Abstract
The process of producing sustainable aviation fuel (SAF) from organic waste involves the use of volatile fatty acids (VFAs) as intermediates that are obtained via arrested anaerobic digestion (AAD) and VFA recovery. This recovery process often requires several steps, including dewatering, filtration, extraction, and purification. The recovery of VFAs is crucial for their upgrading and can pose a challenge in the production of SAF from organic waste due to high costs and compatibility issues. This review discusses various dewatering methods, including centrifuges, belt filter presses, and screw presses, and explores conditioning technologies that can improve dewatering performance. It also introduces filtration technologies, with a focus on dynamic filtration, which shows promise in addressing the issue of membrane fouling. Additionally, the review describes extraction technologies such as electrodialysis, adsorption, and liquid–liquid extraction (LLE). By providing insights into these different techniques, the review aims to contribute to the development of an integrated VFA recovery process with low carbon footprint.
1. Introduction
The International Air Transport Association (IATA), which represents 83% of global air traffic, has committed to achieving net-zero carbon emissions by 2050, aligned with the Paris Agreement goal for global warming not to exceed 1.5 °C [1]. In 2050, industry will need to address the mitigation of approximately 1.8 gigatons of carbon, around 65% of which could be reduced by adopting sustainable aviation fuels (SAF) [1]. Two challenges have been recognized for providing SAF. One is that the jet fuel market has a large and growing size (106 billion gallons in 2019 to 230 billion gallons in 2050), while the current annual production of SAF is only about 2 million gallons due to limited amounts of feedstocks (mainly virgin vegetable oils as well as waste fats, oils, and greases). The other is that SAF has a higher price than petroleum-based jet fuel, which is a hurdle since 20–30% of the operating cost of an airline is fuel [2]. As a result, more abundant and lower-cost feedstock sources are required to meet SAF demand.
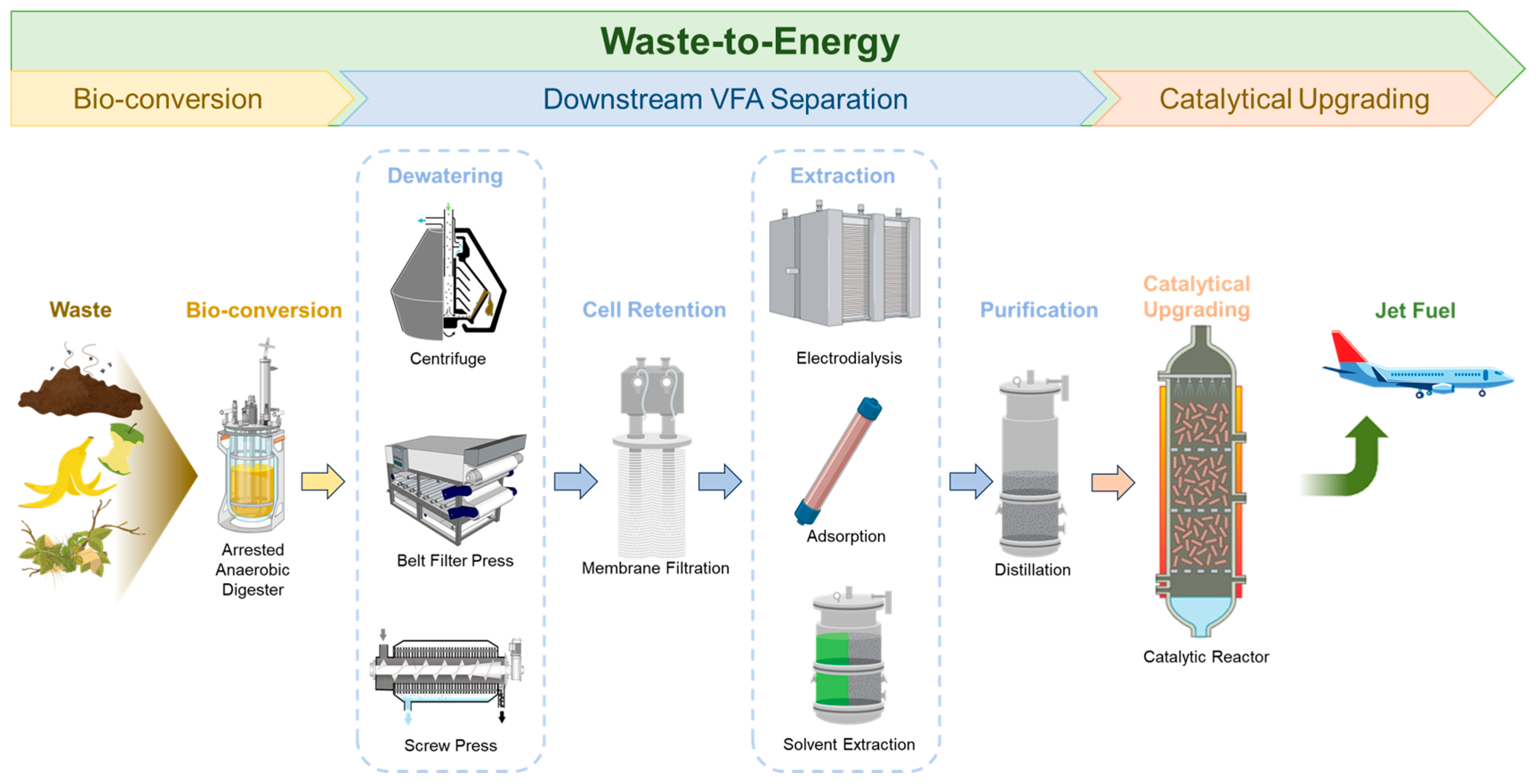
Among the various energy sources, renewable waste substrates are of particular interest for conversion to energy (also named “waste-to-energy (WTE)”). WTE represents an important strategy for waste management, environmental compatibility, the circular economy, and future world stability. Food waste is a major component of municipal solid waste and contributes to 6% of greenhouse emissions. Since food waste has high moisture contents, which limits the use of conventional thermochemical conversion technologies such as pyrolysis and gasification, a bio-conversion pathway to convert waste to SAF has been developed (Figure 1). Biogas production via anaerobic digestion (AD) is the traditional technology to recover energy from food waste [3]. AD is a complicated process that consists of hydrolysis, acidogenesis, acetogenesis, and methanogenesis [4,5]. During hydrolysis, complex organics are solubilized into simple monomeric compounds, which are converted to volatile fatty acids (VFAs) by acidogens and acetogens (via acidogenesis and acetogenesis, respectively). The VFAs are then utilized by methanogens during methanogenesis to produce biogas, mainly methane and CO2 [4,5]. Methanogenesis can be arrested through various methods, such as shortening the retention time, adjusting the pH, providing heat shock, or adding inhibitors (e.g., iodoform) to produce VFAs, which are important precursors for biofuels and biobased chemicals [3,6]. Specifically, VFAs can be catalytically converted to SAF through ketonization and hydrodeoxygenation [3].
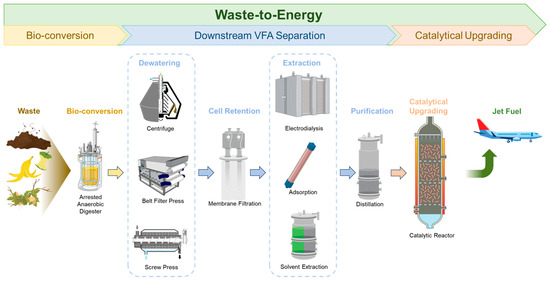
Figure 1.
The bio-conversion pathway to transform waste into sustainable aviation fuel.
