Biohydrogen Gas/Acetone-Butanol-Ethanol Production from Agave Guishe Juice as a Low-Cost Growing Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material Collection
2.2. Guishe Juice Characterization
2.3. Microorganisms
2.4. Experimental Setup
2.5. Analytical Methods
2.6. Data Analyses
3. Results
3.1. Guishe Juice Characterization
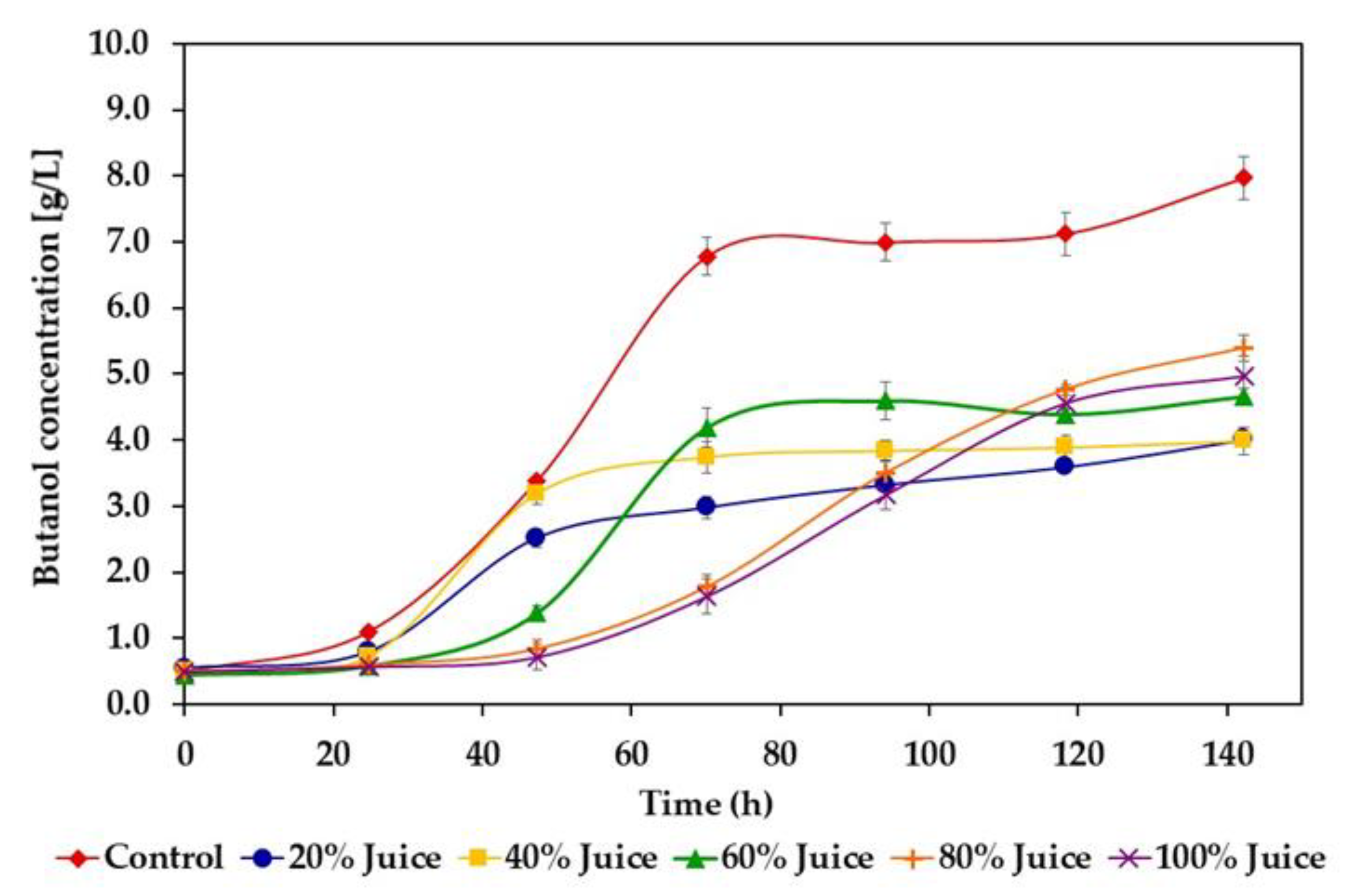
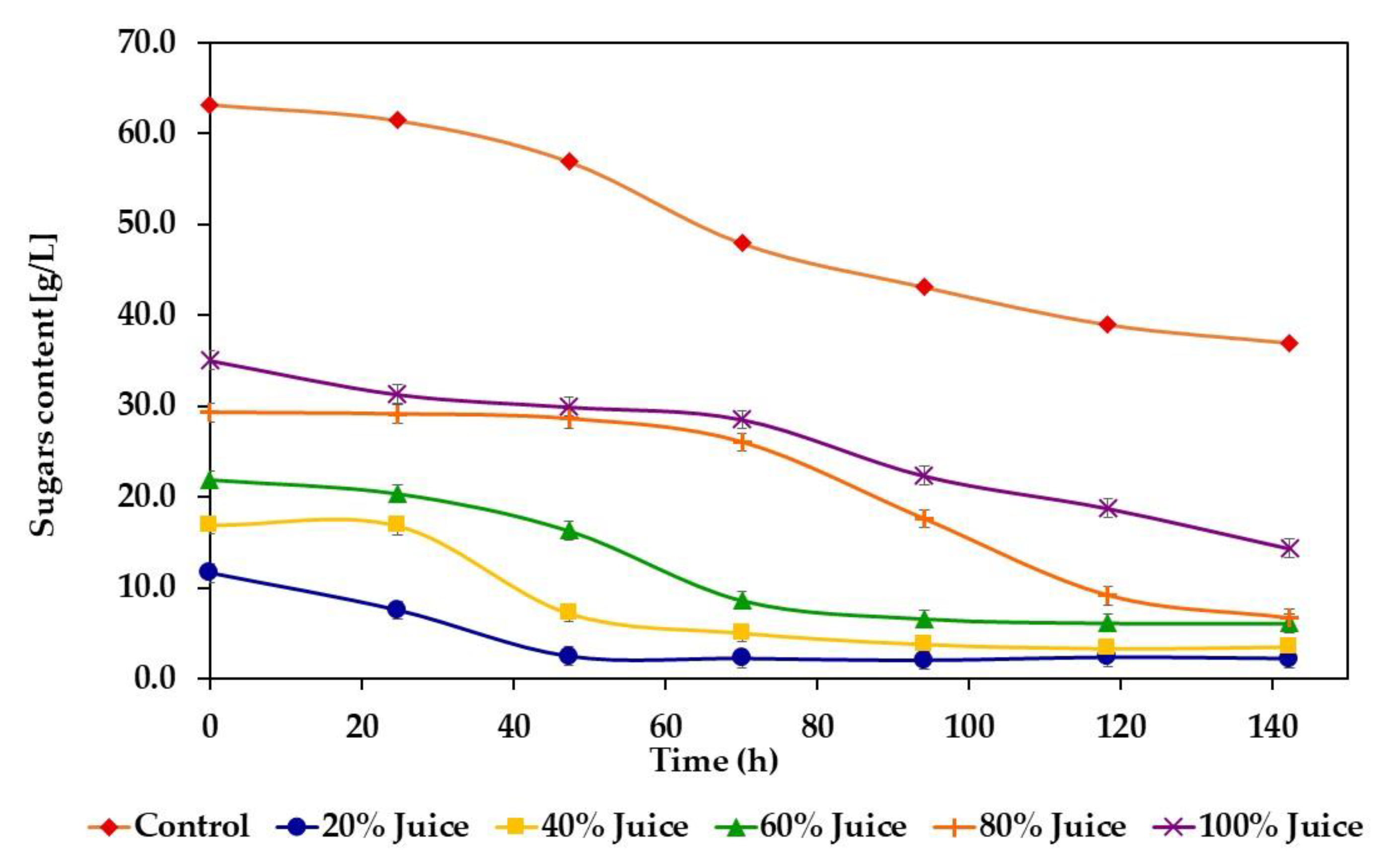
3.2. Butanol and Hydrogen Production
4. Discussion
4.1. Guishe Juice Characterization
4.2. Butanol and Hydrogen Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nosratpour, M.J.; Karimi, K.; Sadegui, M. Improvement of ethanol and biogas production from sugarcane bagasse using sodium alkaline pretreatments. J. Environ. Manag. 2018, 226, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chi, X.; Zhang, Y.; Wang, X. Enhanced coproduction of hydrogen and butanol from rice straw by a novel two-stage fermentation process. Int. Biodeterior. Biodegrad. 2018, 127, 62–68. [Google Scholar] [CrossRef]
- Guerrero, K.; Gallardo, R.; Paredes, I.; Quintero, J.; Mau, S.; Conejeros, R.; Gentina, J.C.; Aroca, G. Continuous biohydrogen production by a degenerated strain of Clostridium acetobutylicum ATCC824. Int. J. Hydrogen Energy 2021, 46, 5100–5111. [Google Scholar] [CrossRef]
- Tondro, H.M.; Musivand, S.; Zilouei, H.; Bazarganipour, M.; Zargoosh, K. Biological production of hydrogen and acetone- butanol-ethanol from sugarcane bagasse and rice straw using co-culture of Enterobacter aerogenes and Clostridium acetobutylicum. Biomass Bioenergy 2020, 142, 105818. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Mathimani, T.; Varjan, S.; Rene, E.R.; Kumar, G.; Kim, S.H.; Ponnusamy, V.K.; Yoon, J.-J. Biobutanol as a promising liquid fuel for the future—Recent updates and perspectives. Fuel 2019, 253, 637–646. [Google Scholar] [CrossRef]
- Ndaba, B.; Chiyanzu, I.; Marx, S. n-Butanol derived from biochemical and chemical routes: A review. Biotechnol. Rep. 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Singh, V.; Singh, H.; Das, D. Optimization of the medium composition for the improvement of hydrogen and butanol production using Clostridium saccharoperbutylacetonicum DSM 14923. Int. J. Hydrogen Energy 2019, 44, 26905–26919. [Google Scholar] [CrossRef]
- Xue, C.; Wu, Y.; Gu, Y.; Jiang, W.; Dong, H.; Zhang, Y.; Zhao, C.; Li, Y. Biofuels and bioenergy: Acetone and butanol. Compr. Biotechnol. 2019, 3, 79–100. [Google Scholar]
- Wang, J.; Yin, Y. Clostridium species for fermentative hydrogen production: An overview. Int. J. Hydrogen Energy 2021, 46, 34599–34625. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, K.; Zhou, K. Using Co-Culture to Functionalize Clostridium Fermentation. Trends Biotechnol. 2021, 39, 914–926. [Google Scholar] [CrossRef]
