Protein Hydrolysates—Production, Effects on Plant Metabolism, and Use in Agriculture
Abstract
1. Introduction
2. Production of Protein Hydrolysates
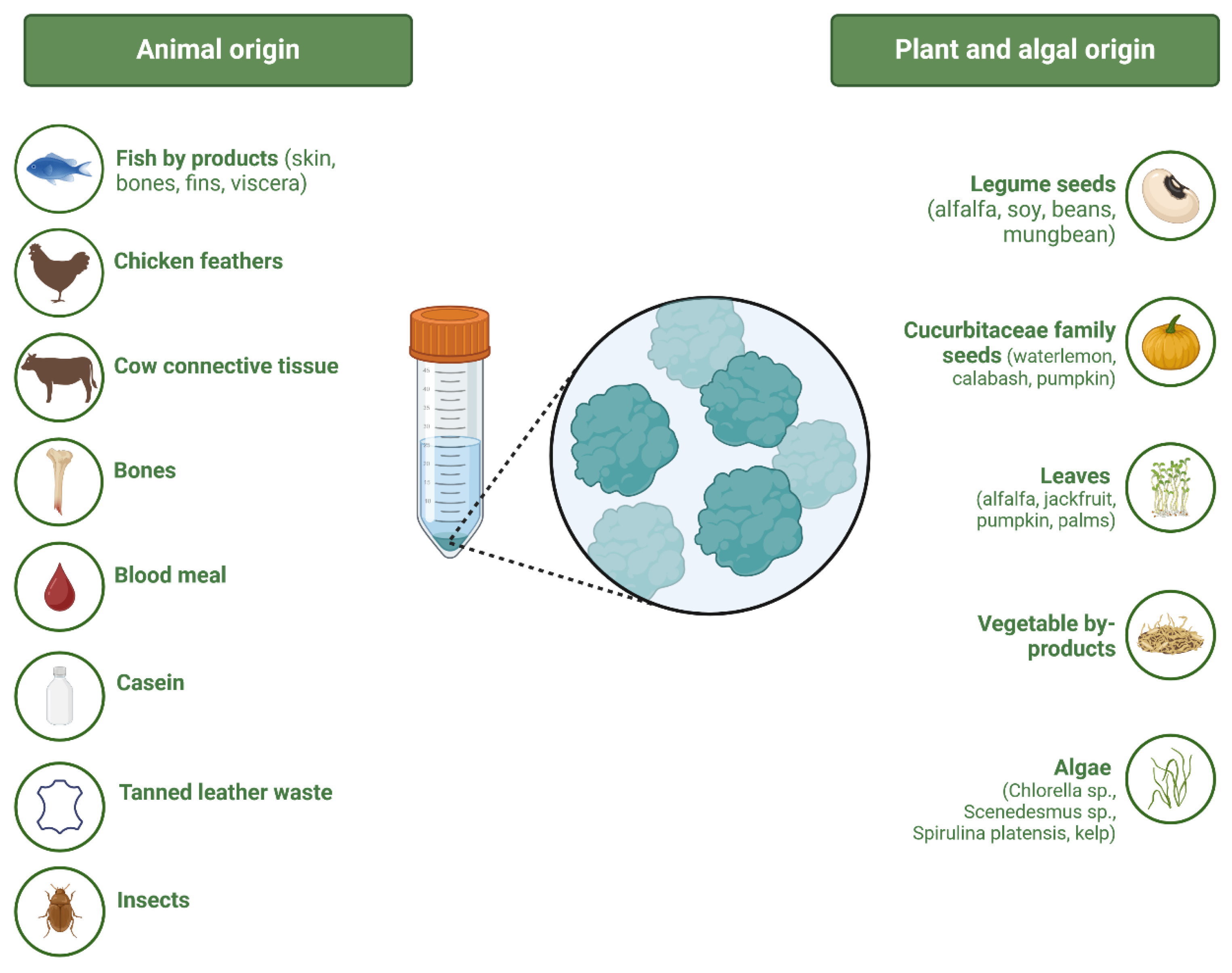
2.1. Sources of Raw Materials for Obtaining Protein Hydrolysates
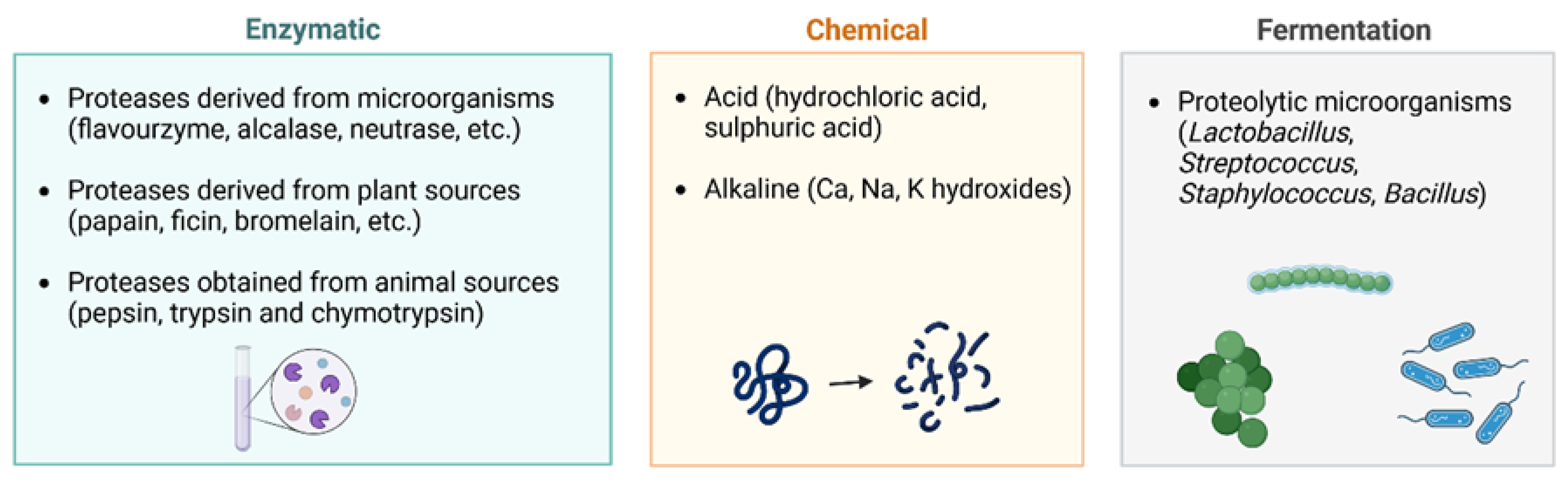
2.2. Type of Hydrolysis
2.2.1. Chemical Hydrolysis
2.2.2. Enzymatic Hydrolysis
3. Mode of Action of PHs on Plants
3.1. Uptake and Translocation
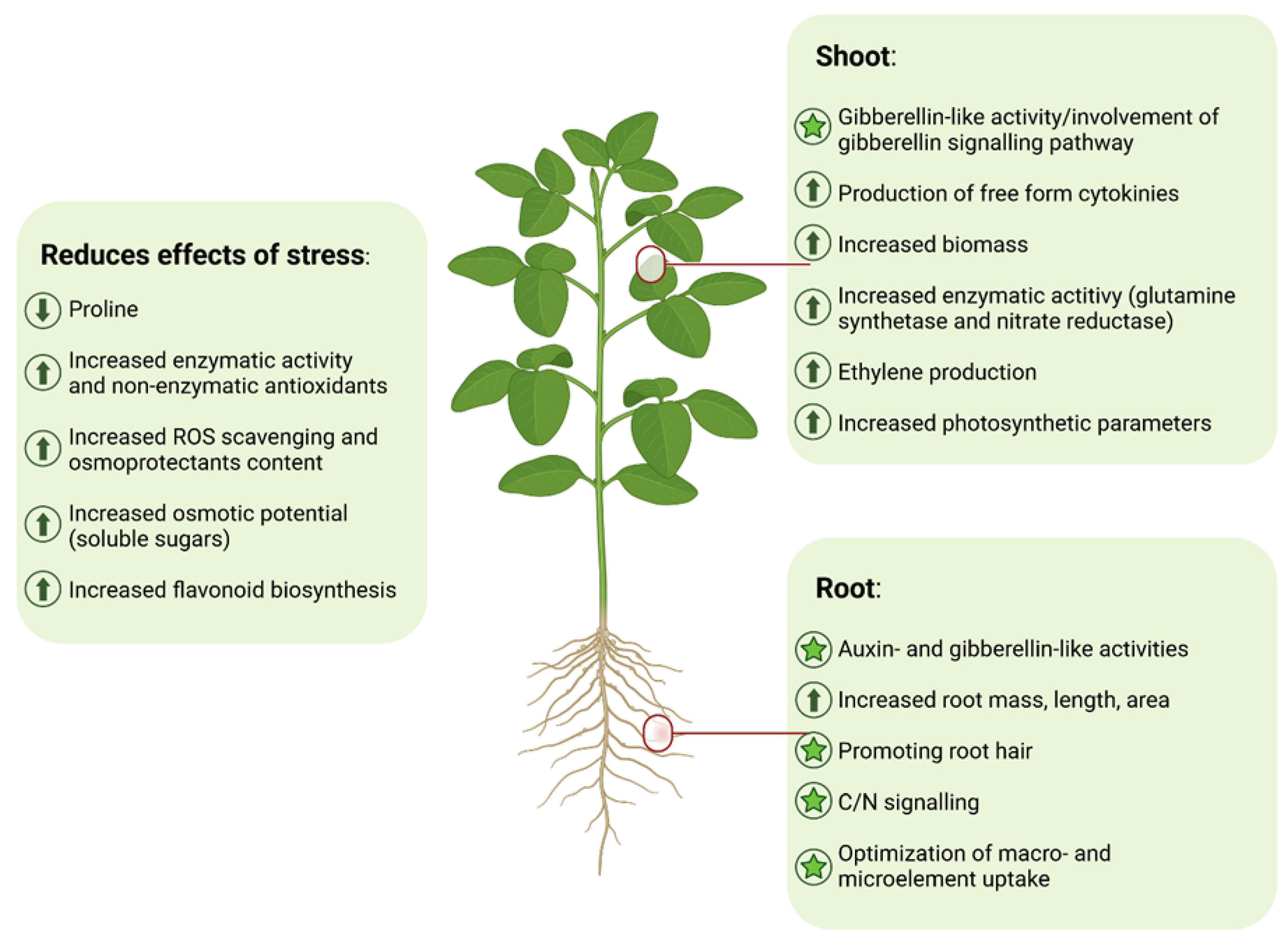
3.2. Effects on Plant Development
3.2.1. Effects on Root Growth and Development
3.2.2. Shoot Growth and Development
4. The Application Effects of PHs on Plants
4.1. Effect on N and C Status in Plants
4.2. Effects on Photosynthesis
4.3. Effects Related to Plant Stress
5. Protein Hydrolysates in Agriculture
6. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a Biostimulant on the Heat Tolerance Associated with Photosynthetic Capacity, Membrane Thermostability, and Polyphenol Production of Perennial Ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Lucini, L.; Canaguier, R.; Stefanoni, W.; Fiorillo, A.; Cardarelli, M. Protein Hydrolysate-Based Biostimulants: Origin, Biological Activity and Application Methods. Acta Hortic. 2016, 1148, 27–34. [Google Scholar] [CrossRef]
- Critchley, A.T.; Critchley, J.S.C.; Norrie, J.; Gupta, S.; Van Staden, J. Perspectives on the Global Biostimulant Market: Applications, Volumes, and Values, 2016 Data and Projections to 2022. In Biostimulants for Crops from Seed Germination to Plant Development; Academic Press: Cambridge, MA, USA, 2021; pp. 289–296. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein Hydrolysates as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- González-Morales, S.; Solís-Gaona, S.; Valdés-Caballero, M.V.; Juárez-Maldonado, A.; Loredo-Treviño, A.; Benavides-Mendoza, A. Transcriptomics of Biostimulation of Plants Under Abiotic Stress. Front. Genet. 2021, 12, 583888. [Google Scholar] [CrossRef] [PubMed]
- Luziatelli, F.; Ficca, A.G.; Colla, G.; Baldassarre Švecová, E.; Ruzzi, M. Foliar Application of Vegetal-Derived Bioactive Compounds Stimulates the Growth of Beneficial Bacteria and Enhances Microbiome Biodiversity in Lettuce. Front. Plant Sci. 2019, 10, 427970. [Google Scholar] [CrossRef]
- Baglieri, A.; Cadili, V.; Mozzetti Monterumici, C.; Gennari, M.; Tabasso, S.; Montoneri, E.; Nardi, S.; Negre, M. Fertilization of Bean Plants with Tomato Plants Hydrolysates. Effect on Biomass Production, Chlorophyll Content and N Assimilation. Sci. Hortic. 2014, 176, 194–199. [Google Scholar] [CrossRef]
- Wilson, H.T.; Amirkhani, M.; Taylor, A.G. Evaluation of Gelatin as a Biostimulant Seed Treatment to Improve Plant Performance. Front. Plant Sci. 2018, 9, 1006. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Lisiecka, J.; Knaflewski, M.; Spiżewski, T.; Frąszczak, B.; Kałużewicz, A.; Krzesiński, W. The effect of animal protein hydrolysate on quantity and quality of strawberry daughter plants cv. ‘Elsanta’. Acta Sci. Pol. Hortorum Cultus 2011, 10, 31–40. [Google Scholar]
- Moe, L.A. Amino Acids in the Rhizosphere: From Plants to Microbes. Am. J. Bot. 2013, 100, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Corte, L.; Dell’Abate, M.T.; Magini, A.; Migliore, M.; Felici, B.; Roscini, L.; Sardella, R.; Tancini, B.; Emiliani, C.; Cardinali, G.; et al. Assessment of Safety and Efficiency of Nitrogen Organic Fertilizers from Animal-Based Protein Hydrolysates—A Laboratory Multidisciplinary Approach. J. Sci. Food Agric. 2014, 94, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Cristofano, F.; El-Nakhel, C.; Pannico, A.; Giordano, M.; Colla, G.; Rouphael, Y. Foliar and Root Applications of Vegetal-Derived Protein Hydrolysates Differentially Enhance the Yield and Qualitative Attributes of Two Lettuce Cultivars Grown in Floating System. Agronomy 2021, 11, 1194. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; Chamorro, S.; Brenes, A. Protein Hydrolysates from Animal Processing By-Products as a Source of Bioactive Molecules with Interest in Animal Feeding: A Review. Food Res. Int. 2015, 73, 204–212. [Google Scholar] [CrossRef]
- Rajarajan, G.; Irshad, A.; Raghunath, B.V.; Mahesh kumar, G.; Punnagaiarasi, A. Utilization of Cheese Industry Whey for Biofuel–Ethanol Production. Integr. Waste Manag. India Status Future Prospect. Environ. Sustain. 2016, 59–64. [Google Scholar] [CrossRef]
- Santi, C.; Zamboni, A.; Varanini, Z.; Pandolfini, T. Growth Stimulatory Effects and Genome-Wide Transcriptional Changes Produced by Protein Hydrolysates in Maize Seedlings. Front. Plant Sci. 2017, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Pati, A. Poultry Feed Based on Protein Hydrolysate Derived from Chrome-Tanned Leather Solid Waste: Creating Value from Waste. Environ. Sci. Pollut. Res. Int. 2016, 23, 8120–8124. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Wang, D.; Sun, L.; Su, R.; Corazzin, M.; Sun, X.; Dou, L.; Zhang, M.; Zhao, L.; Su, L.; et al. Isolation, Purification and Structure Identification of a Calcium-Binding Peptide from Sheep Bone Protein Hydrolysate. Foods 2022, 11, 2655. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Bajaj, R.; Mann, B. Impact of Sequential Enzymatic Hydrolysis on Antioxidant Activity and Peptide Profile of Casein Hydrolysate. J. Food Sci. Technol. 2020, 57, 4562–4575. [Google Scholar] [CrossRef]
- Alves, F.E.D.S.B.; Teixeira, G.L.; Ávila, S.; Ribani, R.H.; Scheer, A.D.P. Functional and Antioxidant Properties of a Chicken Blood Meal Hydrolysate/Propriedades Funcionais e Antioxidantes de Um Hidrolisado de Farinha de Sangue de Frango. Braz. J. Dev. 2020, 6, 21149–21162. [Google Scholar] [CrossRef]
