The Role of Protein Post-Translational Modifications in Fruit Ripening
Abstract
1. Introduction
2. Overview of Fruit Ripening
3. Regulation of Fruit Ripening by Protein Post-Translational Modifications (PTMs)
3.1. Ubiquitination and Fruit Ripening
3.2. Phosphorylation and Fruit Ripening
3.3. Redox Regulation and Fruit Ripening
3.4. Glycosylation and Fruit Ripening
3.5. Other Types of Modifications and Fruit Ripening
4. Crosstalk Regulation of Fruit Ripening among PTMs and Other Regulations
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karlova, R.; Chapman, N.; David, K.; Angenent, G.C.; Seymour, G.B.; de Maagd, R.A. Transcriptional control of fleshy fruit development and ripening. J. Exp. Bot. 2014, 65, 4527–4541. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Gallusci, P.; Lang, Z. Fruit development and epigenetic modifications. New Phytol. 2020, 228, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, J.; Nguyen, C.; Ampofo, B.; Zhong, S.; Fei, Z. The epigenome and transcriptional dynamics of fruit ripening. Annu. Rev. Plant Biol. 2017, 68, 61–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Qin, G.; Tian, S. Regulatory network of fruit ripening: Current understanding and future challenges. New Phytol. 2020, 228, 1219–1226. [Google Scholar] [CrossRef]
- Forlani, S.; Masiero, S.; Mizzotti, C. Fruit ripening: The role of hormones, cell wall modifications, and their relationship with pathogens. J. Exp. Bot. 2019, 70, 2993–3006. [Google Scholar] [CrossRef]
- Brumos, J. Gene regulation in climacteric fruit ripening. Curr. Opin. Plant Biol. 2021, 63, 102042. [Google Scholar] [CrossRef]
- Kapoor, L.; Simkin, A.J.; George, P.D.C.; Siva, R. Fruit ripening: Dynamics and integrated analysis of carotenoids and anthocyanins. BMC Plant Biol. 2022, 22, 27. [Google Scholar] [CrossRef]
- Duran-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar signaling during fruit ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef]
- Li, S.; Chen, K.; Grierson, D. Molecular and hormonal mechanisms regulating fleshy fruit ripening. Cells 2021, 10, 1136. [Google Scholar] [CrossRef]
- Cao, H.; Chen, J.; Yue, M.; Xu, C.; Jian, W.; Liu, Y.; Song, B.; Gao, Y.; Cheng, Y.; Li, Z. Tomato transcriptional repressor MYB70 directly regulates ethylene-dependent fruit ripening. Plant. J. 2020, 104, 1568–1581. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Fan, D.; Wang, W.; Hao, Q.; Jia, W. Do non-climacteric fruits share a common ripening mechanism of hormonal regulation? Front. Plant Sci. 2022, 13, 923484. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Huang, Y.; Shen, Y. The physiological and molecular mechanism of abscisic acid in regulation of fleshy fruit ripening. Front. Plant Sci. 2020, 11, 619953. [Google Scholar] [CrossRef]
- Wang, R.; Wang, G. Protein modification and autophagy activation. Adv. Exp. Med. Biol. 2019, 1206, 237–259. [Google Scholar] [CrossRef]
- Vu, L.D.; Gevaert, K.; De Smet, I. Protein language: Post-translational modifications talking to each other. Trends Plant Sci. 2018, 23, 1068–1080. [Google Scholar] [CrossRef]