- Oliva-Rodríguez, A.G.; Quintero, J.; Medina-Morales, M.A.; Morales-Martínez, T.K.; Rodríguez-De la Garza, J.A.; Moreno-Dávila, M.; Aroca, G.; Ríos-González, L.J. Clostridium strain selection for co-culture with Bacillus subtilis for butanol production from agave hydrolysates. Bioresour. Technol. 2019, 275, 410–415. [Google Scholar] [CrossRef]
- Morales-Martínez, T.K.; Medina-Morales, M.A.; Ortíz-Cruz, A.L.; Rodríguez-De la Garza, J.A.; Moreno-Dávila, M.; López-Badillo, C.M.; Ríos-González, L.J. Consolidated bioprocessing of hydrogen production from agave biomass by Clostridium acetobutylicum and bovine ruminal fluid. Int. J. Hydrogen Energy 2020, 45, 13707–13716. [Google Scholar] [CrossRef]
- Carrillo-Nieves, D.; Rostro, M.J.; De la Cruz, R.; Ruiz, H.A.; Iqbal, H.M.N.; Parra-Saldívar, R. Current status and future trends of bioethanol production from agro-industrial wastes in Mexico. Renew. Sustain. Energy Rev. 2019, 102, 63–74. [Google Scholar] [CrossRef]
- Morreeuw, Z.P.; Castillo-Quiroz, D.; Ríos-González, L.J.; Martínez-Rincón, R.; Estrada, N.; Melchor-Martínez, E.M.; Iqbal, H.M.N.; Parra-Saldívar, E.; Reyes, A.G. High Throughput Profiling of Flavonoid Abundance in Agave lechuguilla Residue-Valorizing under Explored Mexican Plant. Plants 2021, 10, 695. [Google Scholar] [CrossRef]
- Díaz-Jiménez, L.; Carlos-Hernández, S.; Jasso, D.; Rodríguez-García, R. Conceptualization of a biorefinery for guishe revalorization. Ind. Crops Prod. 2019, 138, 111441. [Google Scholar] [CrossRef]
- Morreeuw, Z.P.; Escobedo-Fregoso, C.; Ríos-González, L.J.; Castillo-Quiroz, D.; Reyes, A.G. Transcriptome-based metabolic profiling of flavonoids in Agave lechuguilla waste biomass. Plant Sci. 2021, 305, 110748. [Google Scholar] [CrossRef]
- Figueroa-Díaz, A.B.; Carlos-Hernández, S.; Díaz-Jiménez, L. Crude glycerol/guishe based catalysts for biodiesel production: Conforming a guishe biorefinery. Catalysts 2021, 11, 3. [Google Scholar] [CrossRef]
- Peña-Rodríguez, A.; Pelletier-Morreeuw, Z.; García-Luján, J.; Rodríguez-Jaramillo, M.; Guzmán-Villanueva, L.; Escobedo-Fregoso, C.; Tovar-Ramírez, D.; Reyes, A.G. Evaluation of Agave lechuguilla by-product crude extract as a feed additive for juvenile shrimp Litopenaeus vannamei. Aquac. Res. 2020, 51, 1336–1345. [Google Scholar] [CrossRef]
- Morreeuw, Z.P.; Ríos-González, L.J.; Salinas, C.; Melchor-Martínez, E.M.; Ascacio-Valdés, J.A.; Parra-Saldívar, R.; Iqbal, H.M.N.; Reyes, A.G. Early Optimization stages of Agave lechuguilla bagasse processing toward biorefinement: Drying procedure and enzymatic hydrolysis for flavonoid extraction. Molecules 2021, 26, 7292. [Google Scholar] [CrossRef]