Digestate from food waste is a complex sludge that contains many impurities, such as undigested organic matter, microbial cells, inorganic compounds, and moisture, which can block activated sites of catalysts. As a result, VFAs need to be separated from the digestate and purified prior to the catalysis steps. Currently, the downstream separation of VFAs from digestate remains a major challenge [7] and contributes to ~40% of the total VFA production cost [8] owing to their low concentration (<5%) and the presence of impurities. Various technologies have been investigated for the separation and purification of VFAs from the digestate, including adsorption, gas stripping, liquid–liquid extraction, membrane separation, electrodialysis, distillation, and salting-out [9]. However, as recent research focuses shift more towards in situ VFA recovery to mitigate product inhibition on the microorganisms and achieve downstream VFA separation process intensification and efficiency enhancement [10,11,12,13,14,15], the above unit operation’s compatibility for an integrated separation train needs to be re-evaluated. For example, organic solvents may have toxic effects on the microbial communities and thus should be prevented from entering in direct contact with the cells.
Therefore, the downstream in situ VFA recovery is proposed to involve multiple separation steps, with the purposes of (1) cell retention; (2) VFA separation and concentration; and (3) VFA purification for potential sale in the market or for upgrading. Depending on the requirements of the technologies used for cell retention, dewatering may be needed as an additional pretreatment step to remove most solids with large particle sizes. In this review, technologies in dewatering, membrane filtration and extraction are reviewed, along with discussions of the compatibility of units for an integrated separation train. Perspectives on the potential role of VFAs in SAF production are also discussed.
2. Dewatering
Commonly used dewatering technologies include centrifuges, belt filter presses, and screw presses.
2.1. Centrifuge
Centrifugation uses centrifugal force for solid/liquid separation. During centrifugation, a rotor rotates at high speeds around a fixed axis, and solids that have a greater density than the liquid move outward [16]. Centrifuges are commonly used for dewatering sludge at WWTPs and digester plants. Compared to other dewatering technologies, centrifugation is easy to operate in continuous mode and has a low cost, but it is sensitive to sand and gravel in the sludge due to equipment wear and tear and produces substantial noise while in operation [17].
A relative separation factor is usually used for quantifying the effect of centrifugal separation (Equation (1)) [18].
where ω is the angular velocity of the centrifuge; γ is the rotation radius; and g is the gravitational acceleration.
A higher value of α corresponds to an improved centrifugal effect. Typically, the rate at which sludge particles settle is directly proportional to α; thus, a greater α results in a higher separation rate between solid and liquid components. However, too high an α value can give rise to various issues, including sludge slippage, condensation rupture, mechanical wear, excessive power consumption, and noise. Therefore, it is advisable to select a centrifuge with an α value below 2000 [17].
2.2. Belt Filter Press
Belt filter presses are one of two press filtration technologies (the other is plate-and-frame filter presses) that have been widely used in WWTPs. The belt filter press takes sludge between two filter cloths and squeezes the “sandwich” (two filter cloths with the sludge in between) through rollers. The liquid fraction is pushed through the filter cloths and collected as filtrate, while the solid fraction is scraped off at the discharge end [19].
The dewatering process of the belt filter press follows a principle similar to that of the plate-and-frame filter press. By utilizing the tension of the filter cloth and the pressure exerted by the drum, the belt filter press effectively compresses and dehydrates the sludge [20]. A significant advantage of the belt filter press is its continuous operation, which addresses the shortcomings of plate-and-frame filter presses.
2.3. Screw Press
The screw press has a threaded screw that rotates in a fixed, perforated screen cylinder. Sludge enters the inlet and is pushed toward the outlet as the screw rotates. The liquid fraction is squeezed through the screen and collected as filtrate, while the solid fraction is retained and collected as cake at the outlet [21].
Screw presses have several advantages over belt presses and centrifuges. Firstly, they provide a highly efficient dewatering rate while being compact in size. Additionally, compared to the belt press or centrifuge, screw presses operate at significantly lower noise levels. Furthermore, they consume less energy to produce a cake with a comparable level of dryness to that achieved by centrifuges and belt presses. Moreover, maintenance of the screw press is relatively easy [21].
2.4. Conditioning Technologies for Dewatering
2.4.1. Release of Interstitial Water and Bound Water
According to their existing states, water in sludge can be grouped into three types: bound water, free water, and interstitial water, which account for <10%, >60%, and 10–25% of total water, respectively. The bound water is closely contacted with sludge particles and is difficult to separate. In contrast, free water is free-flowing and thus can be easily separated with conventional dewatering methods, such as centrifuges, belt filter presses, and screw presses. Interstitial water cannot freely flow in a sludge system since it is retained in the hydrophilic radical traps formed by the extracellular polymeric substances (EPS). As a result, one strategy for improving sludge dewatering performance is to release interstitial water and/or bound water via various conditioning technologies that can destroy EPS [17,22].
Chemical oxidation, such as Fenton, persulfate, and potassium ferrate, has been used for the disintegration of EPS. The Fenton reagent is a strong oxidant composed of H2O2 and Fe2+ and achieves the best treatment effect at pH 3. To avoid precipitation of Fe(OH)3, the sludge usually needs to be acidified prior to the Fenton treatment, which is a major drawback of the classical Fenton process. Persulfate is another oxidant reagent that has gained attention for improving the dewaterability of water. Transition metals (such as Fe2+, Ag+, and Co2+) or biochar are usually needed to activate the oxidation process with persulfate. The usage of biochar can significantly reduce the addition of metals and simultaneously remove pollutants, which makes the biochar-based persulfate process highly promising in sludge dewatering. Potassium ferrate is also an attractive reagent for chemical oxidation. Potassium ferrate acts as an oxidant in both acidic and alkaline conditions and is more oxidizing under acidic conditions (stronger than some common oxidants, such as H2O2, O3, and K2MnO4).
Physical treatments, such as thermal, freeze–thaw, microwave, and ultrasonic treatments, can also degrade EPS and break sludge cells, releasing interstitial water and bound water. Heat treatment is a simple and efficient method to break sludge cells and disrupt the colloidal structure. Freeze–thaw treatment is also an effective conditioning method before dewatering. Freezing temperature and freezing time are major factors that affect the dewatering performance. Microwave treatment is more efficient than conventional heating methods since the energy can be directly transferred to the sludge with a fast heating speed and easy control. A drawback of microwaves is their limited penetration ability, which can only treat a small amount of sludge at a time. Safety is another challenge for the application of microwaves on a large scale. Ultrasonic treatment is a relatively new method for sludge conditioning. Ultrasounds form cavitation bubbles, which generate extremely high pressure and temperature at the microscale and destroy sludge cell water, releasing intracellular bound water and interstitial water.
Biological treatments usually use enzymes that degrade EPS under mild conditions [22]. Enzymes (α-amylase, cellulase, acidic protease, neutral protease, and alkaline protease) that selectively act on the main components of EPS (proteins and polysaccharides) have been studied in sludge conditioning for improving dewatering performance [23]. Enzymatic treatment is attractive as an environmentally friendly conditioning process with increased sludge supernatant fractions and decreased sludge water content. However, increased capillary suction time was observed after enzyme conditioning, which limits its potential in dewatering [23].
2.4.2. Coagulation and Flocculation
The sludge particle surface has a high concentration of negative charges, which cause sludge particles to repel each other and prevent the formation of large particles. Coagulants/flocculants have been used to encourage the agglomeration of sludge particles by changing the particle surface charges and reducing the repulsion between particles. The performance of coagulation/flocculation is affected by multiple factors, including charge characteristics, ion characteristics, and functional groups [17].
Both inorganic and organic reagents have been used as coagulants/flocculants to improve dewatering performance. Commonly used inorganic flocculants are iron salt and aluminum salt, including aluminum sulfate (Al2(SO4)3⋅18H2O), alum (Kal(SO4)2⋅12H2O), aluminum chloride (AlCl3), ferric chloride (FeCl3), ferrous chloride (FeCl3), ferric sulfate (Fe2(SO4)3⋅4H2O), polyferric sulfate ([Fe2(OH)n(SO4)3-n/2]m, PFS), and polymeric aluminum ([Al2(OH)nCl6-n]m, PAC) [17]. Organic flocculants can be non-ionic, anionic, or cationic polymers. Cationic polyacrylamide is widely used as a conditioner for sludge dewatering. Cationic flocculants can directly bond with sludge particles that have a negative charge on the surface. Anionic flocculants need metal ions to connect with sludge particles [17].