- Lin, H.; Caroll, K.S. Introduction: Posttranslational protein modification. Chem. Rev. 2018, 118, 887–888. [Google Scholar] [CrossRef]
- Shu, K.; Yang, W. E3 ubiquitin ligases: Ubiquitous actors in plant development and abiotic stress responses. Plant Cell Physiol. 2017, 58, 1461–1476. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, W.; Cai, J.; Zhang, Y.; Qin, G.; Tian, S. Tomato nuclear proteome reveals the involvement of specific E2 ubiquitin-conjugating enzymes in fruit ripening. Genome Biol. 2014, 15, 548. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Shan, W.; Yang, T.W.; Wu, C.J.; Liu, X.C.; Chen, J.Y.; Lu, W.J.; Li, Z.G.; Deng, W.; Kuang, J.F. MaMYB4 is a negative regulator and a substrate of RING-type E3 ligasesMaBRG2/3 in controlling banana fruit ripening. Plant J. 2022, 110, 1651–1669. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Meng, X.; Guo, D.; Yang, S.; Zhang, G.; Liang, Z. Grapevine U-Box E3 ubiquitin ligase VlPUB38 negatively regulates fruit ripening by facilitating abscisic-aldehyde oxidase degradation. Plant Cell Physiol. 2021, 61, 2043–2054. [Google Scholar] [CrossRef]
- Tang, X.; Miao, M.; Niu, X.; Zhang, D.; Cao, X.; Jin, X.; Zhu, Y.; Fan, Y.; Wang, H.; Liu, Y.; et al. Ubiquitin-conjugated degradation of golden 2-like transcription factor is mediated by CUL4-DDB1-based E3 ligase complex in tomato. New Phytol. 2016, 209, 1028–1039. [Google Scholar] [CrossRef]
- Wei, Y.; Jin, J.; Xu, Y.; Liu, W.; Yang, G.; Bu, H.; Li, T.; Wang, A. Ethylene-activated MdPUB24 mediates ubiquitination of MdBEL7 to promote chlorophyll degradation in apple fruit. Plant J. 2021, 108, 169–182. [Google Scholar] [CrossRef] [PubMed]
- An, J.P.; Wang, X.F.; Zhang, X.W.; Xu, H.F.; Bi, S.Q.; You, C.X.; Hao, Y.J. An apple MYB transcription factor regulates cold tolerance and anthocyanin accumulation and undergoes MIEL1-mediated degradation. Plant Biotechnol. J. 2020, 18, 337–353. [Google Scholar] [CrossRef]
- Hu, D.G.; Yu, J.Q.; Han, P.L.; Xie, X.B.; Sun, C.H.; Zhang, Q.Y.; Wang, J.H.; Hao, Y.J. The regulatory module MdPUB29-MdbHLH3 connects ethylene biosynthesis with fruit quality in apple. New Phytol. 2019, 221, 1966–1982. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, Y.; Chen, J.; Lakshmanan, P.; Kuang, J.; Lu, W.; Shan, W. MaNAC029 modulates ethylene biosynthesis and fruit quality and undergoes MaXB3-mediated proteasomal degradation during banana ripening. J. Adv. Res. 2023, 53, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wei, W.; Yang, Y.; Wu, C.; Chen, J.; Lu, W.; Kuang, J.; Shan, W. E3 ligase MaNIP1 degradation of NON-YELLOW COLORING1 at high temperature inhibits banana degreening. Plant Physiol. 2023, 192, 1969–1981. [Google Scholar] [CrossRef]
- Wei, W.; Luo, Q.; Yang, Y.; Wu, C.; Kuang, J.; Chen, J.; Lu, W.; Shan, W. E3 ubiquitin ligase MaRZF1 modulates high temperature-induced green ripening of banana by degrading MaSGR1. Plant Cell Environ. 2024, 47, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Tian, Y.; Hu, Z.; Zhang, L.; Tang, B.; Wang, Y.; Li, J.; Chen, G. Novel translational and phosphorylation modification regulation mechanisms of tomato (Solanum lycopersicum) fruit ripening revealed by integrative proteomics and phosphoproteomics. Int. J. Mol. Sci. 2021, 22, 11782. [Google Scholar] [CrossRef]