- Ortiz-Méndez, O.H.; Morales-Martínez, T.K.; Rios-González, L.J.; Rodríguez-de la Garza, J.A.; Quintero, J.; Aroca, G. Bioethanol production from Agave lechuguilla biomass pretreated by autohydrolysis. Rev. Mex. Ing. Química 2017, 16, 467–476. [Google Scholar]
- Díaz-Blanco, D.I.; de la Cruz, J.R.; López-Linares, J.C.; Morales-Martínez, T.K.; Ruiz, E.; Rios-González, L.J.; Romero, I.; Castro, E. Optimization of dilute acid pretreatment of Agave lechuguilla and ethanol production by co-fermentation with Escherichia coli MM160. Ind. Crops Prod. 2018, 114, 154–163. [Google Scholar] [CrossRef]
- Rios-González, L.J.; Morales-Martínez, T.K.; Hernández-Enríquez, G.G.; Rodríguez de la Garza, J.A.; Moreno-Dávila, M. Hydrogen production by anaerobic digestion from Agave lechuguilla hydrolysates. Bioresources 2018, 13, 7766–7779. [Google Scholar] [CrossRef]
- Reyna-Martínez, R.; Morales-Martínez, T.K.; Castillo-Quiroz, D.; Contreras-Esquivel, J.C.; Ríos-González, L.J. Fungal pretreatment of Agave lechuguilla Torr. biomass to produce ethanol. Rev. Mex. Cienc. For. 2018, 10, 86–106. [Google Scholar]
- Ríos-González, L.J.; Medina-Morales, M.A.; Rodríguez-de la Garza, J.A.; Romero-Galarza, A.; Medina, D.D.; Morales-Martínez, T.K. Comparison of dilute acid pretreatment of agave assisted by microwave versus ultrasound to enhance enzymatic hydrolysis. Bioresour. Technol. 2021, 319, 124099. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial polyphenol-rich extracts: Applications and limitations in the food industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef] [PubMed]
- Zaynab, M.; Sharif, Y.; Abbas, S.; Afzal, M.Z.; Qasim, M.; Khalofah, A.; Ansari, M.J.; Khan, K.A.; Tao, L.; Li, S. Saponin toxicity as key player in plant defense against pathogens. Toxicon 2021, 193, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, P.; Martínez-Amador, S.Y.; Valdez-Aguilar, L.A.; Benavides-Mendoza, A.; Rodríguez-de la Garza, J.A.; Ovando-Medina, V.M. Design and evaluation of a sequential bioelectrochemical system for municipal wastewater treatment and voltage generation. Rev. Mex. Ing. Química 2018, 17, 145–154. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass (Technical Report NREL/TP-510-42622); National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Medina-Morales, M.A.; De la Cruz-Andrade, L.E.; Paredes-Peña, L.A.; Morales-Martínez, T.K.; Rodríguez-De la Garza, J.A.; Moreno-Dávila, I.M.; Tamayo-Ordóñez, M.C.; Ríos-González, L.J. Biohydrogen production from thermochemically pretreated corncob using a mixed culture bioaugmented with Clostridium acetobutylicum. Int. J. Hydrogen Energy 2021, 46, 25974–25984. [Google Scholar] [CrossRef]