Inorganic chemicals have a low cost but may cause corrosion of dewatering equipment and pollution of the environment [17]. Organic flocculants (such as polyacrylamides) are biodegradable and less corrosive than inorganic chemicals, but they are usually expensive and contribute to most of the operation cost of dewatering [17]. Moreover, residual organic flocculants in the liquid fraction could cause membrane fouling issues in the sequential filtration, which limits their application for VFA separation.
3. Membrane Filtration for Cell Retention
The cell retention step is important to protect and recycle the cells in the digestate from the harsh physical and chemical conditions in the downstream in situ VFA recovery process. Microfiltration membranes with a nominal pore size of 0.2 μm have been shown to be effective for cell retention [24]. However, membrane fouling (both biological and organic) remains a major challenge. Membrane fouling refers to the temporary or permanent non-specific surface adsorption/deposition of rejected components on the membrane surface and/or within the pores [25]. Membrane fouling is highly undesirable as it can lead to both flux decline and compromised permeate quality.
Compared to dead-end filtration, crossflow filtration mode is more advantageous for membrane fouling mitigation. However, high crossflow velocity (4–6 m/s) is required to reduce membrane surface concentration polarization (by increasing surface shear rate) and thus minimize the accumulation and/or adsorption of potential foulant molecules onto the membrane surface. Dynamic filtration, which creates shearing by rotating or vibrating, is another approach to mitigate membrane fouling. Compared to conventional crossflow filtration, dynamic filtration does not need a high sludge flow rate with lower pressure drops and is thus more energy efficient. However, it has the drawbacks of structure complexity and additional cost due to moving parts. Alternatively, ceramic disk membranes with a large diameter have become available recently, which makes it practical to scale up membrane modules over 120 m2 [26].
Industrial dynamic filtration modules can be grouped into three types: (i) fixed membranes with rotating disks or rotors, (ii) rotating disk membranes, and (iii) vibrating membranes.
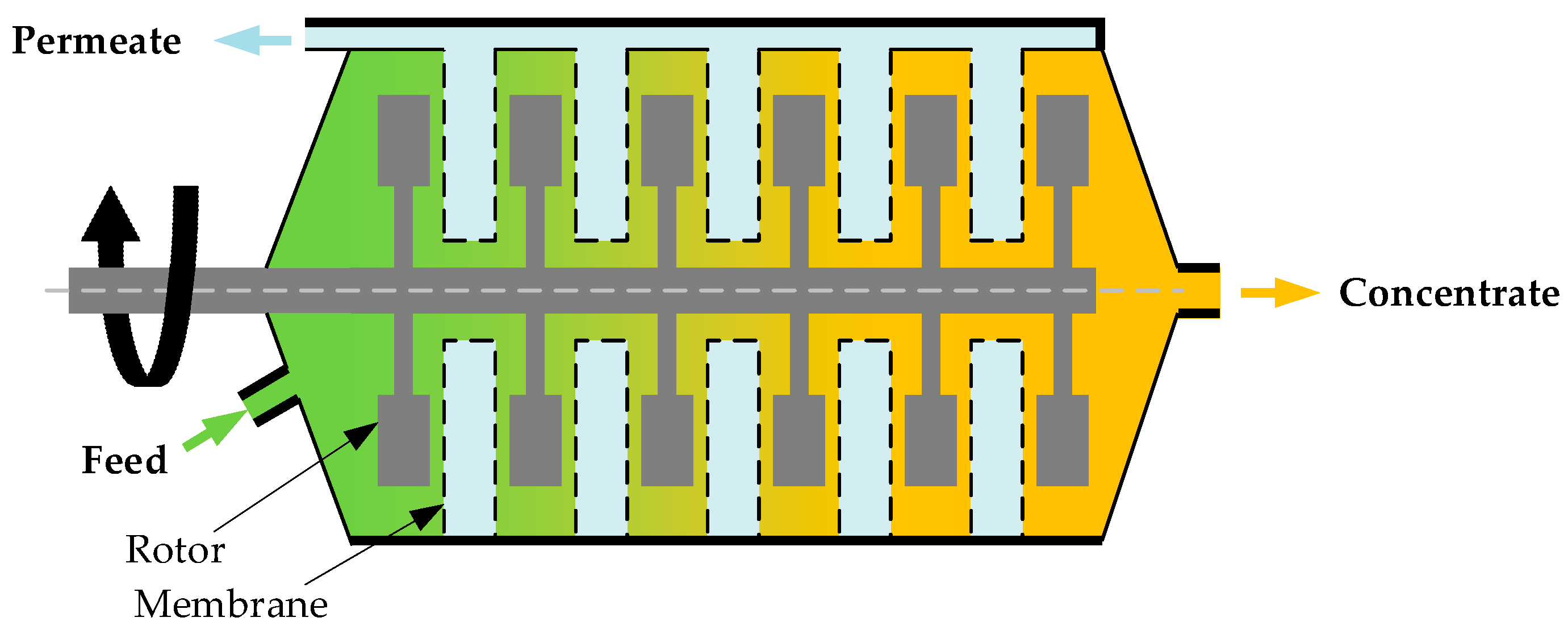
(i) Fixed membranes with rotating disks or rotors (Figure 2)
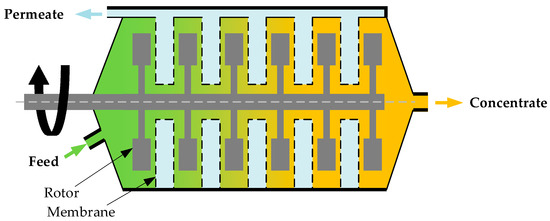
Figure 2.
Fixed membranes with rotating rotors.
The BoCross Dynamic filter is a multiple-chamber system with disk-shaped filter modules and rotors between membranes. The sludge moves through chambers and is concentrated, while the filtrate is discharged out of the chambers through membranes. The rotors spin between membranes and stir the chambers to reduce solid accumulation on the membrane surface [27].
OptiFilter CR, a variation of a fixed membrane dynamic filter, consists of filter cassettes stacked on top of each other. Each cassette has two flat membranes fastened on both sides. A rotor is installed between the membranes of each of the two adjacent cassettes, providing shear force to reduce membrane fouling. OptiFilter CR modules have been used in over 30 plants in the pulp and paper industry [26].
FMX has a stack structure similar to OptiFilter CR, but the rotors are replaced with vortices to prevent membrane fouling. FMX is more suitable than OptiFilter CR for high density, high viscosity, and high solids applications, such as wastewater treatment.
