Lithium: An Element with Potential for Biostimulation and Biofortification Approaches in Plants
Abstract
1. Introduction
2. Abundance and Concentrations in the Environment
3. Uses of and Demand for Li
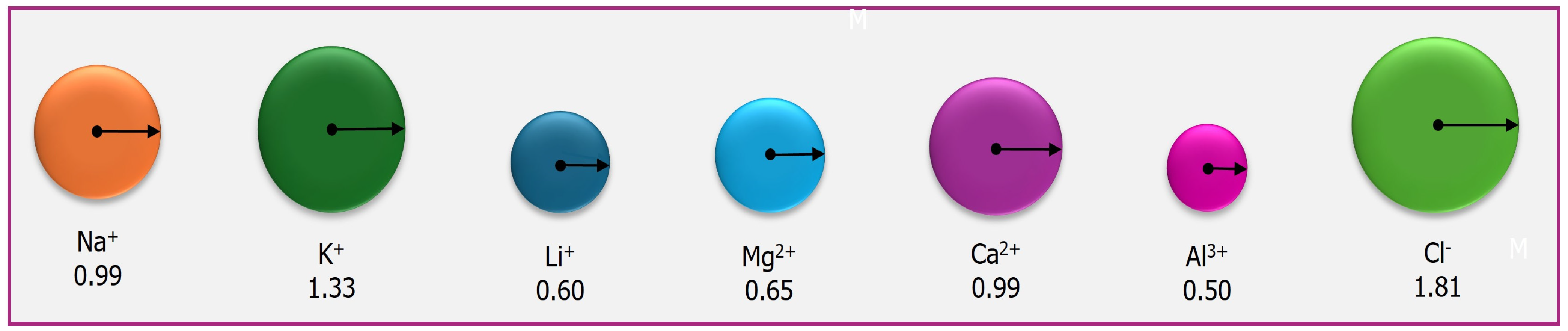
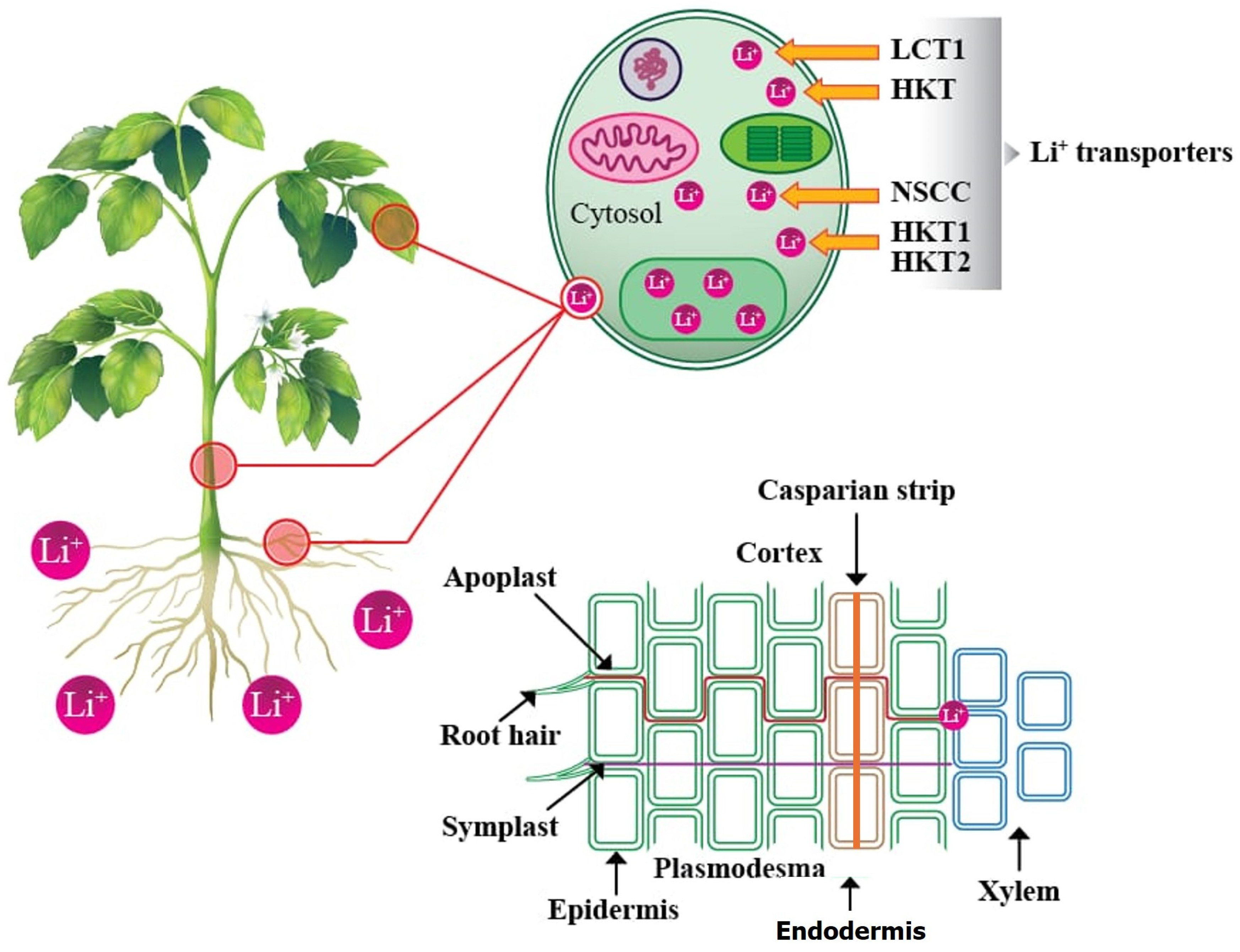
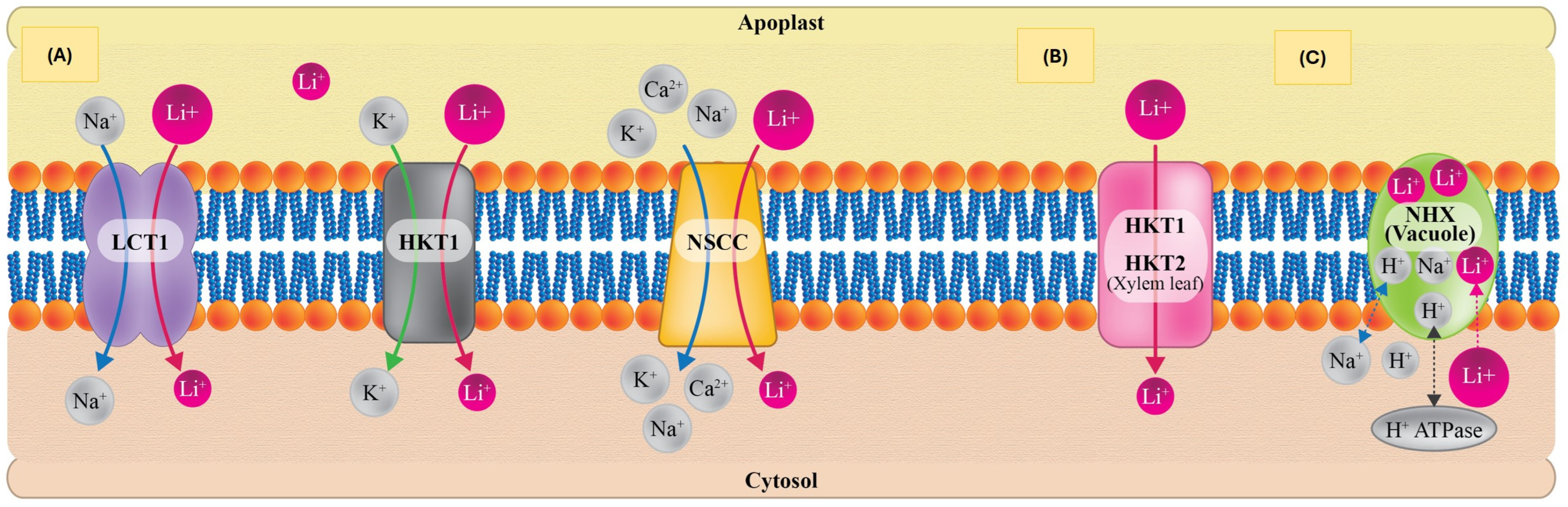
4. Lithium Absorption, Transport, Sequestration, and Translocation in Plants
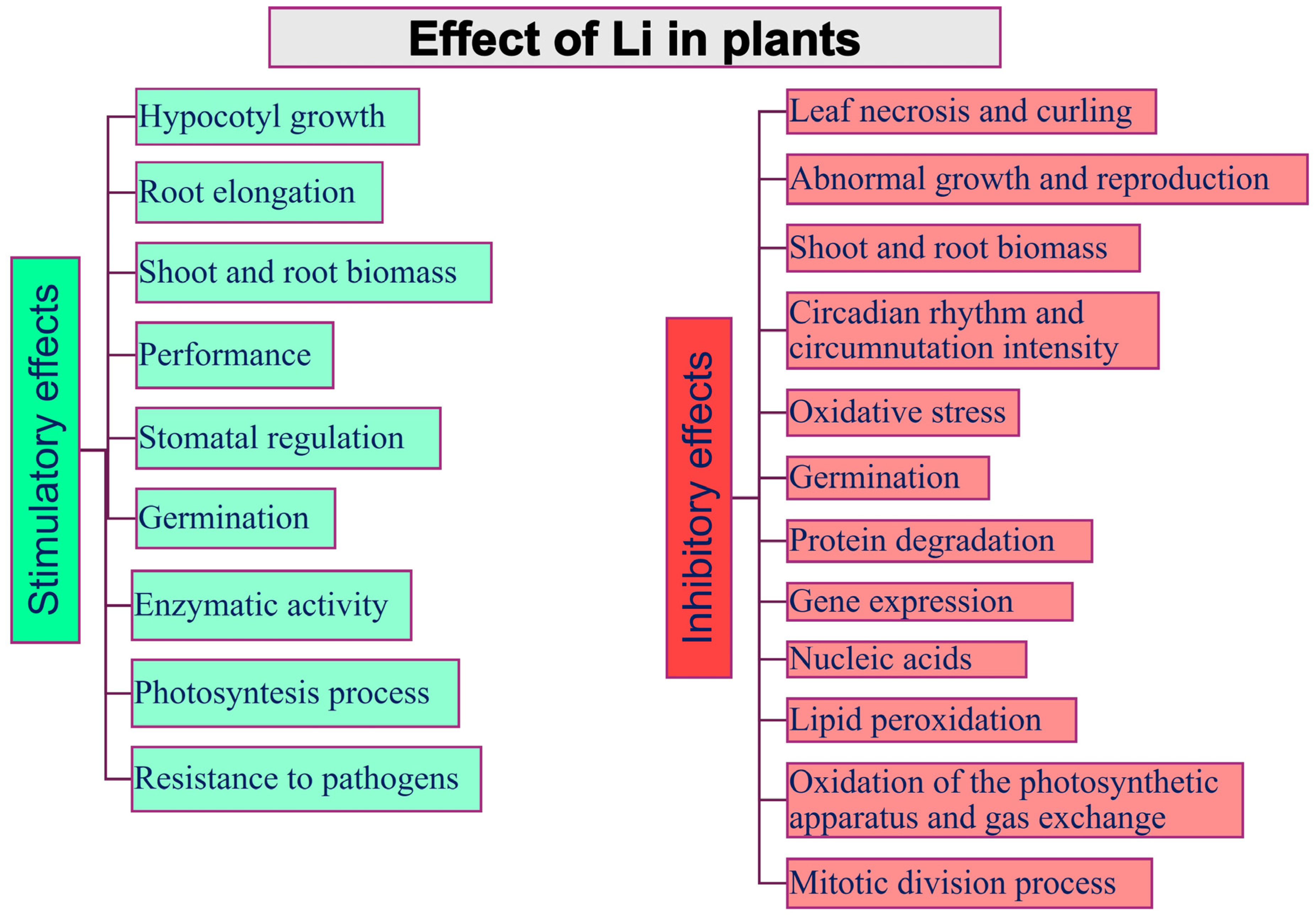
5. Li in Plant Metabolism and Physiology
6. Phytotoxic Effects of Li
7. Interaction of Li with Other Elements, Nutrients, and Biomolecules
8. Ranges of Tolerance, Cytotoxicity, and Genotoxicity of Li
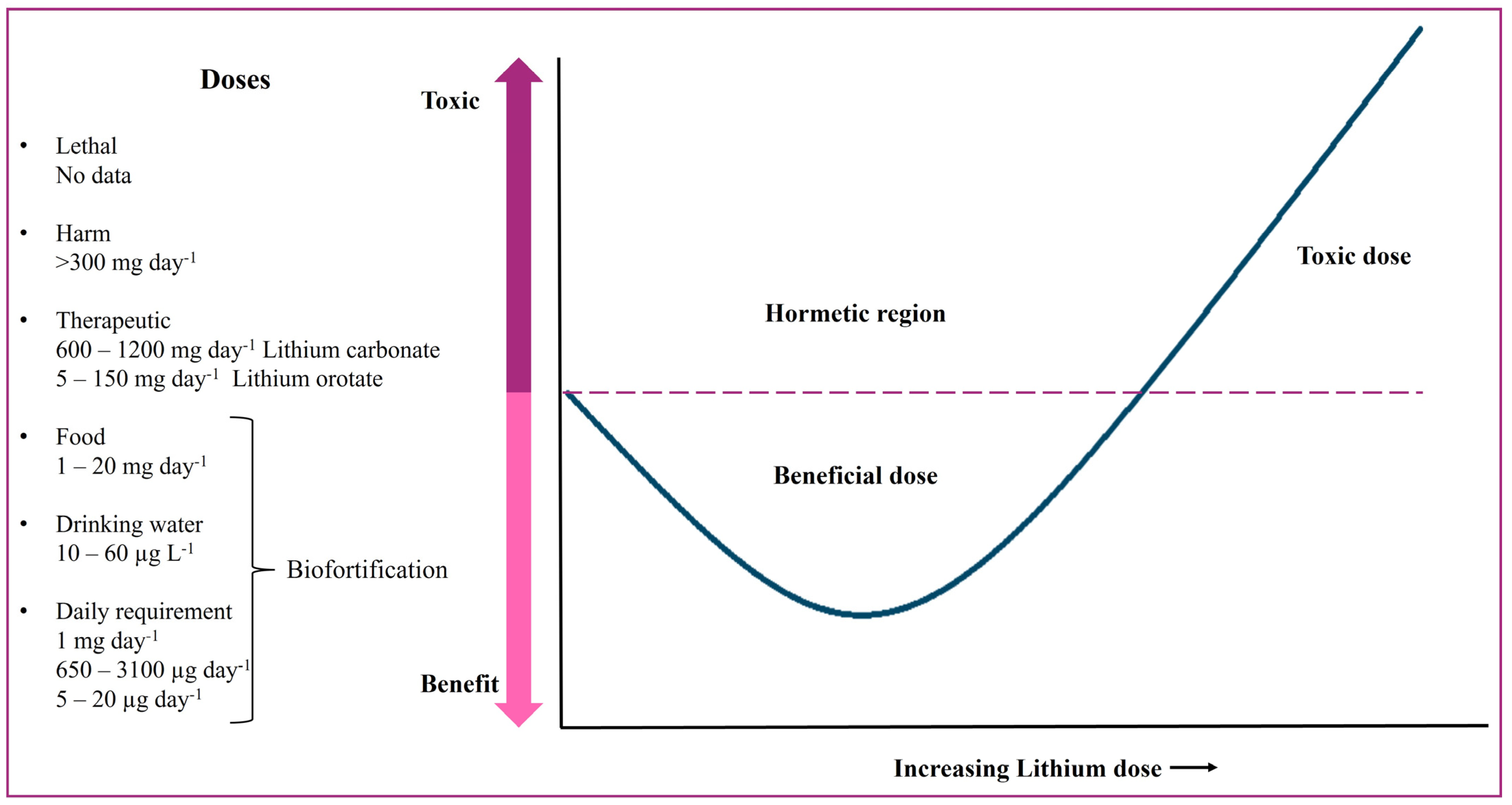
9. Treatment with and Intake of Li in Humans
10. The Future of Li in Agriculture and Animal Production
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habashi, F. Lithium, physical and chemical properties. In Encyclopedia of Metalloproteins, 1st ed.; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 1222–1223. [Google Scholar]
- Baran, E.J. Lithium in plants. In Plant Abiotic Stresses, Physiological Mechanisms Tools and Regulation, 1st ed.; Hemantaranjan, A., Ed.; Scientific Publishers: New Delhi, India, 2019; pp. 151–162. [Google Scholar]
- Sterba, J.; Krzemień, A.; Fernández, P.R.; García-Miranda, C.E.; Valverde, G.F. Lithium mining: Accelerating the transition to sustainable energy. Resour. Policy 2019, 62, 416–426. [Google Scholar] [CrossRef]
- Sobolev, O.I.; Gutyj, B.V.; Darmohray, L.M.; Sobolieva, S.V.; Ivanina, V.V.; Kuzmenko, O.A.; Karkach, P.M.; Fesenko, V.F.; Bilkevych, V.V.; Mashkin1, Y.O.; et al. Lithium in the natural environment and its migration in the trophic chain. Ukr. J. Ecol. 2019, 9, 195–203. [Google Scholar]
- Anderson, C.E. Lithium in plants. In Lithium and Cell Physiology, 1st ed.; Bach, R.O., Gallicchio, V.S., Eds.; Springer: New York, NY, USA, 1990; pp. 25–46. [Google Scholar]
- Bernard, A. Lithium. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Elsevier-Academic Press: San Diego, CA, USA, 2015; Volume 2, pp. 969–974. [Google Scholar]
- Schrauzer, G.N. Lithium in biosphere, distribution. In Encyclopedia of Metalloproteins, 1st ed.; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 1213–1217. [Google Scholar]
