
- Kim, H.; Seomun, S.; Yoon, Y.; Jang, G. Jasmonic acid in plant abiotic stress tolerance and interaction with abscisic acid. Agronomy 2021, 11, 1886. [Google Scholar] [CrossRef]
- Khan, M.; Ahmed, N. Sustainable management of mango nutrition for better yield and quality. Cercet. Agron. Mold. 2020, 4, 473–501. [Google Scholar] [CrossRef]
- Zagzog, O.A.; Gad, M. Improving growth, flowering, fruiting and resistance of malformation of mango trees using nano-zinc. Middle East J. Agric. Res. 2017, 6, 673–681. [Google Scholar]
- Suman, M.; Sangma, P.D.; Singh, D. Role of Micronutrients (Fe, Zn, B, Cu, Mg, Mn and Mo) in Fruit Crops. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 3240–3250. [Google Scholar] [CrossRef]
- Tsonko, T.; Lidon, F. Zinc in plants—An overview. Emir. J. Food Agric. 2012, 24, 322–333. [Google Scholar]
- Faran, M.; Farooq, M.; Rehman, A.; Nawaz, A.; Saleem, M.K.; Ali, N.; Siddique, K.H. High cintrinsic seed Zn concentration improves abiotic stress tolerance in wheat. Plant Soil 2019, 437, 195–213. [Google Scholar] [CrossRef]
- Wang, F.; Adams, C.A.; Shi, Z.; Sun, Y. Combined effects of ZnO NPs and Cd on sweet sorghum as influenced by an arbuscular mycorrhizal fungus. Chemosphere 2018, 209, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Peck, A.W.; McDonald, G.K. Adequate zinc nutrition alleviates the adverse effects of heat stress in bread wheat. Plant Soil. 2010, 337, 355–374. [Google Scholar] [CrossRef]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant Nutrition: An Effective Way to Alleviate Abiotic Stress in Agricultural Crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef]
- Bashir, K.; Rasheed, S.; Kobayashi, T.; Seki, M.; Nishizawa, N.K. Regulating subcellular metal homeostasis: The key to crop improvement. Front. Plant Sci. 2016, 2004, 1192. [Google Scholar] [CrossRef]
- Jalal, A.; Júnior, E.F.; Teixeira Filho, M.C.M. Interaction of Zinc Mineral Nutrition and Plant Growth-Promoting Bacteria in Tropical Agricultural Systems: A Review. Plants 2024, 13, 571. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Surya Ulhas, R.; Sudheer, W.N.; Banadka, A.; Nagella, P.; Aldaej, M.I.; Rezk, A.A.-S.; Shehata, W.F.; Almaghasla, M.I. The Role of Nanoparticles in Response of Plants to Abiotic Stress at Physiological, Biochemical, and Molecular Levels. Plants 2023, 12, 292. [Google Scholar] [CrossRef]
- Khalid, M.F.; Khan, R.I.; Jawaid, M.Z.; Shafqat, W.; Hussain, S.; Ahmed, T.; Rizwan, M.; Ercisli, S.; Pop, O.L.; Marc, R.A. Nanoparticles: The Plant Saviour under Abiotic Stresses. Nanomaterials 2022, 12, 3915. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; The Iowa State University Press: Ames, IA, USA, 1990; p. 593. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Method of Analysis, 21st ed.; AOAC: Washington, DC, USA, 2019. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugars. Anal. Chem. 1972, 31, 426–428. [Google Scholar] [CrossRef]
- Jacob, M.B. The Chemical Analysis of Foods and Food Products, 3rd ed.; D. Van Nastrand Co Inc.: Princeton, NJ, USA, 1958. [Google Scholar]