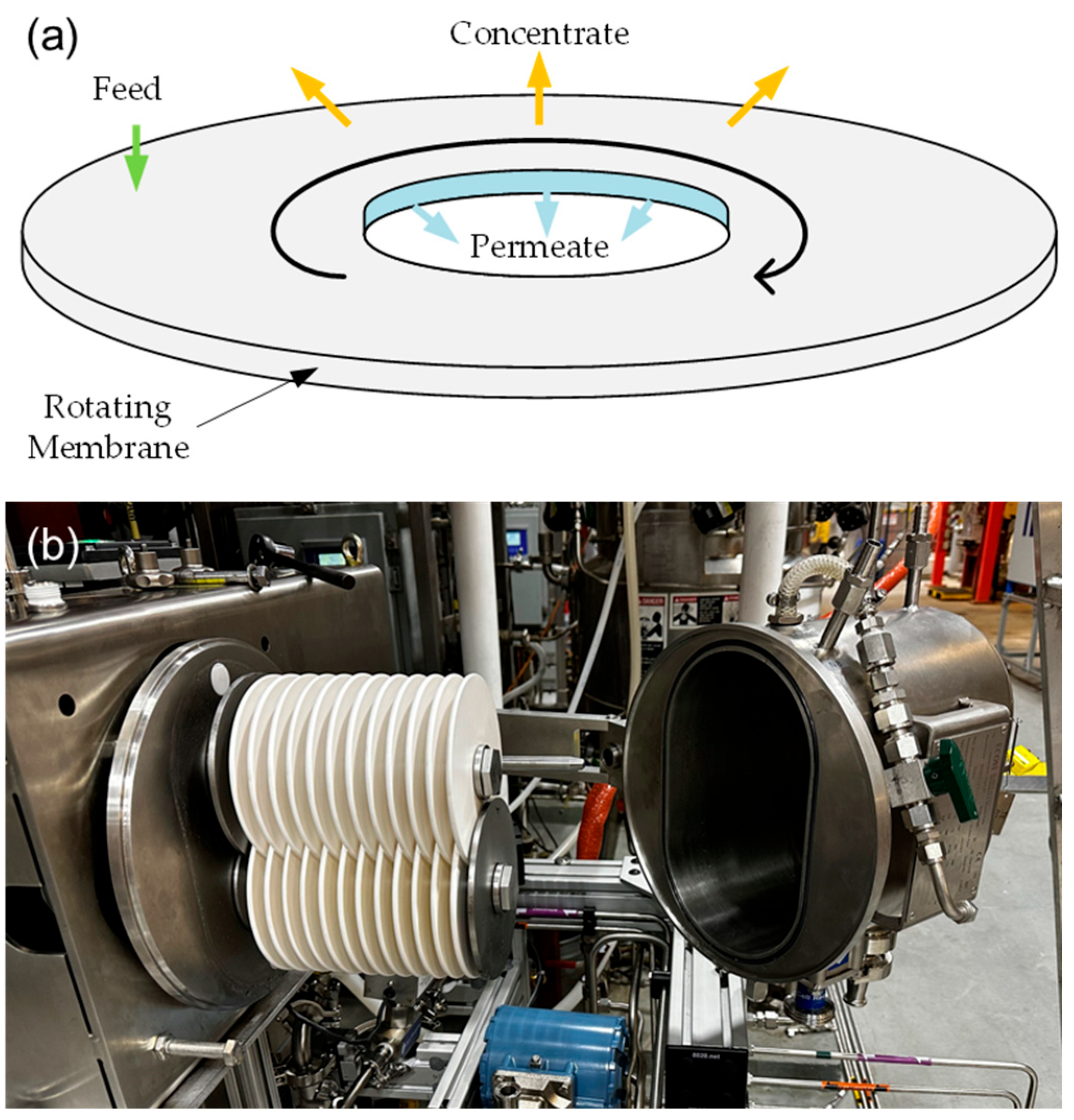
(ii) Rotating Membrane Systems (Figure 3)
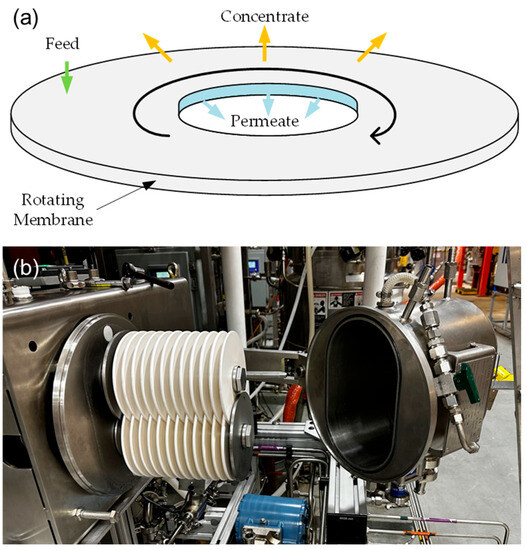
Figure 3.
(a) Illustration of a single rotating membrane disk and (b) picture of a pilot-scale two-shaft rotating membrane system at the National Renewable Energy Laboratory (NREL).
Rotating membrane systems are assembled in single-shaft or multi-shaft designs. A single-shaft disk filter system only has one filter stack rotating on its center shaft, while a multi-shaft disk filter system has two or more filter stacks with overlapping sectors. SpinTek and Novoflow are two single-shaft disk filter systems. The SpinTek system has been used for protein recovery from cheese whey, submicron filtration of lube oil, nonsettable solids concentration, and fine chemical dewatering. Novoflow has also been tested with various media, including wine, juices, tea extracts, plant extracts, algae, laundry wastewater, and slide grinding wastewater [28].
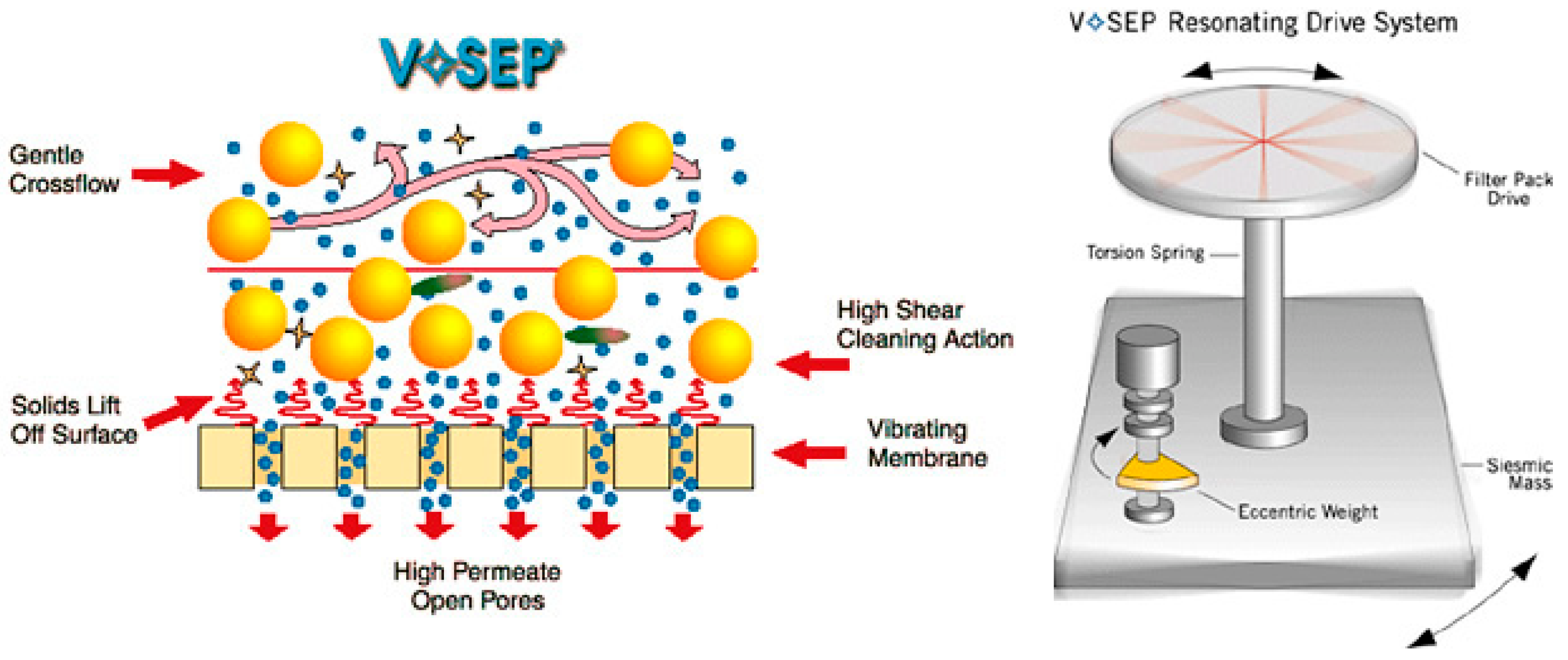
(iii) Vibrating membrane systems
The concept of the vibratory shear-enhanced process (VSEP) was originally invented in 1987. VSEP consists of a stack of circular membranes separated by gaskets and filtrate collectors. The stack of membranes/gaskets/collectors is mounted on a vertical torsion shaft. During VSEP, the shaft spins in azimuthal oscillations of 2–3 cm amplitude on a vibrating base at a resonant frequency of 60.75 Hz (Figure 4). A high shear rate is created by azimuthal vibrations of the membrane, which helps in controlling concentration polarization and preventing membrane fouling. VSEP minimizes the power required for producing vibrations to as low as 9 kW, even for a large scale of 150 m2 [29].