- Naeem, A.; Aslam, M.; Mühling, K.H. Lithium: Perspectives of nutritional beneficence, dietary intake, biogeochemistry, and biofortification of vegetables and mushrooms. Sci. Total Environ. 2021, 798, 149249. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Kalinowska, M.; Szymańska, M. A Study on selected physiological parameters of plants grown under lithium supplementation. Biol. Trace Elem. Res. 2012, 149, 425–430. [Google Scholar] [CrossRef]
- Wietelmann, U.; Steinbild, M. Lithium and lithium compounds. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 1–38. [Google Scholar]
- Franzaring, J.; Schlosser, S.; Damsohn, W.; Fangmeier, A. Regional differences in plant levels and investigations on the phytotoxicity of lithium. Environ. Pollut. 2016, 216, 858–865. [Google Scholar] [CrossRef]
- Miletíc, Z.; Markovíc, M.; Jaríc, S.; Radulovíc, N.; Sekulíc, D.; Mitrovíc, M.; Pavlovíc, P. Lithium and strontium accumulation in native and invasive plants of the Sava River: Implications for bioindication and phytoremediation. Ecotoxicol. Environ. Saf. 2024, 270, 115875. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Hassan, W.; Shah, A.N.; Anjum, S.A.; Cheema, S.A.; Ali, I. Lithium toxicity in plants: Reasons, mechanisms and remediation possibilities–A review. Plant Physiol. Biochem. 2016, 107, 104–115. [Google Scholar] [CrossRef]
- Aral, H.; Vecchio-Sadus, A. Toxicity of lithium to humans and the environment—A literature review. Ecotoxicol. Environ. Saf. 2008, 70, 349–356. [Google Scholar] [CrossRef]
- Gad, S.C.; Pham, T. Lithium. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 94–95. [Google Scholar]
- Shakoor, N.; Adeel, M.; Azeem, I.; Ahmad, M.A.; Zain, M.; Abbas, A.; Zhou, P.; Li, Y.; Ming, X.; Rui, Y. Responses of agricultural plants to lithium pollution: Trends, meta-analysis, and perspectives. BioRxiv 2022. [Google Scholar] [CrossRef]
- Aral, H.; Vecchio-Sadus, A. Lithium: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health, 1st ed.; Nriagu, J.O., Ed.; Elservier: Amsterdam, The Netherlands, 2011; pp. 499–508. [Google Scholar]
- Duff, M.C.; Kuhne, W.W.; Halverson, N.V.; Chang, C.S.; Kitamura, E.; Hawthorn, L.; Martínez, N.E.; Stafford, C.; Milliken, C.E.; Caldwell, E.F.; et al. mRNA Transcript abundance during plant growth and the influence of Li+ exposure. Plant Sci. 2014, 229, 262–279. [Google Scholar] [CrossRef]