- Sánchez, J.H.; Luna, C.F.; Reyes, A.G.; Cruz, M.; Ríos-González, L.J.; Morales-Martínez, T.K.; Ascacio, J.A.; Medina-Morales, M.A. Initial Study of Fungal Bioconversion of guishe (Agave lechuguilla Residue) Juice for Bioherbicide Activity on Model Seeds. Fermentation 2023, 9, 421. [Google Scholar] [CrossRef]
- Tsai, T.-Y.; Lo, Y.-C.; Dong, C.-D.; Nagarajan, D.; Chang, J.-S.; Lee, D.-J. Biobutanol production from lignocellulosic biomass using immobilized Clostridium acetobutylicum. Appl. Energy 2020, 227, 115531. [Google Scholar] [CrossRef]
- Mirfakhar, M.; Asadollahi, M.A.; Amiri, H.; Karimi, K. Co-fermentation of hemicellulosic hydrolysates and starch from sweet sorghum by Clostridium acetobutylicum: A synergistic effect for butanol production. Ind. Crops Prod. 2020, 151, 112459. [Google Scholar] [CrossRef]
- Roussos, A.; Misailidis, N.; Koulouris, A.; Zimbardi, F.; Petrides, D. A Feasibility Study of Cellulosic Isobutanol Production—Process Simulation and Economic Analysis. Processes 2019, 7, 667. [Google Scholar] [CrossRef]
- Cai, D.; Wen, J.; Zhuang, Y.; Huang, T.; Si, Z.; Qin, P.; Chen, H. Review of alternative technologies for acetone-butanol-ethanol separation: Principles, state-of-the-art, and development trends. Sep. Purif. Technol. 2022, 298, 121244. [Google Scholar] [CrossRef]
- Chen, H.; Cai, D.; Chen, H.; Zhang, C.; Wang, J.; Qin, P. Techno-economic analysis of acetone-butanol-ethanol distillation sequences feeding the biphasic condensate after in situ gas stripping separation. Sep. Purif. Technol. 2019, 219, 241–248. [Google Scholar] [CrossRef]
- Du, G.; Wu, Y.; Kang, W.; Xu, Y.; Li, S.; Xue, C. Enhanced butanol production in Clostridium acetobutylicum by manipulating metabolic pathway genes. Process Biochem. 2022, 114, 134–138. [Google Scholar] [CrossRef]
- López-Contreras, A.M.; Claassen, P.A.; Mooibroek, H.; De Vos, W.M. Utilisation of saccharides in extruded domestic organic waste by Clostridium acetobutylicum ATCC 824 for production of acetone, butanol and ethanol. Appl. Microbiol. Biotechnol. 2000, 54, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Kang, H.; Sang, B.-I.; Kim, Y.; Min, J.; Mitchell, R.J.; Lee, J.H. Feasibility of a facile butanol bioproduction using planetary mill pretreatment. Bioresour. Technol. 2016, 199, 283–287. [Google Scholar] [CrossRef]
- Zhao, T.; Tashiro, Y.; Zheng, J.; Sakai, K.; Sonomoto, K. Semi-hydrolysis with low enzyme loading leads to highly effective butanol fermentation. Bioresour. Technol. 2018, 264, 335–342. [Google Scholar] [CrossRef]
