A Review of Liquid and Gaseous Biofuels from Advanced Microbial Fermentation Processes
Abstract
:1. Introduction
2. Classification of Bioconversion Pathways
3. Fermentation-Derived Biofuels
3.1. Bioethanol
3.2. Biobutanol
3.3. Biomethane
3.4. Biohydrogen
3.5. Biodiesel
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pattnaik, F.; Nanda, S.; Kumar, V.; Naik, S.; Dalai, A.K. Subcritical water hydrolysis of Phragmites for sugar extraction and catalytic conversion to platform chemicals. Biomass Bioenergy 2021, 145, 105965. [Google Scholar] [CrossRef]
- Our World in Data. Energy Consumption by Source, World. Available online: https://ourworldindata.org/grapher/energy-consumption-by-source-and-country (accessed on 24 August 2023).
- United States Environmental Protection Agency (USEPA). Global Greenhouse Gas Emissions Data. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 25 August 2023).
- Statista. Average Carbon Dioxide (CO₂) Levels in the Atmosphere Worldwide from 1959 to 2022 (in Parts per Million). Available online: https://www.statista.com/statistics/1091926/atmospheric-concentration-of-co2-historic/ (accessed on 23 August 2023).
- Moioli, E.; Schildhauer, T. Negative CO2 emissions from flexible biofuel synthesis: Concepts, potentials, technologies. Renew. Sustain. Energy Rev. 2022, 158, 112120. [Google Scholar] [CrossRef]
- Podder, J.; Patra, B.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K. A review of carbon capture and valorization technologies. Energies 2023, 16, 2589. [Google Scholar] [CrossRef]
- Nanda, S.; Mohammad, J.; Reddy, S.N.; Kozinski, J.A.; Dalai, A.K. Pathways of lignocellulosic biomass conversion to renewable fuels. Biomass Convers. Bioref. 2014, 4, 157–191. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Bozieva, A.M.; Zharmukhamedov, S.K.; Leong, Y.K.; Lan, J.C.W.; Veziroglu, A.; Nejat Veziroglu, T.; Tomo, T.; Chang, J.S.; Allakhverdiev, S.I. A comprehensive review on lignocellulosic biomass biorefinery for sustainable biofuel production. Int. J. Hydrogen Energy 2022, 47, 1481–1498. [Google Scholar] [CrossRef]
- Nde, D.B.; Foncha, A.C. Optimization methods for the extraction of vegetable oils: A review. Processes 2020, 8, 209. [Google Scholar] [CrossRef]
- Pattnaik, F.; Patra, B.R.; Okolie, J.A.; Nanda, S.; Dalai, A.K.; Naik, S. A review of thermocatalytic conversion of biogenic wastes into crude biofuels and biochemical precursors. Fuel 2022, 320, 123857. [Google Scholar] [CrossRef]
- Song, Y.Q.; Nanda, S.; Cong, W.J.; Sun, J.; Dong, G.H.; Magdziarz, A.; Fang, Z.; Dalai, A.K.; Kozinski, J.A. Hydrogen production from cotton stalk over Ni-La catalysts supported on spent bleaching clay via hydrothermal gasification. Ind. Crops Prod. 2022, 186, 115228. [Google Scholar] [CrossRef]
- Kang, K.; Nanda, S.; Hu, Y. Current trends in biochar application for catalytic conversion of biomass to biofuels. Catal. Today 2022, 404, 3–18. [Google Scholar] [CrossRef]
- Carere, C.R.; Sparling, R.; Cicek, N.; Levin, D.B. Third generation biofuels via direct cellulose fermentation. Int. J. Mol. Sci. 2008, 9, 1342–1360. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. A technical review of bioenergy and resource recovery from municipal solid waste. J. Hazard. Mater. 2021, 403, 123970. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.C.; Wolfram, C.; De Jong, K.P.; Gross, R.; Lewis, N.S.; Boardman, B.; Ragauskas, A.J.; Ehrhardt-Martinez, K.; Crabtree, G.; Ramana, M.V. The frontiers of energy. Nat. Energy 2016, 1, 15020. [Google Scholar] [CrossRef]
- Park, D.; Lee, J. Biological conversion of methane to methanol. Korean J. Chem. Eng. 2013, 30, 977–987. [Google Scholar] [CrossRef]
- Lima, P.J.M.; da Silva, R.M.; Neto, C.A.C.G.; Gomes e Silva, N.C.; Souza, J.E.D.S.; Nunes, Y.L.; Sousa dos Santos, J.C. An overview on the conversion of glycerol to value-added industrial products via chemical and biochemical routes. Biotechnol. Appl. Biochem. 2022, 69, 2794–2818. [Google Scholar] [CrossRef]
- Nanda, S.; Golemi-Kotra, D.; McDermott, J.C.; Dalai, A.K.; Gökalp, I.; Kozinski, J.A. Fermentative production of butanol: Perspectives on synthetic biology. New Biotechnol. 2017, 37, 210–221. [Google Scholar] [CrossRef]
- Elmore, J.R.; Dexter, G.N.; Salvachúa, D.; O’Brien, M.; Klingeman, D.M.; Gorday, K.; Michener, J.K.; Peterson, D.J.; Beckham, G.T.; Guss, A.M. Engineered Pseudomonas putida simultaneously catabolizes five major components of corn stover lignocellulose: Glucose, xylose, arabinose, p-coumaric acid, and acetic acid. Metab. Eng. 2020, 62, 62–71. [Google Scholar] [CrossRef]
- Kamusoko, R.; Jingura, R.M.; Parawira, W.; Chikwambi, Z. Strategies for valorization of crop residues into biofuels and other value-added products. Biofuel Bioprod. Biorefin. 2021, 15, 1950–1964. [Google Scholar] [CrossRef]
- Vyas, S.; Prajapati, P.; Shah, A.V.; Srivastava, V.K.; Varjani, S. Opportunities and knowledge gaps in biochemical interventions for mining of resources from solid waste: A special focus on anaerobic digestion. Fuel 2022, 311, 122625. [Google Scholar] [CrossRef]
- Kukkar, D.; Sharma, P.K.; Kim, K.H. Recent advances in metagenomic analysis of different ecological niches for enhanced biodegradation of recalcitrant lignocellulosic biomass. Environ. Res. 2022, 215, 114369. [Google Scholar] [CrossRef]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef]