- Donohue, S.J.; Aho, D.W. Determination of P, K, Ca, Mg, Mn, Fe, Al, B, Cu, and Zn in plant tissue by inductively coupled plasma (ICP) emission spectroscopy. In Plant Analysis Reference Procedures for the Southern Region of the United States; Plank, C.O., Ed.; Southern Cooperative Series Bulletin: Canberra, Australia, 1992; Volume 368, pp. 37–40. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analyses: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Ranganna, S. Handbook of Analysis and Quality Control for Fruits and Vegetables Products, 2nd ed.; Tata McGraw-Hill Publishing Company Limited: New Delhi, India, 1999. [Google Scholar]
- Lee, S.K.; Mbwambo, Z.H.; Chung, H.; Luyengi, L.; Gamez, E.J.; Mehta, R.G.; Kinghorn, A.D.; Pezzuto, J.M. Evaluation of the antioxidant potential of natural products. Comb. Chem. High Throughput Screen. 1998, 1, 35–46. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1984; 680p. [Google Scholar]
- SAS Institute Inc. The SAS System for Windows, version 9.13; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Farina, V.; Gentile, C.; Sortino, G.; Gianguzzi, G.; Palazzolo, E.; Mazzaglia, A. Tree-Ripe Mango Fruit: Physicochemical Characterization, Antioxidant Properties and Sensory Profile of Six Mediterranean-Grown Cultivars. Agronomy 2020, 10, 884. [Google Scholar] [CrossRef]
- Sivakumar, D.; Jiang, Y.; Yahia, E.M. Maintaining mango (Mangifera indica L.) fruit quality during the export chain. Food Res. Int. 2011, 44, 1254–1263. [Google Scholar] [CrossRef]
- Abdel-Sattar, M.; Al-Obeed, R.S.; Aboukarima, A.M.; Górnik, K.; Eshra, D.H. Improvement of the Physico-Chemical Properties, Nutritional, and Antioxidant Compounds of Pomegranate Fruit cv. ‘Wonderful’ Using Integrated Fertilization. Horticulturae 2023, 9, 195. [Google Scholar] [CrossRef]
- Normand, F.; Lauri, P.E.; Legave, J.M. Climate Change and Its Probable Effects on Mango Production and Cultivation. Acta Hort. ISHS 2015, 1075, 21–31. [Google Scholar] [CrossRef]
- Habibpourmehraban, F.; Wu, Y.; Wu, J.X.; Hamzelou, S.; Masoomi-Aladizgeh, F.; Kamath, K.S.; Amirkhani, A.; Atwell, B.J.; Haynes, P.A. Multiple Abiotic Stresses Applied Simultaneously Elicit Distinct Responses in Two Contrasting Rice Cultivars. Int. J. Mol. Sci. 2022, 23, 1739. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mittler, R.; Blumwald, E.; Zandalinas, S.I. Developing climate-resilient crops: Improving plant tolerance to stress combination. Plant J. 2022, 109, 373–389. [Google Scholar] [CrossRef]
- Gerbaud, P. Mango. FruiTrop 2012, 197, 9–54. [Google Scholar]
- Christensen, J.H.; Hewitson, B.; Busuioc, A.; Chen, A.; Gao, X.; Held, I.; Jones, R.; Kolli, R.K.; Kwon, W.T.; Laprise, R.; et al. Regional climate projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Halaji, B.; Haghighi, M.; Amiri, A.; Kappel, N. Efects of Potassium and Nanocapsule of Potassium on Pepper Growth and Physiological Changes in High-Temperature Stress. J. Soil Sci. Plant Nutr. 2023, 23, 6317–6330. [Google Scholar] [CrossRef]
