Preparation of Self-Releasing Carbon Biofilm Carrier Based on Corncob and Denitrification Properties
Abstract
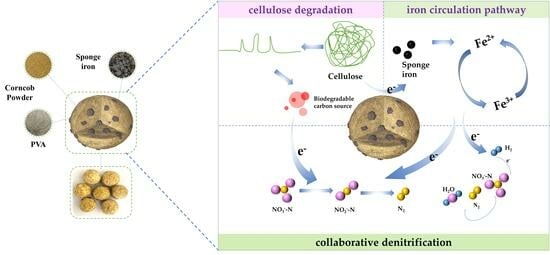
:1. Introduction
2. Materials and Methods
2.1. Self-Releasing Carbon Biofilm Carrier Preparation
2.2. Experimental Setup and Operation
2.3. Analytical Methods
2.3.1. Determination of Indicators
2.3.2. Data Analysis
2.3.3. Analysis of Dissolved Organic Matter
3. Results and Discussion
3.1. Static Release Performance of Biofilm Carrier
3.2. Static Denitrification Performance of Biofilm Carriers
3.2.1. Denitrification Performance
3.2.2. Carbon Source Use Characteristics
3.3. MCA Biofilm Carrier Surface Characteristics
3.4. Microorganism Community Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, W.; Song, G.; Wang, Y.; Wang, Q.; Duan, P.; Liu, C.; Zhang, X.; Rao, Z. Advances in Research into and Applications of Heterotrophic Nitrifying and Aerobic Denitrifying Microorganisms. Front. Environ. Sci. 2022, 10, 887093. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Q.; Chen, X.; Zhu, Y.; Yuan, C.; Zhang, C. The influence mechanism of DO on the microbial community and carbon source metabolism in two solid carbon source systems. Environ. Res. 2022, 206, 112410. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Wang, F.; Guan, Y.; Meng, D.; Li, X.; Zhou, H.; Li, X.; Chen, Y.; Tan, Z. Response of performance, antibiotic resistance genes and bacterial community exposure to compound antibiotics stress: Full nitrification to shortcut nitrification and denitrification. Chem. Eng. J. 2023, 451, 138750. [Google Scholar] [CrossRef]
- Fernández-Nava, Y.; Marañón, E.; Soons, J.; Castrillón, L. Denitrification of high nitrate concentration wastewater using alternative carbon sources. J. Hazard. Mater. 2010, 173, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Maken, S.; Jang, J.-H.; Park, K.; Park, J.-W. Development of physicochemical nitrogen removal process for high strength industrial wastewater. Water Res. 2006, 40, 975–980. [Google Scholar] [CrossRef]
- Qiu, T.; Liu, L.; Gao, M.; Zhang, L.; Tursun, H.; Wang, X. Effects of solid-phase denitrification on the nitrate removal and bacterial community structure in recirculating aquaculture system. Biodegradation 2016, 27, 165–178. [Google Scholar] [CrossRef]
- Mahmoud, A.; Hamza, R.A.; Elbeshbishy, E. Enhancement of denitrification efficiency using municipal and industrial waste fermentation liquids as external carbon sources. Sci. Total Environ. 2022, 816, 151578. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chu, L. Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biotechnol. Adv. 2016, 34, 1103–1112. [Google Scholar] [CrossRef]
- Mehrotra, T.; Dev, S.; Banerjee, A.; Chatterjee, A.; Singh, R.; Aggarwal, S. Use of immobilized bacteria for environmental bioremediation: A review. J. Environ. Chem. Eng. 2021, 9, 105920. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Y.; Su, J.; He, C.; Shi, J.; Yan, H.; Wei, H. Simultaneous removal of nitrate, lead, and tetracycline by a fixed−biofilm reactor assembled with kapok fiber and sponge iron: Comparative analysis of operating conditions and biotic community. Environ. Res. 2023, 219, 115163. [Google Scholar] [CrossRef]
- Jiang, J.; Liang, D.; Hu, Y. Solid slow-release carbon sources improve the simultaneous nitrification and denitrification processes in low carbon resource wastewater. Bioresour. Technol. 2022, 365, 128148. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Jing, Z.; Tao, Z.; Luo, H.; Zuo, S. Improvements of nitrogen removal and electricity generation in microbial fuel cell-constructed wetland with extra corncob for carbon-limited wastewater treatment. J. Clean. Prod. 2021, 297, 126639. [Google Scholar] [CrossRef]