- Zhang, M.; Su, J.; Zhang, Y.; Xu, J.; Zhang, S. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr. Opin. Plant Biol. 2018, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Zhang, Z.; Herold, S.; Kassem, S.; Wu, X.N.; Schulze, W.X. Phosphorylation site motifs in plant protein kinases and their substrates. Methods Mol Biol 2021, 2358, 1–16. [Google Scholar] [CrossRef]
- Liang, B.; Sun, Y.; Wang, J.; Zheng, Y.; Zhang, W.; Xu, Y.; Li, Q.; Leng, P. Tomato protein phosphatase 2C influences the onset of fruit ripening and fruit glossiness. J. Exp. Bot. 2021, 72, 2403–2418. [Google Scholar] [CrossRef]
- Tatsuki, M.; Mori, H. Phosphorylation of tomato 1-aminocyclopropane-1-carboxylic acid synthase, LE-ACS2, at the C-terminal region. J. Biol. Chem. 2001, 276, 28051–28057. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Roy, S.; Sengupta, D.N. A Ser/Thr protein kinase phosphorylates MA-ACS1 (Musa acuminata 1-aminocyclopropane-1-carboxylic acid synthase 1) during banana fruit ripening. Planta 2012, 236, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.J.; Shan, W.; Liu, X.C.; Zhu, L.S.; Wei, W.; Yang, Y.Y.; Guo, Y.F.; Bouzayen, M.; Chen, J.Y.; Lu, W.J.; et al. Phosphorylation of transcription factor bZIP21 by MAP kinase MPK6-3 enhances banana fruit ripening. Plant Physiol. 2022, 188, 1665–1685. [Google Scholar] [CrossRef]
- Wu, C.; Shan, W.; Liang, S.; Zhu, L.; Guo, Y.; Chen, J.; Lu, W.; Li, Q.; Su, X.; Kuang, J. MaMPK2 enhances MabZIP93-mediated transcriptional activation of cell wall modifying genes during banana fruit ripening. Plant Mol. Biol. 2019, 101, 113–127. [Google Scholar] [CrossRef]
- Jia, H.F.; Lu, D.; Sun, J.H.; Li, C.L.; Xing, Y.; Qin, L.; Shen, Y.Y. Type 2C protein phosphatase ABI1 is a negative regulator of strawberry fruit ripening. J. Exp. Bot. 2013, 64, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, T.; Li, Q.; Xu, C.; Tian, J.; Wang, Y.; Zhang, X.; Xu, X.; Han, Z.; Wu, T. Phosphorylation of MdERF17 by MdMPK4 promotes apple fruit peel degreening during light/dark transitions. Plant Cell 2022, 34, 1980–2000. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Li, X.; Wang, W.; Li, T.; Dai, Z.; Chen, Y.; Zhang, K.; Zhu, H.; Mao, W.; Feng, Q.; et al. SnRK2 subfamily I protein kinases regulate ethylene biosynthesis by phosphorylating HB transcription factors to induce ACO1 expression in apple. New Phytol. 2022, 234, 1262–1277. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Z.; Zhu, H.; Jiang, Y.; Jiang, G.; Qu, H. Energy homeostasis mediated by the LcSnRK1alpha-LcbZIP1/3 signaling pathway modulates litchi fruit senescence. Plant J. 2022, 111, 698–712. [Google Scholar] [CrossRef]
- Huang, F.; Sun, M.; Yao, Z.; Zhou, J.; Bai, Q.; Chen, X.; Huang, Y.; Shen, Y. Protein kinase SnRK2.6 phosphorylates transcription factor bHLH3 to regulate anthocyanin homeostasis during strawberry fruit ripening. J. Exp. Bot. 2024, 75, 5627–5640. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zuo, J.; Ma, Q.; Zhang, X.; Xu, Y.; Ding, S.; Wang, J.; Luo, Q.; Li, Y.; Wu, C.; et al. Phytosulfokine promotes fruit ripening and quality via phosphorylation of transcription factor DREB2F in tomato. Plant Physiol. 2024, 194, 2739–2754. [Google Scholar] [CrossRef]