- Zhai, R.; Hu, J.; Jin, M. Towards efficient enzymatic saccharification of pretreated lignocellulose: Enzyme inhibition by lignin-derived phenolics and recent trends in mitigation strategies. Biotechnol. Adv. 2022, 61, 108044. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.K.; Dixit, M.; Kapoor, R.K.; Shukla, P. Xylanolytic enzymes in pulp and paper industry: New technologies and perspectives. Mol. Biotechnol. 2022, 64, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Iqbal, H.M.N.; Cardullo, N.; Muccilli, V.; Fernández-Lucas, J.; Schmidt, J.E.; Jesionowski, T.; Bilal, M. Structural insights, biocatalytic characteristics, and application prospects of lignin-modifying enzymes for sustainable biotechnology. Int. J. Biol. Macromol. 2023, 242, 124968. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Junior, N.L.; Bento, H.B.S.; de Carvalho, A.K.F.; de Souza Vandenberghe, L.P.; Soccol, C.R.; Aminabhavi, T.M.; Chandel, A.K. Process strategies to reduce cellulase enzyme loading for renewable sugar production in biorefineries. Chem. Eng. J. 2023, 451, 138690. [Google Scholar] [CrossRef]
- Keshav, P.K.; Banoth, C.; Kethavath, S.N.; Bhukya, B. Lignocellulosic ethanol production from cotton stalk: An overview on pretreatment, saccharification and fermentation methods for improved bioconversion process. Biomass Convers. Bioref. 2023, 13, 4477–4493. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Lukk, T.; Tuohy, M.G.; Gong, L.; Nguyen-Tri, P.; Goddard, A.D.; Bill, R.M.; Nayak, S.C.; et al. Lignocellulosic biorefineries: The current state of challenges and strategies for efficient commercialization. Renew. Sustain. Energy Rev. 2021, 148, 111258. [Google Scholar] [CrossRef]
- Tang, S.; Yu, Y.L.; Liu, R.; Wei, S.; Zhang, Q.; Zhao, J.; Li, S.; Dong, Q.; Li, Y.B.; Wang, Y. Enhancing ethylene glycol and ferric chloride pretreatment of rice straw by low-pressure carbon dioxide to improve enzymatic saccharification. Bioresour. Technol. 2023, 369, 128391. [Google Scholar] [CrossRef]
- Adsul, M.; Sandhu, S.K.; Singhania, R.R.; Gupta, R.; Puri, S.K.; Mathur, A. Designing a cellulolytic enzyme cocktail for the efficient and economical conversion of lignocellulosic biomass to biofuels. Enz. Microb. Technol. 2020, 133, 109442. [Google Scholar] [CrossRef]
- Reihani, S.F.S.; Khosravi-Darani, K. Influencing factors on single-cell protein production by submerged fermentation: A review. Electr. J. Biotechnol. 2019, 37, 34–40. [Google Scholar] [CrossRef]
- Martău, G.A.; Unger, P.; Schneider, R.; Venus, J.; Vodnar, D.C.; López-Gómez, J.P. Integration of solid state and submerged fermentations for the valorization of organic municipal solid waste. J. Fungi 2021, 7, 766. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Wang, R.; Liu, L.; Zhang, Y.; Bao, H.; Jang, J.M.; Wang, E.; Yuan, H. Comparative characterization of extracellular enzymes secreted by Phanerochaete chrysosporium during solid-state and submerged fermentation. Int. J. Biol. Macromol. 2020, 152, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Namnuch, N.; Thammasittirong, A.; Thammasittirong, S.N.R. Lignocellulose hydrolytic enzymes production by Aspergillus flavus KUB2 using submerged fermentation of sugarcane bagasse waste. Mycology 2021, 12, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Velasco, L.M.; Favela-Torres, E.; Théatre, A.; Arguelles-Arias, A.; Saucedo-Castañeda, J.G.; Jacques, P. Relationship between lipopeptide biosurfactant and primary metabolite production by Bacillus strains in solid-state and submerged fermentation. Bioresour. Technol. 2022, 345, 126556. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Zhang, M.; Liu, L.; Dong, L.; Ma, Y.; Wei, Z.; Chi, J.; Zhang, R. Co-culture submerged fermentation by Lactobacillus and yeast more effectively improved the profiles and bioaccessibility of phenolics in extruded brown rice than single-culture fermentation. Food Chem. 2020, 326, 126985. [Google Scholar] [CrossRef]
- Saeed, S.; Baig, U.U.R.; Tayyab, M.; Altaf, I.; Irfan, M.; Raza, S.Q.; Nadeem, F.; Mehmood, T. Valorization of banana peels waste into biovanillin and optimization of process parameters using submerged fermentation. Biocatal. Agric. Biotechnol. 2021, 36, 102154. [Google Scholar] [CrossRef]
- Abdollahi, F.; Jahadi, M.; Ghavami, M. Thermal stability of natural pigments produced by Monascus purpureus in submerged fermentation. Food Sci. Nutr. 2021, 9, 4855–4862. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, Y.; Zhang, Y.; He, J.; Xie, Y. Enhanced exopolysaccharide production in submerged fermentation of Ganoderma lucidum by Tween 80 supplementation. Bioproc. Biosyst. Eng. 2021, 44, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Carboué, Q.; Saucedo-Castaneda, G.; de Jesús Cázares-Marinero, J. Biosurfactants: The green generation of speciality chemicals and potential production using solid-state fermentation (SSF) technology. Bioresour. Technol. 2021, 320, 124222. [Google Scholar] [CrossRef]
- Leite, P.; Sousa, D.; Fernandes, H.; Ferreira, M.; Costa, A.R.; Filipe, D.; Gonçalves, M.; Peres, H.; Belo, I.; Salgado, J.M. Recent advances in production of lignocellulolytic enzymes by solid-state fermentation of agro-industrial wastes. Curr. Opin. Green Sustain. Chem. 2021, 27, 100407. [Google Scholar] [CrossRef]
- Roasa, J.; De Villa, R.; Mine, Y.; Tsao, R. Phenolics of cereal, pulse and oilseed processing by-products and potential effects of solid-state fermentation on their bioaccessibility, bioavailability and health benefits: A review. Trend Food Sci. Technol. 2021, 116, 954–974. [Google Scholar] [CrossRef]