- Romero, P.; Botia, P.; Keller, M. Hydraulics and gas exchange recover more rapidly from severe drought stress in small pot-grown grapevines than in field-grown plants. J. Plant Physiol. 2017, 216, 58–73. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K. Impact of micronutrients in mitigation of abiotic stresses in soils and plants—A progressive step toward crop security and nutritional quality. Adv. Agron. 2022, 173, 1–78. [Google Scholar] [CrossRef]
- Beak, D.; Cha, J.Y.; Kang, S.; Park, B.; Lee, H.J.; Hong, H. The Arabidopsis a zinc finger domain protein ARS1 is essential for seed germination and ROS homeostasis in response to ABA and oxidative stress. Front. Plant Sci. 2015, 6, 963. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Hassan, M.U.; Nawaz, M.; Mahmood, A.; Shah, A.A.; Shah, A.N.; Muhammad, F.; Batool, M.; Rasheed, A.; Jaremko, M.; Abdelsalam, N.R.; et al. The role of zinc to mitigate heavy metals toxicity in crops. Front. Environ. Sci. 2022, 10, 990223. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Guoqin, H. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- El-Gioushy, S.F.; Sami, R.; Al-Mushhin, A.A.M.; Abou El-Ghit, H.M.S.; Gawish, M.; Ismail, K.A.; Zewail, R.M.Y. Foliar Application of ZnSO4 and CuSO4 Affects the Growth, Productivity, and Fruit Quality of Washington Navel Orange Trees (Citrus sinensis L.) Osbeck. Horticulturae 2021, 7, 233. [Google Scholar] [CrossRef]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef]
- Almohammedi, O.M.; Sekhi, Y.S.; Ismail, M.H. A Review of Nano fertilization and its role on growth, yield and quality characteristics of fruit trees. Tikrit J. Agric. Sci. 2023, 23, 158–167. [Google Scholar] [CrossRef]
- Zhang, Q.; Ackah, M.; Wang, M.; Amoako, F.K.; Shi, Y.; Wang, L.; Dari, L.; Li, J.; Jin, X.; Jiang, Z.; et al. The impact of boron nutrient supply in mulberry (Morus alba) response to metabolomics, enzyme activities, and physiological parameters. Plant Physiol. Biochem. 2023, 200, 107649. [Google Scholar] [CrossRef] [PubMed]
- Mahdieh, M.; Sangi, M.R.; Bamdad, F.; Ghanem, A. Effect of seed and foliar application of nano-zinc oxide, zinc chelate, and zinc sulphate rates on yield and growth of pinto bean (Phaseolus vulgaris) cultivars. J. Plant Nutr. 2018, 41, 2401–2412. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Saleem, M.; Khan, T.A.; Hayat, S. Zinc as a Versatile Element in Plants: An Overview on Its Uptake, Translocation, Assimilatory Roles, Deficiency and Toxicity Symptoms. In Microbial Biofertilizers and Micronutrient Availability; Khan, S.T., Malik, A., Eds.; Springer Nature: Cham, Switzerland, 2022; pp. 137–158. [Google Scholar] [CrossRef]
- Maklad, T.N.; El-Sawwah, O.A.O.; Nassar, S.A. Effect of Calcium, Zinc and Boron Treatments on Flowering, Yield and Fruit Quality of Mango Ewais Cultivar. J. Plant Prod. Mansoura Univ. 2020, 11, 1463–1468. [Google Scholar] [CrossRef]
- Beede, R.H.; Brown, P.H.; Kallsen, C.; Weinbaum, S.A. Diagnosing and correcting nutrient deficiencies. In Pistachio Production Manual, 4th ed.; University of California: Oakland, CA, USA, 2005; pp. 147–157. [Google Scholar]