- Huang, Y.-R.; Liu, Q.-Q.; Fan, Y.-Z.; Li, H.-Z. A comparative study on the use of palm bark as a supplementary carbon source in partially saturated vertical constructed wetland: Organic matter characterization, release-adsorption kinetics, and pilot-scale performance. Chemosphere 2020, 253, 126663. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Wei, J.; Jiang, L.; Zhang, Q.; Mao, Y.; Liu, C.; Liao, Y.; Ji, F. Denitrification performance and characteristics of untreated corncob for enhanced nitrogen removal of municipal sewage with low C/N ratio. Environ. Res. 2022, 213, 113673. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, X.; Yuan, R.; Chen, Z.; Zhou, B.; Chen, H. Carbon sources derived from corncobs enhanced nitrogen removal in SBBR treating low C/N domestic sewage. Process Saf. Environ. Prot. 2022, 166, 628–637. [Google Scholar] [CrossRef]
- Li, J.; Feng, Y.; Qiu, Y.; Chen, D.; Liang, D.; Zhou, J.; Liu, G. Recovery of electron and carbon source from agricultural waste corncob by microbial electrochemical system to enhance wastewater denitrification. Sci. Total Environ. 2023, 878, 162926. [Google Scholar] [CrossRef]
- Wang, H.; Chen, N.; Feng, C.; Deng, Y. Insights into heterotrophic denitrification diversity in wastewater treatment systems: Progress and future prospects based on different carbon sources. Sci. Total Environ. 2021, 780, 146521. [Google Scholar] [CrossRef]
- Wang, H.; Feng, C.; Deng, Y. Effect of potassium on nitrate removal from groundwater in agricultural waste-based heterotrophic denitrification system. Sci. Total Environ. 2020, 703, 134830. [Google Scholar] [CrossRef]
- Brar, K.K.; Kaur, S.; Chadha, B.S. A novel staggered hybrid SSF approach for efficient conversion of cellulose/hemicellulosic fractions of corncob into ethanol. Renew. Energy 2016, 98, 16–22. [Google Scholar] [CrossRef]
- Feng, L.; Chen, K.; Han, D.; Zhao, J.; Lu, Y.; Yang, G.; Mu, J.; Zhao, X. Comparison of nitrogen removal and microbial properties in solid-phase denitrification systems for water purification with various pretreated lignocellulosic carriers. Bioresour. Technol. 2017, 224, 236–245. [Google Scholar] [CrossRef]
- Lian, Z.; Ye, L. Effect of PEO on the network structure of PVA hydrogels prepared by freezing/thawing method. J. Appl. Polym. Sci. 2013, 128, 3325–3329. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Yan, G.; Dong, W.; Chu, Z.; Wang, H.; Chang, Y.; Ling, Y.; Zhang, Y. Initial carbon release characteristics, mechanisms and denitrification performance of a novel slow release carbon source. J. Environ. Sci. 2022, 118, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, D.; Yang, X.; Cai, Q.; Peng, S.; Peng, X.; Yao, H.; Xie, B. Novel characteristics on micro-electrolysis mediated Fe(0)-oxidizing autotrophic denitrification with aeration: Efficiency, iron-compounds transformation, N2O and NO2− accumulation, and microbial characteristics. Chem. Eng. J. 2020, 387, 123409. [Google Scholar] [CrossRef]
- Si, Z.; Song, X.; Wang, Y.; Cao, X.; Wang, Y.; Zhao, Y.; Ge, X.; Sand, W. Untangling the nitrate removal pathways for a constructed wetland-sponge iron coupled system and the impacts of sponge iron on a wetland ecosystem. J. Hazard. Mater. 2020, 393, 122407. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Ni, B.-J. Zero valent iron simultaneously enhances methane production and sulfate reduction in anaerobic granular sludge reactors. Water Res. 2015, 75, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Yong, K.; Duujong, L.; Ying, F. Bioaugmented sulfate reduction using enriched anaerobic microflora in the presence of zero valent iron. Chemosphere 2008, 73, 1436–1441. [Google Scholar] [CrossRef]