- Sheikha, A.F.E.; Ray, R.C. Bioprocessing of horticultural wastes by solid-state fermentation into value-added/innovative bioproducts: A review. Food Rev. Int. 2022, 39, 3009–3065. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Kozinski, J.A. Butanol and ethanol production from lignocellulosic feedstock: Biomass pretreatment and bioconversion. Energy Sci. Eng. 2014, 2, 138–148. [Google Scholar] [CrossRef]
- Nanda, S.; Azargohar, R.; Dalai, A.K.; Kozinski, J.A. An assessment on the sustainability of lignocellulosic biomass for biorefining. Renew. Sustain. Energy Rev. 2015, 50, 925–941. [Google Scholar] [CrossRef]
- Jha, S.; Okolie, J.A.; Nanda, S.; Dalai, A.K. A review of biomass resources and thermochemical conversion technologies. Chem. Eng. Technol. 2022, 45, 791–799. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K.; Kumar, J.; Ahluwalia, V. A critical review on current strategies and trends employed for removal of inhibitors and toxic materials generated during biomass pretreatment. Bioresour. Technol. 2020, 299, 122633. [Google Scholar] [CrossRef] [PubMed]
- Gopinarayanan, V.E.; Nair, N.U. Pentose metabolism in Saccharomyces cerevisiae: The need to engineer global regulatory systems. Biotechnol. J. 2019, 14, e1800364. [Google Scholar] [CrossRef] [PubMed]
- Donato, P.D.; Finore, I.; Poli, A.; Nicolaus, B.; Lama, L. The production of second generation bioethanol: The biotechnology potential of thermophilic bacteria. J. Clean. Prod. 2019, 233, 1410–1417. [Google Scholar] [CrossRef]
- Mladenovska, Z.; Dabrowski, S. Thermophilic Fermentative Bacterium Producing Butanol and/or Hydrogen from Glycerol. World Intellectual Property Organization International Patent Application NO. WO2010031793A2, 25 March 2010. [Google Scholar]
- Singh, N.; Gupta, R.P.; Puri, S.K.; Mathur, A.S. Bioethanol production from pretreated whole slurry rice straw by thermophilic co-culture. Fuel 2021, 301, 121074. [Google Scholar] [CrossRef]
- Zuliani, L.; Serpico, A.; De Simone, M.; Frison, N.; Fusco, S. Biorefinery gets hot: Thermophilic enzymes and microorganisms for second-generation bioethanol production. Processes 2021, 9, 1583. [Google Scholar] [CrossRef]
- Jeffries, T.W. Ethanol fermentation on the move. Nat. Biotechnol. 2005, 23, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Raud, M.; Rocha-Meneses, L.; Lane, D.J.; Sippula, O.; Shurpali, N.J.; Kikas, T. Utilization of barley straw as feedstock for the production of different energy vectors. Processes 2021, 9, 726. [Google Scholar] [CrossRef]
- Sivarathnakumar, S.; Jayamuthunagai, J.; Baskar, G.; Praveenkumar, R.; Selvakumari, I.A.E.; Bharathiraja, B. Bioethanol production from woody stem Prosopis juliflora using thermo tolerant yeast Kluyveromyces marxianus and its kinetics studies. Bioresour. Technol. 2019, 293, 122060. [Google Scholar] [CrossRef] [PubMed]
- Raita, M.; Ibenegbu, C.; Champreda, V.; Leak, D.J. Production of ethanol by thermophilic oligosaccharide utilising Geobacillus thermoglucosidasius TM242 using palm kernel cake as a renewable feedstock. Biomass Bioenergy 2016, 95, 45–54. [Google Scholar] [CrossRef]
- Prasad, S.; Kumar, S.; Yadav, K.K.; Choudhry, J.; Kamyab, H.; Bach, Q.V.; Sheetal, K.R.; Kannojiya, S.; Gupta, N. Screening and evaluation of cellulytic fungal strains for saccharification and bioethanol production from rice residue. Energy 2020, 190, 116422. [Google Scholar] [CrossRef]
- Qu, C.; Dai, K.; Fu, H.; Wang, J. Enhanced ethanol production from lignocellulosic hydrolysates by Thermoanaerobacterium aotearoense SCUT27/ΔargR1864 with improved lignocellulose-derived inhibitors tolerance. Renew. Energy 2021, 173, 652–661. [Google Scholar] [CrossRef]
- Prasoulas, G.; Gentikis, A.; Konti, A.; Kalantzi, S.; Kekos, D.; Mamma, D. Bioethanol production from food waste applying the multienzyme system produced on-site by Fusarium oxysporum F3 and mixed microbial cultures. Fermentation 2020, 6, 39. [Google Scholar] [CrossRef]
- Khoshkho, S.M.; Mahdavian, M.; Karimi, F.; Karimi-Maleh, H.; Razaghi, P. Production of bioethanol from carrot pulp in the presence of Saccharomyces cerevisiae and beet molasses inoculum; a biomass-based investigation. Chemosphere 2022, 286, 131688. [Google Scholar] [CrossRef]
- Periyasamy, S.; Isabel, J.B.; Kavitha, S.; Karthik, V.; Mohamed, B.A.; Gizaw, D.G.; Sivashanmugam, P.; Aminabhavi, T.M. Recent advances in consolidated bioprocessing for conversion of lignocellulosic biomass into bioethanol—A review. Chem. Eng. J. 2023, 453, 139783. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Recent advances in second generation ethanol production by thermophilic bacteria. Energies 2015, 8, 1–30. [Google Scholar] [CrossRef]
- Tse, T.J.; Wiens, D.J.; Reaney, M.J. Production of bioethanol—A review of factors affecting ethanol yield. Fermentation 2021, 7, 268. [Google Scholar] [CrossRef]
- Plaza, P.E.; Gallego-Morales, L.J.; Peñuela-Vásquez, M.; Lucas, S.; García-Cubero, M.T.; Coca, M. Biobutanol production from brewer’s spent grain hydrolysates by Clostridium beijerinckii. Bioresour. Technol. 2017, 244, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Seo, S.O.; Lu, T. System-level modeling of acetone–butanol–ethanol fermentation. FEMS Microbiol. Lett. 2016, 363, fnw074. [Google Scholar] [CrossRef]