- Öztürk, B.; Özkan, Y.; Yıldız, K.; Özkan, A.; Kılıç, K.; Uçar, M.; Karakaya, M.; Karakaya, O. The Role of Pre-Harvest Aminoethoxyvinylglycine Treatments on Fruit Quality of Braeburn Apple during Cold Storage. In Proceedings of the International Mesopotamia Agriculture Congress, Diyarbakır, Turkey, 22–25 September 2014. [Google Scholar]
- Suganya, A.; Saravanan, A.; Manivannan, N. Role of zinc nutrition for increasing zinc availability, uptake, yield and quality of maize (Zea mays L.) grains: An Overview. Comm. Soil Sci. Plant. Anal. 2020, 51, 2001–2021. [Google Scholar] [CrossRef]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in habanero peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef]
- Okoth, D.; Sila, E.; Onyango, C.; Owino, W.; Musyimi, S.; Mathooko, F.M. Evaluation of chemical and nutritional quality attributes of selected mango varieties at three stages of ripeness, grown in lower Eastern province of Kenya—Part 2. J. Anim. Plant Sci. 2013, 17, 2619–2630. [Google Scholar]
- Montaño-Herrera, A.; Santiago-Saenz, Y.O.; López-Palestina, C.U.; Cadenas-Pliego, G.; Pinedo-Guerrero, Z.H.; Hernández-Fuentes, A.D.; Pinedo-Espinoza, J.M. Effects of Edaphic Fertilization and Foliar Application of Se and Zn Nanoparticles on Yield and Bioactive Compounds in Malus domestica L. Horticulturae 2022, 8, 542. [Google Scholar] [CrossRef]
- Sindhu, S.S.; Sharma, R.; Sindhu, S.; Phour, M. Plant Nutrient Management Through Inoculation of Zinc-Solubilizing Bacteria for Sustainable Agriculture. Biofertil. Sustain. Agric. Environ. 2019, 55, 173–201. [Google Scholar]
- Kour, R.; Singh, M.; Gill, P.P.S.; Jawandha, S.K. Ripening quality of Dusehri mango in relation to harvest time. J. Food Sci. Technol. 2018, 55, 2395–2400. [Google Scholar] [CrossRef] [PubMed]
- Hatung, W. Plant response to stress: Abscisic acid fluxes. In Encyclopedia of Plant and Crop Science; Goodman, R.M., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 540–640. [Google Scholar]
- Yahia, E.M.; De Jesu’s Ornelas-Paz, J.; Gonzalez-Aguilar, G.A. Nutritional and health-promoting properties of tropical and subtropical fruits. In Postharvest Biology and Technology of Tropical and Subtropical Fruits. Volume 1. Fundamental Issues; Woodhead Publishing: Sawston, UK, 2011; pp. 21–78. [Google Scholar]
- USDA. National nutrient database for standard reference. U.S. Department of Agriculture, Agricultural Research Service. 2018. Available online: http://www.ars.usda.gov/nutrientdata (accessed on 15 July 2023).
- Yahia, E.M.; Ornelas-Paz, J.J.; Brecht, J.K.; Garcı´a-Solı´s, P.; Maldonado Celis, M.E. The contribution of mango fruit (Mangifera indica L.) to human nutrition and health. Arab. J. Chem. 2023, 16, 104860. [Google Scholar] [CrossRef]
- Verma, N.; Kaushal, P.; Gahalot, D.; Sidhu, A.K.; Kaur, K. Mechanistic Aspect of Zinc Oxide Nanoparticles in Alleviating Abiotic Stress in Plants—A Sustainable Agriculture Approach. BioNanoScience 2023. [Google Scholar] [CrossRef]