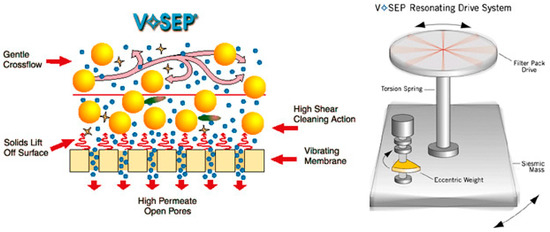
Figure 4.
Vibrating membrane system (Reprinted with permission from New Logic Research New Logic Research Technology [30]).
Currently, dynamic filtration systems are mainly used in the paper and food industries, with few applications in AD plants. Most recently, NREL has coupled the dynamic filtration technology to arrested AD and a VFA separation/purification system for SAF production and validated the integrated system at a lab scale for handling a high solid stream (Patent Application No. 63/020,598). This system will further be evaluated at a pilot scale at Quasar Energy Group’s facility in Ohio under the support of a DOE grant (DE-EE0009765) [31].
4. Extraction of VFAs
Due to product toxicity and possible product degradation, VFA extraction steps were strategized to selectively recover (and potentially concentrate) the targeted VFAs and achieve simultaneous impurity and contaminant removal. Numerous methods have been employed to extract VFAs from the aqueous phase, including electrodialysis, adsorption, and liquid–liquid extraction (LLE) [32]. It is noted that the extraction steps are often followed by a secondary purification stage, using technologies including distillation, evaporation, and crystallization, to reach the targeted purity for potential sale in the market or downstream upgrading [33].
4.1. Electrodialysis
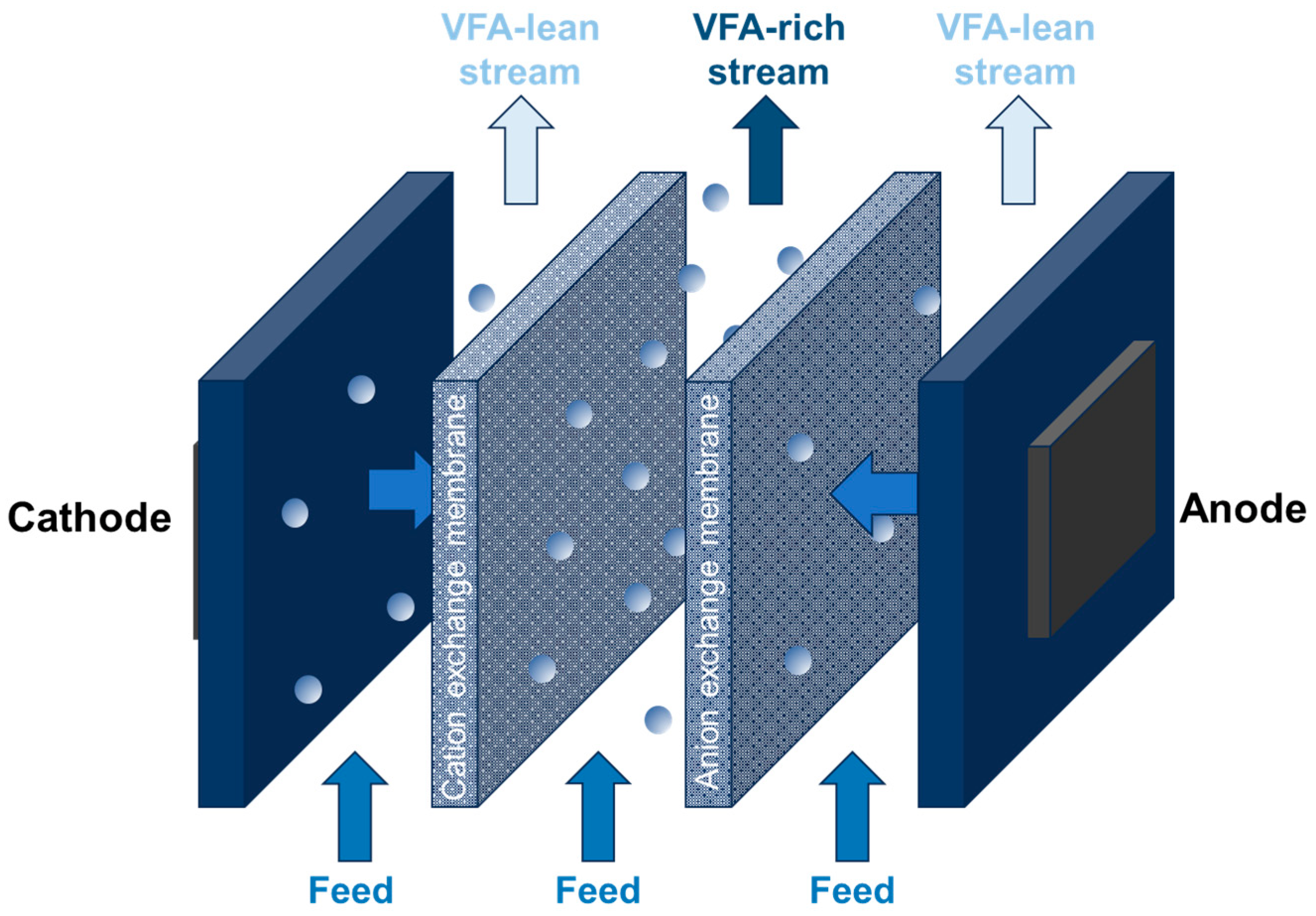
Electrodialysis (ED) is a membrane-based separation process that utilizes an electric field to drive ions across a membrane, achieving their separation and concentration (Figure 5). Unlike traditional methods that rely on concentration gradients, ED harnesses the power of an electric field. There are two main types of ED: conventional ED (CED) and ED using a bipolar membrane (BMED).
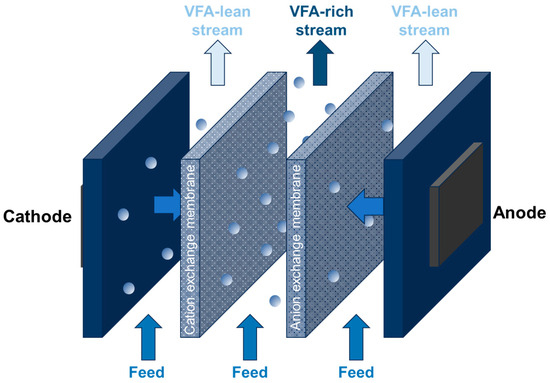
Figure 5.
Principle of using electrodialysis for VFA concentration.
In both CED and BMED, a stack of cationic and anionic exchange membranes is positioned between the anode and cathode. This configuration enables the selective separation of ions. However, the BMED method, while effective, can be expensive due to the challenges involved in recovering and removing inhibitory volatile fatty acids.