- Li, T.; Liu, Z.; Lv, T.; Xu, Y.; Wei, Y.; Liu, W.; Wei, Y.; Liu, L.; Wang, A. Phosphorylation of MdCYTOKININ RESPONSE FACTOR4 suppresses ethylene biosynthesis during apple fruit ripening. Plant Physiol. 2023, 191, 694–714. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjarvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, M.; Papolu, P.K.; Satish, L.; Vinod, K.K.; Wei, Q.; Sharma, A.; Emamverdian, A.; Zou, L.H.; Zhou, M. Redox status of the plant cell determines epigenetic modifications under abiotic stress conditions and during developmental processes. J. Adv. Res. 2022, 42, 99–116. [Google Scholar] [CrossRef]
- Jiang, G.; Zeng, J.; Li, Z.; Song, Y.; Yan, H.; He, J.; Jiang, Y.; Duan, X. Redox regulation of the NOR transcription factor is involved in the regulation of fruit ripening in tomato. Plant Physiol. 2020, 183, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, L.; Wu, C.; Shan, W.; Cai, D.; Lin, Z.; Wei, W.; Chen, J.; Lu, W.; Kuang, J. Methionine oxidation and reduction of the ethylene signalling component MaEIL9 are involved in banana fruit ripening. J. Integr. Plant Biol. 2022, 65, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Jiang, G.; Wu, F.; Li, Z.; Xiao, L.; Jiang, Y.; Duan, X. Sulfoxidation regulation of transcription factor NAC42 influences its functions in relation to stress-induced fruit ripening in banana. J. Exp. Bot. 2021, 72, 682–699. [Google Scholar] [CrossRef]
- Xiao, L.; Jiang, G.; Yan, H.; Lai, H.; Su, X.; Jiang, Y.; Duan, X. Methionine sulfoxide reductase B regulates the activity of ascorbate peroxidase of banana fruit. Antioxidants 2021, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Xiao, L.; Yan, H.; Zhang, D.; Wu, F.; Liu, X.; Su, X.; Dong, X.; Wang, J.; Duan, X.; et al. Redox regulation of methionine in calmodulin affects the activity levels of senescence-related transcription factors in litchi. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wu, F.; Li, Z.; Li, T.; Gupta, V.K.; Duan, X.; Jiang, Y. Sulfoxidation Regulation of Musa acuminata Calmodulin (MaCaM) Influences the Functions of MaCaM-Binding Proteins. Plant Cell Physiol. 2018, 59, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Jiang, G.; Yan, H.; Xiao, L.; Liang, H.; Zhang, D.; Jiang, Y.; Duan, X. Redox regulation of glutathione peroxidase by thioredoxin in longan fruit in relation to senescence and quality deterioration. Food Chem. 2021, 345, 128664. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Vargas, M.A.; Gonzalez-Gordo, S.; Canas, A.; Lopez-Jaramillo, J.; Palma, J.M.; Corpas, F.J. Endogenous hydrogen sulfide (H2S) is up-regulated during sweet pepper (Capsicum annuum L.) fruit ripening. In vitro analysis shows that NADP-dependent isocitrate dehydrogenase (ICDH) activity is inhibited by H2S and NO. Nitric Oxide-Biol. Chem. 2018, 81, 36–45. [Google Scholar] [CrossRef]