- Tanveer, M.; Hasanuzzaman, M.; Wang, L. Lithium in environment and potential targets to reduce lithium toxicity in plants. J. Plant Growth Regul. 2019, 38, 1574–1586. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Jasiewicz, C.; Koncewicz-Baran, M.; Bączek-Kwinta, R. Determination of lithium bioretention by maize under hydroponic conditions. Arch. Environ. Prot. 2017, 43, 94–104. [Google Scholar] [CrossRef]
- Environment Protection Regulation 2005 (EPR). Available online: https://www.legislation.act.gov.au/sl/2005-38 (accessed on 12 August 2024).
- Balaram, V.; Santosh, M.; Satyanarayanan, M.; Srinivas, N.; Gupta, H. Lithium: A review of applications, occurrence, exploration, extraction, rercycling, analysis, and environmental impact. Geosci. Front. 2024, 15, 101868. [Google Scholar] [CrossRef]
- Adeel, M.; Zain, M.; Shakoor, N.; Ahmad, M.A.; Azeem, I.; Aziz, M.A.; Tulcan, R.X.S.; Rathore, A.; Tahir, M.; Horton, R.; et al. Global navigation of lithium in water bodies and emerging human health crisis. npj Clean Water 2023, 6, 33. [Google Scholar] [CrossRef]
- Kuloğlu, S.S.; Yalçin, E.; Çavuşoğlu, K.; Acar, A. Dose-dependent toxicity profile and genotoxicity mechanism of lithium carbonate. Sci. Rep. 2022, 12, 13504. [Google Scholar] [CrossRef]
- Kalinowska, M.; Hawrylak-Nowak, B.; Szymańska, M. The influence of two lithium forms on the growth, L-ascorbic acid content and lithium accumulation in lettuce plants. Biol. Trace Elem. Res. 2013, 152, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, L.; Keohane, J.; Cabellos, G.G.; Lloyd, A.; Cleary, J. Induced plant accumulation of lithium. Geosciences 2018, 8, 56. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements of Group 12 (Previously Group IIb). In Trace Elements from Soil to Human, 1st ed.; Springer: Berlin, Germany, 2007; pp. 283–319. [Google Scholar]
- Ammari, T.G.; Al-Zu’bi, Y.; Abu-Baker, S.; Dababneh, B.; Gnemat, W.; Tahboub, A. The occurrence of lithium in the environment of the Jordan Valley and its transfer into the food chain. Environ. Geochem. Health 2011, 33, 427–437. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Piasentin, S.; Furini, A. Nutrient metal elements in plants. Metallomics 2014, 6, 1770–1788. [Google Scholar] [CrossRef]
- Qiao, L.; Tanveer, M.; Wang, L.; Tian, C. Subcellular distribution and chemical forms of lithium in Li-accumulator Apocynum venetum. Plant Physiol. Biochem. 2018, 132, 341–344. [Google Scholar] [CrossRef]