- Ou, C.; Shen, J.; Zhang, S.; Mu, Y.; Han, W.; Sun, X.; Li, J.; Wang, L. Coupling of iron shavings into the anaerobic system for enhanced 2,4-dinitroanisole reduction in wastewater. Water Res. 2016, 101, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Li, Y.; Zhang, L.; Qiao, S.; Yang, F.; Quan, X. Enhancement of nitrogen removal in a novel anammox reactor packed with Fe electrode. Bioresour. Technol. 2012, 114, 102–108. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Z.; Guan, Y.; Xu, S. Role and application of iron in water treatment for nitrogen removal: A review. Chemosphere 2018, 204, 51–62. [Google Scholar] [CrossRef]
- Watanabe, K.; Manefield, M.; Lee, M.; Kouzuma, A. Electron shuttles in biotechnology. Curr. Opin. Biotechnol. 2009, 20, 633–641. [Google Scholar] [CrossRef]
- Xu, L.; Su, J.; Huang, T.; Li, G.; Ali, A.; Shi, J. Simultaneous removal of nitrate and diethyl phthalate using a novel sponge–based biocarrier combined modified walnut shell biochar with Fe3O4 in the immobilized bioreactor. J. Hazard. Mater. 2021, 414, 125578. [Google Scholar] [CrossRef]
- Li, J.; Ali, A.; Su, J.; Huang, T.; Zhai, Z.; Xu, L. Synergistic removal of nitrate by a cellulose-degrading and denitrifying strain through iron loaded corn cobs filled biofilm reactor at low C/N ratio: Capability, enhancement and microbiome analysis. Bioresour. Technol. 2023, 369, 128433. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Yang, X.-Y.; Meng, H.-S.; Zhang, Y.-C.; Li, X.-Y.; Xu, J. Enhanced denitrification by nano α-Fe2O3 induced self-assembled hybrid biofilm on particle electrodes of three-dimensional biofilm electrode reactors. Environ. Int. 2019, 125, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, N.; Feng, C. Performance and enhancement mechanism of corncob guiding chromium (VI) bioreduction. Water Res. 2021, 197, 117057. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhou, D.; Zhong, D.; Li, J.; Zhang, J.; Su, P.; Liu, X.; Dong, J.; Zhang, S.; Du, X. Study of nitrogen removal efficiency of the filled bed reactors using alkali-treated corncobs-sulfur (mixotrophic) for treating the effluent from simulated urban wastewater plants. Bioresour. Technol. 2022, 349, 126630. [Google Scholar] [CrossRef]
- Sobeck, D.C.; Higgins, M.J. Examination of three theories for mechanisms of cation-induced bioflocculation. Water Res. 2002, 36, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Pan, Z.; Feng, H.; Cheng, X.; Guo, T.; Yan, A.; Li, J. Biofilm coupled micro-electrolysis of waste iron shavings enhanced iron and hydrogen autotrophic denitrification and phosphate accumulation for wastewater treatment. J. Environ. Chem. Eng. 2022, 10, 108959. [Google Scholar] [CrossRef]
- Wang, Z.-P.; Zhang, T. Characterization of soluble microbial products (SMP) under stressful conditions. Water Res. 2010, 44, 5499–5509. [Google Scholar] [CrossRef]
- Zou, L.; Zhou, M.; Luo, Z.; Zhang, H.; Yang, Z.; Cheng, H.; Li, R.; He, Q.; Ai, H. Selection and synthesization of multi–carbon source composites to enhance simultaneous nitrification–denitrification in treating low C/N wastewater. Chemosphere 2022, 288, 132567. [Google Scholar] [CrossRef]
- Lv, D.; Zhou, X.; Zhou, J.; Liu, Y.; Li, Y.; Yang, K.; Lou, Z.; Baig, S.A.; Wu, D.; Xu, X. Design and characterization of sulfide-modified nanoscale zerovalent iron for cadmium(II) removal from aqueous solutions. Appl. Surf. Sci. 2018, 442, 114–123. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Li, B.; Hao, R.-X. Comparison and optimization of cellulose carbon source for denitrification filter. Huan Jing Ke Xue Huanjing Kexue 2013, 34, 1428–1434. [Google Scholar] [PubMed]