- Heikens, A.; Panaullah, G.M.; Meharg, A.A. Arsenic behaviour from groundwater and soil to crops: Impacts on agriculture and food safety. Rev. Environ. Contam. Toxicol. 2007, 189, 43–87. [Google Scholar] [CrossRef]
- Flora, S.J.S. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxidative Med. Cell. Longev. 2009, 2, 191–206. [Google Scholar] [CrossRef]
- Sogi, D.; Siddiq, M.; Roidoung, S.; Dolan, K. Total phenolics, carotenoids, ascorbic acid, and antioxidant properties of fresh cut mango (Mangifera indica L., cv. Tommy Atkins) as affected by infrared heat treatment. J. Food Sci. 2012, 77, C1197–C1202. [Google Scholar] [CrossRef]
- Siddiq, M.; Sogi, D.; Dolan, K. Antioxidant properties, total phenolics, and quality of fresh-cut ‘Tommy Atkins’ mangoes as affected by different pre-treatments. LWT-Food Sci. Technol. 2013, 53, 156–162. [Google Scholar] [CrossRef]
- Almeida, M.M.; de Sousa, P.H.; Arriaga, Â.M.; do Prado, G.M.; de Carvalho Magalhães, C.E.; Maia, G.A.; de Lemos, T.L. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res. Int. 2011, 44, 2155–2159. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Sendra, E.; Sayas-Barberá, E.; Pérez-Álvarez, J.A. Antioxidant properties of pomegranate (Punica granatum L.) bagasses obtained as co-product in the juice extraction. Food Res. Int. 2011, 44, 1217–1223. [Google Scholar] [CrossRef]
- Pierson, J.T.; Dietzgen, R.G.; Shaw, P.N.; Roberts-Thomson, S.J.; Monteith, G.R.; Gidley, M.J. Major Australian tropical fruits biodiversity: Bioactive compounds and their bioactivities. Mol. Nutr. Food Res. 2011, 56, 357–387. [Google Scholar] [CrossRef]
- Rufino, M.d.S.M.; Alves, R.E.; De Brito, E.S.; Pérez-Jiménezc, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Stanislas, G.; Douraguia, E.; Marie-Paule Gonthier, M.-P. Evaluation of nutritional and antioxidant properties of the tropical fruits banana, litchi, mango, papaya, passion fruit and pineapple cultivated in Réunion French Island. Food Chem. 2016, 212, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Barrios, D.L.; Cruz-Alvarez, O.; Sánchez-Chavez, E.; Juan, P. Ciscomani-Larios, J.P. Effect of foliar application of zinc on annual productivity, foliar nutrients, bioactive compounds and oxidative metabolism in pecan. Folia Hort. 2023, 35, 179–192. [Google Scholar] [CrossRef]
- Du, J.; Tang, J.; Xu, S.; Ge, J.; Dong, Y.; Li, H.; Jin, M. ZnO nanoparticles: Recent advances in ecotoxicity and risk assessment. Drug Chem. Toxicol. 2018, 43, 322–333. [Google Scholar] [CrossRef]
- Hezaveh, T.A.; Rahmani, F.; Alipour, H.; Pourakbar, L. Effects of Foliar Application of ZnO Nanoparticles on Secondary Metabolite and Micro-Elements of Camelina (Camelina sativa L.) Under Salinity Stress. J. Stress Physiol. Biochem. 2020, 16, 54–69. [Google Scholar]
- Liu, L.; Nian, H.; Lian, T. Plants and rhizospheric environment: Affected by zinc oxide nanoparticles (ZnO NPs). A review. Plant Physiol. Biochem. 2022, 185, 91–100. [Google Scholar] [CrossRef]