- He, C.-R.; Huang, C.-L.; Lai, Y.-C.; Li, S.-Y. The utilization of sweet potato vines as carbon sources for fermenting bio-butanol. J. Taiwan Inst. Chem. Eng. 2017, 79, 7–13. [Google Scholar] [CrossRef]
- Khedkar, M.A.; Nimbalkar, P.R.; Gaikwad, S.G.; Chavan, P.V.; Bankar, S.B. Sustainable biobutanol production from pineapple waste by using Clostridium acetobutylicum B 527: Drying kinetics study. Bioresour. Technol. 2017, 225, 359–366. [Google Scholar] [CrossRef]
- Hassan, E.A.; Abd-Alla, M.H.; Bagy, M.M.K.; Morsy, F.M. In situ hydrogen, acetone, butanol, ethanol and microdiesel production by Clostridium acetobutylicum ATCC 824 from oleaginous fungal biomass. Anaerobe 2015, 34, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cheng, C.; Cao, G.; Yang, S.T.; Ren, N. Potential of hydrogen production from sugarcane juice by Ethanoligenens harbinense Yuan-3. J. Clean. Prod. 2019, 237, 117552. [Google Scholar] [CrossRef]
- Contreras-Dávila, C.A.; Méndez-Acosta, H.O.; Arellano-García, L.; Alatriste-Mondragón, F.; Razo-Flores, E. Continuous hydrogen production from enzymatic hydrolysate of Agave tequilana bagasse: Effect of the organic loading rate and reactor configuration. Chem. Eng. J. 2017, 313, 671–679. [Google Scholar] [CrossRef]
- Montiel-Corona, V.; Razo-Flores, E. Continuous hydrogen and methane production from Agave tequilana bagasse hydrolysate by sequential process to maximize energy recovery efficiency. Bioresour. Technol. 2018, 249, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Cervantes, A.; Arreola-Vargas, J.; Elias-Palacios, S.V.; Marino-Marmolejo, E.N.; Dávila-Vázquez, G.; González-Álvarez, V.; Méndez-Acosta, H.O. Evaluation of semi-continuous hydrogen production from enzymatic hydrolysates of Agave tequilana bagasse: Insight into the enzymatic cocktail effect over the co-production of methane. Int. J. Hydrogen Energy 2018, 43, 14193–14201. [Google Scholar] [CrossRef]
- Montoya-Rosales, J.d.J.; Olmos-Hernández, D.K.; Palomo-Briones, R.; Montiel-Corona, V.; Mari, A.G.; Razo-Flores, E. Improvement of continuous hydrogen production using individual and binary enzymatic hydrolysates of agave bagasse in suspended-culture and biofilm reactors. Bioresour. Technol. 2019, 283, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Corona, V.; Palomo-Briones, R.; Razo-Flores, E. Continuous thermophilic hydrogen production from an enzymatic hydrolysate of agave bagasse: Inoculum origin, homoacetogenesis and microbial community analysis. Bioresour. Technol. 2020, 306, 123087. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Páez, K.M.; Alvarado-Michi, E.L.; Moreno-Andrade, I.; Buitrón, G.; Valdez-Vazquez, I. Comparison of suspended and granular cell anaerobic bioreactors for hydrogen production from acid agave bagasse hydrolyzates. Int. J. Hydrogen Energy 2020, 45, 275–285. [Google Scholar] [CrossRef]