- Callegaro, K.; Welter, N.; Daroit, D.J. Feathers as Bioresource: Microbial Conversion into Bioactive Protein Hydrolysates. Process. Biochem. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from Fish By-Product Protein Hydrolysates and Its Functional Properties: An Overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.B.; Kim, J.G.; Je, J.Y. Purification and Antioxidant Properties of Octapeptide from Salmon Byproduct Protein Hydrolysate by Gastrointestinal Digestion. Food Chem. 2014, 147, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Slizyte, R.; Rommi, K.; Mozuraityte, R.; Eck, P.; Five, K.; Rustad, T. Bioactivities of Fish Protein Hydrolysates from Defatted Salmon Backbones. Biotechnol. Rep. 2016, 11, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Tejpal, C.S.; Vijayagopal, P.; Elavarasan, K.; Linga Prabu, D.; Lekshmi, R.G.K.; Asha, K.K.; Anandan, R.; Chatterjee, N.S.; Mathew, S. Antioxidant, Functional Properties and Amino Acid Composition of Pepsin-Derived Protein Hydrolysates from Whole Tilapia Waste as Influenced by Pre-Processing Ice Storage. J. Food Sci. Technol. 2017, 54, 4257–4267. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Baraniak, B.; Karaś, M. Antioxidant and Anti-Inflammatory Activities of Hydrolysates and Peptide Fractions Obtained by Enzymatic Hydrolysis of Selected Heat-Treated Edible Insects. Nutrients 2017, 9, 970. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Diao, X.; Jiang, F.; Han, J.; Kong, B. The Enzymatic Hydrolysis of Soy Protein Isolate by Corolase PP under High Hydrostatic Pressure and Its Effect on Bioactivity and Characteristics of Hydrolysates. Food Chem. 2018, 245, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Sonklin, C.; Laohakunjit, N.; Kerdchoechuen, O.; Ratanakhanokchai, K. Volatile Flavour Compounds, Sensory Characteristics and Antioxidant Activities of Mungbean Meal Protein Hydrolysed by Bromelain. J. Food Sci. Technol. 2018, 55, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Evangelho, J.A.D.; Vanier, N.L.; Pinto, V.Z.; Berrios, J.J.D.; Dias, A.R.G.; Zavareze, E. da R. Black Bean (Phaseolus vulgaris L.) Protein Hydrolysates: Physicochemical and Functional Properties. Food Chem. 2017, 214, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Ertani, A.; Nardi, S. Effects of an Alfalfa Protein Hydrolysate on the Gene Expression and Activity of Enzymes of the Tricarboxylic Acid (TCA) Cycle and Nitrogen Metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. [Google Scholar] [CrossRef]
- Dash, P.; Ghosh, G. Amino Acid Composition, Antioxidant and Functional Properties of Protein Hydrolysates from Cucurbitaceae Seeds. J. Food Sci. Technol. 2017, 54, 4162–4172. [Google Scholar] [CrossRef]
- Jacks, T.J.; Hensarling, T.P.; Yatsu, L.Y. Cucurbit Seeds: I. Characterizations and Uses of Oils and Proteins. A Review. Econ. Bot. 1972, 26, 135–141. [Google Scholar] [CrossRef]
- Popović, L.; Peričin, D.; Vaštag, Ž.; Popović, S.; Krimer, V.; Torbica, A. Antioxidative and Functional Properties of Pumpkin Oil Cake Globulin Hydrolysates. J. Am. Oil Chem. Soc. 2013, 90, 1157–1165. [Google Scholar] [CrossRef]
- Xie, Z.; Huang, J.; Xu, X.; Jin, Z. Antioxidant Activity of Peptides Isolated from Alfalfa Leaf Protein Hydrolysate. Food Chem. 2008, 111, 370–376. [Google Scholar] [CrossRef]
- Calderón-Chiu, C.; Calderón-Santoyo, M.; Herman-Lara, E.; Ragazzo-Sánchez, J.A. Jackfruit (Artocarpus heterophyllus Lam) Leaf as a New Source to Obtain Protein Hydrolysates: Physicochemical Characterization, Techno-Functional Properties and Antioxidant Capacity. Food Hydrocoll. 2021, 112, 106319. [Google Scholar] [CrossRef]
- Famuwagun, A.A.; Alashi, A.M.; Gbadamosi, S.O.; Taiwo, K.A.; Oyedele, D.J.; Adebooye, O.C.; Aluko, R.E. In Vitro Characterization of Fluted Pumpkin Leaf Protein Hydrolysates and Ultrafiltration of Peptide Fractions: Antioxidant and Enzyme-Inhibitory Properties. Pol. J. Food Nutr. Sci. 2020, 70, 429–443. [Google Scholar] [CrossRef]
- Hau, E.H.; Teh, S.S.; Yeo, S.K.; Chua, B.L.; Owatworakit, A.; Xiao, J.; Mah, S.H. Physicochemical and Functional Properties of Flavourzyme-Extracted Protein Hydrolysate from Oil Palm Leaves. Biomass Convers. Biorefin. 2022, 1–15. [Google Scholar] [CrossRef]
- Ortega, M.L.S.; Orellana-Palacios, J.C.; Garcia, S.R.; Rabanal-Ruiz, Y.; Moreno, A.; Hadidi, M. Olive Leaf Protein: Extraction Optimization, in Vitro Digestibility, Structural and Techno-Functional Properties. Int. J. Biol. Macromol. 2024, 256, 128273. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Barberán, M.; Lerma-García, M.J.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Use of an Enzyme-Assisted Method to Improve Protein Extraction from Olive Leaves. Food Chem. 2015, 169, 28–33. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant Activity of Two Protein Hydrolyzates in the Growth and Nitrogen Metabolism of Maize Seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Dinesh Kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish Protein Hydrolysates: Proximate Composition, Amino Acid Composition, Antioxidant Activities and Applications: A Review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Fathollahy, I.; Farmani, J.; Kasaai, M.R.; Hamishehkar, H. Characteristics and Functional Properties of Persian Lime (Citrus latifolia) Seed Protein Isolate and Enzymatic Hydrolysates. LWT 2021, 140, 110765. [Google Scholar] [CrossRef]
- Azman, A.T.; Isa, N.S.M.; Zin, Z.M.; Abdullah, M.A.A.; Aidat, O.; Zainol, M.K. Protein Hydrolysate from Underutilized Legumes: Unleashing the Potential for Future Functional Foods. Prev. Nutr. Food Sci. 2023, 28, 209–223. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein Content and Amino Acid Composition of Commercially Available Plant-Based Protein Isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Hempel, N.; Petrick, I.; Behrendt, F. Biomass Productivity and Productivity of Fatty Acids and Amino Acids of Microalgae Strains as Key Characteristics of Suitability for Biodiesel Production. J. Appl. Phycol. 2012, 24, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Hegde, A.S.; Sharma, K.; Parmar, P.; Srivatsan, V. Microalgae as a Sustainable Source of Edible Proteins and Bioactive Peptides—Current Trends and Future Prospects. Food Res. Int. 2022, 157, 111338. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- González-Pérez, B.K.; Rivas-Castillo, A.M.; Valdez-Calderón, A.; Gayosso-Morales, M.A. Microalgae as Biostimulants: A New Approach in Agriculture. World J. Microbiol. Biotechnol. 2022, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Celus, I.; Brijs, K.; Delcour, J.A. Enzymatic Hydrolysis of Brewers’ Spent Grain Proteins and Technofunctional Properties of the Resulting Hydrolysates. J. Agric. Food Chem. 2007, 55, 8703–8710. [Google Scholar] [CrossRef]