- Li, S.; Huang, L.; Ke, C.; Pang, Z.; Liu, L. Pathway dissection, regulation, engineering and application: Lessons learned from biobutanol production by solventogenic clostridia. Biotechnol. Biofuels 2020, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Sonomoto, K.; Oshiro, M.; Hanada, K. Method of Producing Butanol. U.S. Patent US8420359B2, 16 April 2013. [Google Scholar]
- Yang, X.; Xu, M.; Yang, S.T. Metabolic and process engineering of Clostridium cellulovorans for biofuel production from cellulose. Metab. Eng. 2015, 32, 39–48. [Google Scholar] [CrossRef]
- Branska, B.; Fořtová, L.; Dvořáková, M.; Liu, H.; Patakova, P.; Zhang, J.; Melzoch, M. Chicken feather and wheat straw hydrolysate for direct utilization in biobutanol production. Renew. Energy 2020, 145, 1941–1948. [Google Scholar] [CrossRef]
- Wen, Z.; Minton, N.P.; Zhang, Y.; Li, Q.; Liu, J.; Jiang, Y.; Yang, S. Enhanced solvent production by metabolic engineering of a twin-clostridial consortium. Metab. Eng. 2017, 39, 38–48. [Google Scholar] [CrossRef]
- Tang, C.; Chen, Y.; Liu, J.; Shen, T.; Cao, Z.; Shan, J.; Zhu, C.; Ying, H. Sustainable biobutanol production using alkali-catalyzed organosolv pretreated cornstalks. Ind. Crops Prod. 2017, 95, 383–392. [Google Scholar] [CrossRef]
- Xue, C.; Zhao, J.; Lu, C.; Yang, S.T.; Bai, F.; Tang, I.C. High-titer n-butanol production by Clostridium acetobutylicum JB200 in fed-batch fermentation with intermittent gas stripping. Biotechnol. Bioeng. 2012, 109, 2746–2756. [Google Scholar] [CrossRef]
- Rajagopalan, G.; He, J.; Yang, K.L. One-pot fermentation of agricultural residues to produce butanol and hydrogen by Clostridium strain BOH3. Renew. Energy 2016, 85, 1127–1134. [Google Scholar] [CrossRef]
- Tsai, T.Y.; Lo, Y.C.; Dong, C.D.; Nagarajan, D.; Chang, J.S.; Lee, D.J. Biobutanol production from lignocellulosic biomass using immobilized Clostridium acetobutylicum. Appl. Energy 2020, 277, 115531. [Google Scholar] [CrossRef]
- Su, C.; Qi, L.; Cai, D.; Chen, B.; Chen, H.; Zhang, C.; Si, Z.; Wang, Z.; Li, G.; Qin, P. Integrated ethanol fermentation and acetone-butanol-ethanol fermentation using sweet sorghum bagasse. Renew. Energy 2020, 162, 1125–1131. [Google Scholar] [CrossRef]
- Mondal, S.; Santra, S.; Rakshit, S.; Halder, S.K.; Hossain, M.; Mondal, K.C. Saccharification of lignocellulosic biomass using an enzymatic cocktail of fungal origin and successive production of butanol by Clostridium acetobutylicum. Bioresour. Technol. 2022, 343, 126093. [Google Scholar] [CrossRef]
- Narueworanon, P.; Laopaiboon, L.; Phukoetphim, N.; Laopaiboon, P. Impacts of initial sugar, nitrogen and calcium carbonate on butanol fermentation from sugarcane molasses by Clostridium beijerinckii. Energies 2020, 13, 694. [Google Scholar] [CrossRef]
- Allen, S.D.; Rusnack, M.R. Process for Improving the Yield and Efficiency of an Ethanol Fermentation Plant. U.S. Patent 8,927,239 B2, 6 January 2015. [Google Scholar]
- Josse, J.C.; Benedek, A. Syngas Biomethanation Process and Anaerobic Digestion System. U.S. Patent 9.284,203 B2, 15 March 2016. [Google Scholar]
- Wang, P.; Wang, H.; Qiu, Y.; Ren, L.; Jiang, B. Microbial characteristics in anaerobic digestion process of food waste for methane production—A review. Bioresour. Technol. 2018, 248, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Nozhevnikova, A.N.; Russkova, Y.I.; Litti, Y.V.; Parshina, S.N.; Zhuravleva, E.A.; Nikitina, A.A. Syntrophy and interspecies electron transfer in methanogenic microbial communities. Microbiology 2020, 89, 129–147. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Yuan, Z.; Wang, R.; Angelidaki, I.; Zhu, G. Syntrophy mechanism, microbial population, and process optimization for volatile fatty acids metabolism in anaerobic digestion. Chem. Eng. J. 2023, 452, 139137. [Google Scholar] [CrossRef]
- Venkateshkumar, R.; Shanmugam, S.; Veerappan, A.R. Experimental investigation on the effect of anaerobic co-digestion of cotton seed hull with cow dung. Biomass Convers. Biorefin. 2021, 11, 1255–1262. [Google Scholar] [CrossRef]
- Wu, Y.; Song, K. Anaerobic co-digestion of waste activated sludge and fish waste: Methane production performance and mechanism analysis. J. Clean. Prod. 2021, 279, 123678. [Google Scholar] [CrossRef]
- Elsayed, M.; Diab, A.; Soliman, M. Methane production from anaerobic co-digestion of sludge with fruit and vegetable wastes: Effect of mixing ratio and inoculum type. Biomass Convers. Biorefin. 2021, 11, 989–998. [Google Scholar] [CrossRef]
- Riggio, V.; Comino, E.; Rosso, M. Energy production from anaerobic co-digestion processing of cow slurry, olive pomace and apple pulp. Renew. Energy 2015, 83, 1043–1049. [Google Scholar] [CrossRef]
- Bouaita, R.; Derbal, K.; Panico, A.; Iasimone, F.; Pontoni, L.; Fabbricino, M.; Pirozzi, F. Methane production from anaerobic co-digestion of orange peel waste and organic fraction of municipal solid waste in batch and semi-continuous reactors. Biomass Bioenergy 2022, 160, 106421. [Google Scholar] [CrossRef]
- Azevedo, A.; Gominho, J.; Duarte, E. Performance of anaerobic co-digestion of pig slurry with pineapple (Ananas comosus) bio-waste residues. Waste Biomass Valor. 2021, 12, 303–311. [Google Scholar] [CrossRef]
- Adeleye, T.; Yeo, H.; Seth, R.; Hafez, H.; Biswas, N. Influence of mix ratio of potato peel and pig manure on reaction kinetics and methane recovery from anaerobic co-digestion. Can. J. Civ. Eng. 2022, 49, 675–682. [Google Scholar] [CrossRef]