- Sadati, S.Y.; Godehkahriz, S.J.; Ebadi, A.; Sedghi, M. Zinc oxide nanoparticles enhance drought tolerance in wheat via physio-biochemical changes and stress genes expression. Iran. J. Biotechnol. 2022, 20, e3027. [Google Scholar] [CrossRef]
| Months | Relative Humidity (%) | Minimum Temperature (°C) | Maximum Temperature (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Season | Season | Season | ||||||||||
| 2012 | 2013 | 2022 | 2023 | 2012 | 2013 | 2022 | 2023 | 2012 | 2013 | 2022 | 2023 | |
| January | 55.94 | 50.15 | 64.38 | 53.85 | 13.57 | 15.25 | 15.05 | 18.94 | 33.28 | 32.31 | 30.48 | 30.20 |
| February | 42.75 | 43.00 | 55.44 | 48.40 | 16.11 | 15.67 | 16.90 | 19.76 | 34.78 | 36.61 | 33.62 | 31.95 |
| March | 43.56 | 43.19 | 44.25 | 48.33 | 18.12 | 19.89 | 19.21 | 21.49 | 37.64 | 36.91 | 37.22 | 32.74 |
| April | 46.75 | 47.15 | 29.62 | 45.96 | 22.17 | 20.74 | 20.31 | 24.40 | 37.10 | 36.84 | 40.80 | 35.29 |
| May | 33.44 | 32.00 | 25.75 | 48.39 | 24.87 | 22.82 | 23.63 | 26.06 | 43.51 | 42.42 | 43.54 | 35.90 |
| June | 27.38 | 25.31 | 31.94 | 49.90 | 25.68 | 25.02 | 25.55 | 26.12 | 44.21 | 42.70 | 44.44 | 37.09 |
| July | 41.69 | 41.31 | 54.94 | 49.14 | 25.12 | 25.03 | 22.90 | 27.36 | 44.19 | 40.21 | 38.65 | 37.43 |
| August | 47.06 | 55.31 | 71.81 | 47.17 | 24.82 | 22.24 | 22.27 | 27.54 | 43.28 | 39.67 | 39.01 | 38.28 |
| September | 33.35 | 35.00 | 46.75 | 33.26 | 23.37 | 24.26 | 22.49 | 27.37 | 41.87 | 42.05 | 40.86 | 41.28 |
| October | 30.12 | 33.12 | 32.56 | 42.43 | 20.21 | 20.94 | 20.33 | 23.63 | 39.58 | 39.61 | 39.98 | 36.60 |
| November | 41.62 | 48.12 | 42.44 | 52.56 | 19.57 | 16.64 | 18.86 | 21.17 | 36.48 | 36.91 | 36.40 | 32.87 |
| December | 53.94 | 48.44 | 49.25 | 39.94 | 17.17 | 14.10 | 15.87 | 18.59 | 32.49 | 33.87 | 33.65 | 31.92 |
| Average | 41.47 | 41.84 | 45.76 | 46.61 | 20.9 | 20.22 | 20.28 | 23.54 | 39.03 | 38.34 | 38.22 | 35.13 |
| pH | CaCo3 % | EC dS/m | O.M | Textural class | Sand % | Silt % | Clay % | ||
| 8.03 | 16.70 | 1.71 | 1.56 | Sandy loam | 43.92 | 49.27 | 6.81 | ||
| Nutrients (mg/kg) | Soluble anions (meq/L) | Soluble cations (meq/L) | |||||||
| P | K | N | HCO3− | Cl− | SO42− | Ca2+ | Mg2+ | Na+ | K+ |
| 45.4 | 71.8 | 56.02 | 6.00 | 5.1 | 6.20 | 6.00 | 3.00 | 5.95 | 2.09 |
| Season | Treatment | Fruit Weight (g) | Seed Weight (g) | Peel Weight (g) | Pulp Weight (g) | Pulp/Fruit Ratio | Fruit Length (cm) | Fruit Width (cm) | Fruit Shape Index | Fruit Firmness (Ib/Inch2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2022 | T, Control | 296.00 j | 25.70 j | 23.50 j | 246.80 h | 0.83 a | 9.46 j | 6.06 j | 1.56 e | 16.14 j |
| T2, A + A | 394.00 a | 54.55 a | 41.30 a | 298.15 a | 0.76 i | 13.80 a | 7.93 a | 1.74 a | 18.90 a | |
| T3, B + B | 359.25 e | 40.65 e | 32.03 e | 286.58 d | 0.80 e | 11.42 e | 7.33 e | 1.56 e | 17.49 e | |
| T4, C + C | 316.50 i | 28.85 i | 25.10 i | 262.55 g | 0.83 b | 10.16 i | 6.55 i | 1.55 ef | 16.45 i | |
| T5, A + B | 384.75 b | 51.55 b | 39.35 b | 293.85 b | 0.76 h | 13.20 b | 7.73 b | 1.71 b | 18.43 b | |
| T6, A + C | 376.75 c | 47.00 c | 36.88 c | 292.88 bc | 0.78 g | 12.76 c | 7.57 c | 1.69 c | 18.19 c | |