- Pasupuleti, V.K.; Braun, S. State of the Art Manufacturing of Protein Hydrolysates. Protein Hydrolysates Biotechnol. 2010, 11–32. [Google Scholar] [CrossRef]
- Driskell, J.A.; Wolinsky, I. Energy-Yielding Macronutrients and Energy Metabolism in Sports Nutrition; Cambridge University Press & Assessment: Cambridge, MA, USA, 2000; p. 337. [Google Scholar]
- Forsum, O.; Svennerstam, H.; Ganeteg, U.; Näsholm, T. Capacities and Constraints of Amino Acid Utilization in Arabidopsis. New Phytol. 2008, 179, 1058–1069. [Google Scholar] [CrossRef]
- Chen, X.L.; Peng, M.; Li, J.; Tang, B.L.; Shao, X.; Zhao, F.; Liu, C.; Zhang, X.Y.; Li, P.Y.; Shi, M.; et al. Preparation and Functional Evaluation of Collagen Oligopeptide-Rich Hydrolysate from Fish Skin with the Serine Collagenolytic Protease from Pseudoalteromonas sp. SM9913. Sci. Rep. 2017, 7, 15716. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Hernández, J.M.; Benítez-García, I.; Mazorra-Manzano, M.A.; Ramírez-Suárez, J.C.; Sánchez, E. Strategies for Production, Characterization and Application of Protein-Based Biostimulants in Agriculture: A Review. Chil. J. Agric. Res. 2020, 80, 274–289. [Google Scholar] [CrossRef]
- Clemente, A. Enzymatic Protein Hydrolysates in Human Nutrition. Trends Food Sci. Technol. 2000, 11, 254–262. [Google Scholar] [CrossRef]
- Luna-Vital, D.A.; Mojica, L.; González de Mejía, E.; Mendoza, S.; Loarca-Piña, G. Biological Potential of Protein Hydrolysates and Peptides from Common Bean (Phaseolus vulgaris L.): A Review. Food Res. Int. 2015, 76, 39–50. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Control of the Proteolytic Reaction and of the Level of Bitterness in Protein Hydrolysis Processes. J. Chem. Technol. Biotechnol. 1984, 34, 215–222. [Google Scholar] [CrossRef]
- dos Santos, S.D.A.; Martins, V.G.; Salas-Mellado, M.; Prentice, C. Evaluation of Functional Properties in Protein Hydrolysates from Bluewing Searobin (Prionotus punctatus) Obtained with Different Microbial Enzymes. Food Bioprocess Technol. 2011, 4, 1399–1406. [Google Scholar] [CrossRef]
- Gao, R.; Shen, Y.; Shu, W.; Bai, F.; Jin, W.; Wang, J.; Yuan, L. Optimization of Enzymatic Conditions of Sturgeon Muscles and Their Anti-Inflammatory Potential. J. Food Qual. 2020, 2020, 9698134. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-Derived Peptidic Antioxidants: A Review of Their Production, Assessment, and Potential Applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Ramírez Fuentes, L.; Richard, C.; Chen, L. Sequential Alcalase and Flavourzyme Treatment for Preparation of α-Amylase, α-Glucosidase, and Dipeptidyl Peptidase (DPP)-IV Inhibitory Peptides from Oat Protein. J. Funct. Foods 2021, 87, 104829. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Fish Protein Hydrolysates: Production, Biochemical, and Functional Properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Y. The Physiological Response of Photosynthesis to Nitrogen Deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Sun, X.; Chen, F.; Yuan, L.; Mi, G. The Physiological Mechanism Underlying Root Elongation in Response to Nitrogen Deficiency in Crop Plants. Planta 2020, 251, 84. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M.; Nogueira, M.A. Nitrogen Fixation. In Marschner’s Mineral Nutrition of Plants, 4th ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 615–650. [Google Scholar] [CrossRef]
- Ganeteg, U.; Ahmad, I.; Jämtgård, S.; Aguetoni-Cambui, C.; Inselsbacher, E.; Svennerstam, H.; Schmidt, S.; Näsholm, T. Amino Acid Transporter Mutants of Arabidopsis Provides Evidence That a Non-Mycorrhizal Plant Acquires Organic Nitrogen from Agricultural Soil. Plant Cell Environ. 2017, 40, 413–423. [Google Scholar] [CrossRef]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Soares, J.N.; Reichardt, K.; Neto, D.D. Seed and Foliar Application of Amino Acids Improve Variables of Nitrogen Metabolism and Productivity in Soybean Crop. Front. Plant Sci. 2018, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.R.; Nzokou, P.; Güney, D.; Kulaç, Ş. Growth Response and Nitrogen Use Physiology of Fraser Fir (Abies fraseri), Red Pine (Pinus resinosa), and Hybrid Poplar under Amino Acid Nutrition. New For. 2013, 44, 281–295. [Google Scholar] [CrossRef]
- Lipson, D.; Näsholm, T. The Unexpected Versatility of Plants: Organic Nitrogen Use and Availability in Terrestrial Ecosystems. Oecologia 2001, 128, 305–316. [Google Scholar] [CrossRef]
- Fischer, W.N.; Loo, D.D.F.; Koch, W.; Ludewig, U.; Boorer, K.J.; Tegeder, M.; Rentsch, D.; Wright, E.M.; Frommer, W.B. Low and High Affinity Amino Acid H+-Cotransporters for Cellular Import of Neutral and Charged Amino Acids. Plant J. 2002, 29, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Svennerstam, H.; Ganeteg, U.; Näsholm, T. Root Uptake of Cationic Amino Acids by Arabidopsis Depends on Functional Expression of Amino Acid Permease 5. New Phytol. 2008, 180, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Hirner, A.; Ladwig, F.; Stransky, H.; Okumoto, S.; Keinath, M.; Harms, A.; Frommer, W.B.; Koch, W. Arabidopsis LHT1 Is a High-Affinity Transporter for Cellular Amino Acid Uptake in Both Root Epidermis and Leaf Mesophyll. Plant Cell 2006, 18, 1931–1946. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Healey, J.R.; Willett, V.B.; Farrar, J.F.; Hodge, A. Dissolved Organic Nitrogen Uptake by Plants—An Important N Uptake Pathway? Soil Biol. Biochem. 2005, 37, 413–423. [Google Scholar] [CrossRef]
- Tejada, M.; Rodríguez-Morgado, B.; Paneque, P.; Parrado, J. Effects of Foliar Fertilization of a Biostimulant Obtained from Chicken Feathers on Maize Yield. Eur. J. Agron. 2018, 96, 54–59. [Google Scholar] [CrossRef]