Both CED and BMED have found applications as in situ separation methods for carboxylic acids during fermentation processes, offering a way to efficiently separate and recover valuable products. For example, a VFA recovery efficiency range of 69–99% with a concentration factor range of 1.2–4 was reported in the literature [15,34].
4.2. Adsorption
An alternative method for product recovery in solid–liquid separation is through adsorption. This approach involves the use of a solid material, often a resin, that exhibits an affinity for the target product. The fermentation fluid is brought into contact with the solid material, and the product of interest is adsorbed onto the solid, effectively removing it from the solution. This process continues until the resin is fully loaded with the product.
During the loading cycle, various compounds such as solvents, acids, fine chemicals, aromas, steroids, secondary metabolites, and organic acids produced during fermentation can be absorbed from the solution by utilizing a suitable absorbent material with an affinity for the desired compound. Typically, this absorbent material is packed into a column, and the fermentation broth is passed through it. The VFA absorption is based on ion exchange when the acids are in the dissociated form and hydrophobic interactions in the free acid form [15]. A 11–85% VFA recovery efficiency range was reported [15,33] for the adsorption method, depending on the resin selection.
Once the absorbent material has reached its maximum capacity for product loading, a desorption cycle is employed to recover the product from the solid material. The product is eluted into an eluent, allowing for the regeneration of the solid material and subsequent reuse.
Although absorbents offer potential energy savings compared to methods like gas stripping, they suffer from high resin costs and chemical-intensive desorption and regeneration cycles. Another major concern is fouling, which occurs when the absorbent becomes contaminated with cells, cell debris, nutrients, substrates, by-products, and acids present in the fermentation broth. Over time, this fouling can diminish the effectiveness of the absorbent material.
However, implementing a perfusion recycle loop that retains cells can help mitigate the issue of absorbent fouling in an integrated fermentation process that incorporates adsorption. By recycling cells through the perfusion loop, the risk of fouling the absorbent with cells is minimized. This approach helps maintain the efficacy of the adsorbent and supports efficient product recovery within the fermentation process.
4.3. Liquid–Liquid Extraction (LLE)
Solvent LLE is an effective and economical separation technique for downstream VFA recovery that depends on the different solubilities of the target product in two immiscible liquid phases. The liquid used as the extracting solvent is commonly called the extractant, while the other liquid phase is known as the raffinate. During LLE, VFAs are partitioned from the digestate (raffinate phase) to the solvent phase, and the partition coefficient relies on multiple factors, such as digestate pH, initial VFA concentration, and any other solutes in the digestate [32]. However, solvent selection is particularly important for extraction performance. Specific requirements for good solvents for LLE include [32]:
- (1)
- Excellent selectivity in separating VFAs, with a notable separation factor;
- (2)
- A considerable partition coefficient, with a significant affinity for partitioning between the two liquid phases;
- (3)
- Good accessibility in large quantities and economic viability;
- (4)
- Non-toxicity to microorganisms and chemical inertness towards other nutrients and components present in the digestate;
- (5)
- Immiscibility with aqueous solutions and forms a distinct and clear phase separation;
- (6)
- Ability to be effectively recovered from the solvent, allowing for a regeneration and recycling process.
Commonly used extractants for VFA extraction are tributyl phosphate (TBP), trioctylamine (TOA), trioctyl phosphine oxide (TOPO), 2-pentanol, 1-butanol, n-octanol, and Aliquat 336. Moreover, 2-undecanone (UD) and Cyanex 923 have gained significant interest [35,36]. A wide range of VFA recovery efficiency, from 32% to 98%, has been reported in the literature, depending on the solvent composition and fermentation broth pH [15,34].
The traditional LLE is carried out in overlayers but suffers from its batch operating mode, low mass transfer rate, and difficult separation of emulsions. Membrane extraction (ME) was recently investigated as an extension of LLE to allow continuous operation and address the difficulty of demulsification. In ME, hydrophobic porous membranes are used as barriers between the aqueous and organic phases [15], which are allowed to come into contact only through the membrane pores to avoid dispersion and the formation of emulsions. However, the limited contact area between the extractant and raffinate (due to 30% or less membrane porosity) led to a low mass transfer rate of the acids into the organic extractant phase (0.26–10 g/m2·h; [25]). Another challenge in typical ME systems is the low stability of the extractant-filled membrane pores—phase breakthrough and emulsion formation are still inevitable in the long run.
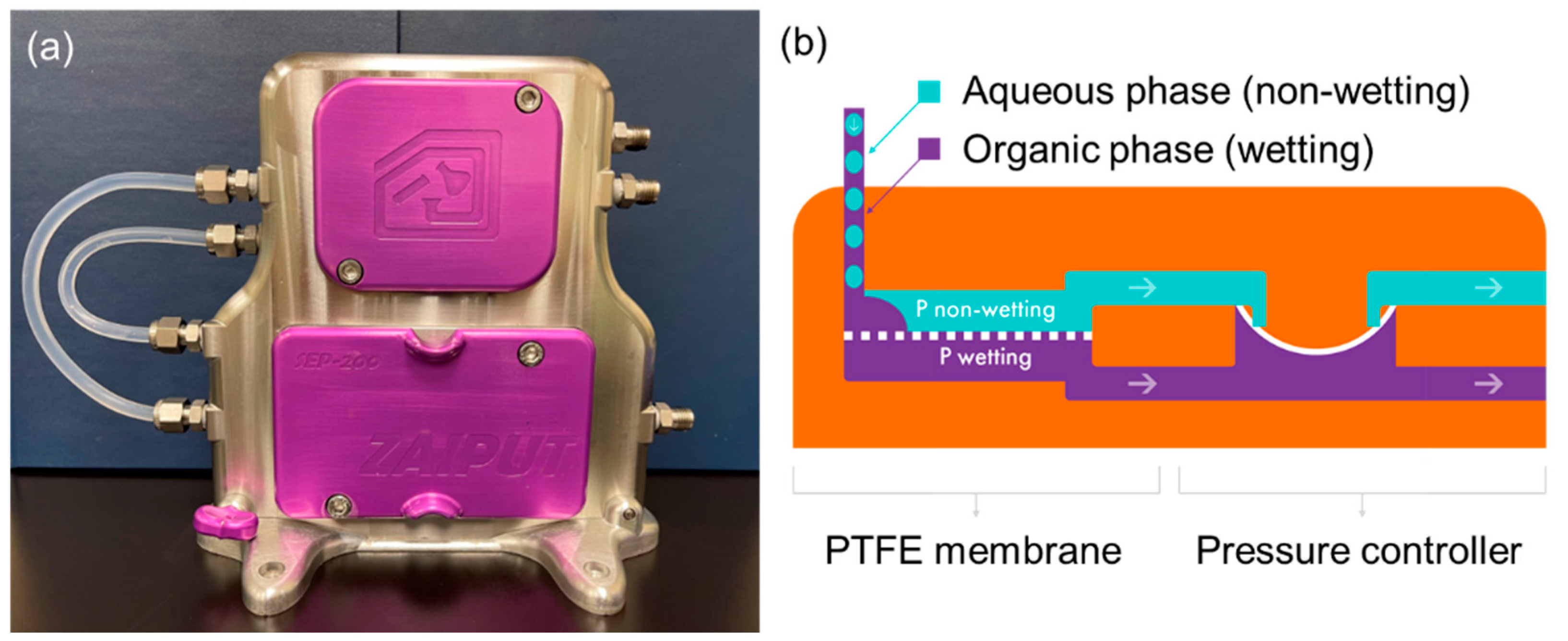
Zaiput Flow Technologies’ patented liquid–liquid separators, on the other hand, offer solutions to both low mass transfer and difficult separation of emulsions [37]. These innovative devices utilize an in-line mixer to sufficiently mix the extractant and raffinate to form an emulsion and accelerate VFA partitioning, followed by membrane technology to leverage surface tension as a driving force for phase separation. When a two-phase stream consisting of an aqueous and an organic liquid enters the separator, one phase (referred to as the “wetting” phase) exhibits an affinity for the membrane and fills the pores. The other phase (the “non-wetting” phase) is repelled and does not fill the pores. Once the membrane pores are filled with the wetting phase, a precisely controlled pressure differential is applied across the membrane. Zaiput’s proprietary internal pressure controller fine-tunes the pressure differential, ensuring that only the wetting phase is pushed through the pores while preventing the non-wetting phase from passing through (as depicted in Figure 6). By maintaining a constant pressure differential across designated flow rates, the separator functions as a modular unit that can be easily integrated into existing systems.