| T7, B + A | 367.25 d | 44.60 d | 33.93 d | 288.73 cd | 0.79 f | 12.15 d | 7.41 d | 1.64 d | 17.92 d | |
| T8, B + C | 347.25 f | 37.65 f | 29.08 f | 280.53 e | 0.81 dc | 11.21 f | 7.24 f | 1.55 ef | 17.23 f | |
| T9, C + A | 337.75 g | 34.25 g | 28.03 g | 275.48 f | 0.82 c | 10.93 g | 7.14 g | 1.54 f | 17.04 g | |
| T10, C + B | 330.00 h | 31.70 h | 26.45 h | 271.85 f | 0.82 bc | 10.47 h | 6.93 h | 1.51 g | 16.80 h | |
| LSD0.05 | 3.86 | 1.13 | 0.42 | 4.22 | 0.006 | 0.09 | 0.05 | 0.017 | 0.05 | |
| 2023 | T, Control | 294.50 j | 22.60 j | 24.13 j | 247.78 g | 0.84 a | 9.54 j | 6.02 j | 1.59 de | 16.14 j |
| T2, A + A | 386.60 a | 41.00 a | 53.10 a | 292.50 a | 0.76 h | 13.88 a | 7.95 a | 1.75 a | 18.90 a | |
| T3, B + B | 345.75 e | 32.60 e | 37.35 e | 275.80 c | 0.80 e | 11.69 e | 7.35 e | 1.59 d | 17.49 e | |
| T4, C + C | 312.20 i | 24.28 i | 25.95 i | 261.98 f | 0.84 a | 10.20 i | 6.51 i | 1.57 e | 16.45 i | |
| T5, A + B | 372.70 b | 39.40 b | 51.03 b | 282.28 b | 0.76 h | 13.17 b | 7.79 b | 1.69 b | 18.43 b | |
| T6, A + C | 361.25 c | 37.40 c | 47.10 c | 276.75 c | 0.77 g | 12.83 c | 7.54 c | 1.70 b | 18.19 c | |
| T7, B + A | 355.40 d | 34.15 d | 41.90 d | 279.35 bc | 0.79 f | 12.23 d | 7.44 d | 1.64 c | 17.92 d | |
| T8, B + C | 337.15 f | 30.05 f | 35.60 f | 271.50 d | 0.81 d | 11.20 f | 7.25 f | 1.55 f | 17.23 f | |
| T9, C + A | 328.15 g | 28.55 g | 31.40 g | 268.20 de | 0.82 c | 10.93 g | 7.13 g | 1.53 f | 17.04 g | |
| T10, C + B | 321.75 h | 26.50 h | 28.40 h | 266.85 e | 0.83 b | 10.62 h | 6.89 h | 1.54 f | 16.80 h | |
| LSD0.05 | 3.63 | 0.35 | 0.68 | 3.75 | 0.004 | 0.09 | 0.06 | 0.020 | 0.05 |
| Treatment | N (g/kg) | P (g/kg) | K (g/kg) | Ca (mg/kg) | Mg (mg/kg) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| T, Control | 6.89 g | 6.77 j | 0.78 j | 0.77 j | 8.18 j | 8.24 j | 0.52 j | 0.54 j | 0.85 j | 0.86 j |
| T2, A + A | 11.13 a | 11.57 a | 1.65 a | 1.77 a | 12.28 a | 12.12 a | 1.62 a | 1.66 a | 1.95 a | 1.85 a |
| T3, B + B | 9.48 c | 9.86 e | 1.28 e | 1.39 e | 10.77 e | 10.86 e | 1.16 e | 1.18 e | 1.36 e | 1.45 e |
| T4, C + C | 8.32 f | 8.55 i | 0.83 i | 0.93 i | 8.96 i | 9.17 i | 0.66 i | 0.65 i | 0.94 i | 1.06 i |
| T5, A + B | 10.77 b | 11.17 b | 1.51 b | 1.64 b | 11.72 b | 11.84 b | 1.53 b | 1.54 b | 1.85 b | 1.78 b |
| T6, A + C | 10.54 b | 10.85 c | 1.47 c | 1.59 c | 11.49 c | 11.68 c | 1.45 c | 1.45 c | 1.74 c | 1.65 c |
| T7, B + A | 9.66 c | 10.27 d | 1.33 d | 1.44 d | 10.93 d | 11.17 d | 1.24 d | 1.27 d | 1.55 d | 1.56 d |
| T8, B + C | 9.18 d | 9.45 f | 1.14 f | 1.21 f | 10.34 f | 10.45 f | 0.99 f | 1.07 f | 1.26 f | 1.36 f |
| T9, C + A | 8.99 d | 9.14 g | 1.03 g | 1.18 g | 9.87 g | 9.93 g | 0.85 g | 0.93 g | 1.16 g | 1.27 g |
| T10, C + B | 8.62 e | 8.84 h | 1.00 h | 1.03 h | 9.36 h | 9.57 h | 0.76 h | 0.76 h | 1.05 h | 1.16 h |
| LSD0.05 | 0.26 | 0.05 | 0.19 | 0.01 | 0.03 | 0.05 | 0.04 | 0.03 | 0.04 | 0.04 |
| Season | Treatment | Cu | Fe | Mn | Zn | Na | B | Ni |
|---|---|---|---|---|---|---|---|---|