- Chris Stiegler, J.; Richardson, M.D.; Karcher, D.E.; Roberts, T.L.; Norman, R.J. Foliar Absorption of Various Inorganic and Organic Nitrogen Sources by Creeping Bentgrass. Crop Sci. 2013, 53, 1148–1152. [Google Scholar] [CrossRef]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The Molecular-Physiological Functions of Mineral Macronutrients and Their Consequences for Deficiency Symptoms in Plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef] [PubMed]
- Pramanick, B.; Dubey, R.; Kesarwani, A.; Bera, A.; Bhutia, K.L.; Kumar, M.; Maitra, S. Advancement in Mitigating the Effects of Waterlogging Stress in Wheat. In Abiotic Stresses in Wheat; Academic Press: Cambridge, MA, USA, 2023; pp. 339–355. [Google Scholar] [CrossRef]
- Ertani, A.; Nardi, S.; Francioso, O.; Sanchez-Cortes, S.; Di Foggia, M.; Schiavon, M. Effects of Two Protein Hydrolysates Obtained from Chickpea (Cicer arietinum L.) and Spirulina platensis on Zea mays (L.) Plants. Front. Plant Sci. 2019, 10, 954. [Google Scholar] [CrossRef] [PubMed]
- Polo, J.; Mata, P. Evaluation of a Biostimulant (Pepton) Based in Enzymatic Hydrolyzed Animal Protein in Comparison to Seaweed Extracts on Root Development, Vegetative Growth, Flowering, and Yield of Gold Cherry Tomatoes Grown under Low Stress Ambient Field Conditions. Front. Plant Sci. 2018, 8, 2261. [Google Scholar] [CrossRef]
- De Lucia, B.; Vecchietti, L. Type of Bio-Stimulant and Application Method Effects on Stem Quality and Root System Growth in L.A. Lily. Eur. J. Hortic. Sci. 2012, 77, 1611–4426. [Google Scholar]
- Raguraj, S.; Kasim, S.; Jaafar, N.M.; Nazli, M.H. Growth of Tea Nursery Plants as Influenced by Different Rates of Protein Hydrolysate Derived from Chicken Feathers. Agronomy 2022, 12, 299. [Google Scholar] [CrossRef]
- Tütüncü, M. Effects of Protein Hydrolysate Derived from Anchovy By-Product on Plant Growth of Primrose and Root System Architecture Analysis with Machine Learning. Horticulturae 2024, 10, 400. [Google Scholar] [CrossRef]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Giordano, M.; El-Nakhel, C.; Cuciniello, A.; Cenvinzo, V.; Colla, G.; Rouphael, Y. Protein Hydrolysate or Plant Extract-Based Biostimulants Enhanced Yield and Quality Performances of Greenhouse Perennial Wall Rocket Grown in Different Seasons. Plants 2019, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa Plant-Derived Biostimulant Stimulate Short-Term Growth of Salt Stressed Zea mays L. Plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant Action of a Plant-Derived Protein Hydrolysate Produced through Enzymatic Hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef]
- Matsumiya, Y.; Kubo, Y.M.A.M. Soybean Peptide: Novel Plant Growth Promoting Peptide from Soybean. In Soybean and Nutrition; InTechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Paul, T.; Halder, S.K.; Das, A.; Bera, S.; Maity, C.; Mandal, A.; Das, P.S.; Mohapatra, P.K.D.; Pati, B.R.; Mondal, K.C. Exploitation of Chicken Feather Waste as a Plant Growth Promoting Agent Using Keratinase Producing Novel Isolate Paenibacillus Woosongensis TKB2. Biocatal. Agric. Biotechnol. 2013, 2, 50–57. [Google Scholar] [CrossRef]
- Marfà, O.; Cáceres, R.; Polo, J.; Ródenas, J. Animal Protein Hydrolysate as a Biostimulant for Transplanted Strawberry Plants Subjected to Cold Stress. Acta Hortic. 2009, 842, 315–318. [Google Scholar] [CrossRef]
- Francesca, S.; Arena, C.; Hay Mele, B.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The Use of a Plant-Based Biostimulant Improves Plant Performances and Fruit Quality in Tomato Plants Grown at Elevated Temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef]
- Rouphael, Y.; Lucini, L.; Miras-Moreno, B.; Colla, G.; Bonini, P.; Cardarelli, M. Metabolomic Responses of Maize Shoots and Roots Elicited by Combinatorial Seed Treatments With Microbial and Non-Microbial Biostimulants. Front. Microbiol. 2020, 11, 664. [Google Scholar] [CrossRef]
- Carillo, P.; De Micco, V.; Ciriello, M.; Formisano, L.; El-Nakhel, C.; Giordano, M.; Colla, G.; Rouphael, Y. Morpho-Anatomical, Physiological, and Mineral Composition Responses Induced by a Vegetal-Based Biostimulant at Three Rates of Foliar Application in Greenhouse Lettuce. Plants 2022, 11, 2030. [Google Scholar] [CrossRef]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The Yin-Yang of Hormones: Cytokinin and Auxin Interactions in Plant Development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, A.V.; Miras-Moreno, B.; Buffagni, V.; Senizza, B.; Pii, Y.; Cardarelli, M.; Rouphael, Y.; Colla, G.; Lucini, L. Foliar Application of Different Vegetal-Derived Protein Hydrolysates Distinctively Modulates Tomato Root Development and Metabolism. Plants 2021, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Reynaud, H.; Canaguier, R.; Trtílek, M.; Panzarová, K.; et al. Understanding the Biostimulant Action of Vegetal-Derived Protein Hydrolysates by High-Throughput Plant Phenotyping and Metabolomics: A Case Study on Tomato. Front. Plant Sci. 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Schaller, G.E. Ethylene and the Regulation of Plant Development. BMC Biol. 2012, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Small, C.C.; Degenhardt, D. Plant Growth Regulators for Enhancing Revegetation Success in Reclamation: A Review. Ecol. Eng. 2018, 118, 43–51. [Google Scholar] [CrossRef]