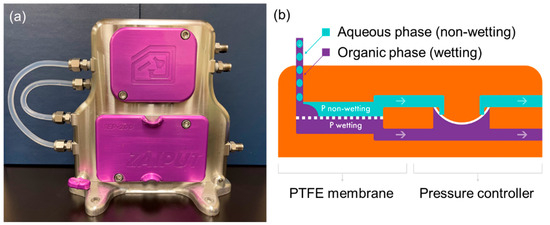
Figure 6.
(a) The picture of the Zaiput separator at NREL and (b) the schematic of its phase separation mechanism [38].
5. Perspectives on the Potential Role of VFAs in SAF Production
VFAs, derived from organic waste, present a renewable feedstock for SAF. By utilizing waste materials that might otherwise end up in landfills or incineration, VFAs contribute to waste valorization. This waste-to-fuel concept aligns with circular economy principles. Different from conventional jet fuels, which contribute to higher carbon emissions, fuels from organic waste are believed to reduce aviation’s carbon footprint with a negative carbon intensity. While VFA-based SAF holds promise, challenges remain in scaling up the whole process. Besides efficient and cost-effective methods for VFA production and recovery, technologies for converting VFAs into aviation-grade fuels are also needed to meet industry demands.
Recent progress has developed pathways enabling mixed VFA upgrading without the need to separate the individual acids upfront [33]. VFA chain lengths ranging from C3 to C8 recovered from AAD in their neat acid form can be catalytically upgraded to SAF by carbon coupling and deoxygenation. The VFA carbon backbone elongation can be achieved via (cross-)ketonization, followed by ketone hydro-deoxygenation, to produce normal paraffins identical to those in petroleum. It is noted that, depending on the reacted VFA chain length distribution, a second carbon coupling reaction may be needed prior to hydrodeoxygenation to produce paraffin-rich hydrocarbons within the C8 to C18 range of jet fuel. For example, a simplified ketonization kinetic model indicates that a C4/C6 mixture leads to only a 44–47% yield of ketones with chain lengths C8 suitable for direct hydrodeoxygenation, while a C6/C8 VFA mixture can increase the yield to 95% [3]. Although the chemistry of ketonization has been well known and studied over the years, technical bottlenecks exist, including the complex reaction mechanisms and low control of selective VFA conversions, as well as the low VFA titer and productivity in AAD and the costly downstream VFA separation process. If overcoming the above challenges, life-cycle assessment and techno-economic analysis predict that VFA-SAF has the potential to provide up to 165% reduced greenhouse gas (GHG) emissions relative to fossil jet fuel and reach a minimum fuel selling price of USD 2.50/gallon if VFAs can be produced at USD 0.30/kg [3].
6. Conclusions
Several dewatering technologies (centrifuge, belt filter press, or screw press) can be used as the first step for VFA recovery to remove most of the large solid impurities. Organic flocculants, which can easily cause membrane fouling in sequential filtration, are not suitable for the dewatering step for VFA recovery. Alternatively, inorganic chemicals (below their inhibiting levels) can be considered for improving dewatering performance. Compared to other dynamic filtration systems, vibrating membranes have the advantages of simple structure and low energy consumption at large scales, which makes them a promising secondary solids removal unit during the VFA recovery process. Moreover, LLE is believed to be more effective and economical than other extraction methods, such as electrodialysis and adsorption. However, a foreseeable challenge for LLE could be screening and selecting suitable solvents that can meet multiple requirements, i.e., high selectivity, high affinity, low cost, low toxicity, easy separation from the aqueous phase, and easy regeneration. Lastly, extensive studies are needed to develop and validate integrated VFA recovery systems with compatible dewatering, filtration, and extraction units.
Author Contributions
Conceptualization, X.G. and Y.L.; Investigation, X.G. and Y.C.; Writing—original draft preparation, X.G. and Y.C.; Writing—review and editing, X.G. and Y.C.; Visualization, X.G. and Y.C.; Supervision, X.G., V.S.i.N. and Y.L.; Project administration, X.G., V.S.i.N. and Y.L.; Funding acquisition, X.G., V.S.i.N. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the U.S. Department of Energy (Grant Number DE-EE0009765) and the U.S. Department of Agriculture (grant number 2023-79000-38973). This work was authored in part by the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC, for the U.S. Department of Energy (DOE) under Contract No. DE-AC36-08GO28308. The U.S. Government retains and the publisher, by accepting the article for publication, acknowledges that the U.S. Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for U.S. Government purposes.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IATA Net-Zero Carbon Emissions by 2050. 2021. Available online: https://www.iata.org/en/pressroom/pressroom-archive/2021-releases/2021-10-04-03/ (accessed on 3 July 2023).
- Holladay, J.; Abdullah, Z.; Heyne, J. Sustainable Aviation Fuel: Review of Technical Pathways Report. 2020. Available online: https://www.energy.gov/sites/default/files/2020/09/f78/beto-sust-aviation-fuel-sep-2020.pdf (accessed on 3 July 2023).
