Lactic Acid Bacteria and Cellulase Improve the Fermentation Characteristics, Aerobic Stability and Rumen Degradation of Mixed Silage Prepared with Amaranth and Rice Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Silage Preparation
2.2. Experimental Design
2.3. Chemical Composition and Fermentation Quality Analysis of Mixed Silage
2.4. Microbial Composition and Aerobic Stability Analysis of Mixed Silage
2.5. Rumen Degradability of Mixed Silage
2.6. Statistical Analysis
3. Results
3.1. Fermentation Characteristics of Amaranth and Rice-Straw Mixed Silage
3.2. Chemical Composition of Amaranth and Rice-Straw Mixed Silage
3.3. Microbial Population of Amaranth and Rice-Straw Mixed Silage
3.4. Aerobic Stability of Amaranth and Rice-Straw Mixed Silage
3.5. Ruminal Dry-Matter Degradation Characteristics of Amaranth and Rice-Straw Mixed Silage
3.6. Ruminal Crude-Protein Degradation Characteristics of Amaranth and Rice-Straw Mixed Silage
3.7. Ruminal Neutral-Detergent-Fiber Degradation Characteristics of Amaranth and Rice-Straw Mixed Silage
3.8. Ruminal Acid-Detergent-Fiber Degradation Characteristics of Amaranth and Rice-Straw Mixed Silage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, C.; Wu, Y.; Li, S.; Cao, Z.; Wang, Y.; Mao, J.; Shi, H.; Shi, R.; Su, X.; Zhen, K.; et al. Effects of different forage types on rumen fermentation, microflora, and production performance in peak-lactation dairy cows. Fermentation 2022, 8, 507. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, Q.; Li, F.; Xu, C.; Cai, Y. Comparative analysis of ensiling characteristics and protein degradation of alfalfa silage prepared with corn or sweet sorghum in semiarid region of Inner Mongolia. Anim. Sci. J. 2020, 91, e13321. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, M.; Rouzbhan, Y.; Rezaei, J. Influence of diets containing different levels of Salicornia bigelovii forage on digestibility, ruminal and blood variables and antioxidant capacity of Shall male sheep. Anim. Feed Sci. Technol. 2021, 281, 115085. [Google Scholar] [CrossRef]
- Xie, Y.; Du, E.; Yao, Y.; Wang, W.; Huang, X.; Sun, H.; Zheng, Y.; Cheng, Q.; Wang, C.; Chen, C.; et al. Effect of epiphytic microflora after aerobic enrichment and reconstitution on fermentation quality and microbial community of corn stalk silage and Pennisetum sinese silage. Front. Microbiol. 2022, 13, 1078408. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sun, G.; Shah, A.M.; Fan, X.; Li, S.; Yu, X. Effects of different growth stages of amaranth silage on the rumen degradation of dairy cows. Animals 2019, 9, 793. [Google Scholar] [CrossRef]
- Rahjerdi, N.K.; Rouzbehan, Y.; Fazaeli, H.; Rezaei, J. Chemical composition, fermentation characteristics, digestibility, and degradability of silages from two amaranth varieties (kharkovskiy and sem), corn, and an amaranth-corn combination. J. Anim. Sci. 2015, 93, 5781–5790. [Google Scholar] [CrossRef]
- Rezaei, J.; Rouzbehan, Y.; Zahedifar, M.; Fazaeli, H. Effects of dietary substitution of maize silage by amaranth silage on feed intake, digestibility, microbial nitrogen, blood parameters, milk production and nitrogen retention in lactating Holstein cows. Anim. Feed Sci. Technol. 2015, 202, 32–41. [Google Scholar] [CrossRef]