- Consentino, B.B.; Virga, G.; la Placa, G.G.; Sabatino, L.; Rouphael, Y.; Ntatsi, G.; Iapichino, G.; la Bella, S.; Mauro, R.P.; D’anna, F.; et al. Celery (Apium graveolens L.) Performances as Subjected to Different Sources of Protein Hydrolysates. Plants 2020, 9, 1633. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-Wide Identification of Differentially Expressed Genes in Solanum lycopersicon L. In Response to an Alfalfa-Protein Hydrolysate Using Microarrays. Front. Plant Sci. 2017, 8, 1159. [Google Scholar] [CrossRef]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein Hydrolysate Stimulates Growth in Tomato Coupled with N-Dependent Gene Expression Involved in N Assimilation. Front. Plant Sci. 2018, 9, 364202. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Gordon-Weeks, R.; Shen, Q.; Miller, A.J. Glutamine Transport and Feedback Regulation of Nitrate Reductase Activity in Barley Roots Leads to Changes in Cytosolic Nitrate Pools. J. Exp. Bot. 2006, 57, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Fan, X.; Shen, Q.; Smith, S.J. Amino Acids and Nitrate as Signals for the Regulation of Nitrogen Acquisition. J. Exp. Bot. 2008, 59, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, Y.; Sakagami, Y. Peptide Hormones in Plants. Annu. Rev. Plant Biol. 2006, 57, 649–674. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; El-Nakhel, C.; Corrado, G.; Rouphael, Y. Biostimulatory Action of a Plant-Derived Protein Hydrolysate on Morphological Traits, Photosynthetic Parameters, and Mineral Composition of Two Basil Cultivars Grown Hydroponically under Variable Electrical Conductivity. Horticulturae 2022, 8, 409. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Drench Application of Fish-Derived Protein Hydrolysates Affects Lettuce Growth, Chlorophyll Content, and Gas Exchange. Horttechnology 2017, 27, 539–543. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Hammad, S.A.R.; Ali, O.A.M. Physiological and Biochemical Studies on Drought Tolerance of Wheat Plants by Application of Amino Acids and Yeast Extract. Ann. Agric. Sci. 2014, 59, 133–145. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The Significance of Amino Acids and Amino Acid-Derived Molecules in Plant Responses and Adaptation to Heavy Metal Stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M.N.V. Heavy Metal-Induced Oxidative Damage, Defense Reactions, and Detoxification Mechanisms in Plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Francesca, S.; Cirillo, V.; Raimondi, G.; Maggio, A.; Barone, A.; Rigano, M.M. A Novel Protein Hydrolysate-Based Biostimulant Improves Tomato Performances under Drought Stress. Plants 2021, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Agliassa, C.; Mannino, G.; Molino, D.; Cavalletto, S.; Contartese, V.; Bertea, C.M.; Secchi, F. A New Protein Hydrolysate-Based Biostimulant Applied by Fertigation Promotes Relief from Drought Stress in Capsicum annuum L. Plant Physiol. Biochem. 2021, 166, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Leporino, M.; Rouphael, Y.; Bonini, P.; Colla, G.; Cardarelli, M. Protein Hydrolysates Enhance Recovery from Drought Stress in Tomato Plants: Phenomic and Metabolomic Insights. Front. Plant Sci. 2024, 15, 1357316. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.S.; Yamamoto, S.I.; Biswas, M.S.; Sakurai, C.; Isoai, H.; Mano, J. Histidine-Containing Dipeptides Mitigate Salt Stress in Plants by Scavenging Reactive Carbonyl Species. J. Agric. Food Chem. 2022, 70, 11169–11178. [Google Scholar] [CrossRef] [PubMed]
- Liatile, P.C.; Potgieter, G.; Moloi, M.J. A Natural Bio-Stimulant Consisting of a Mixture of Fish Protein Hydrolysates and Kelp Extract Enhances the Physiological, Biochemical and Growth Responses of Spinach under Different Water Levels. Plants 2022, 11, 3374. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, M.Z.; Desoky, E.S.M.; Osman, A.; Rady, M.M. Pumpkin Seed Protein Hydrolysate Treatment Alleviates Salt Stress Effects on Phaseolus Vulgaris by Elevating Antioxidant Capacity and Recovering Ion Homeostasis. Sci. Hortic. 2020, 271, 109495. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Rady, M.O.A.; Marey, R.A.; Abd El-Mageed, T.A. Exogenously Applied Proline Enhances Growth and Productivity of Drought Stressed Onion by Improving Photosynthetic Efficiency, Water Use Efficiency and up-Regulating Osmoprotectants. Sci. Hortic. 2020, 272, 109580. [Google Scholar] [CrossRef]
- Mamatha, B.C.; Rudresh, K.; Karthikeyan, N.; Kumar, M.; Das, R.; Taware, P.B.; Khapte, P.S.; Soren, K.R.; Rane, J.; Gurumurthy, S. Vegetal Protein Hydrolysates Reduce the Yield Losses in Off-Season Crops under Combined Heat and Drought Stress. Physiol. Mol. Biol. Plants 2023, 29, 1049–1059. [Google Scholar] [CrossRef]
- Schiavon, M.; Pizzeghello, D.; Muscolo, A.; Vaccaro, S.; Francioso, O.; Nardi, S. High Molecular Size Humic Substances Enhance Phenylpropanoid Metabolism in Maize (Zea mays L.). J. Chem. Ecol. 2010, 36, 662–669. [Google Scholar] [CrossRef]
- Shetty, K.; Wahlqvist, M. A Model for the Role of the Proline-Linked Pentose-Phosphate Pathway in Phenolic Phytochemical Bio-Synthesis and Mechanism of Action for Human Health and Environmental Applications. Asia Pac. J. Clin. Nutr. 2004, 13, 1–24. [Google Scholar] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, F.; Tian, L.; Huang, M.; Deng, R.; Li, X.; Chen, W.; Wu, P.; Li, M.; Jiang, H.; et al. The Phenylalanine Ammonia Lyase Gene LjPAL1 Is Involved in Plant Defense Responses to Pathogens and Plays Diverse Roles in Lotus japonicus-Rhizobium Symbioses. Mol. Plant Microbe Interact 2017, 30, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Solekha, R.; Susanto, F.A.; Joko, T.; Nuringtyas, T.R.; Purwestri, Y.A. Phenylalanine Ammonia Lyase (PAL) Contributes to the Resistance of Black Rice against Xanthomonas oryzae Pv. oryzae. J. Plant Pathol. 2020, 102, 359–365. [Google Scholar] [CrossRef]
- Singh, D.P.; Bahadur, A.; Sarma, B.K.; Maurya, S.; Singh, H.B.; Singh, U.P. Exogenous Application of L-Phenylalanine and Ferulic Acid Enhance Phenylalanine Ammonia Lyase Activity and Accumulation of Phenolic Acids in Pea (Pisum sativum) to Offer Protection against Erysiphe pisi. Arch. Phytopathol. Plant Prot. 2010, 43, 1454–1462. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Andreotti, C. Use of Biostimulants for Organic Apple Production: Effects on Tree Growth, Yield, and Fruit Quality at Harvest and during Storage. Front. Plant Sci. 2018, 9, 1342. [Google Scholar] [CrossRef]
- Gurav, R.G.; Jadhav, J.P. A Novel Source of Biofertilizer from Feather Biomass for Banana Cultivation. Environ. Sci. Pollut. Res. Int. 2013, 20, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Consentino, B.B.; Vultaggio, L.; Sabatino, L.; Ntatsi, G.; Rouphael, Y.; Bondì, C.; De Pasquale, C.; Guarino, V.; Iacuzzi, N.; Capodici, G.; et al. Combined Effects of Biostimulants, N Level and Drought Stress on Yield, Quality and Physiology of Greenhouse-Grown Basil. Plant Stress 2023, 10, 100268. [Google Scholar] [CrossRef]