- Chan, G.A.H.; dos Santos, G.R.; Dias, M.A.R.; da Silva, D.B.; Ramos, D.P.; Rodrigues, L.U.; Barilli, J.; Moreira, P.S.; Mendez, D.F.S.; Ferrari, J.M.; et al. Chia biofortification with lithium sources applied by foliar fertilization. Am. Acad. Sci. Res. J. Eng. Technol. Sci. 2021, 75, 121–137. [Google Scholar]
- Gayathri, N.; Sailesh, A.R.; Srinivas, N. Effect of lithium on seed germination and plant growth of Amaranthus viridis. J. Appl. Nat. Sci. 2022, 14, 133–139. [Google Scholar] [CrossRef]
- Kent, N.L. Absorption, translocation and ultimate fate of lithium in the wheat plant. New Phytol. 1941, 40, 291–298. [Google Scholar] [CrossRef]
- Alcántar, G.G.; Trejo-Téllez, L.I.; Gómez-Merino, F.C. Nutrición de Cultivos, 2nd ed.; Colegio de Postgraduados: Guadalajara, Jal, Mexico, 2016. [Google Scholar]
- Bahamonde, H.A.; Fernández, V.; Gyenge, J.; Mattenet, F.; Peri, P.L. Essential nutrient and trace element foliar resorption of two co-existing Nothofagus species grown under different environmental conditions in southern Patagonia. Front. Plant Sci. 2019, 10, 1542. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, W.E. The action on the growth of crops of small percentages of certain metallic compounds when applied with ordinary artificial fertilisers. J. Agric. Sci. 1932, 22, 704–735. [Google Scholar] [CrossRef]
- Liang, X.; Shen, N.F.; Theologis, A. Li+-regulated 1-aminocyclopropane-1-carboxylate synthase gene expression in Arabidopsis thaliana. Plant J. 1996, 10, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, M.A.; Romero, C.; Bellés, J.M.; Montesinos, C.; Vicente, O.; Serrano, R. Lithium treatment induces a hypersensitive-like response in tobacco. Planta 2003, 217, 417–424. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Gantait, S.; Mitra, M.; Yang, Y.; Li, X. Role of ethylene crosstalk in seed germination and early seedling development: A review. Plant Physiol. Biochem. 2020, 151, 124–131. [Google Scholar] [CrossRef]
- Squassina, A.; Manchia, M.; Chillotti, C.; Deiana, V.; Congiu, D.; Paribello, F.; Roncada, P.; Soggiu, A.; Piras, C.; Urbani, A.; et al. Differential effect of lithium on spermidine/spermine N1-acetyltransferase expression in suicidal behaviour. Int. J. Neuropsychopharmacol. 2013, 16, 2209–2218. [Google Scholar] [CrossRef]
- Gilad, G.M.; Gilad, V.H. Overview of the brain polyamine-stress-response: Regulation, development, and modulation by lithium and role in cell survival. Cell Mol. Neurobiol. 2003, 23, 637–649. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.R.; de Faria, A.J.G.; Alexandrino, G.d.C.; Ribeiro, E.A.; dos Santos, A.C.M.; Deusdara, T.T.; do Nascimento, I.R.; Nascimento, V.L. Enrichment of lithium in lettuce plants through agronomic biofortification. J. Plant Nutr. 2019, 42, 2102–2113. [Google Scholar] [CrossRef]