- Latifi, P.; Karrabi, M.; Danesh, S. Anaerobic co-digestion of poultry slaughterhouse wastes with sewage sludge in batch-mode bioreactors (effect of inoculum-substrate ratio and total solids). Renew. Sustain. Energy Rev. 2019, 107, 288–296. [Google Scholar] [CrossRef]
- Kang, K.; Azargohar, R.; Dalai, A.K.; Wang, H. Hydrogen production from lignin, cellulose and waste biomass via supercritical water gasification: Catalyst activity and process optimization study. Energy Convers. Manag. 2016, 117, 528–537. [Google Scholar] [CrossRef]
- Kovač, A.; Paranos, M.; Marciuš, D. Hydrogen in energy transition: A review. Int. J. Hydrogen Energy 2021, 46, 10016–10035. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.; Setiabudi, H.D.; Nanda, S.; Vo, D.V.N. Advanced synthesis strategies of mesoporous SBA-15 supported catalysts for catalytic reforming applications: A state-of-the-art review. Appl. Catal. A Gen. 2018, 559, 57–74. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Hu, Y.H. Steam reforming of methane: Current states of catalyst design and process upgrading. Renew. Sustain. Energy Rev. 2021, 149, 111330. [Google Scholar] [CrossRef]
- Nanda, S.; Rana, R.; Zheng, Y.; Kozinski, J.A.; Dalai, A.K. Insights on pathways for hydrogen generation from ethanol. Sustain. Energy Fuels 2017, 1, 1232–1245. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Huang, R.; Zhang, W.; He, W.; Deng, Z.; Han, Y.; Xiao, B.; Luo, H.; Qu, W. Effects of temperature and total solid content on biohydrogen production from dark fermentation of rice straw: Performance and microbial community characteristics. Chemosphere 2022, 286, 131655. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Liu, W.; Guo, J.; Xian, M. Debottlenecking the biological hydrogen production pathway of dark fermentation: Insight into the impact of strain improvement. Microb. Cell Fact. 2022, 21, 166. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, P.K.; Nanda, S. Biohydrogen production through dark fermentation. Chem. Eng. Technol. 2020, 43, 601–612. [Google Scholar] [CrossRef]
- Lopez-Hidalgo, A.M.; Smoliński, A.; Sanchez, A. A meta-analysis of research trends on hydrogen production via dark fermentation. Int. J. Hydrogen Energy 2022, 47, 13300–13339. [Google Scholar] [CrossRef]
- Qu, X.; Zeng, H.; Gao, Y.; Mo, T.; Li, Y. Bio-hydrogen production by dark anaerobic fermentation of organic wastewater. Front. Chem. 2022, 10, 978907. [Google Scholar] [CrossRef] [PubMed]
- Laurent, B.; Serge, H.; Julien, M.; Christopher, H.; Philippe, T. Effects of hydrogen partial pressure on fermentative biohydrogen production by a chemotropic Clostridium bacterium in a new horizontal rotating cylinder reactor. Energy Proc. 2012, 29, 34–41. [Google Scholar] [CrossRef]
- Kim, D.H.; Yoon, J.J.; Kim, S.H.; Park, J.H. Acceleration of lactate-utilizing pathway for enhancing biohydrogen production by magnetite supplementation in Clostridium butyricum. Bioresour. Technol. 2022, 359, 127448. [Google Scholar] [CrossRef]
- Mohanraj, S.; Pandey, A.; Mohan, S.V.; Anbalagan, K.; Kodhaiyolii, S.; Pugalenthi, V. Metabolic engineering and molecular biotechnology of biohydrogen production. In Biohydrogen, 2nd ed.; Pandey, A., Mohan, S.V., Chang, J.S., Hallenbeck, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 413–434. [Google Scholar]
- Schut, G.J.; Adams, M.W.W. The iron-hydrogenase of Thermotoga maritima utilizes ferredoxin and NADH synergistically: A new perspective on anaerobic hydrogen production. J. Bacteriol. 2009, 191, 4451–4457. [Google Scholar] [CrossRef]
- Losey, N.A.; Mus, F.; Peters, J.W.; Le, H.M.; McInerney, M.J. Syntrophomonas wolfei uses an NADH-dependent, ferredoxin-independent [FeFe]-hydrogenase to reoxidize NADH. Environ. Microbiol. 2017, 83, e01335-17. [Google Scholar] [CrossRef]
- Willquist, K.; Zeidan, A.A.; van Niel, E.W. Physiological characteristics of the extreme thermophile Caldicellulosiruptor saccharolyticus: An efficient hydrogen cell factory. Microb. Cell Fact. 2010, 9, 89. [Google Scholar] [CrossRef]
- Pradhan, N.; Dipasquale, L.; d’Ippolito, G.; Panico, A.; Lens, P.N.L.; Esposito, G.; Fontana, A. Hydrogen production by the thermophilic bacterium Thermotoga neapolitana. Int. J. Mol. Sci. 2015, 16, 12578–12600. [Google Scholar] [CrossRef]
- Auria, R.; Boileau, C.; Davidson, S.; Casalot, L.; Christen, P.; Liebgott, P.P.; Combet-Blanc, Y. Hydrogen production by the hyperthermophilic bacterium Thermotoga maritima Part II: Modeling and experimental approaches for hydrogen production. Biotechnol. Biofuels 2016, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Byrne, E.; Björkmalm, J.; Bostick, J.P.; Sreenivas, K.; Willquist, K.; van Niel, E.W.J. Characterization and adaptation of Caldicellulosiruptor strains to higher sugar concentrations, targeting enhanced hydrogen production from lignocellulosic hydrolysates. Biotechnol. Biofuels 2021, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Bielen, A.A.M.; Verhaart, M.R.A.; van der Oost, J.; Kengen, S.W.M. Biohydrogen production by the thermophilic bacterium Caldicellulosiruptor saccharolyticus: Current status and perspectives. Life 2013, 3, 52–85. [Google Scholar] [CrossRef]