- García-Santiago, J.C.; Lozano Cavazos, C.J.; González-Fuentes, J.A.; Zermeño-González, A.; Rascón Alvarado, E.; Rojas Duarte, A.; Preciado-Rangel, P.; Troyo-Diéguez, E.; Peña Ramos, F.M.; Valdez-Aguilar, L.A.; et al. Effects of Fish-Derived Protein Hydrolysate, Animal-Based Organic Fertilisers and Irrigation Method on the Growth and Quality of Grape Tomatoes. Biol. Agric. Hortic. 2021, 37, 107–124. [Google Scholar] [CrossRef]
- Bavaresco, L.; Lucini, L.; Squeri, C.; Zamboni, M.; Frioni, T. Protein Hydrolysates Modulate Leaf Proteome and Metabolome in Water-Stressed Grapevines. Sci Hortic 2020, 270, 109413. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, W.; Wang, W.; Lv, H.; Liang, B.; Li, J. Exogenous Pig Blood-Derived Protein Hydrolysates as a Promising Method for Alleviation of Salt Stress in Tomato (Solanum lycopersicum L.). Sci. Hortic. 2022, 294, 110779. [Google Scholar] [CrossRef]
- Ambrosini, S.; Sega, D.; Santi, C.; Zamboni, A.; Varanini, Z.; Pandolfini, T. Evaluation of the Potential Use of a Collagen-Based Protein Hydrolysate as a Plant Multi-Stress Protectant. Front. Plant Sci. 2021, 12, 600623. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Z.; Wang, Y.; Cheng, W.; Mou, H. Production of a Water-Soluble Fertilizer Containing Amino Acids by Solid-State Fermentation of Soybean Meal and Evaluation of Its Efficacy on the Rapeseed Growth. J. Biotechnol. 2014, 187, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Colla, G.; Cardarelli, M.; Kim, H.J. Effects of Plant-Derived Protein Hydrolysates on Yield, Quality, and Nitrogen Use Efficiency of Greenhouse Grown Lettuce and Tomato. Agronomy 2022, 12, 1018. [Google Scholar] [CrossRef]
- Osman, A.; Merwad, A.R.M.; Mohamed, A.H.; Sitohy, M. Foliar Spray with Pepsin-and Papain-Whey Protein Hydrolysates Promotes the Productivity of Pea Plants Cultivated in Clay Loam Soil. Molecules 2021, 26, 2805. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar Applications of a Legume-Derived Protein Hydrolysate Elicit Dose-Dependent Increases of Growth, Leaf Mineral Composition, Yield and Fruit Quality in Two Greenhouse Tomato Cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Barrada, A.; Delisle-Houde, M.; Nguyen, T.T.A.; Tweddell, R.J.; Dorais, M. Drench Application of Soy Protein Hydrolysates Increases Tomato Plant Fitness, Fruit Yield, and Resistance to a Hemibiotrophic Pathogen. Agronomy 2022, 12, 1761. [Google Scholar] [CrossRef]
- Visconti, F.; de Paz, J.M.; Bonet, L.; Jordà, M.; Quiñones, A.; Intrigliolo, D.S. Effects of a Commercial Calcium Protein Hydrolysate on the Salt Tolerance of Diospyros kaki L. Cv. “Rojo Brillante” Grafted on Diospyros lotus L. Sci. Hortic. 2015, 185, 129–138. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Elia, A. Foliar Application of Protein Hydrolysates on Baby-Leaf Spinach Grown at Different n Levels. Agronomy 2022, 12, 36. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Cristofano, F.; Cardarelli, M.; Colla, G. Effects of Vegetal- versus Animal-Derived Protein Hydrolysate on Sweet Basil Morpho-Physiological and Metabolic Traits. Sci. Hortic. 2021, 284, 110123. [Google Scholar] [CrossRef]
- Iqbal, W.; Ayyub, C.M.; Jahangir, M.M.; Ahmad, R. Effect of Foliar Application of Bio-Stimulants on Growth, Yield and Nutritional Quality of Broccoli. Braz. J. Biol. 2023, 83, e263302. [Google Scholar] [CrossRef] [PubMed]
- Bettoni, M.M.; Fabbrin, E.G.D.S.; Olinik, J.R.; Mógor, Á.F. Efeito Da Aplicação Foliar de Hidrolisado Protéico Sob a Produtividade de Cultivares de Brócolis. Rev. Agro@ Mbiente On-line 2013, 7, 179. [Google Scholar] [CrossRef]
- Almadi, L.; Paoletti, A.; Cinosi, N.; Daher, E.; Rosati, A.; Di Vaio, C.; Famiani, F. A Biostimulant Based on Protein Hydrolysates Promotes the Growth of Young Olive Trees. Agriculture 2020, 10, 618. [Google Scholar] [CrossRef]
- Elwaziri, E.; Ismail, H.; El-Khairl, E.S.A.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Abd Elgawad, H.G.; Omar, W.A.; Abdelaal, K.; Osman, A. Biostimulant Application of Whey Protein Hydrolysates and Potassium Fertilization Enhances the Productivity and Tuber Quality of Sweet Potato. Not. Bot. Horti Agrobot. Cluj Napoca 2023, 51, 13122. [Google Scholar] [CrossRef]
- Nurdiawati, A.; Suherman, C.; Maxiselly, Y.; Akbar, M.A.; Purwoko, B.A.; Prawisudha, P.; Yoshikawa, K. Liquid Feather Protein Hydrolysate as a Potential Fertilizer to Increase Growth and Yield of Patchouli (Pogostemon cablin Benth) and Mung Bean (Vigna radiata). Int. J. Recycl. Org. Waste Agric. 2019, 8, 221–232. [Google Scholar] [CrossRef]
- Mironenko, G.A.; Zagorskii, I.A.; Bystrova, N.A.; Kochetkov, K.A. The Effect of a Biostimulant Based on a Protein Hydrolysate of Rainbow Trout (Oncorhynchus mykiss) on the Growth and Yield of Wheat (Triticum aestivum L.). Molecules 2022, 27, 6663. [Google Scholar] [CrossRef] [PubMed]
- El-Sanatawy, A.M.; Ash-Shormillesy, S.M.A.I.; El-Yazied, A.A.; Abd El-Gawad, H.G.; Azab, E.; Gobouri, A.A.; Sitohy, M.; Osman, A. Enhancing Grain Yield and Nitrogen Accumulation in Wheat Plants Grown under a Mediterranean Arid Environment by Foliar Spray with Papain-Released Whey Peptides. Agronomy 2021, 11, 1913. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Altissimo, A.; Nardi, S. Use of Meat Hydrolyzate Derived from Tanning Residues as Plant Biostimulant for Hydroponically Grown Maize. J. Plant Nutr. Soil Sci. 2013, 176, 287–295. [Google Scholar] [CrossRef]
- Cristiano, G.; De Lucia, B. Petunia Performance Under Application of Animal-Based Protein Hydrolysates: Effects on Visual Quality, Biomass, Nutrient Content, Root Morphology, and Gas Exchange. Front. Plant Sci. 2021, 12, 640608. [Google Scholar] [CrossRef] [PubMed]
- Engel, D.C.H.; Feltrim, D.; Rodrigues, M.; Baptistella, J.L.C.; Mazzafera, P. Application of Protein Hydrolysate Improved the Productivity of Soybean under Greenhouse Cultivation. Agriculture 2024, 14, 1205. [Google Scholar] [CrossRef]