- Chu-Puga, A.; Gonzalez-Gordo, S.; Rodriguez-Ruiz, M.; Palma, J.M.; Corpas, F.J. NADPH Oxidase (Rboh) activity is up regulated during sweet pepper (Capsicum annuum L.) fruit ripening. Antioxidants 2019, 8, 9. [Google Scholar] [CrossRef]
- Gonzalez-Gordo, S.; Rodriguez-Ruiz, M.; Lopez-Jaramillo, J.; Munoz-Vargas, M.A.; Palma, J.M.; Corpas, F.J. Nitric oxide (NO) differentially modulates the ascorbate peroxidase (APX) isozymes of sweet pepper (Capsicum annuum L.) fruits. Antioxidants 2022, 11, 765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, K.; Ma, L.; Geng, M.; Zhang, C.; Yao, G.; Zhang, H. Persulfidation and phosphorylation of transcription factor SlWRKY6 differentially regulate tomato fruit ripening. Plant Physiol. 2024, 196, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yao, G.F.; Li, L.X.; Li, T.T.; Zhao, Y.Q.; Hu, K.D.; Zhang, C.; Zhang, H. E3 ligase BRG3 persulfidation delays tomato ripening by reducing ubiquitination of the repressor WRKY71. Plant Physiol. 2023, 192, 616–632. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Yanez, A.; Ramos, P.; Morales-Quintana, L. Role of glycoproteins during fruit ripening and seed development. Cells 2021, 10, 2095. [Google Scholar] [CrossRef]
- Bose, S.K.; He, Y.; Howlader, P.; Wang, W.; Yin, H. The N-glycan processing enzymes beta-D-N-acetylhexosaminidase are involved in ripening-associated softening in strawberry fruit. J. Food Sci. Technol.-Mysore 2021, 58, 621–631. [Google Scholar] [CrossRef]
- Mendez-Yanez, A.; Beltran, D.; Campano-Romero, C.; Molinett, S.; Herrera, R.; Moya-Leon, M.A.; Morales-Quintana, L. Glycosylation is important for FcXTH1 activity as judged by its structural and biochemical characterization. Plant Physiol. Biochem. 2017, 119, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Meli, V.S.; Ghosh, S.; Prabha, T.N.; Chakraborty, N.; Chakraborty, S.; Datta, A. Enhancement of fruit shelf life by suppressing N-glycan processing enzymes. Proc. Natl. Acad. Sci. USA 2010, 107, 2413–2418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, H.; Du, H.; Bao, Z.; Shi, Q. Sugar metabolic and N-glycosylated profiles unveil the regulatory mechanism of tomato quality under salt stress. Environ. Exp. Bot. 2020, 177, 104145. [Google Scholar] [CrossRef]
- Xu, J.; Liu, S.; Cai, L.; Wang, L.; Dong, Y.; Qi, Z.; Yu, J.; Zhou, Y. SPINDLY interacts with EIN2 to facilitate ethylene signaling-mediated fruit ripening in tomato. Plant Biotechnol. J. 2022, 21, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, G.; Liu, X.; Ding, X.; Zhang, D.; Wang, X.; Zhou, Y.; Yan, H.; Li, T.; Wu, K.; et al. Histone demethylase SlJMJ6 promotes fruit ripening by removing H3K27 methylation of ripening-related genes in tomato. New Phytol. 2020, 227, 1138–1156. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Liu, X.; Jiang, G.; Li, Z.; Song, Y.; Zhang, D.; Jiang, Y.; Duan, X. SlJMJ7 orchestrates tomato fruit ripening via crosstalk between H3K4me3 and DML2-mediated DNA demethylation. New Phytol. 2022, 233, 1202–1219. [Google Scholar] [CrossRef]