- Li, X.; Sui, K.; Zhang, J.; Liu, X.; Xu, Q.; Wang, D.; Yang, Q. Revealing the mechanisms of rhamnolipid enhanced hydrogen production from dark fermentation of waste activated sludge. Sci. Total Environ. 2022, 806, 150347. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Singh, R.; Syed, A.; Pal, D.B.; Elgorban, A.M.; Kushwaha, D.; Mishra, P.K.; Gupta, V.K. Co-fermentation of residual algal biomass and glucose under the influence of Fe3O4 nanoparticles to enhance biohydrogen production under dark mode. Bioresour. Technol. 2021, 342, 126034. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Basak, N. Optimization and modelling of dark fermentative hydrogen production from cheese whey by Enterobacter aerogenes 2822. Int. J. Hydrogen Energy 2021, 46, 1777–1800. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Yang, J.; Li, Z.; Zhang, J.; Zhao, W.; Zang, L. Cobalt ferrate nanoparticles improved dark fermentation for hydrogen evolution. J. Clean. Prod. 2021, 316, 128275. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Zhang, J.; Zhou, C.; Pei, Y.; Zang, L. Improved biohydrogen evolution through calcium ferrite nanoparticles assisted dark fermentation. Bioresour. Technol. 2022, 361, 127676. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, G.; Sage, V.; Xu, J.; Sun, G.; He, J.; Sun, Y. Optimization of dark fermentation for biohydrogen production using a hybrid artificial neural network (ANN) and response surface methodology (RSM) approach. Environ. Prog. Sustain. Energy 2021, 40, e13485. [Google Scholar] [CrossRef]
- Cieciura-Włoch, W.; Borowski, S.; Domański, J. Dark fermentative hydrogen production from hydrolyzed sugar beet pulp improved by iron addition. Bioresour. Technol. 2020, 314, 123713. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Shobana, S.; Nguyen, D.D.; Banu, J.R.; Sivagurunathan, P.; Chang, S.W.; Ponnusamy, V.K.; Kumar, G. Application of nanotechnology (nanoparticles) in dark fermentative hydrogen production. Int. J. Hydrogen Energy 2019, 44, 1431–1440. [Google Scholar] [CrossRef]
- Gouda, S.P.; Dhakshinamoorthy, A.; Rokhum, S.L. Metal-organic framework as a heterogeneous catalyst for biodiesel production: A review. Chem. Eng. J. Adv. 2022, 2022, 100415. [Google Scholar] [CrossRef]
- Dias, A.L.B.; de Aguiar, A.C.; Rostagno, M.A. Extraction of natural products using supercritical fluids and pressurized liquids assisted by ultrasound: Current status and trends. Ultrason. Sonochem. 2021, 74, 105584. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Zaidi, S. A review of the feedstocks, catalysts, and intensification techniques for sustainable biodiesel production. J. Environ. Chem. Eng. 2020, 8, 104523. [Google Scholar] [CrossRef]
- Taher, H.; Giwa, A.; Abusabiekeh, H.; Al-Zuhair, S. Biodiesel production from Nannochloropsis gaditana using supercritical CO2 for lipid extraction and immobilized lipase transesterification: Economic and environmental impact assessments. Fuel Process. Technol. 2020, 198, 106249. [Google Scholar] [CrossRef]
- Bashir, M.A.; Wu, S.; Zhu, J.; Krosuri, A.; Khan, M.U.; Aka, R.J.N. Recent development of advanced processing technologies for biodiesel production: A critical review. Fuel Process. Technol. 2022, 227, 107120. [Google Scholar] [CrossRef]
- Khandelwal, K.; Boahene, P.; Nanda, S.; Dalai, A.K. Hydrogen production from supercritical water gasification of model compounds of crude glycerol from biodiesel industries. Energies 2023, 16, 3746. [Google Scholar] [CrossRef]
- El-Bakry, M.; Abraham, J.; Cerda, A.; Barrena, R.; Ponsá, S.; Gea, T.; Sánchez, A. From wastes to high value added products: Novel aspects of SSF in the production of enzymes. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1999–2042. [Google Scholar] [CrossRef]
- Bharathi, D.; Rajalakshmi, G. Microbial lipases: An overview of screening, production and purification. Biocatal. Agric. Biotechnol. 2019, 22, 101368. [Google Scholar] [CrossRef]
- Bhan, C.; Singh, J. Role of microbial lipases in transesterification process for biodiesel production. Environ. Sustain. 2020, 3, 257–266. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Puna, J.; Gomes, J. A review on bio-based catalysts (immobilized enzymes) used for biodiesel production. Energies 2020, 13, 3013. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. Production of methyl ester from two microalgae by two-step transesterification and direct transesterification. Environ. Sci. Pollut. Res. 2017, 24, 4950–4963. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Gerken, H.; Zhang, C.; Hu, Q.; Li, Y. Highly-efficient enzymatic conversion of crude algal oils into biodiesel. Bioresour. Technol. 2014, 172, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Da Rós, P.C.M.; e Silva, W.C.; Grabauskas, D.; Perez, V.H.; de Castro, H.F. Biodiesel from babassu oil: Characterization of the product obtained by enzymatic route accelerated by microwave irradiation. Ind. Crops Prod. 2014, 52, 313–320. [Google Scholar] [CrossRef]
- Korkut, I.; Bayramoglu, M. Ultrasound assisted biodiesel production in presence of dolomite catalyst. Fuel 2016, 180, 624–629. [Google Scholar] [CrossRef]
- Du, L.; Ding, S.; Li, Z.; Lv, E.; Lu, J.; Ding, J. Transesterification of castor oil to biodiesel using NaY zeolite-supported La2O3 catalysts. Energy Convers. Manag. 2018, 173, 728–734. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, S.; Lu, C.; Gong, Z.; Hu, X. Catalytic performance of NaAlO2/γ-Al2O3 as heterogeneous nanocatalyst for biodiesel production: Optimization using response surface methodology. Energy Convers. Manag. 2020, 203, 112263. [Google Scholar] [CrossRef]
- Putra, M.D.; Irawan, C.; Ristianingsih, Y.; Nata, I.F. A cleaner process for biodiesel production from waste cooking oil using waste materials as a heterogeneous catalyst and its kinetic study. J. Clean. Prod. 2018, 195, 1249–1258. [Google Scholar] [CrossRef]