- Di Mola, I.; Conti, S.; Cozzolino, E.; Melchionna, G.; Ottaiano, L.; Testa, A.; Sabatino, L.; Rouphael, Y.; Mori, M. Plant-Based Protein Hydrolysate Improves Salinity Tolerance in Hemp: Agronomical and Physiological Aspects. Agronomy 2021, 11, 342. [Google Scholar] [CrossRef]
- Vernieri, P.; Borghesi, E.; Tognoni, F.; Serra, G.; Ferrante, A.; Piaggesi, A. Use of Biostimulants for Reducing Nutrient Solution Concentration in Floating System. Acta Hortic. 2006, 718, 477–484. [Google Scholar] [CrossRef]
- Basile, B.; Brown, N.; Valdes, J.M.; Cardarelli, M.; Scognamiglio, P.; Mataffo, A.; Rouphael, Y.; Bonini, P.; Colla, G. Plant-Based Biostimulant as Sustainable Alternative to Synthetic Growth Regulators in Two Sweet Cherry Cultivars. Plants 2021, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Pati, A.; Chaudhary, R. Soybean Plant Growth Study Conducted Using Purified Protein Hydrolysate-Based Fertilizer Made from Chrome-Tanned Leather Waste. Environ. Sci. Pollut. Res. Int. 2015, 22, 20316–20321. [Google Scholar] [CrossRef]
- Kocira, S. Effect of Amino Acid Biostimulant on the Yield and Nutraceutical Potential of Soybean. Chil. J. Agric. Res. 2019, 79, 17–25. [Google Scholar] [CrossRef]

| Plant Species | Type of PH | Application | Experimental Conditions | Effects | References |
|---|---|---|---|---|---|
| Lettuce (Lactuca sativa L.) | Legume-based (Trainer®) | Foliar | Greenhouse | Growth stimulation of epiphytic bacteria with plant growth-promoting activity and/or biological-control activity against pathogens | [7] |
| Legume-based (Trainer®) | Foliar and root | Greenhouse | Higher yield and increase in SPAD index and photosynthetic parameters. Responses were cultivar-specific | [14] | |
| Animal-based (pig blood hydrolysate) | Foliar | Hydroponic system | Increased phenolic content, antioxidant activity, and upregulation of genes related to phenolic biosynthesis | [130] | |
| Legume-based (Trainer®) | Foliar | Pot trial in greenhouse | Increased nutrient-use efficiency, root dry weight, and leaf area. Dose-dependent positive effects on photosynthetic activity and root growth | [93] | |
| Legume-based (Trainer®) | Foliar and root | Pot trial in greenhouse | Improvement in nutrient use and uptake efficiency; increase in water-use efficiency, chlorophyll content, and antioxidant activity; and enhanced yield and quality | [133] | |
| Pea (Pisum sativum L.) | Legume-based (Trainer®) | Foliar | Greenhouse | On average, 33% increase in shoot length in gibberellin-deficient plants; increase in plant biomass, SPAD index, and leaf nitrogen content | [87] |
| Whey protein hydrolysate (hydrolyzed by papain and pepsin) | Foliar | Field trial | Improvement in nutrient status (N, P, and K); and increased content of photosynthetic pigments and, ultimately, yield | [134] | |
| Tomato (Solanum lycopersicum L.) | Legume-based (Trainer®) | Fertigation | Greenhouse | Increase in SPAD index and leaf nitrogen content; and increase in shoot, root, and total dry biomass | [87] |
| Sugar-cane molasses and yeast extract (CycoFlow®) | Fertigation | Field trial in tunnels | Significant stimulation of growth and number of fruits under heat stress, as well as increase in antioxidant content in leaf and fruit | [91] | |
| Legume-based (Trainer®) | Foliar | Field experiment | Increase in marketable yield (cultivar specific), increase net assimilation of CO2, improved nutritional status seen as increase in K and Mg, and increase in antioxidant activity and total soluble solids and bioactive molecules | [135] | |
| Legume-based (Trainer®) | Immersion | Laboratory bioassay | Auxin-like effect; and increase in root and shoot dry weight, root length, and area by 21, 35, 24, and 26% | [87] | |
| Vegetable-based, soy meal | Substrate drenching | Greenhouse, pot and plastic plug tray trials | Increased plant growth and fruit production; enhanced glycine content; and increase in expression levels of defense-related genes | [136] | |
| Legume-based (Trainer®) | Foliar and root | Pot trial in greenhouse | Enhanced shoot dry weight and marketable yield; and increased chlorophyll content and antioxidant activity | [133] | |
| Animal-based (PEPTON 85/16®) and seaweed-based (Acadian Suelo®) | Combination of foliar spray and irrigation | Field trial | Increases in plant height, stem diameter, leaf length, number of leaves per plant, root length and diameter, and yield. PEPTON 85/16 showed larger positive effect on yield than Acadian | [81] | |
| Banana (Musa sp. Cv. G9) | Derived from chicken feathers | Foliar and root | Field trial | Increased content of proteins, amino acids, reducing sugars, total chlorophyll, and proline content. Promotes earlier flowering | [126] |
| Wall rocket (Diplotaxis tenuifolia L.) | Legume-based (Trainer®) and tropical-plant extract (Auxim®) | Foliar | Greenhouse | Enhanced growth and productivity; increased leaf dry matter content, oxalic and citric acids, concentrations of Ca and P, phenols and ascorbic acid, and antioxidant activity | [85] |
| Strawberry (Fragaria × ananassa cv. Diamante) | Animal-based (PEPTON 85/16®) | Soil injection | Field trial in tunnels | When exposed to cold stress, application of PH increased root biomass, earlier flowering, and early production of fruit | [90] |
| Grapevine (Vitis vinifera L.) | Legume-based (Trainer® and Stimtide®) | Foliar | Pot trial | Induction of changes in leaf proteome and metabolome, delay of physiological maturity, and maintained higher acidity in water-stressed plants | [129] |
| Persimmon (Diospyros kaki L.) | Animal origin, PH stabilized with calcium salts (Stressal®) | Irrigation | Orchard | Lower chloride uptake and resulting decreased level of necrosis and lower leaf-water potential under salt stress. Increased synthesis of proline, glycine betaine, and salt stress-response proteins | [137] |
| Spinach (Spinacia oleracea L.) | Legume-based (Trainer®) and animal based (Isabion®) | Foliar | Soilless cultivation system in climate chamber | Increased growth and yield, modulation of root architecture, and increase in N uptake and photosynthetic activity, but dependent on the available N levels | [138] |