| 2022 | T, Control | 9.62 j | 15.22 j | 6.21 j | 5.25 j | 0.28 j | 1.75 j | 0.67 j |
| T2, A + A | 18.61 a | 34.83 a | 12.19 a | 14.35 a | 1.26 a | 2.34 a | 1.55 a | |
| T3, B + B | 14.37 e | 25.76 e | 9.81 e | 10.80 e | 0.84 e | 2.09 e | 1.14 e | |
| T4, C + C | 10.24 i | 17.25 i | 7.83 i | 6.91 i | 0.43 i | 1.87 i | 0.73 i | |
| T5, A + B | 17.84 b | 32.56 b | 11.29 b | 13.68 b | 1.18 b | 2.28 b | 1.45 b | |
| T6, A + C | 16.42 c | 30.84 c | 10.73 c | 12.84 c | 1.05 c | 2.18 c | 1.35 c | |
| T7, B + A | 15.86 d | 28.47 d | 10.10 d | 11.50 d | 0.95 d | 2.12 d | 1.26 d | |
| T8, B + C | 13.84 f | 23.45 f | 9.58 f | 9.63 f | 0.77 f | 2.02 f | 1.03 f | |
| T9, C + A | 12.28 g | 21.39 g | 8.96 g | 8.50 g | 0.63 g | 1.98 g | 0.96 g | |
| T10, C + B | 11.88 h | 19.69 h | 8.27 h | 7.45 h | 0.56 h | 1.92 h | 0.84 h | |
| LSD0.05 | 0.11 | 0.21 | 0.08 | 0.23 | 0.07 | 0.02 | 0.03 | |
| 2023 | T, Control | 9.66 j | 16.82 j | 6.40 j | 5.77 j | 0.35 j | 1.72 j | 0.67 j |
| T2, A + A | 19.83 a | 36.56 a | 12.91 a | 15.14 a | 1.37 a | 2.31 a | 1.53 a | |
| T3, B + B | 14.61 f | 26.76 f | 9.65 f | 10.89 f | 0.84 f | 1.92 f | 1.05 f | |
| T4, C + C | 11.71 i | 19.31 i | 7.68 i | 7.44 i | 0.55 i | 1.79 i | 0.75 i | |
| T5, A + B | 18.58 b | 34.45 b | 11.69 b | 14.41 b | 1.27 b | 2.24 b | 1.47 b | |
| T6, A + C | 17.27 c | 32.20 c | 10.65 c | 13.91 c | 1.15 c | 2.16 c | 1.36 c | |
| T7, B + A | 16.08 d | 30.84 d | 10.25 d | 12.78 d | 1.04 d | 2.05 d | 1.25 d | |
| T8, B + C | 13.42 g | 24.88 g | 9.04 g | 9.38 g | 0.73 g | 1.88 g | 0.96 g | |
| T9, C + A | 15.93 e | 28.17 e | 9.84 e | 11.30 e | 0.93 e | 1.98 e | 1.16 e | |
| T10, C + B | 12.16 h | 21.70 h | 8.50 h | 8.23 h | 0.65 h | 1.82 h | 0.86 h | |
| LSD0.05 | 0.07 | 0.22 | 0.08 | 0.07 | 0.04 | 0.03 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Sattar, M.; Makhasha, E.; Al-Obeed, R.S. Conventional and Nano-Zinc Foliar Spray Strategies to Improve the Physico-Chemical Properties and Nutritional and Antioxidant Compounds of Timor Mango Fruits under Abiotic Stress. Horticulturae 2024, 10, 1096. https://doi.org/10.3390/horticulturae10101096
Abdel-Sattar M, Makhasha E, Al-Obeed RS. Conventional and Nano-Zinc Foliar Spray Strategies to Improve the Physico-Chemical Properties and Nutritional and Antioxidant Compounds of Timor Mango Fruits under Abiotic Stress. Horticulturae. 2024; 10(10):1096. https://doi.org/10.3390/horticulturae10101096
Chicago/Turabian StyleAbdel-Sattar, Mahmoud, Essa Makhasha, and Rashid S. Al-Obeed. 2024. "Conventional and Nano-Zinc Foliar Spray Strategies to Improve the Physico-Chemical Properties and Nutritional and Antioxidant Compounds of Timor Mango Fruits under Abiotic Stress" Horticulturae 10, no. 10: 1096. https://doi.org/10.3390/horticulturae10101096
APA StyleAbdel-Sattar, M., Makhasha, E., & Al-Obeed, R. S. (2024). Conventional and Nano-Zinc Foliar Spray Strategies to Improve the Physico-Chemical Properties and Nutritional and Antioxidant Compounds of Timor Mango Fruits under Abiotic Stress. Horticulturae, 10(10), 1096. https://doi.org/10.3390/horticulturae10101096