- Rezaei, J.; Rouzbehan, Y.; Fazaeli, H.; Zahedifar, M. Effects of substituting amaranth silage for corn silage on intake, growth performance, diet digestibility, microbial protein, nitrogen retention and ruminal fermentation in fattening lambs. Anim. Feed Sci. Technol. 2014, 192, 29–38. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, C.; Zu, X.; Wang, X.; Yu, X.; Chen, H.; Xu, L.; Wang, M.; Li, Q. Effect of mixing peanut vine on fermentation quality, nitrogen fraction and microbial community of high-moisture alfalfa silage. Fermentation 2023, 9, 713. [Google Scholar] [CrossRef]
- Guan, H.; Li, H.; Gan, L.; Chen, S.; Yan, Y.; Jia, Z.; Liu, W.; Wei, X.; Ma, X.; Zhou, Q. The effects of native lactic acid bacteria on the microbiome, fermentation profile, and nutritive value of napier grass silage prepared with different legume ratios. Front. Microbiol. 2023, 13, 1112058. [Google Scholar] [CrossRef]
- Ding, H.; Han, Z.; Li, J.; Li, X.; Dong, Z.; Zhao, J.; Wang, S.; Shao, T. Effect of fibrolytic enzymes, cellulolytic fungi and lactic acid bacteria on fermentation characteristics, structural carbohydrate composition and in vitro digestibility of rice straw silage. Fermentation 2022, 8, 709. [Google Scholar] [CrossRef]
- Stirling, S.; Díaz, J.E.; Repetto, J.L.; Pla, M.; Arroyo, J.M.; Cajarville, C. Growth stage and ensiling: Impact on chemical composition, conservation quality and in situ ruminal degradability of whole-crop oat. J. Sci. Food Agric. 2022, 102, 2783–2791. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Dang, S.; Liu, S.; Jing, L.; Wang, Y.; Zhang, W. Determination of the appropriate ratio of sample size to nylon bag area for in situ nylon bag technique evaluation of rumen digestibility of feedstuffs in sheep. Livest. Sci. 2020, 241, 104254. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburgs, MD, USA, 2000. [Google Scholar]
- Murphy, R.P. A method for the extraction of plant samples and the determination of total soluble carbohydrates. J. Sci. Food Agric. 1958, 9, 714–717. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zheng, M.; Zhou, Y.; Gao, L.; Zhou, W.; Wang, M.; Zhu, Y.; Xu, W. Improving the quality of napier grass silage with pyroligneous acid: Fermentation, aerobic stability, and microbial communities. Front. Microbiol. 2022, 13, 1034198. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Na, N.; Li, X.; Li, Z.; Wang, C.; Wu, X.; Xiao, Y.; Yin, G.; Liu, S.; Liu, Z.; et al. Impact of packing density on the bacterial community, fermentation, and in vitro digestibility of whole-crop barley silage. Agriculture 2021, 11, 672. [Google Scholar] [CrossRef]
- de Melo, N.N.; Carvalho-Estrada, P.A.; Tavares, Q.G.; Pereira, L.M.; Delai Vigne, G.L.; Camargo Rezende, D.M.L.; Schmidt, P. The effects of short-time delayed sealing on fermentation, aerobic stability and chemical composition on maize silages. Agronomy 2023, 13, 223. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy of Sciences: Washington, DC, USA, 2001.
- Øskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighed according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Dong, C.; Liu, P.; Wang, X.; Zhang, W.; He, L. Effects of phenyllactic acid on fermentation parameters, nitrogen fractions and bacterial community of high-moisture stylo silage. Fermentation 2023, 9, 572. [Google Scholar] [CrossRef]
- Chen, X. Economic potential of biomass supply from crop residues in China. Appl. Energy 2016, 166, 141–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Huang, R.; Wang, X.; Ma, C.; Zhang, F. Effects of Lactiplantibacillus plantarum and Lactiplantibacillus brevis on fermentation, aerobic stability, and the bacterial community of paper mulberry silage. Front. Microbiol. 2022, 13, 1063914. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Li, J.; Xin, Y.; Wang, T.; Chen, C.; Zhong, Y.; Zhang, L.; Liu, H.; He, Y.; Wen, X.; et al. Response of fermentation quality and microbial community of oat silage to homofermentative lactic acid bacteria inoculation. Front. Microbiol. 2023, 13, 1091394. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Ge, G.; Wang, Z.; Xiao, Y.; Zhao, M.; Sun, L.; Wang, Y.; Zhang, J.; Jia, Y.; Du, S. Effect of isolated lactic acid bacteria on the quality and bacterial diversity of native grass silage. Front. Plant Sci. 2023, 14, 1160369. [Google Scholar] [CrossRef]
- Si, Q.; Wang, Z.; Liu, W.; Liu, M.; Ge, G.; Jia, Y.; Du, S. Influence of cellulase or Lactiplantibacillus plantarum on the ensiling performance and bacterial community in mixed silage of alfalfa and Leymus chinensis. Microorganisms 2023, 11, 426. [Google Scholar] [CrossRef]
- Agustinho, B.C.; Daniel, J.L.P.; Zeoula, L.M.; Ferraretto, L.F.; Monteiro, H.F.; Pupo, M.R.; Ghizzi, L.G.; Agarussi, M.C.N.; Heinzen, C.; Lobo, R.R.; et al. Effects of lignocellulolytic enzymes on the fermentation profile, chemical composition, and in situ ruminal disappearance of whole-plant corn silage. J. Anim. Sci. 2021, 99, skab295. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Wang, Q.; Wang, Y.; Zhang, Z. Effects of cellulase and xylanase on fermentative profile, bacterial diversity, and in vitro degradation of mixed silage of agro-residue and alfalfa. Chem. Biol. Technol. Agric. 2023, 10, 40. [Google Scholar] [CrossRef]