| Chickpea (Cicer arietinum L.) | Animal-based, chicken feathers | PH mixed with soil | Pot trial | Increased germination rate, seedling growth, and nodule formation. Improved soil fertility (alteration of N, P, K, and C/N ratio) | [89] |
| Sweet basil (Ocimum basilicum L.) | Legume-based (Trainer®) and collagen-based Siapton® | Foliar | Greenhouse | Improvement of water-use efficiency, CO2 assimilation, and fresh weight with legume-based PH. Negative effects were observed using collagen-based PH at higher doses (decrease in photosynthetic parameters, growth, and biomass production) | [139] |
| Broccoli (Brassica oleracea L.) | Animal-based (Isabion®) and seaweed extract (Wokozim®) | Foliar | Field trial | Enhanced growth and yield, and increase in the content of antioxidant enzymes | [140] |
| Vegetable-based | Foliar | Field trial | Increase in fresh and dry mass, head diameter, and broccoli yield | [141] | |
| Olive (Olea europaea L.) | Vegetable-based (Sinergon Bio®) | Fertigation | Pot and field trial | Increased plant growth; higher leaf photosynthetic rates and stomatal conductance; and increased biomass of roots, stem, shoots, and leaves | [142] |
| Sweet potato (Ipomoea batatas [L.] Lam.) | Animal-based and whey-protein hydrolysate (hydrolyzed by trypsin) | Foliar | Field trial | Combination with potassium fertilization increased shoot dry weight; N, P, and K uptake; average tuber root weight; yield per plant; and marketable yield | [143] |
| Mung bean (Vigna radiata L.) | Animal-based, feathers | Soil application | Field trial | Increase in pod size and seed weight, and higher yields | [144] |
| Tea (Camellia sinensis L.) | Derived from chicken feathers | Soil drench | Trial in poly bags | Increase in plant height, leaf and dry biomass, root length and surface, dry root biomass, and chlorophyll a and b; increase in photosynthetic rate and stomatal conductance; and increase in leaf nitrogen, phosphorous, manganese, and copper contents | [83] |
| Wheat (Triticum aestivum L.) | Fish-based protein hydrolysate (Agromoree®) | Mixed with soil | Pot trial | Increase in wheat ears length, quantity of grains and seed weight, increase in the numbers of productive stems, increase in yield, increase in gluten content and mass fraction of protein | [145] |
| Used as fertilizer | Field trial | ||||
| Whey-protein hydrolysate | Foliar | Field trial | Increase in grain yield, yield attributes, nitrogen accumulation, flag leaf area, and spike number | [146] | |
| Maize (Zea mays L.) | Vegetable-based, alfalfa | Not specified | Hydroponic in climate chamber | Gibberellin- and auxin-like activity, enhanced plant growth and leaf sugar accumulation, and induction of enzymes involved in carbon metabolism | [32] |
| Based on tanning residues | Root | Hydroponic in climate chamber | Enhancement of plant growth and microelement concentrations in maize seedlings; and increased activity of enzymes involved in nitrogen and carbon metabolism | [147] | |
| Plant-based, alfalfa | Root | Hydroponic in climate chamber | Increased activity of enzymes involved in nitrogen metabolism; and increase in plant biomass, antioxidant enzymes’ activity, and phenolics production | [86] | |
| Chickpea and Spirulina platensis | Root | Hydroponic in climate chamber | Auxin- and gibberellin-like activity, positive influence on plant growth and nitrogen assimilation, increased activity of peroxidase and esterase, and increased accumulation of micro- and macroelements | [80] | |
| Legume-based (Trainer®) | Immersion | Laboratory bioassay | Auxin-like effect and significant elongation of the coleoptile | [87] | |
| Primrose (Primula acaulis cv. Danova F1) | Animal-based, anchovy head and viscera | Root | Pot trial | Increase in dry weight and leaf area, chlorophyll content, and root area | [84] |
| Petunia (Petunia × hybrida Hort.) | Animal-based, erythrocyte hydrolysate (Hydrostim®) | Foliar and root | Greenhouse | Depending on the mode of application, a significant effect on visual quality has been observed (increased number of flowers and leaves and leaf area), increase in total aboveground weight, P and K content, photosynthetic parameters, root length, and total root surface area number of root tips and crossings | [148] |
| Patchouli (Pogostemon cablin Benth) | Animal-based, feathers | Soil application | Poly bags, field trial | When used with 50% fertilizer, increased leaf area, dry weight, and chlorophyll content | [144] |
| Soybean (Glycine max [L.] Merril) | Animal-based (acid hydrolysis of collagen, alkalized with KOH) | Foliar | Pot trial | Increased nitrate, amino acid, and ureides content in soybean leaves; altered gene expression; and increased plant productivity | [149] |
| Hemp (Cannabis sativa L.) | Legume-derived | Foliar | Pot trial | Protective effect under saline irrigation, improvement of seed yield and residual biomass (fiber production) | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasković, I.; Popović, L.; Pongrac, P.; Polić Pasković, M.; Kos, T.; Jovanov, P.; Franić, M. Protein Hydrolysates—Production, Effects on Plant Metabolism, and Use in Agriculture. Horticulturae 2024, 10, 1041. https://doi.org/10.3390/horticulturae10101041
Pasković I, Popović L, Pongrac P, Polić Pasković M, Kos T, Jovanov P, Franić M. Protein Hydrolysates—Production, Effects on Plant Metabolism, and Use in Agriculture. Horticulturae. 2024; 10(10):1041. https://doi.org/10.3390/horticulturae10101041
Chicago/Turabian StylePasković, Igor, Ljiljana Popović, Paula Pongrac, Marija Polić Pasković, Tomislav Kos, Pavle Jovanov, and Mario Franić. 2024. "Protein Hydrolysates—Production, Effects on Plant Metabolism, and Use in Agriculture" Horticulturae 10, no. 10: 1041. https://doi.org/10.3390/horticulturae10101041
APA StylePasković, I., Popović, L., Pongrac, P., Polić Pasković, M., Kos, T., Jovanov, P., & Franić, M. (2024). Protein Hydrolysates—Production, Effects on Plant Metabolism, and Use in Agriculture. Horticulturae, 10(10), 1041. https://doi.org/10.3390/horticulturae10101041