- Yang, X.-L.; Jiang, Q.; Song, H.-L.; Gu, T.-T.; Xia, M.-Q. Selection and application of agricultural wastes as solid carbon sources and biofilm carriers in MBR. J. Hazard. Mater. 2015, 283, 186–192. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Sun, G.; Wan, J.; Sun, Y.; Li, H.; Chang, C.; Wang, Y. Enhanced removal of nitrate and refractory organic pollutants from bio-treated coking wastewater using corncobs as carbon sources and biofilm carriers. Chemosphere 2019, 237, 124520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cui, B.; Yuan, B.; Zhang, A.; Feng, J.; Zhang, J.; Han, X.; Pan, L.; Li, L. Denitrification mechanism and artificial neural networks modeling for low-pollution water purification using a denitrification biological filter process. Sep. Purif. Technol. 2021, 257, 117918. [Google Scholar] [CrossRef]
- Cao, S.; Li, B.; Du, R.; Ren, N.; Peng, Y. Nitrite production in a partial denitrifying upflow sludge bed (USB) reactor equipped with gas automatic circulation (GAC). Water Res. 2016, 90, 309–316. [Google Scholar] [CrossRef]
- Kim, I.; Cha, D.K. Effect of low temperature on abiotic and biotic nitrate reduction by zero-valent Iron. Sci. Total Environ. 2021, 754, 142410. [Google Scholar] [CrossRef]
- Hu, X.; Chen, H.; Zhang, S.; Song, W.; Li, J.; Wang, K. Study on performance of carbon source released from fruit shells and the effect on biological denitrification in the advanced treatment. Chemosphere 2022, 307, 136173. [Google Scholar] [CrossRef]
- Tang, L.; Li, J.; Li, Y.; Zhang, X.; Shi, X. Mixotrophic denitrification processes based on composite filler for low carbon/nitrogen wastewater treatment. Chemosphere 2022, 286, 131781. [Google Scholar] [CrossRef]
- Li, R.; Guan, M.; Wang, W. Simultaneous arsenite and nitrate removal from simulated groundwater based on pyrrhotite autotrophic denitrification. Water Res. 2021, 189, 116662. [Google Scholar] [CrossRef]
- Xu, J.; Hao, Z.; Xie, C.; Lv, X.; Yang, Y.; Xu, X. Promotion effect of Fe2+ and Fe3O4 on nitrate reduction using zero-valent iron. Desalination 2012, 284, 9–13. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Dong, W.; Chang, Y.; Yan, G.; Chu, Z.; Ling, Y.; Wang, Z.; Fan, T.; Li, C. Nitrogen removal and microbial community for the treatment of rural domestic sewage with low C/N ratio by A/O biofilter with Arundo donax as carbon source and filter media. J. Water Process Eng. 2020, 37, 101509. [Google Scholar] [CrossRef]
- Wang, W.; Cao, L.; Tan, H.; Zhang, R. Nitrogen removal from synthetic wastewater using single and mixed culture systems of denitrifying fungi, bacteria, and actinobacteria. Appl. Microbiol. Biotechnol. 2016, 100, 9699–9707. [Google Scholar] [CrossRef]
- Du, Y.; Yu, D.; Wang, X.; Zhen, J.; Bi, C.; Gong, X.; Zhao, J. Achieving simultaneous nitritation, anammox and denitrification (SNAD) in an integrated fixed-biofilm activated sludge (IFAS) reactor: Quickly culturing self-generated anammox bacteria. Sci. Total Environ. 2021, 768, 144446. [Google Scholar] [CrossRef]
- Xia, L.; Li, X.; Fan, W.; Wang, J. Denitrification performance and microbial community of bioreactor packed with PHBV/PLA/rice hulls composite. Sci. Total Environ. 2022, 803, 150033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, Y.; Guo, L.; Hu, F.; Zhao, Y.; Jin, C.; She, Z.; Gao, M.; Wang, G. Elucidating salinity adaptation and shock loading on denitrification performance: Focusing on microbial community shift and carbon source evaluation. Bioresour. Technol. 2020, 305, 123030. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, Y.; Zhu, W.; Xie, T.; Li, L. Effect of COD/NO3−-N ratio on nitrite accumulation and microbial behavior in glucose-driven partial denitrification system. Heliyon 2023, 9, e14920. [Google Scholar] [CrossRef]
- Qiu, W.; Li, W.; He, J.; Zhao, H.; Liu, X.; Yuan, Y. Variations regularity of microorganisms and corrosion of cast iron in water distribution system. J. Environ. Sci. 2018, 74, 177–185. [Google Scholar] [CrossRef]