- Bai, B.; Qiu, R.; Wang, Z.; Liu, Y.; Bao, J.; Sun, L.; Liu, T.; Ge, G.; Jia, Y. Effects of cellulase and lactic acid bacteria on ensiling performance and bacterial community of Caragana korshinskii silage. Microorganisms 2023, 11, 337. [Google Scholar] [CrossRef]
- Nicola, L.H.; Jörg, S.; Claudia, D.; Hans-Joachim, N.; Hans, O. Influence on lactic acid content in maize silage variations by manganese supplementation. Ind. Crops Prod. 2016, 79, 146–151. [Google Scholar]
- Zhao, C.; Wang, L.; Ma, G.; Jiang, X.; Yang, J.; Lv, J.; Zhang, Y. Cellulase interacts with lactic acid bacteria to affect fermentation quality, microbial community, and ruminal degradability in mixed silage of soybean residue and corn stover. Animals 2021, 11, 334. [Google Scholar] [CrossRef]
- Vicente, F.; Rodríguez, M.L.; Martínez-Fernández, A.; Soldado, A.; Argamentería, A.; Peláez, M.; Roza-Delgado, B. Subclinical ketosis on dairy cows in transition period in farms with contrasting butyric acid contents in silages. Sci. World J. 2014, 2014, 279614. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Z.; Du, S.; Sun, L.; Bao, J.; Hao, J.; Ge, G. Lactobacillus plantarum and propionic acid improve the fermentation quality of high-moisture amaranth silage by altering the microbial community composition. Front. Microbiol. 2022, 13, 1066641. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ke, W.; Zhang, Q.; Undersander, D.; Zhang, G. Effects of Bacillus coagulans and Lactobacillus plantarum on the fermentation quality, aerobic stability and microbial community of triticale silage. Chem. Biol. Technol. Agric. 2023, 10, 79. [Google Scholar] [CrossRef]
- Kaewpila, C.; Khota, W.; Gunun, P.; Kesorn, P.; Cherdthong, A. Strategic addition of different additives to improve silage fermentation, aerobic stability and in vitro digestibility of napier grasses at late maturity stage. Agriculture 2020, 10, 262. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Zhang, Q.; Jiang, Y.; Wu, Z.; Yu, Z. Effects of mixing red clover with alfalfa at different ratios on dynamics of proteolysis and protease activities during ensiling. J. Dairy Sci. 2018, 101, 8954–8964. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, R.; Wang, C.; Dong, W.; Zhang, Z.; Zhao, L.; Zhang, X. Effects of cellulase and Lactobacillus plantarum on fermentation quality, chemical composition, and microbial community of mixed silage of whole-plant corn and peanut vines. Appl. Biochem. Biotechnol. 2022, 194, 2465–2480. [Google Scholar] [CrossRef]