- Huq, N.A.; Hafenstine, G.R.; Huo, X.; Nguyen, H.; Tifft, S.M.; Conklin, D.R.; Stück, D.; Stunkel, J.; Yang, Z.; Heyne, J.S.; et al. Toward Net-Zero Sustainable Aviation Fuel with Wet Waste-Derived Volatile Fatty Acids. Proc. Natl. Acad. Sci. USA 2021, 118, e2023008118. [Google Scholar] [CrossRef] [PubMed]
- Grady, C.P.L.J.; Daigger, G.T.; Lim, H.C. Biological Wastewater Treatment, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 1999; ISBN 0824789199. [Google Scholar]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications, 1st ed.; McGraw-Hill Education: New York, NY, USA, 2001; ISBN 0072345535. [Google Scholar]
- Wu, H.; Dalke, R.; Mai, J.; Holtzapple, M.; Urgun-Demirtas, M. Arrested Methanogenesis Digestion of High-Strength Cheese Whey and Brewery Wastewater with Carboxylic Acid Production. Bioresour. Technol. 2021, 332, 125044. [Google Scholar] [CrossRef]
- Zhu, X.; Leininger, A.; Jassby, D.; Tsesmetzis, N.; Ren, Z.J. Will Membranes Break Barriers on Volatile Fatty Acid Recovery from Anaerobic Digestion? ACS EST Eng. 2021, 1, 141–153. [Google Scholar] [CrossRef]
- Inyang, V.; Lokhat, D. Butyric Acid Reactive Extraction Using Trioctylamine in 1-Decanol: Response Surface Methodology Parametric Optimization Technique. Arab. J. Sci. Eng. 2021, 46, 6567–6577. [Google Scholar] [CrossRef]
- Tharani, D.; Ananthasubramanian, M. Process Intensification in Separation and Recovery of Biogenic Volatile Fatty Acid Obtained through Acidogenic Fermentation of Organics-Rich Substrates. Chem. Eng. Process.-Process. Intensif. 2021, 169, 108592. [Google Scholar] [CrossRef]
- Gössi, A.; Burgener, F.; Kohler, D.; Urso, A.; Kolvenbach, B.A.; Riedl, W.; Schuur, B. In-Situ Recovery of Carboxylic Acids from Fermentation Broths through Membrane Supported Reactive Extraction Using Membrane Modules with Improved Stability. Sep. Purif. Technol. 2020, 241, 116694. [Google Scholar] [CrossRef]
- Burgé, G.; Chemarin, F.; Moussa, M.; Saulou-Bérion, C.; Allais, F.; Spinnler, H.É.; Athès, V. Reactive Extraction of Bio-Based 3-Hydroxypropionic Acid Assisted by Hollow-Fiber Membrane Contactor Using TOA and Aliquat 336 in n-Decanol. J. Chem. Technol. Biotechnol. 2016, 91, 2705–2712. [Google Scholar] [CrossRef]
- Yang, S.T.; Huang, H.; Tay, A.; Qin, W.; Guzman, L.D.; Nicolas, E.C.S. Extractive Fermentation for the Production of Carboxylic Acids. In Bioprocessing for Value-Added Products from Renewable Resources: New Technologies and Applications; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Burgé, G.; Moussa, M.; Athes-Dutour, V.; Saulou-Berion, C.; Spinnler, H.E. In Situ Extraction of 3-Hydroxyproionic Acid Assisted by Membrane Congractor. In Proceedings of the 4th International Congress on Green Process Engineering, Sevilla, Spain, 7–10 April 2014; Volume 3. [Google Scholar]
- Saboe, P.O.; Manker, L.P.; Michener, W.E.; Peterson, D.J.; Brandner, D.G.; Deutch, S.P.; Kumar, M.; Cywar, R.M.; Beckham, G.T.; Karp, E.M. In Situ Recovery of Bio-Based Carboxylic Acids. Green Chem. 2018, 20, 1791–1804. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current Perspectives on Acidogenic Fermentation to Produce Volatile Fatty Acids from Waste. Rev. Environ. Sci. Biotechnol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- Ge, X.; Sheets, J.P.; Li, Y.; Mani, S. Algae-Based Feedstocks. In Bioenergy: Principles and Applications; Li, Y., Khanal, S.K., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Zhang, X.; Ye, P.; Wu, Y. Enhanced Technology for Sewage Sludge Advanced Dewatering from an Engineering Practice Perspective: A Review. J. Environ. Manag. 2022, 321, 115938. [Google Scholar] [CrossRef]
- Oshima, K.; Nakamura, K.; Guo, H.; Smith, R.L. Mini-Review on Application of Analytical Centrifugation, Ultracentrifugation and Centrifugal Devices to Phase Equilibria and Separation Processes. Fluid Phase Equilib. 2022, 558, 113457. [Google Scholar] [CrossRef]
- EPA. Biosolids Technology Fact Sheet: Belt Filter Press; EPA: Washington, DC, USA, 2000. Available online: https://www.epa.gov/sites/default/files/2018-11/documents/belt-filter-press-factsheet.pdf (accessed on 3 July 2023).
- Wakeman, R.J. Separation Technologies for Sludge Dewatering. J. Hazard. Mater. 2007, 144, 614–619. [Google Scholar] [CrossRef] [PubMed]
- El idrissi, B.; Loranger, É.; Lanouette, R.; Bousquet, J.P.; Martinez, M. Dewatering Parameters in a Screw Press and Their Influence on the Screw Press Outputs. Chem. Eng. Res. Des. 2019, 152, 300–308. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, T.; Zhang, W.; Wang, D. Enhanced Technology Based for Sewage Sludge Deep Dewatering: A Critical Review. Water Res. 2021, 189, 116650. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Li, C.; Ding, W.; Ma, Y.; Gao, S.; Zhou, X.; Chen, Y.; Liu, W.; Jiang, G. Optimization of Operating Conditions in the Biological Enzymes for Efficient Waste Activated Sludge Dewatering. Process Saf. Environ. Prot. 2023, 170, 545–552. [Google Scholar] [CrossRef]
- Suchecka, T.; Biernacka, E.; Piatkiewicz, W. Microorganism Retention on Microfiltration Membranes. Filtr. Sep. 2003, 40, 50–55. [Google Scholar] [CrossRef]
- Chen, Y. Performance Tuning of Ultrafiltration and Reverse Osmosis Membranes Surface Nano-Structured with Tethered Poly (Acrylic Acid) Chains. Ph.D. Thesis, UCLA, Los Angeles, CA, USA, 2022. [Google Scholar]
- Jaffrin, M.Y. Dynamic Filtration with Rotating Disks, and Rotating and Vibrating Membranes: An Update. Curr. Opin. Chem. Eng. 2012, 1, 171–177. [Google Scholar] [CrossRef]
- BOKELA BOKELA Filtration Technologies. Available online: http://www.bokela.de/de/technologien/cross-flow-filtration.html (accessed on 10 December 2022).
- Novoflow Dynamic Cross-Flow Filtration. Available online: https://www.novoflow.com/en/dynamische-cross-flow-filtration (accessed on 10 December 2022).
- Ding, L.; Jaffrin, M.Y.; Luo, J. Dynamic Filtration with Rotating Disks, and Rotating or Vibrating Membranes. In Progress in Filtration and Separation; Tarleton, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 27–59. [Google Scholar]
- New Logic Research New Logic Research Technology. Available online: https://www.vsep.com/technology (accessed on 10 December 2022).
- DOE. High Solids In Situ Product Recovery. In The Next Generation of Arrested Anaerobic Digestion Technology; DOE: Washington, DC, USA. Available online: https://www.energy.gov/sites/default/files/2021-09/2396-1628_Quasar_Energy_Group_Summary.pdf#:~:text=High%20Solids%20In%20Situ%20Product%20Recovery%3B%20The%20Next,%3E%201%20kg%20of%20Sustainable%20Aviation%20Fuel%20%28SAF%29 (accessed on 3 July 2023).
- Teke, G.M.; Tai, S.L.; Pott, R.W.M. Extractive Fermentation Processes: Modes of Operation and Application. ChemBioEng Rev. 2022, 9, 146–163. [Google Scholar] [CrossRef]
- Giduthuri, A.T.; Ahring, B.K. Current Status and Prospects of Valorizing Organic Waste via Arrested Anaerobic Digestion: Production and Separation of Volatile Fatty Acids. Fermentation 2023, 9, 13. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-Based Volatile Fatty Acid Production and Recovery from Waste Streams: Current Status and Future Challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- James, G.; Görgens, J.F.; Pott, R.W.M. Co-Production of Volatile Fatty Acids and Biogas from an Anaerobic Digestion System Using in Situ Extraction. Sep. Purif. Technol. 2021, 257, 117891. [Google Scholar] [CrossRef]
- Salvachúa, D.; Saboe, P.O.; Nelson, R.S.; Singer, C.; McNamara, I.; del Cerro, C.; Chou, Y.C.; Mohagheghi, A.; Peterson, D.J.; Haugen, S.; et al. Process Intensification for the Biological Production of the Fuel Precursor Butyric Acid from Biomass. Cell Rep. Phys. Sci. 2021, 2, 100587. [Google Scholar] [CrossRef]
- Chen, Y.; Saboe, P.; Kruger, J.; Hengge, N.; Berninghaus, A.; Nogue, V.S.I.; Karp, E.; Linger, J.; Beckham, G. Hydrophobic Polytetrafluoroethylene (PTFE) Membrane-Based Emulsion Separator for Butyric Acid Recovery from Clostridia Tyrobutyricum Fermentation Broth. In Proceedings of the 45th Symposium on Biomaterials, Fuels and Chemicals, SIMB, Portland, OR, USA, 30 April–3 May 2023. [Google Scholar]
- Zaiput Flow Technologies. Liquid-Liquid/Liquid-Gas Separators Providing in-Line Separation for Batch/Flow Chemistry; Zaiput Flow Technologies: Waltham, MA, USA, 2023; Available online: https://www.zaiput.com/technical_notes/Separators-TechnicalSpecification.pdf (accessed on 3 July 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).