- Makus, D.J.; Zibilske, L.; Lester, G. Effect of light intensity, soil type, and lithium addition on spinach and mustard greens leaf constituents. Subtrop. Plant Sci. 2006, 58, 35–41. [Google Scholar]
- Bonaventura, R.; Costa, C.; Deidda, I.; Zito, F.; Russo, R. Gene expression analysis of the stress response to lithium, nickel, and zinc in Paracentrotus lividus Embryos. Toxics 2022, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, N.; Adeel, M.; Azeem, I.; Ahmad, M.A.; Zain, M.; Abbas, A.; Hussain, M.; Jiang, Y.; Zhou, P.; Li, Y.; et al. Interplay of higher plants with lithium pollution: Global trends, meta-analysis, and perspectives. Chemosphere 2023, 310, 136663. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.; Zhang, L.; Tian, C. Tolerance and accumulation of lithium in Apocynum pictum Schrenk. PeerJ 2018, 6, e5559. [Google Scholar] [CrossRef]
- Zonia, L.E.; Tupý, J. Lithium treatment of Nicotiana tabacum microspores blocks polar nuclear migration, disrupts the partitioning of membrane-associated Ca2+, and induces symmetrical mitosis. Sex. Plant Reprod. 1995, 8, 152–160. [Google Scholar] [CrossRef]
- Allender, W.J.; Cresswell, G.C.; Kaldor, J.; Kennedy, I.R. Effect of lithium and lanthanum on herbicide induced hormesis in hydroponically-grown cotton and corn. J. Plant Nutr. 1997, 20, 81–95. [Google Scholar] [CrossRef]
- Bingham, F.T.; Bradford, G.R.; Page, A.L. Toxicity of lithium to plants. Calif. Agric. 1964, 18, 6–7. [Google Scholar]
- McStay, N.G.; Rogers, H.H.; Anderson, C.E. Effects of lithium on Phaseolus vulgaris L. Sci. Total Environ. 1980, 16, 185–191. [Google Scholar] [CrossRef]
- Hara, T.; Sonoda, Y.; Iwai, I. Growth response of cabbage plants to lithium, sodium, and rubidium under water culture conditions. Soil Sci. Plant Nutr. 1977, 23, 531–539. [Google Scholar] [CrossRef][Green Version]
- Magalhães, J.R.; Wilcox, G.E.; Rocha, A.N.F.; Silva, F.L.I.M. Research on lithium-phytological metabolism and recovery of hypo-lithium. Pesqui. Agropecu. Bras. 1990, 25, 1781–1787. [Google Scholar]
- Li, X.; Gao, P.; Gjetvaj, B.; Westcott, N.; Gruber, M.Y. Analysis of the metabolome and transcriptome of Brassica carinata seedlings after lithium chloride exposure. Plant Sci. 2009, 177, 68–80. [Google Scholar] [CrossRef]