- Jia, T.; Yun, Y.; Yu, Z. Propionic acid and sodium benzoate affected biogenic amine formation, microbial community, and quality of oat silage. Front. Microbiol. 2021, 12, 750920. [Google Scholar] [CrossRef]
- Ran, Q.; Guan, H.; Li, H.; He, W.; Zhu, R.; Zhang, L.; Huang, Y.; Xu, Y.; Fan, Y. Effect of formic acid and inoculants on microbial community and fermentation profile of wilted or un-wilted Italian ryegrass silages during ensiling and aerobic exposure. Fermentation 2022, 8, 755. [Google Scholar] [CrossRef]
- Liao, C.; Tang, X.; Li, M.; Lu, G.; Huang, X.; Li, L.; Zhang, M.; Xie, Y.; Chen, C.; Li, P. Effect of lactic acid bacteria, yeast, and their mixture on the chemical composition, fermentation quality, and bacterial community of cellulase-treated Pennisetum sinese silage. Front. Microbiol. 2022, 13, 1047072. [Google Scholar] [CrossRef]
- Ursula, A.A.; Esemu, S.N.; Ndip, R.N.; Ndip, L.M. Prevalence and risk factors of coliform-associated mastitis and antibiotic resistance of coliforms from lactating dairy cows in North West Cameroon. PLoS ONE 2022, 17, e0268247. [Google Scholar]
- Ling, W.; Zhang, L.; Feng, Q.; Degen, A.A.; Li, J.; Qi, Y.; Li, Y.; Zhou, Y.; Liu, Y.; Yang, F.; et al. Effects of different additives on fermentation quality, microbial communities, and rumen degradation of alfalfa silage. Fermentation 2022, 8, 660. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, S.; Zhao, Y.; Yang, J.; Lv, L.; Zheng, Z.; Lu, H.; Ren, Y. Comparative study of the nutritional value and degradation characteristics of amaranth hay in the rumen of goats at different growth stages. Animals 2023, 13, 25. [Google Scholar] [CrossRef]
- Cardozo, P.W.; Calsamiglia, S.; Ferret, A.; Kamel, C. Effects of natural plant extracts on ruminal protein degradation and fermentation profiles in continuous culture. J. Anim. Sci. 2004, 82, 3230–3236. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hao, Y.; Luo, C.; Wang, Q.; Wang, Z.; Li, D.; Yuan, J.; Cao, Z.; Yang, H.; Li, S. Effects of different additives on the chemical composition, fermentation profile, in vitro and in situ digestibility of paper mulberry silage. Fermentation 2022, 8, 435. [Google Scholar] [CrossRef]