- Kumar, V.; Thakur, J.K.; Prasad, M. Histone acetylation dynamics regulating plant development and stress responses. Cell Mol. Life Sci. 2021, 78, 4467–4486. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Han, Z.; Wang, T.; Li, H.; Li, Q.; Wang, S.; Tian, J.; Wang, Y.; Zhang, X.; Xu, X.; et al. Ethylene response factor MdERF4 and histone deacetylase MdHDA19 suppress apple fruit ripening through histone deacetylation of ripening-related genes. Plant Physiol. 2022, 188, 2166–2181. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.E.; Hu, Z.; Yu, X.; Li, A.; Li, F.; Wang, Y.; Tian, S.; Chen, G. A histone deacetylase gene, SlHDA3, acts as a negative regulator of fruit ripening and carotenoid accumulation. Plant Cell Rep. 2018, 37, 125–135. [Google Scholar] [CrossRef]
- Guo, J.E. Histone deacetylase gene SlHDT1 regulates tomato fruit ripening by affecting carotenoid accumulation and ethylene biosynthesis. Plant Sci. 2022, 318, 111235. [Google Scholar] [CrossRef]
- Guo, J.; Hu, Z.; Zhu, M.; Li, F.; Zhu, Z.; Lu, Y.; Chen, G. The tomato histone deacetylase SlHDA1 contributes to the repression of fruit ripening and carotenoid accumulation. Sci. Rep. 2017, 7, 7930. [Google Scholar] [CrossRef] [PubMed]
- Leutert, M.; Entwisle, S.W.; Villen, J. Decoding post-translational modification crosstalk with proteomics. Mol. Cell. Proteom. 2021, 20, 100129. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Z.; Lv, T.; Xu, Y.; Wei, Y.; Liu, W.; Liu, L.; Wang, A.; Li, T. Ethylene enhances MdMAPK3-mediated phosphorylation of MdNAC72 to promote apple fruit softening. Plant Cell 2023, 35, 2887–2909. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Chen, T.; Qin, G.; Tian, S. Current insights into posttranscriptional regulation of fleshy fruit ripening. Plant Physiol. 2023, 192, 1785–1798. [Google Scholar] [CrossRef] [PubMed]
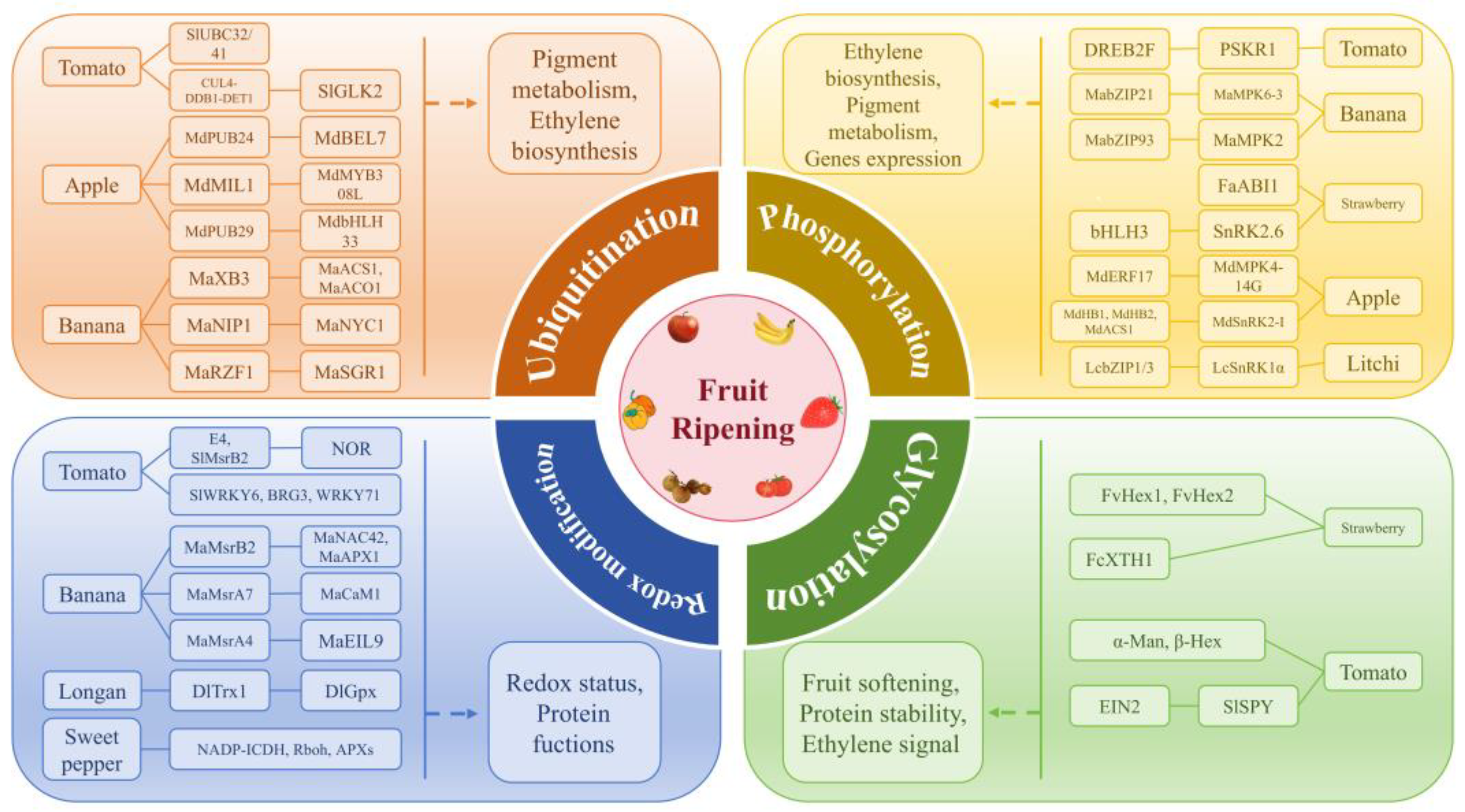
| Type | Fruit | Enzyme | Substrate | Site | Regulatory Effects | Reference |
|---|---|---|---|---|---|---|
| Ubiquitination | Tomato | SlUBC32/41 | Regulates pigment metabolism | [18] | ||
| CUL4-DDB1-DET1 | SlGLK2 | K11, K253 | Regulates chloroplast development and pigment metabolism | [21] | ||
| Apple | MdPUB24 | MdBEL7 | Promotes chlorophyll degradation | [22] | ||
| MdMIL1 | MdMYB308L | Negatively regulates anthocyanin accumulation | [23] | |||
| MdPUB29 | MdbHLH33 | Inhibits ethylene biosynthesis and anthocyanin accumulation | [24] | |||
| Banana | MaXB3 | MaACS1, MaACO1 | Negatively regulates ethylene biosynthesis | [25] | ||
| MaNIP1 | MaNYC1 | Negatively regulates chlorophyll catabolism | [26] | |||
| MaRZF1 | MaSGR1 | Negatively regulates chlorophyll catabolism | [27] | |||
| Phosphorylation | Tomato | SlPP2C1 | Inhibits fruit ripening | [31] | ||
| LeACS2 | S460 | Participates in ethylene signal transduction | [32] | |||
| PSKR1 | DREB2F | Y30 | Increases transcription levels of ripening-related genes | [41] | ||
| Banana | Protein kinases | MaACS1 | S476, S479 | Regulates ethylene biosynthesis | [33] | |
| MaMPK6-3 | MabZIP21 | T318, S436 | Enhances the transcriptional activation ability of ripening-related genes and accelerates fruit ripening | [34] | ||