- Muñoz-Páez, K.M.; Buitrón, G. Role of xylose from acidic hydrolysates of agave bagasse during biohydrogen production. Water Sci. Technol. 2021, 84, 656–666. [Google Scholar] [CrossRef]
- Rios-Del Toro, E.E.; Arreola-Vargas, J.; Cárdenas-López, R.L.; Valdez-Guzmán, B.E.; Toledo-Cervantes, A.; González-Álvarez, V.; Méndez-Acosta, H.O. Two-stage semi-continuous hydrogen and methane production from undetoxified and detoxified acid hydrolysates of agave bagasse. Biomass Bioenergy 2021, 150, 106130. [Google Scholar] [CrossRef]
- Valencia-Ojeda, C.; Montoya-Rosales, J.d.J.; Palomo-Briones, R.; Montiel-Corona, V.; Celis, L.B.; Razo-Flores, E. Saccharification of agave bagasse with cellulase 50 XL is an effective alternative to highly specialized lignocellulosic enzymes for continuous hydrogen production. J. Environ. Chem. Eng. 2021, 9, 105448. [Google Scholar] [CrossRef]
- Tapia-Rodríguez, A.; Ibarra-Faz, E.; Razo-Flores, E. Hydrogen and methane production potential of agave bagasse enzymatic hydrolysates and comparative technoeconomic feasibility implications. Int. J. Hydrogen Energy 2019, 44, 17792–17801. [Google Scholar] [CrossRef]
- Zheng, X.; Gallot, G. Dynamics of cell membrane permeabilization by saponins using terahertz attenuated total reflection. In Proceedings of the European Conference on Biomedical Optics, Virtual Event, 20–24 June 2021; pp. 749–755. [Google Scholar]
- Zhu, Z.; Wen, Y.; Yi, J.; Cao, Y.; Liu, F.; McClements, D.J. Comparison of natural and synthetic surfactants at forming and stabilizing nanoemulsions: Tea saponin, Quillaja saponin, and Tween 80. J. Colloid. Interface Sci. 2019, 536, 80–87. [Google Scholar] [CrossRef]
- Rai, S.; Acharya-Siwakoti, E.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Plant-Derived Saponins: A Review of Their Surfactant Properties and Applications. Sci 2021, 3, 44. [Google Scholar] [CrossRef]
- Xin, F.; Liu, J.; He, M.; Wu, B.; Ni, Y.; Dong, W.; Zhang, W.; Hu, G.; Jiang, M. High biobutanol production integrated with in situ extraction in the presence of Tween 80 by Clostridium acetobutylicum. Process Biochem. 2018, 67, 113–117. [Google Scholar] [CrossRef]
- Alcázar-Valle, E.B. Caracterización de Saponinas de Agave durangensis y salmiana, y Su Efecto en la Pared y Membrana Celular de Kluyveromyces marxianus y Saccharomyces cerevisiae. 2016. Available online: http://ciatej.repositorioinstitucional.mx/jspui/handle/1023/421/ (accessed on 1 December 2021).