- Liu, W.; Si, Q.; Sun, L.; Wang, Z.; Liu, M.; Du, S.; Ge, G.; Jia, Y. Effects of cellulase and xylanase addition on fermentation quality, aerobic stability, and bacteria composition of low water-soluble carbohydrates oat silage. Fermentation 2023, 9, 638. [Google Scholar] [CrossRef]
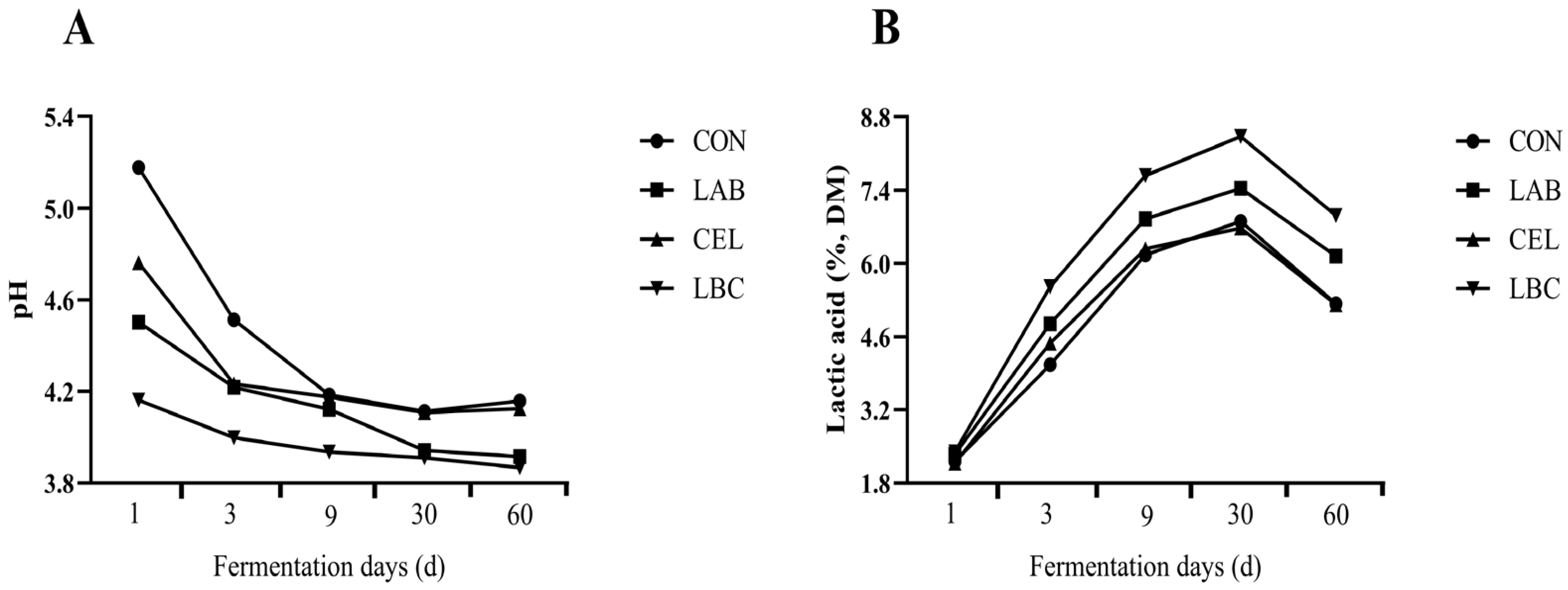
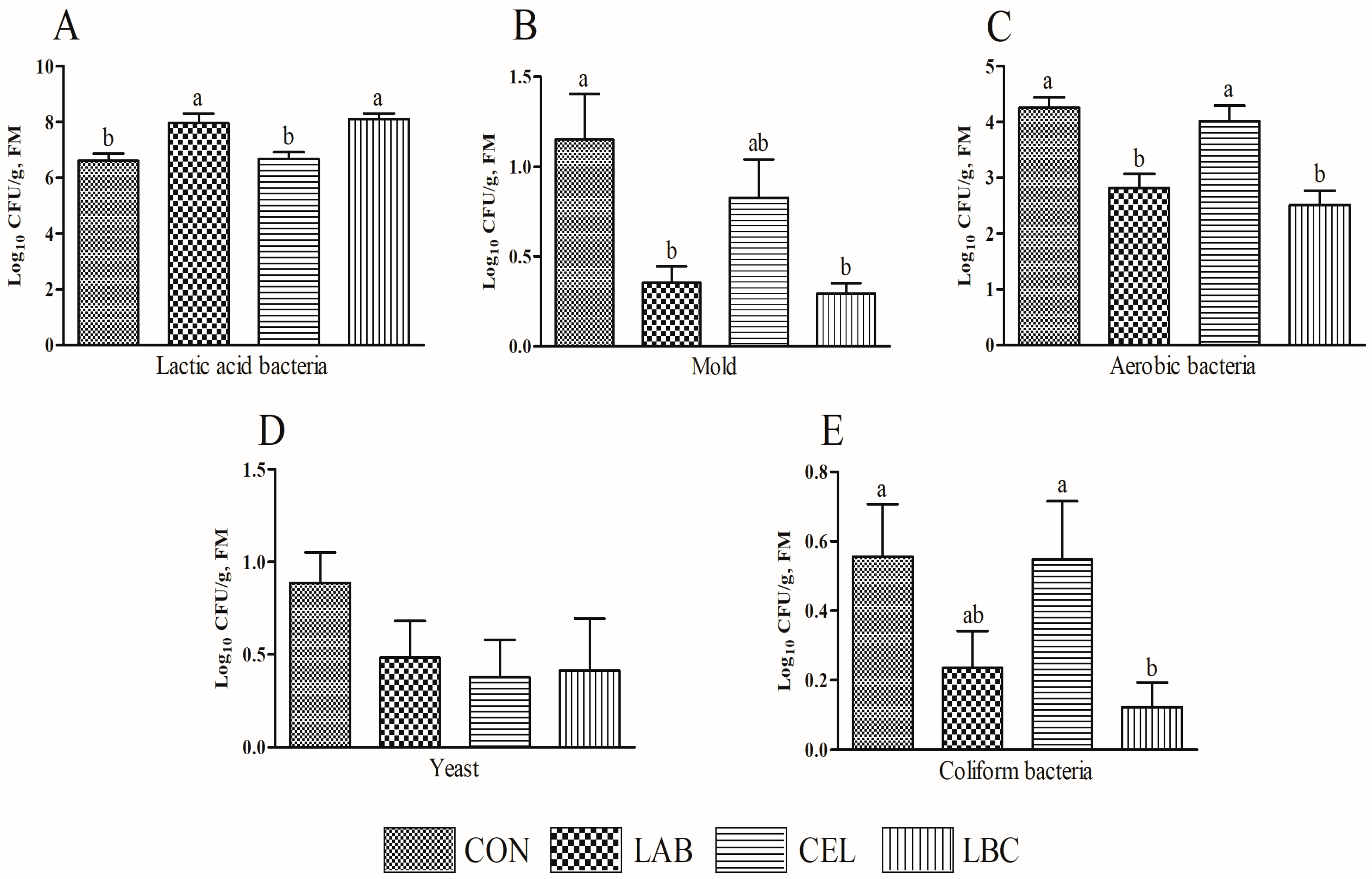

| Items | Treatments | SEM | p Value | |||
|---|---|---|---|---|---|---|
| CON | LAB | CEL | LBC | |||
| pH | 4.16 a | 3.92 ab | 4.13 a | 3.87 b | 0.048 | 0.049 |
| NH3-N/total nitrogen | 4.57 a | 3.43 b | 2.97 b | 2.81 b | 0.198 | <0.001 |
| Lactic acid (%, DM) | 5.23 c | 6.14 b | 5.21 c | 6.91 a | 0.192 | <0.001 |
| Acetic acid (%, DM) | 1.39 a | 1.08 b | 1.11 b | 0.77 c | 0.064 | <0.001 |
| Propionic acid (%, DM) | 0.032 a | 0.010 bc | 0.018 b | 0.007 c | 0.003 | 0.001 |
| Butyric acid (%, DM) | 0.005 | ND | 0.002 | ND | 0.001 | 0.055 |
| Lactic acid/Acetic acid | 3.79 c | 5.73 b | 4.76 bc | 9.27 a | 0.582 | <0.001 |
| Items | Treatments | SEM | p Value | |||
|---|---|---|---|---|---|---|
| CON | LAB | CEL | LBC | |||
| DM | 24.44 b | 28.89 a | 25.02 b | 28.40 a | 0.539 | <0.001 |
| OM | 86.65 | 87.06 | 84.13 | 88.56 | 0.763 | 0.231 |
| CP | 6.78 | 7.29 | 6.95 | 7.57 | 0.119 | 0.068 |
| WSC | 2.18 | 1.84 | 2.03 | 2.27 | 0.092 | 0.419 |
| NDF | 59.64 a | 57.96 ab | 54.07 bc | 53.30 c | 0.897 | 0.013 |
| ADF | 41.58 a | 38.53 ab | 34.58 c | 35.61 bc | 0.892 | 0.007 |
| Items | Treatments | SEM | p Value | |||
|---|---|---|---|---|---|---|
| CON | LAB | CEL | LBC | |||
| Time point (%) | ||||||
| 4 | 23.95 b | 28.91 ab | 27.28 ab | 31.63 a | 1.009 | 0.032 |
| 8 | 31.20 b | 35.65 ab | 34.41 b | 40.94 a | 1.212 | 0.017 |
| 16 | 40.47 c | 47.70 ab | 43.56 bc | 51.78 a | 1.402 | 0.008 |
| 24 | 52.36 b | 57.52 ab | 52.55 b | 60.63 a | 1.325 | 0.052 |
| 36 | 59.19 | 62.28 | 60.84 | 64.88 | 0.943 | 0.176 |
| 48 | 61.51 | 64.22 | 64.82 | 67.15 | 0.941 | 0.212 |
| 72 | 63.54 b | 69.13 a | 66.44 ab | 70.89 a | 1.031 | 0.041 |
| Ruminal degradation parameters | ||||||
| a (%) | 19.68 c | 23.46 ab | 21.47 bc | 23.97 a | 0.552 | 0.006 |