- Stolarz, M.; Król, E.; Dziubińska, H. Lithium distinguishes between growth and circumnutation and augments glutamate-induced excitation of Helianthus annuus seedlings. Acta Physiol. Plant. 2015, 37, 69. [Google Scholar] [CrossRef]
- Epstein, E. Calcium–lithium competition in absorption by plant roots. Nature 1960, 185, 705–706. [Google Scholar] [CrossRef]
- Monreal, J.A.; López-Baena, F.J.; Vidal, J.; Echevarría, C.; García-Mauriño, S. Effect of LiCl on phosphoenolpyruvate carboxylase kinase and the phosphorylation of phosphoenolpyruvate carboxylase in leaf disks and leaves of Sorghum vulgare. Planta 2007, 225, 801–812. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Lithium: Occurrence, dietary intakes, nutritional essentiality. J. Am. Coll. Nutr. 2002, 21, 14–21. [Google Scholar] [CrossRef]
- Marshall, T.M. Lithium as a nutrient. J. Am. Physicians Surg. 2015, 20, 104–109. [Google Scholar]
- Jefferson, J.W.; Greist, J.H. Lithium in psychiatry—A review. CNS Drugs 1994, 1, 448–464. [Google Scholar] [CrossRef]
- Szklarska, D.; Rzymski, P. Is lithium a micronutrient? From biological activity and epidemiological observation to food fortification. Biol. Trace Elem. Res. 2019, 189, 18–27. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Pressman, P.; Hayes, A.W.; Dhawan, G.; Kapoor, R.; Agathokleous, E.; Calabrese, V. Lithium and hormesis: Enhancement of adaptive responses and biological performance via hormetic mechanisms. J. Trace Elem. Med. Biol. 2023, 78, 127156. [Google Scholar] [CrossRef] [PubMed]
- Kling, M.A.; Manowitz, P.; Pollack, I.W. Rat brain and serum lithium concentrations after acute injections of lithium carbonate and orotate. J. Pharm. Pharmacol. 1978, 30, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, O.; Borshch, O.O.; Riznychuk, I.; Kyshlaly, O. Fortification of meat products of geese farming with lithium by introducing it into poultry mixed feed. Agraarteadus J. Agric. Sci. 2022, 33, 154–161. [Google Scholar]
- Thibon, F.; Weppe, L.; Vigier, N.; Churlaud, C.; Lacoue-Labarthe, T.; Metian, M.; Cherel, Y.; Bustamante, P. Large-scale survey of lithium concentrations in marine organisms. Sci. Total Environ. 2021, 751, 141453. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, X.; Read, P.; Loseke, B.; Gamet, S.; Li, W.; Xu, C. Biofortification with selenium and lithium improves nutraceutical properties of major winery grapes in the Midwestern United States. Int. J. Food Sci. Tech. 2021, 56, 825–837. [Google Scholar] [CrossRef]