| Parameter | Value | |
|---|---|---|
| Chemical analysis | COD (g/L) | 300 ± 7.1 |
| BOD (g/L) | 276 ± 8.3 | |
| * TPC (mg GAE/g) | 11.4 ± 0.7 | |
| * TFC (mg QE/g) | 7.6 ± 0.4 | |
| ** Sugar content (g/L) | 35.0 ± 2.8 | |
| Ash (% w/w) | 12.0 ± 0.9 | |
| Elemental analysis | Ca (% w/w) | 5.4 ± 0.2 |
| K (% w/w) | 4.1 ± 0.3 | |
| Mg (% w/w) | 1.9 ± 0.1 |
| Juice Concentration (%) | Solvent Concentration (g/L) | Total ABE Concentration (g/L) | Butanol Productivity (mg/L h−1) | Butanol Yield (g/g Sugars Consumed) | ||
|---|---|---|---|---|---|---|
| Acetone | Butanol | Ethanol | ||||
| Control | 2.83 ± 0.12 | 7.97 ± 0.30 | 0.17 ± 0.01 | 10.97 ± 0.43 | 90.3 ± 3.7 | 0.30 ± 0.01 |
| 20 | 0.52 ± 0.01 E | 4.01 ± 0.08 C | 0.04 ± 0.01 C | 4.57 ± 0.10 E | 25.3 ± 1.4 E | 0.43 ± 0.02 A |
| 40 | 0.97 ± 0.08 D | 3.98 ± 0.26 C | 0.08 ± 0.01 B | 5.03 ± 0.35 D | 52.1 ± 3.2 A | 0.30 ± 0.01 B |
| 60 | 1.26 ± 0.06 C | 4.66 ± 0.13 B | 0.12 ± 0.01 A | 6.04 ± 0.20 C | 51.2 ± 3.6 B | 0.30 ± 0.02 B |
| 80 | 1.55 ± 0.06 A | 5.39 ± 0.19 A | 0.14 ± 0.00 A | 7.09 ± 0.25 A | 38.9 ± 1.3 C | 0.24 ± 0.01 C |
| 100 | 1.42 ± 0.13 B | 4.96 ± 0.18 B | 0.14 ± 0.01 A | 6.52 ± 0.32 B | 36 ± 2.2 D | 0.24 ± 0.01 C |
| Juice Concentration (%) | Productivity (L H2/L d−1) | Productivity (mmol H2/L h−1) | Hydrogen Yield (mL/g Sugars Consumed) |
|---|---|---|---|
| Control | 0.75 ± 0.00 | 1.28 ± 0.01 | 10.9 ± 0.13 |
| 20 | 1.10 ± 0.01 A | 1.88 ± 0.03 B | 17.4 ± 0.30 B |
| 40 | 1.16 ± 0.02 A | 1.99 ± 0.04 A | 18.4 ± 0.39 A |
| 60 | 1.08 ± 0.04 A | 1.86 ± 0.07 B | 18.6 ± 0.74 A |
| 80 | 0.91 ± 0.01 B | 1.56 ± 0.01 C | 15.5 ± 0.17 D |
| 100 | 0.86 ± 0.03 B | 1.48 ± 0.05 D | 16.1 ± 0.59 C |
| Juice Concentration (%) | Sugar Consumption (g/L) | Sugar Consumption Yield (%) |
|---|---|---|
| Control | 26.2 ± 0.55 | 41.6 ± 2.1 |
| 20 | 9.4 ± 0.10 E | 81.1 ± 1.1 A |
| 40 | 13.4 ± 0.33 D | 79.2 ± 2.5 B |
| 60 | 15.8 ± 0.26 C | 72.1 ± 1.7 D |
| 80 | 22.6 ± 0.29 A | 77.3 ± 1.3 C |
| 100 | 20.6 ± 0.37 B | 59.1 ± 1.8 E |
| Substrate | Microorganism | Butanol (g/L) | Butanol Yield (g/g Sugars Consumed) | Butanol Productivity (g/L h−1) | Ref. |
|---|---|---|---|---|---|
| Rice straw hydrolysate | C. acetobutylicum ATCC 824 | 9.10 | 0.17 | 0.79 | [31] |
| Sugar cane bagasse hydrolysate | C. acetobutylicum ATCC 824 | 8.40 | 0.16 | 0.80 | [31] |