| b (%) | 40.79 b | 46.47 a | 42.99 b | 47.46 a | 0.821 | 0.002 |
| c (%/h) | 0.038 | 0.039 | 0.046 | 0.041 | 0.001 | 0.154 |
| a + b (%) | 60.46 c | 69.93 a | 64.46 b | 71.43 a | 1.266 | <0.001 |
| Effective degradability (%) | 42.02 c | 49.07 ab | 47.06 b | 51.00 a | 0.956 | <0.001 |
| Items | Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON | LAB | CEL | LBC | |||
| Time point (%) | ||||||
| 4 | 34.25 b | 35.14 b | 39.77 a | 40.30 a | 0.789 | <0.001 |
| 8 | 46.37 | 46.99 | 50.62 | 52.20 | 0.965 | 0.075 |
| 16 | 56.02 b | 58.14 b | 59.08 b | 63.58 a | 0.971 | 0.020 |
| 24 | 63.57 c | 66.29 bc | 68.73 ab | 72.06 a | 1.052 | 0.011 |
| 36 | 66.37 c | 68.66 bc | 70.76 ab | 73.67 a | 0.884 | 0.007 |
| 48 | 70.10 b | 73.09 ab | 74.17 ab | 76.69 a | 0.835 | 0.023 |
| 72 | 73.81 b | 75.77 ab | 77.35 ab | 79.47 a | 0.781 | 0.047 |
| Ruminal degradation parameters | ||||||
| a (%) | 35.45 | 37.44 | 39.11 | 40.28 | 0.702 | 0.062 |
| b (%) | 38.01 | 38.44 | 39.82 | 39.98 | 0.607 | 0.624 |
| c (%/h) | 0.048 | 0.056 | 0.051 | 0.055 | 0.001 | 0.088 |
| a + b (%) | 73.46 b | 75.87 ab | 78.92 a | 80.25 a | 0.982 | 0.041 |
| Effective degradability (%) | 58.40 b | 62.17 ab | 63.74 a | 65.86 a | 0.948 | 0.019 |
| Items | Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| CON | LAB | CEL | LBC | |||
| Time point (%) | ||||||
| 4 | 6.05 c | 6.84 bc | 9.77 a | 8.57 ab | 0.497 | 0.015 |
| 8 | 10.55 b | 12.13 ab | 14.22 a | 15.05 a | 0.641 | 0.033 |
| 16 | 15.31 b | 16.63 b | 19.58 a | 20.51 a | 0.677 | 0.004 |
| 24 | 23.87 b | 26.32 b | 30.05 a | 32.07 a | 0.957 | 0.001 |
| 36 | 33.90 b | 37.02 ab | 39.81 a | 38.91 a | 0.803 | 0.025 |
| 48 | 42.28 c | 45.61 bc | 48.84 ab | 50.10 a | 0.952 | 0.003 |
| 72 | 47.02 b | 49.50 ab | 51.95 a | 53.12 a | 0.796 | 0.013 |
| Ruminal degradation parameters | ||||||
| a (%) | 3.82 | 3.86 | 4.08 | 4.64 | 0.355 | 0.867 |
| b (%) | 43.74 b | 45.76 ab | 47.71 ab | 49.41 a | 0.798 | 0.047 |
| c (%/h) | 0.039 | 0.041 | 0.045 | 0.044 | 0.001 | 0.294 |
| a + b (%) | 47.55 c | 49.62 bc | 51.80 ab | 54.05 a | 0.772 | 0.004 |
| Effective degradability (%) | 28.12 c | 29.94 bc | 32.26 ab | 33.45 a | 0.666 | 0.005 |
| Items | Treatments | SEM | p Value | |||
|---|---|---|---|---|---|---|
| CON | LAB | CEL | LBC | |||