- Deng, S.; Li, D.; Yang, X.; Xing, W.; Li, J.; Zhang, Q. Biological denitrification process based on the Fe(0)–carbon micro-electrolysis for simultaneous ammonia and nitrate removal from low organic carbon water under a microaerobic condition. Bioresour. Technol. 2016, 219, 677–686. [Google Scholar] [CrossRef]
- Pishgar, R.; Dominic, J.A.; Sheng, Z.; Tay, J.H. Denitrification performance and microbial versatility in response to different selection pressures. Bioresour. Technol. 2019, 281, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Sun, C.; Jin, Z.; Wang, M.; Yu, Q.; Zhao, Z.; Zhang, Y. Magnetite-mediated electrically connected community for shortening startup of methane-dependent denitrification in a membrane biofilm reactor. Chem. Eng. J. 2022, 428, 132004. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Q.; Sun, H.; Jia, L.; Zhao, L.; Wu, W. Metagenomic analyses of microbial structure and metabolic pathway in solid-phase denitrification systems for advanced nitrogen removal of wastewater treatment plant effluent: A pilot-scale study. Water Res. 2021, 196, 117067. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Li, G.-X. Sulfur-Driven Iron Reduction Coupled to Anaerobic Ammonium Oxidation. Environ. Sci. Technol. 2017, 51, 6691–6698. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, W.; Liu, T.; Chen, L.; Chen, P.; Li, F. Changes in the composition and diversity of microbial communities during anaerobic nitrate reduction and Fe(II) oxidation at circumneutral pH in paddy soil. Soil Biol. Biochem. 2016, 94, 70–79. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.-H.; Lee, J.-H.; Hur, H.-G. Sphaerotilus natans encrusted with nanoball-shaped Fe(III) oxide minerals formed by nitrate-reducing mixotrophic Fe(II) oxidation. FEMS Microbiol. Ecol. 2014, 90, 68–77. [Google Scholar] [CrossRef]
- Mielcarek, A.; Rodziewicz, J.; Janczukowicz, W.; Dulski, T.; Ciesielski, S.; Thornton, A. Denitrification aided by waste beer in anaerobic sequencing batch biofilm reactor (AnSBBR). Ecol. Eng. 2016, 95, 384–389. [Google Scholar] [CrossRef]
- Ueki, A.; Akasaka, H.; Suzuki, D.; Ueki, K. Paludibacter propionicigenes gen. nov., sp. nov., a novel strictly anaerobic, Gram-negative, propionate-producing bacterium isolated from plant residue in irrigated rice-field soil in Japan. Int. J. Syst. Evol. Microbiol. 2006, 56, 39–44. [Google Scholar] [CrossRef]






| Biofilm Carrier | Second-Order Kinetics Equation | Ritger–Peppas Equation | ||||||
|---|---|---|---|---|---|---|---|---|
| Fitting Formula | R2 | Cm | K | t1/2 | Fitting Formula | R2 | N | |
| MC | t/Ct = 0.00191t + 0.01604 | 0.995 | 523.56 | 62.34 | 8.40 | Ct = 151.798t0.279 | 0.996 | 0.279 |
| MCA | t/Ct = 0.00150t + 0.01418 | 0.998 | 666.67 | 70.52 | 9.45 | Ct = 165.619t0.316 | 0.975 | 0.316 |
| MCB | t/Ct = 0.00244t + 0.00768 | 0.999 | 409.84 | 130.21 | 3.15 | Ct = 213.452t0.155 | 0.940 | 0.155 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Liu, J.; Li, P.; Chen, X.; Zhang, X.; Wen, C. Preparation of Self-Releasing Carbon Biofilm Carrier Based on Corncob and Denitrification Properties. Fermentation 2023, 9, 796. https://doi.org/10.3390/fermentation9090796
Wang B, Liu J, Li P, Chen X, Zhang X, Wen C. Preparation of Self-Releasing Carbon Biofilm Carrier Based on Corncob and Denitrification Properties. Fermentation. 2023; 9(9):796. https://doi.org/10.3390/fermentation9090796
Chicago/Turabian StyleWang, Baoshan, Jie Liu, Pengcheng Li, Xiaojie Chen, Xu Zhang, and Chengcheng Wen. 2023. "Preparation of Self-Releasing Carbon Biofilm Carrier Based on Corncob and Denitrification Properties" Fermentation 9, no. 9: 796. https://doi.org/10.3390/fermentation9090796
APA StyleWang, B., Liu, J., Li, P., Chen, X., Zhang, X., & Wen, C. (2023). Preparation of Self-Releasing Carbon Biofilm Carrier Based on Corncob and Denitrification Properties. Fermentation, 9(9), 796. https://doi.org/10.3390/fermentation9090796