| Domestic organic waste | C. acetobutylicum ATCC 824 | 7.80 | ----- | 0.065 | [37] |
| Wood waste (Pinus rigida) | C. beijerinckii NCIMB 8052 | 6.91 | 0.25 | ----- | [38] |
| Rice straw hydrolysate | C. saccharoperbutylacetonicum ATCC 13564 | 6.68 | 0.09 | 0.28 | [39] |
| Sweet potato vine | C. acetobutylicum ATCC 824 | 6.40 | 0.18 | 0.09 | [40] |
| A. lechuguilla hydrolysate | C. acetobutylicum ATCC 824 | 6.10 | 0.27 | 0.073 | [11] |
| A. lechuguilla juice | C. acetobutylicum ATCC 824 | 5.39 | 0.24 | 0.04 | This study |
| Pineapple waste hydrolysate | C. acetobutylicum B 517 | 5.23 | 0.15 | 0.05 | [41] |
| Microalgae biomass | C. acetobutylicum ATCC 824 | 4.36 | ----- | ----- | [31] |
| Oleaginous fungal biomass (Cunninghamella echinulate) | C. acetobutylicum ATCC 824 | 2.19 | 0.45 | 0.072 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva-Rodríguez, A.G.; Cervantes-Güicho, V.d.J.; Morales-Martínez, T.K.; Rodríguez-De la Garza, J.A.; Medina-Morales, M.A.; Martínez-Amador, S.Y.; Reyes, A.G.; Ríos-González, L.J. Biohydrogen Gas/Acetone-Butanol-Ethanol Production from Agave Guishe Juice as a Low-Cost Growing Medium. Fermentation 2023, 9, 811. https://doi.org/10.3390/fermentation9090811
Oliva-Rodríguez AG, Cervantes-Güicho VdJ, Morales-Martínez TK, Rodríguez-De la Garza JA, Medina-Morales MA, Martínez-Amador SY, Reyes AG, Ríos-González LJ. Biohydrogen Gas/Acetone-Butanol-Ethanol Production from Agave Guishe Juice as a Low-Cost Growing Medium. Fermentation. 2023; 9(9):811. https://doi.org/10.3390/fermentation9090811
Chicago/Turabian StyleOliva-Rodríguez, Alejandra G., Vianey de J. Cervantes-Güicho, Thelma K. Morales-Martínez, José A. Rodríguez-De la Garza, Miguel A. Medina-Morales, Silvia Y. Martínez-Amador, Ana G. Reyes, and Leopoldo J. Ríos-González. 2023. "Biohydrogen Gas/Acetone-Butanol-Ethanol Production from Agave Guishe Juice as a Low-Cost Growing Medium" Fermentation 9, no. 9: 811. https://doi.org/10.3390/fermentation9090811
APA StyleOliva-Rodríguez, A. G., Cervantes-Güicho, V. d. J., Morales-Martínez, T. K., Rodríguez-De la Garza, J. A., Medina-Morales, M. A., Martínez-Amador, S. Y., Reyes, A. G., & Ríos-González, L. J. (2023). Biohydrogen Gas/Acetone-Butanol-Ethanol Production from Agave Guishe Juice as a Low-Cost Growing Medium. Fermentation, 9(9), 811. https://doi.org/10.3390/fermentation9090811