| Time point (%) | ||||||
| 4 | 5.62 | 5.48 | 5.41 | 6.07 | 0.429 | 0.960 |
| 8 | 8.75 | 9.08 | 8.92 | 10.00 | 0.443 | 0.792 |
| 16 | 10.93 | 11.54 | 12.19 | 12.99 | 0.396 | 0.311 |
| 24 | 19.91 | 21.68 | 23.10 | 22.58 | 0.488 | 0.087 |
| 36 | 27.52 b | 30.79 ab | 33.22 a | 34.08 a | 0.792 | 0.002 |
| 48 | 36.92 b | 38.51 ab | 42.73 ab | 44.18 a | 1.020 | 0.016 |
| 72 | 39.71 b | 41.55 ab | 44.96 ab | 48.19 a | 1.149 | 0.024 |
| Ruminal degradation parameters | ||||||
| a (%) | 3.08 | 3.14 | 3.25 | 3.45 | 0.267 | 0.971 |
| b (%) | 37.74 b | 38.36 b | 41.69 ab | 46.30 a | 1.096 | 0.005 |
| c (%/h) | 0.027 | 0.030 | 0.034 | 0.036 | 0.002 | 0.372 |
| a + b (%) | 40.81 b | 41.50 b | 44.94 b | 49.75 a | 1.029 | <0.001 |
| Effective degradability (%) | 20.34 b | 21.90 ab | 25.00 ab | 28.06 a | 1.023 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Fan, X.; Wu, T.; Zhou, J.; Huang, H.; Qiu, T.; Xing, Z.; Zhao, Z.; Yin, F.; Gan, S. Lactic Acid Bacteria and Cellulase Improve the Fermentation Characteristics, Aerobic Stability and Rumen Degradation of Mixed Silage Prepared with Amaranth and Rice Straw. Fermentation 2023, 9, 853. https://doi.org/10.3390/fermentation9090853
Ma J, Fan X, Wu T, Zhou J, Huang H, Qiu T, Xing Z, Zhao Z, Yin F, Gan S. Lactic Acid Bacteria and Cellulase Improve the Fermentation Characteristics, Aerobic Stability and Rumen Degradation of Mixed Silage Prepared with Amaranth and Rice Straw. Fermentation. 2023; 9(9):853. https://doi.org/10.3390/fermentation9090853
Chicago/Turabian StyleMa, Jian, Xue Fan, Tingting Wu, Jiaxin Zhou, Haozhan Huang, Tianzhen Qiu, Zhewei Xing, Zhihui Zhao, Fuquan Yin, and Shangquan Gan. 2023. "Lactic Acid Bacteria and Cellulase Improve the Fermentation Characteristics, Aerobic Stability and Rumen Degradation of Mixed Silage Prepared with Amaranth and Rice Straw" Fermentation 9, no. 9: 853. https://doi.org/10.3390/fermentation9090853
APA StyleMa, J., Fan, X., Wu, T., Zhou, J., Huang, H., Qiu, T., Xing, Z., Zhao, Z., Yin, F., & Gan, S. (2023). Lactic Acid Bacteria and Cellulase Improve the Fermentation Characteristics, Aerobic Stability and Rumen Degradation of Mixed Silage Prepared with Amaranth and Rice Straw. Fermentation, 9(9), 853. https://doi.org/10.3390/fermentation9090853