| MaMPK2 | MabZIP93 | Enhances the transcriptional activation of cell wall modification-related genes and promotes fruit ripening | [35] | |||
| Strawberry | FaABI1 | Regulates the expression of ripening-related genes | [36] | |||
| SnRK2.6 | bHLH3 | Suppresses binding to UFGT promoter and negatively regulates fruit coloring. | [40] | |||
| Apple | MdMPK4-14G | MdERF17 | T67 | Promotes chlorophyll degradation | [37] | |
| MdSnRK2-I | MdHB1, MdHB2, MdACS1 | Regulates ethylene biosynthesis | [38] | |||
| MdCRF4 | Suppresses ethylene biosynthesis | [42] | ||||
| Litchi | LcSnRK1α | LcbZIP1/3 | Activates the expression of metabolic reprogramming genes and maintains energy and redox homeostasis | [39] | ||
| Redox modification | Tomato | E4, SlMsrB2 | NOR | M138 | Reduces the oxidized NOR and restores its function | [45] |
| Banana | MaMsrB2 | MaNAC42 | M134, M135 | Reduces the oxidized MaNAC42 and restores its function | [47] | |
| MaMsrB2 | MaAPX1 | M36 | Possibly regulates the redox status of banana fruit during ripening and senescence | [48] | ||
| MaMsrA7 | MaCaM1 | M77 M110 | Reduces the oxidized MaCaM1 and affects its binding activity with target proteins | [50] | ||
| MaMsrA4 | MaEIL9 | M129, M130 M282 | Reduces the oxidized MaEIL9 and restores its function | [46] | ||
| Longan | DlTrx1 | DlGpx | C90 | Regulates the redox state during fruit senescence and quality deterioration | [51] | |
| Sweet pepper | NADP-ICDH | C133, Y450 | Regulates NADPH production, affects cell redox state | [52] | ||
| Rboh | Involved in nitro-oxidative stress | [53] | ||||
| APXs | C32, Y235 | [54] | ||||
| Tomato | SlWRKY6 | C396 | Suppresses the expression of SlSGR1 and SlSAG12 | [55] | ||
| BRG3 | C206, C212 | Reduces the degradation of the ripening repressor WRKY71 | [56] | |||
| WRKY71 | Enhances binding and transcriptional repression of CAS1, delaying ripening | [56] | ||||
| Glycosylation | Strawberry | FvHex1, FvHex2 | Promotes fruit softening | [58] | ||
| FcXTH1 | Affects the stability of protein–ligand complexes | [59] | ||||
| Tomato | α-Man, β-Hex | Promotes fruit softening | [60] | |||
| SlSPY | EIN2 | S771, T821 | Enhances protein stability and affects ethylene signal transduction | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Zeng, J.; Yang, X.; Garcia-Caparros, P.; Duan, X. The Role of Protein Post-Translational Modifications in Fruit Ripening. Horticulturae 2024, 10, 1042. https://doi.org/10.3390/horticulturae10101042
Li T, Zeng J, Yang X, Garcia-Caparros P, Duan X. The Role of Protein Post-Translational Modifications in Fruit Ripening. Horticulturae. 2024; 10(10):1042. https://doi.org/10.3390/horticulturae10101042
Chicago/Turabian StyleLi, Ting, Jing Zeng, Xinquan Yang, Pedro Garcia-Caparros, and Xuewu Duan. 2024. "The Role of Protein Post-Translational Modifications in Fruit Ripening" Horticulturae 10, no. 10: 1042. https://doi.org/10.3390/horticulturae10101042
APA StyleLi, T., Zeng, J., Yang, X., Garcia-Caparros, P., & Duan, X. (2024). The Role of Protein Post-Translational Modifications in Fruit Ripening. Horticulturae, 10(10), 1042. https://doi.org/10.3390/horticulturae10101042