| Name (Formula) | Atomic Mass (g mol−1) | Oxidation State | Boiling Point (°C) | Melting Point (°C) | Density (g cm−3) | Solubility | n-Octanol/Water Partition Coefficient (Log KOW) |
|---|---|---|---|---|---|---|---|
| Lithium (Li+) | 6.94 | +1 | 1342 | 180.54 | 0.53 | --- | --- |
| Li molybdate (Li2MoO4) | 173.82 | +1 | --- | 700.00 | 2.66 | Highly water soluble | --- |
| Li hydroxide (LiOH) | 23.95 | +1 | 450 | 924.00 | 1.46 | Water soluble | ≈0.00 a |
| Li chloride (LiCl) | 42.39 | +1 | 1382 | 614.00 | 2.07 | Water soluble | −2.66 |
| Li carbonate (Li2CO3) | 70.02 | +1 | --- | 726.00 | 2.10 | Water soluble | −6.19 |
| Plant | Concentration | Effects | References |
|---|---|---|---|
| Cabbage (Brassica oleracea var. capitata L.) cv. Nagaoka | 1 and 10 meq Li L−1 | Total dry weight decreased by 50% | [52] |
| Common bean (Phaseolus vulgaris L.) Bush Blue Lake 290 | 4 mg LiNO3 kg−1 >4 mg LiNO3 kg−1 | Increases in plant height, fresh weight, and leaf area. Partial closure of stomata and an effect on water relations | [51] |
| Radish (Raphanus sativus L.) | 1 mM LiNO3 2 mM LiNO3 | Increase in dry weight of leaves and bulb Reduction in dry weight of leaves and bulb | [53] |
| Lettuce (Lactuca sativa L.) | 1–2 mM LiNO3 | Significant increase in dry weight of leaves | [53] |
| Watercress (Nasturtium officinale L.) | 1 mM LiNO3 | Significant increase in dry weight of leaves | [53] |
| Tobacco (Nicotiana tabacum L.) | 50 mM LiCl | Growth inhibition and leaf necrosis | [38] |
| Spinach (Spinacia oleracea) cv. Samich | 40 mg Li2SO4 kg−1 | Increase in biomass | [44] |
| Mustard greens (Brassica juncea) cv. Florida Broadleaf | 40 mg Li2SO4 kg−1 | Increase in biomass | [44] |
| Abyssinian mustard (Brassica carinata) | 0.03–0.3 mM LiCl 30–120 mM LiCl | Increase in radicle length at germination and fresh weight Reduces germination rate, fresh weight, and chlorophyll concentration Increases anthocyanins | [54] |
| Sunflower (Helianthus annuus L.) | 50 mg LiCl dm−3 | Necrosis in leaves, reduction of dry biomass in shoots and leaf area Increased levels of lipid peroxidation | [9] |