- Jayaraman, J.; Alagu, K.; Appavu, P.; Joy, N.; Jayaram, P.; Mariadoss, A. Enzymatic production of biodiesel using lipase catalyst and testing of an unmodified compression ignition engine using its blends with diesel. Renew. Energy 2020, 145, 399–407. [Google Scholar] [CrossRef]
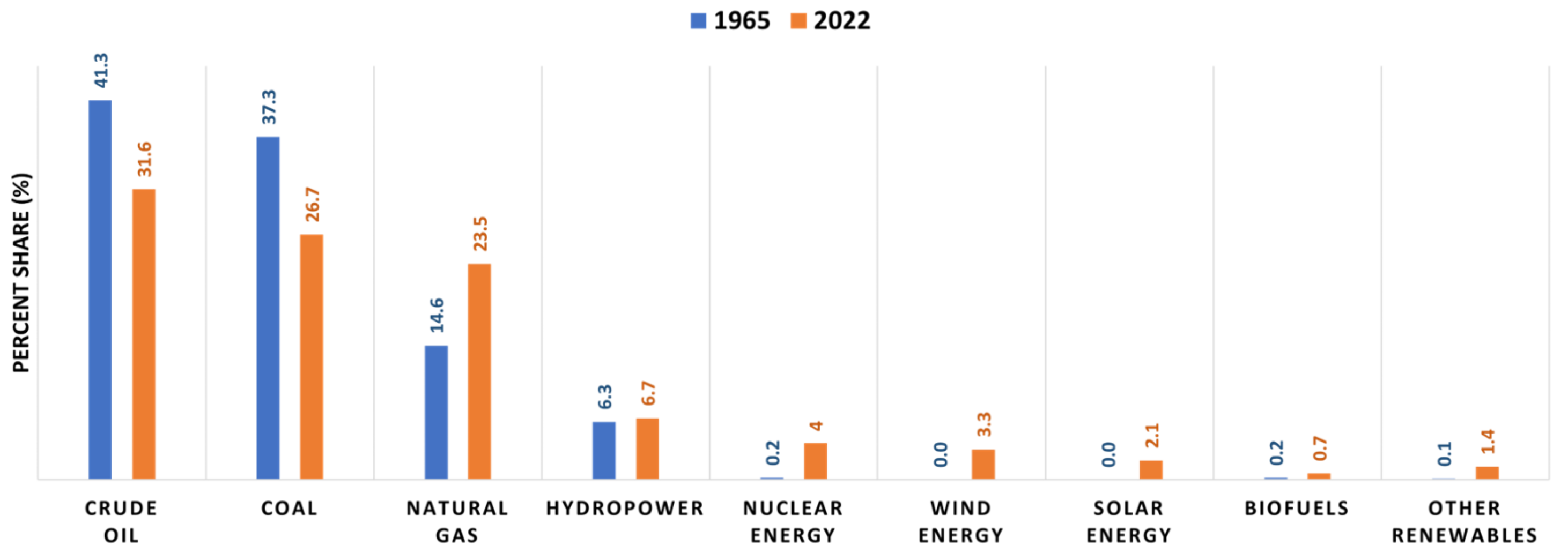
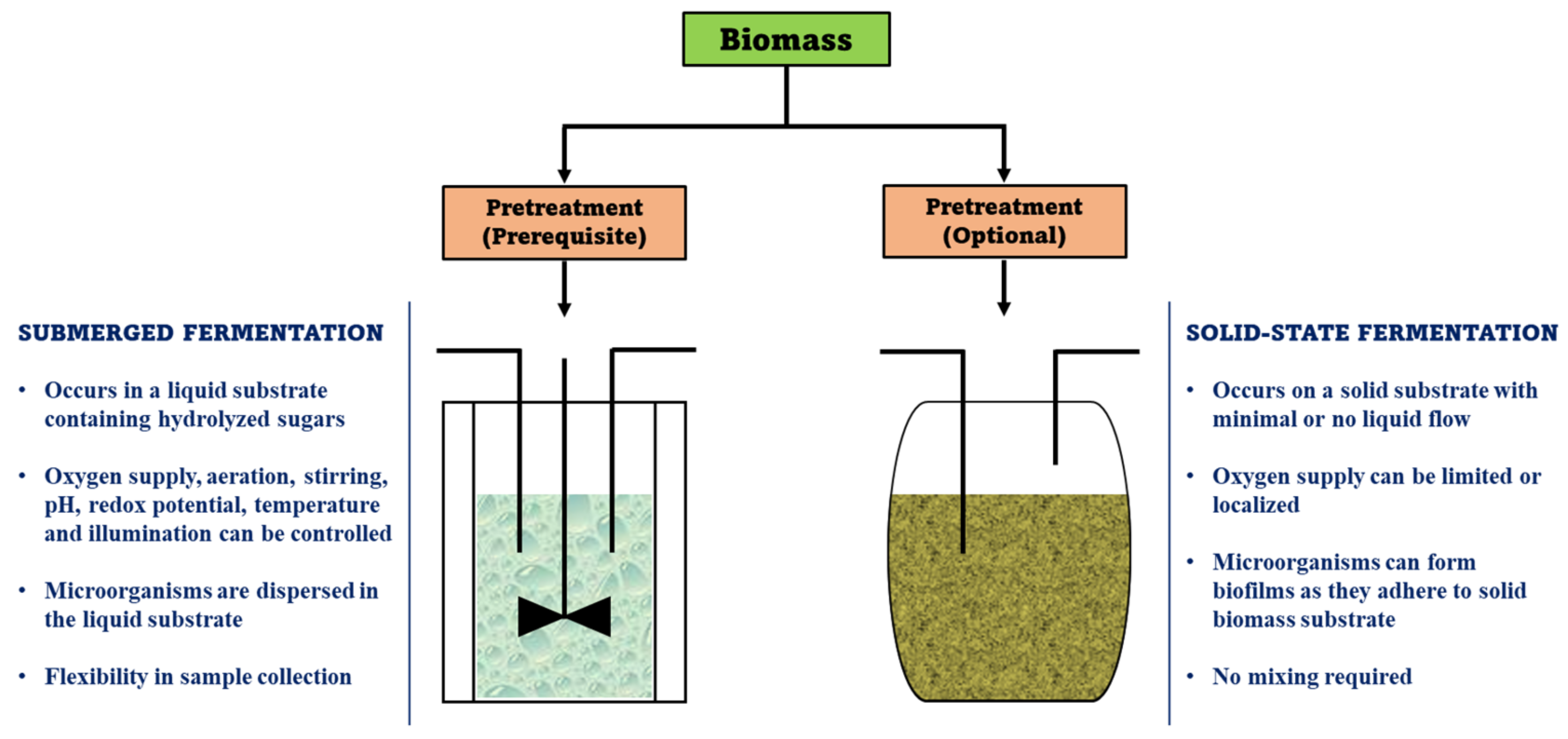

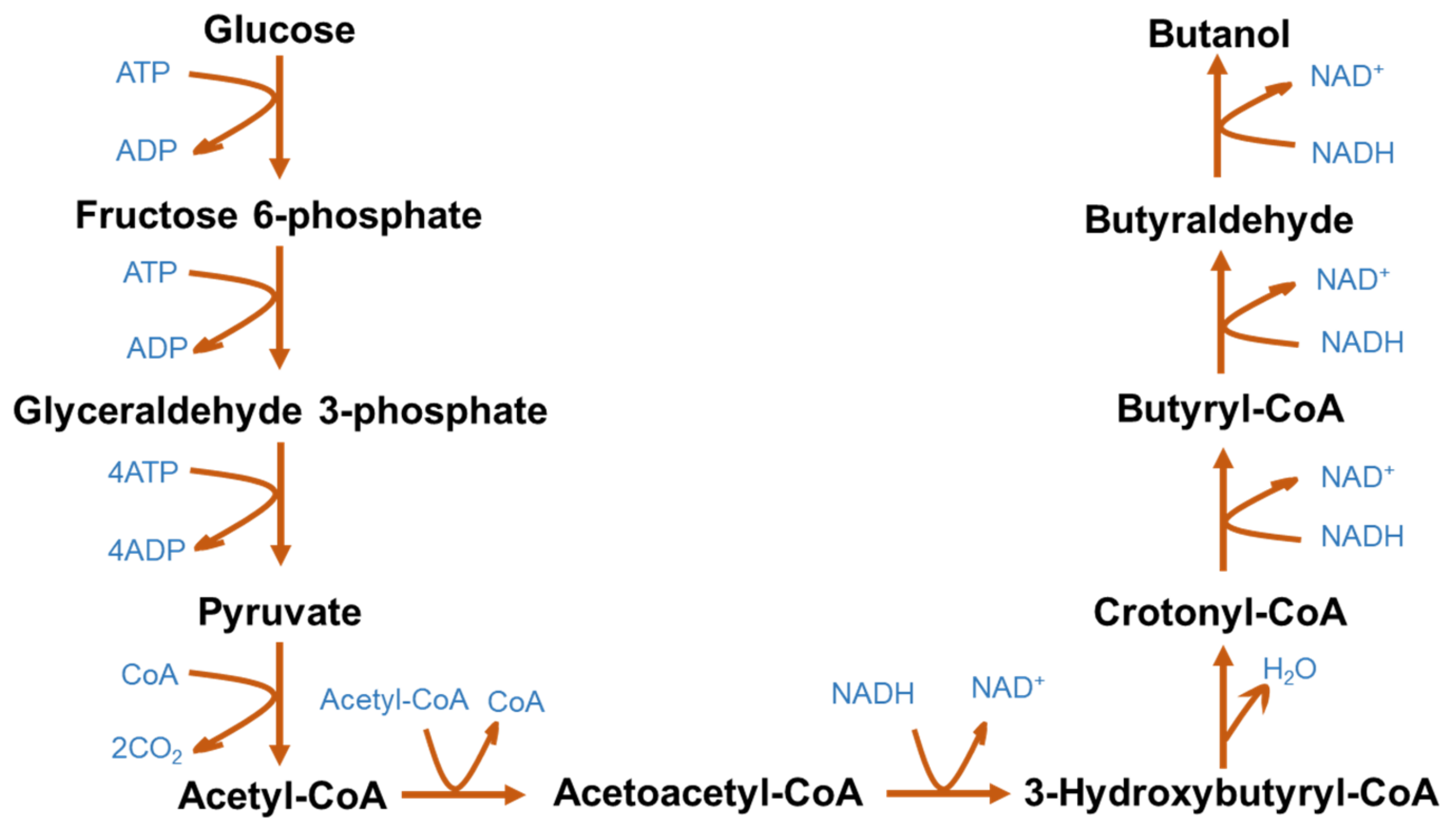

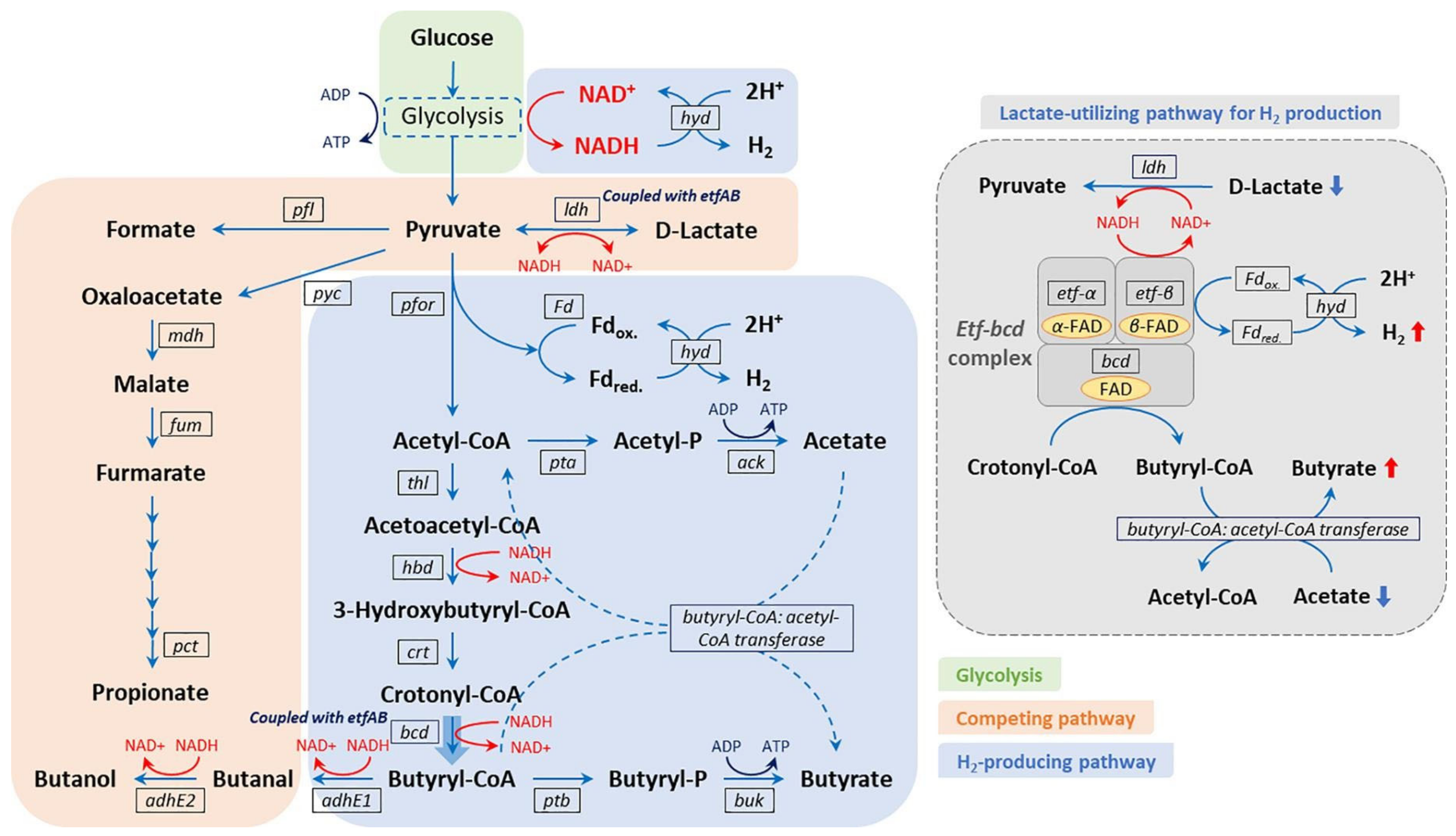
| Biomass | Pretreatment | Process Parameters | Ethanol Yield (g/g) | Reference |
|---|---|---|---|---|
| Barley straw | • Decompression pretreatment • Temperature: 125–175 °C • N2 pressure: 3 MPa | • Hydrolysis (Accellerase 1500, 30 FPU/g cellulase, temperature: 50 °C, agitation: 250 rpm, time: 72 h) • Fermentation: Saccharomyces cerevisiae (1 g per 200 mL, temperature: 22 °C, time: 7 days) | 0.43–0.9 | Raud et al. [55] |
| Mesquite stem (Prosopis juliflora) | • Mild acid pretreatment • 3 vol/vol% HNO3 • Temperature: 121 °C • Biomass-to-liquid ratio: 1:10 • Pressure: 0.01 MPa | • Simultaneous saccharification and fermentation (commercial cellulases 12 FPU/g of biomass, Kluyveromyces marxianus MTCC 1389, inoculum concentration: 3 vol/vol%, pH 4.9, temperature: 41 °C, substrate concentration: 5 wt/vol%, time: 72 h) | 0.67 | Sivarathnakumar et al. [56] |
| Palm kernel cake | • Steam explosion • Pressure: 0.45 MPa • Reaction time: 15 min | • Hydrolysis (SEB mannanase 17.9 U/g mannan, CTec2 cellulase 10.4 FPU/g glucan, temperature: 50 °C, time: 72 h, agitation: 250 rpm) • Fermentation (Geobacillus thermoglucosidasius TM242, temperature: 60 °C, time: 48 h, agitation: 250 rpm) | 0.47 | Raita et al. [57] |
| Rice straw | • Microwave-assisted NaOH pretreatment • Temperature: 100–140 °C • Reaction time: 1–2 min | • Hydrolysis (cellulolytic enzymes produced from Trichoderma reesei NCIM-1052: 20 FPU/g of biomass, time: 64 h), pH 4.8, temperature: 55 °C, fermentation time: 72 h, agitation: 150 rpm) • Fermentation (Pichia stipites NCIM 3499 and Saccharomyces cerevisiae 3186, temperature: 30 °C, time: 74 h, pH 4.5) | 0.44 | Prasad et al. [58] |
| Sorghum stalk | • Mild acid pretreatment (H2SO4) | • Fermentation (Thermoanaerobacterium aotearoense SCUT27/ΔargR1864, total sugar: 15 g/L, temperature: 55 °C, agitation: 150 rpm) | 0.36 | Qu et al. [59] |
| Soybean straw | • Mild acid pretreatment • 0.1 M H2SO4 • Temperature: 121 °C • Reaction time: 30 min | • Fermentation (Thermoanaerobacterium aotearoense SCUT27/ΔargR1864, total sugar: 15 g/L, temperature: 55 °C, agitation: 150 rpm) | 0.34 | Qu et al. [59] |
| Starch-based food waste | • Mild acid pretreatment (H2SO4) • Feedstock concentration: 30 wt/vol% | • Simultaneous saccharification and fermentation (enzyme was produced inside the reactor using Saccharomyces cerevisiae and Fusarium oxysporum, inoculum-to-feedstock: 1:10, pH 6, temperature: 30 °C, time: 94 h, agitation: 80 rpm) | 0.1 | Prasoulas et al. [60] |
| Biomass | Pretreatment | Process Parameters | Biobutanol Concentration, Yield and Productivity | Reference |
|---|---|---|---|---|
| Cellulose | - | • Clostridium cellulovorans (overexpressing adhE2) | • Concentration: 1.42 g/L, yield: 0.195 g/g, productivity: 5.9 mg/L/h | Yang et al. [69] |
| Chicken feather and wheat straw | • Alkaline pretreatment • 0.6% NaOH • Temperature: 80 °C • Time: 20 h • Agitation: 130 rpm | • Enzyme: CellicCtec2 enzyme (temperature: 50 °C, pH: 6.3, time: 20 h, agitation: 130 rpm) • Fermentation: Clostridium beijerinckii strain NCIMB 8052 (temperature: 37 °C, time: 48 h) | • Concentration: 4.6 g/L, yield: 0.054 g/g | Branska et al. [70] |
| Corn cob | • Alkali pretreatment | • Clostridium cellulovorans DSM 743B and Clostridium beijerinckii NCIMB 8052 | • Concentration: 11.8 g/L, yield: 0.14 g/g, productivity: 5.9 mg/L/h | Wen et al. [71] |
| Corn stalk | • Ethanol-assisted alkali pretreatment • 4% NaOH • 60 vol/vol% ethanol • Temperature: 110 °C • Time: 90 min | • Enzymes: Cellulase and xylanase (temperature: 50 °C, agitation: 150 rpm) • Fermentation: Clostridium beijerinckii NCIMB 4110 | • Concentration: 12.8 g/L, yield: 0.43 g/g, productivity: 0.18 g/L/h | Tang et al. [72] |
| Glucose | - | • Fed-batch fermentation (Clostridium acetobutylicum JB200 in 78 h) | • Concentration: 19.1 g/L, yield: 0.21 g/g, productivity: 0.24 g/L/h | Xue et al. [73] |
| Rice bran and sesame oil cake | • Autoclaved at 121 °C for 20 min | • Clostridium sp. BOH3 | • Concentration: 13.5 g/L, yield: 0.1 g/g | Rajagopalan et al. [74] |
| Rice straw | • H2O2 (0.2 wt/vol%) assisted NaOH (1.5%) pretreatment | • Enzyme (Accellerase 1500) • Fermentation (polyvinyl alcohol-immobilized Clostridium acetobutylicum ATCC 824) | • Concentration: 13.8 g/L, yield: 0.23 g/g, productivity: 0.9 g/L/h | Tsai et al. [75] |
| Sweet sorghum bagasse | • Alkali pretreatment • 2% NaOH • Feedstock-to-liquid ratio: 1:10 • Temperature: 120 °C • Time: 1 h | • Enzyme: (Cellulase 30 FPU/g, temperature: 50 °C, time 96 h, agitation: 180 rpm) • Fermentation (bioethanol production by Saccharomyces cerevisiae M3013 followed by ABE fermentation by Clostridium acetobutylicum ABE 1201) | • Ethanol yield: 0.144 g/g, butanol yield: 0.02 g/g | Su et al. [76] |
| Wheat bran, sugarcane bagasse and orange peel | • Microwave-assisted acid and surfactant-based pretreatment | • Enzyme (Aspergillus niger SKN1 and Trametes hirsuta SKH1) • Fermentation (Clostridium acetobutylicum ATCC 824) | • Concentration: 16.5 g/L, yield: 0.24 g/g | Mondal et al. [77] |