| Maize (Zea mays L.) var. saccharata Kcke, cv. Zlota Karlowa | 50 mg LiCl dm−3 5 mg LiCl dm−3 | Necrosis in leaves, reduction in dry biomass, decrease in photosynthetic pigment content, and increase in lipid peroxidation levels Increased aboveground biomass and leaf area | [9] |
| Lettuce (Lactuca sativa L.) var. capitata cv. Justyna | 100 mg LiOH or LiCl dm−3 50 mg LiOH dm−3 2.5 mg LiOH dm−3 | Necrosis, inhibition of growth and yield Reduction in fresh weight of shoots and roots Increased fresh root weight | [25] |
| Sunflower (Helianthus annuus L.) | 0.2, 0.5, 5, 10, 60, and 80 mM LiCl | Reduction in hypocotyl length | [55] |
| Kendyr (Apocynum pictum Schrenk) | 25 mmol LiCl 100–400 mmol LiCl 200–400 mmol LiCl | Increased seed germination percentage Decrease in germination rate and percentage Reduction in dry weight of leaves, stems, and roots Decrease in chlorophyll a and b and carotene content | [47] |
| Lettuce (Lactuca sativa L.) | 7.5 to 22 mg Li2SO4 or LiOH dm−3 40 mg Li2SO4 dm−3 | Increases dry root weight, specific leaf area, and stem diameter Reduced stem diameter, root dry weight, total leaf area, plant height, and leaf dry weight | [43] |
| Onion (Allium cepa L.) | 100 mg Li2SO4 L−1 | Decreased germination rate and root length | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buendía-Valverde, M.d.l.L.; Gómez-Merino, F.C.; Fernández-Pavía, Y.L.; Mateos-Nava, R.A.; Trejo-Téllez, L.I. Lithium: An Element with Potential for Biostimulation and Biofortification Approaches in Plants. Horticulturae 2024, 10, 1022. https://doi.org/10.3390/horticulturae10101022
Buendía-Valverde MdlL, Gómez-Merino FC, Fernández-Pavía YL, Mateos-Nava RA, Trejo-Téllez LI. Lithium: An Element with Potential for Biostimulation and Biofortification Approaches in Plants. Horticulturae. 2024; 10(10):1022. https://doi.org/10.3390/horticulturae10101022
Chicago/Turabian StyleBuendía-Valverde, María de la Luz, Fernando Carlos Gómez-Merino, Yolanda Leticia Fernández-Pavía, Rodrigo Aníbal Mateos-Nava, and Libia Iris Trejo-Téllez. 2024. "Lithium: An Element with Potential for Biostimulation and Biofortification Approaches in Plants" Horticulturae 10, no. 10: 1022. https://doi.org/10.3390/horticulturae10101022
APA StyleBuendía-Valverde, M. d. l. L., Gómez-Merino, F. C., Fernández-Pavía, Y. L., Mateos-Nava, R. A., & Trejo-Téllez, L. I. (2024). Lithium: An Element with Potential for Biostimulation and Biofortification Approaches in Plants. Horticulturae, 10(10), 1022. https://doi.org/10.3390/horticulturae10101022