| Yeast extract, rice bran, soybean waste, dried spent yeast, urea, ammonium sulfate | - | • Clostridium beijerinckii TISTR 1461 | • Concentration: 11.4 g/L, yield: 0.4 g/g, productivity: 0.32 g/L/h | Narueworanon et al. [78] |
| Biomass | Pretreatment | Process Parameters | Biomethane or Biogas Yield | Reference |
|---|---|---|---|---|
| Cow dung (CD) and cotton seed hull (CSH) | Biogas plant slurry | • 500 mL batch reactor • 300 mL of inoculum • CD/CSH ratio: 100:0, 0:100, 50:50, 75:25 and 25:75 • Temperature: 35 °C • pH: 7.5 • Reaction time: 45 days • Stirring: 90 rpm | • CD: 193 mL/g VS • CSH: 33 mL/g VS • CD/CSH (50:50 ratio): 37 mL/g VS • CD/CSH (75:25 ratio): 86 mL/g VS • CD/CSH (25:75 ratio): 23 mL/g VS | Venkateshkumar et al. [84] |
| Fish waste and activated sludge | Anaerobic digested sludge | • 300 mL batch reactor • Temperature: 37 °C • Reaction time: 50 days • Fish waste loading: 0, 1.5, 3, 6 and 10 wt% • Inoculum/substrate ratio: 1:8 | • 1.5 wt% fish waste: 410 mL/g VS • 3 wt% fish waste: 684 mL/g VS | Wu and Song [85] |
| Fruit and vegetable waste (FVW) and primary sludge (PS) | Activated sludge | • 500 mL batch reactor • FVW/PS ratio: 50:50 • Temperature: 37 °C • Reaction time: 30 days • Inoculum/substrate ratio (50:50 FVW/PS): 2 | • Without inoculum: 141 mL/g VS • With inoculum: 295 mL/g VS | Elsayed et al. [86] |
| Olive pomace (OP) and apple pulp (AP) | Cow slurry (CS) | • 128 L pilot scale reactor • Temperature: 35 °C • pH: 7.5 • Reaction time: 40 days • Stirring: 90 rpm • Feedstock composition: 85 wt% CS, 10 wt% OP and 5 wt% AP | • 216 mL/g VS (CH4 fraction: 52%) | Riggio et al. [87] |
| Orange peel waste (OPW) and organic fraction of the municipality waste (OFMSW) | Degassed sewage sludge | • 150 mL batch reactor • Temperature: 37 °C and 55 °C • pH: 7.5 • Reaction time: 35 days • OPW/OFMSW ratio: 50:50 • Inoculum/substrate ratio: 1:3 | • At 55 °C (thermophilic condition): 432 mL/g VS • At 37 °C (mesophilic condition): 294.6 mL/g VS | Bouaita et al. [88] |
| Pineapple peel (PP) | Pig slurry (PS) | • 4.8 L continuous stirred reactor • Organic loading rate: 1.46 g VS/L/day • PS/PP ratio: 80:20 • Temperature: 37 °C • pH: 7.5 • Reaction time: 16 days | • 580 mL/g VS | Azevedo et al. [89] |
| Potato peel (PP) and pig manure (PM) | Anaerobically digested sludge | • 150 mL batch reactor • Temperature: 38 °C • Reaction time: 27 days • Stirring: 150 rpm • Feedstock composition: 50 wt% PP and 50 wt% PM | • 380 mL/g VS | Adeleye et al. [90] |
| Slaughterhouse waste and sewage sludge | Sludge from the secondary treatment plant (Activated sludge) | • 1 L batch reactor • Total solids: 5% • Inoculum/substrate ratio: 4 • Temperature: 34 °C | • Biogas yield: 631 mL/g VS • CH4 yield: 462 mL/g VS | Latifi et al. [91] |
| Biomass | Microorganism | Bioreactor Type | Biohydrogen Yield or Productivity | Reference |
|---|---|---|---|---|
| Activated sludge | Actinobacteria, Bacterioidetes, Chloroflexi, Firmicutes and Proteobacteria | Plexiglass bottles | 11 mL/g | Li et al. [112] |
| Algae and glucose | Clostridium pasteurianum | Serum bottles | 67 mL/g | Srivastava et al. [113] |
| Cheese whey | Enterobacter aerogenes 2822 | Double-walled cylindrical bioreactor | 0.75 mL/g/h | Rao and Basak [114] |
| Glucose | Clostridium sensu stricto 1 | Glass bioreactors | 205 mL/g | Zhang et al. [115] |
| Glucose | Firmicutes, Chloroflexi, Proteobacteria, Synergistetes and Bacteroidetes | Serum vials | 250.1 mL/g | Zhang et al. [116] |
| Potato peel | Clostridium propionicum | Transfusion bottle submerged in a water bath | 106.2 mL/g | Wang et al. [117] |
| Sugar beet pulp | Clostridia and Coriobacteriia | Glass bottles (batch tests), cylindrical glass reactors (semi-continuous tests) | 58.9 mL/g | Cieciura-Włoch et al. [118] |
| Feedstock | Catalyst or Biocatalyst | Biodiesel Yield | Reference |
|---|---|---|---|
| Algae | NaOH | 92% | Sivaramakrishnan et al. [131] |
| Algal oil | Lipase from Candida antarctica | 99.1% | Wang et al. [132] |
| Babassu oil | Lipase from Burkholderia cepacia | >99% | Da Rós et al. [133] |
| Canola oil | CaO calcined dolomite | 99.4% | Korkut and Bayramoglu [134] |
| Castor oil | La2O3/Na–Y-600 | 85% | Du et al. [135] |
| Palm oil | NaAlO2/γ-Al2O3 | 97.7% | Zhang et al. [136] |
| Waste cooking oil | CaO/SiO2 | 91% | Putra et al. [137] |
| Waste cooking oil | Lipase | >99% | Jayaraman et al. [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanda, S.; Pattnaik, F.; Patra, B.R.; Kang, K.; Dalai, A.K. A Review of Liquid and Gaseous Biofuels from Advanced Microbial Fermentation Processes. Fermentation 2023, 9, 813. https://doi.org/10.3390/fermentation9090813
Nanda S, Pattnaik F, Patra BR, Kang K, Dalai AK. A Review of Liquid and Gaseous Biofuels from Advanced Microbial Fermentation Processes. Fermentation. 2023; 9(9):813. https://doi.org/10.3390/fermentation9090813
Chicago/Turabian StyleNanda, Sonil, Falguni Pattnaik, Biswa R. Patra, Kang Kang, and Ajay K. Dalai. 2023. "A Review of Liquid and Gaseous Biofuels from Advanced Microbial Fermentation Processes" Fermentation 9, no. 9: 813. https://doi.org/10.3390/fermentation9090813
APA StyleNanda, S., Pattnaik, F., Patra, B. R., Kang, K., & Dalai, A. K. (2023). A Review of Liquid and Gaseous Biofuels from Advanced Microbial Fermentation Processes. Fermentation, 9(9), 813. https://doi.org/10.3390/fermentation9090813








