Use of a Highly Specialized Biocatalyst to Produce Lactate or Biohydrogen and Butyrate from Agro-Industrial Resources in a Dual-Phase Dark Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inoculum
2.2. Feedstocks
2.3. Experimental Setup and DF Conditions
2.4. Oxygen Depletion Test
2.5. Self-Fermentation Test
2.6. Analytical Methods
3. Results and Discussion
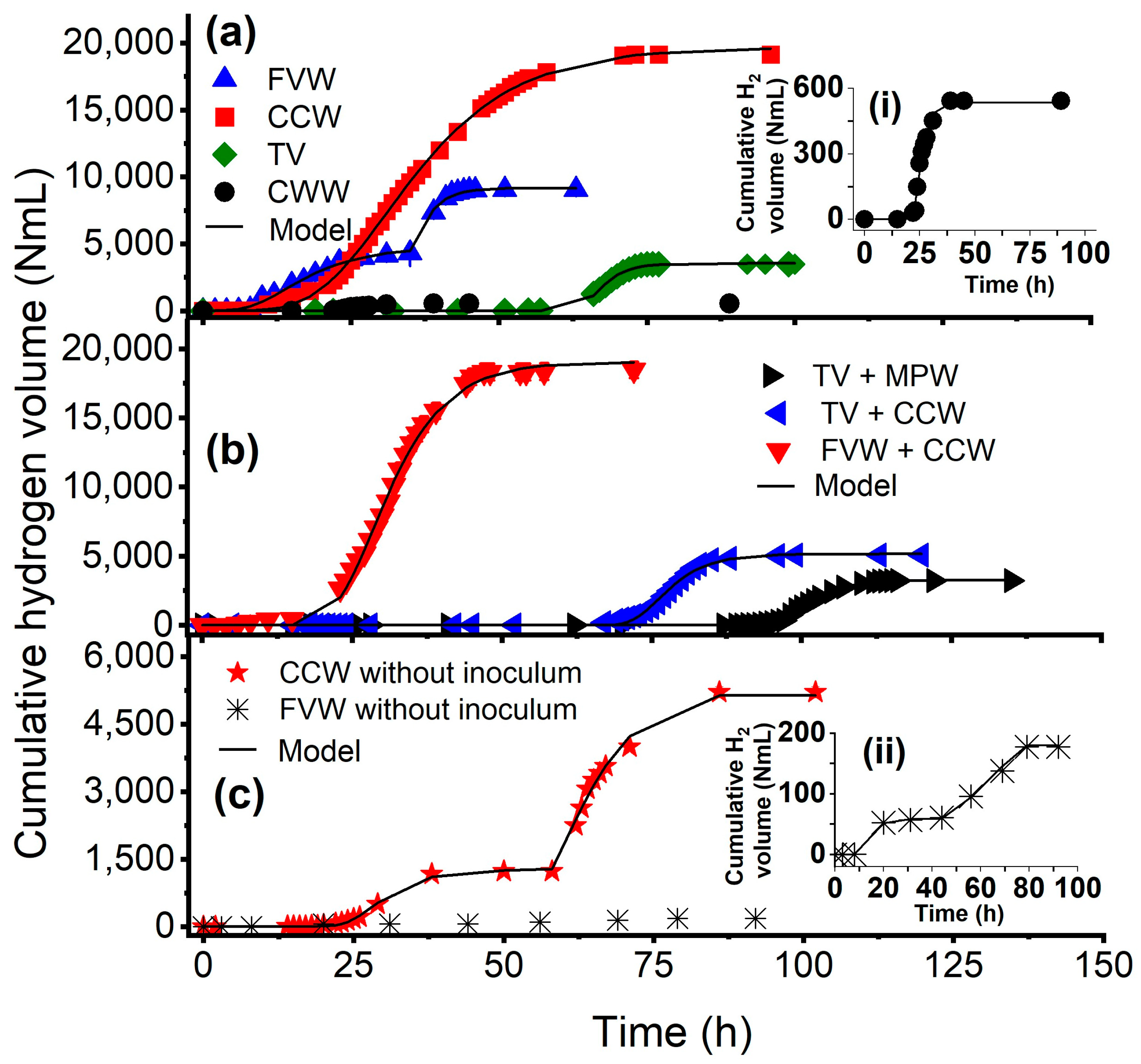
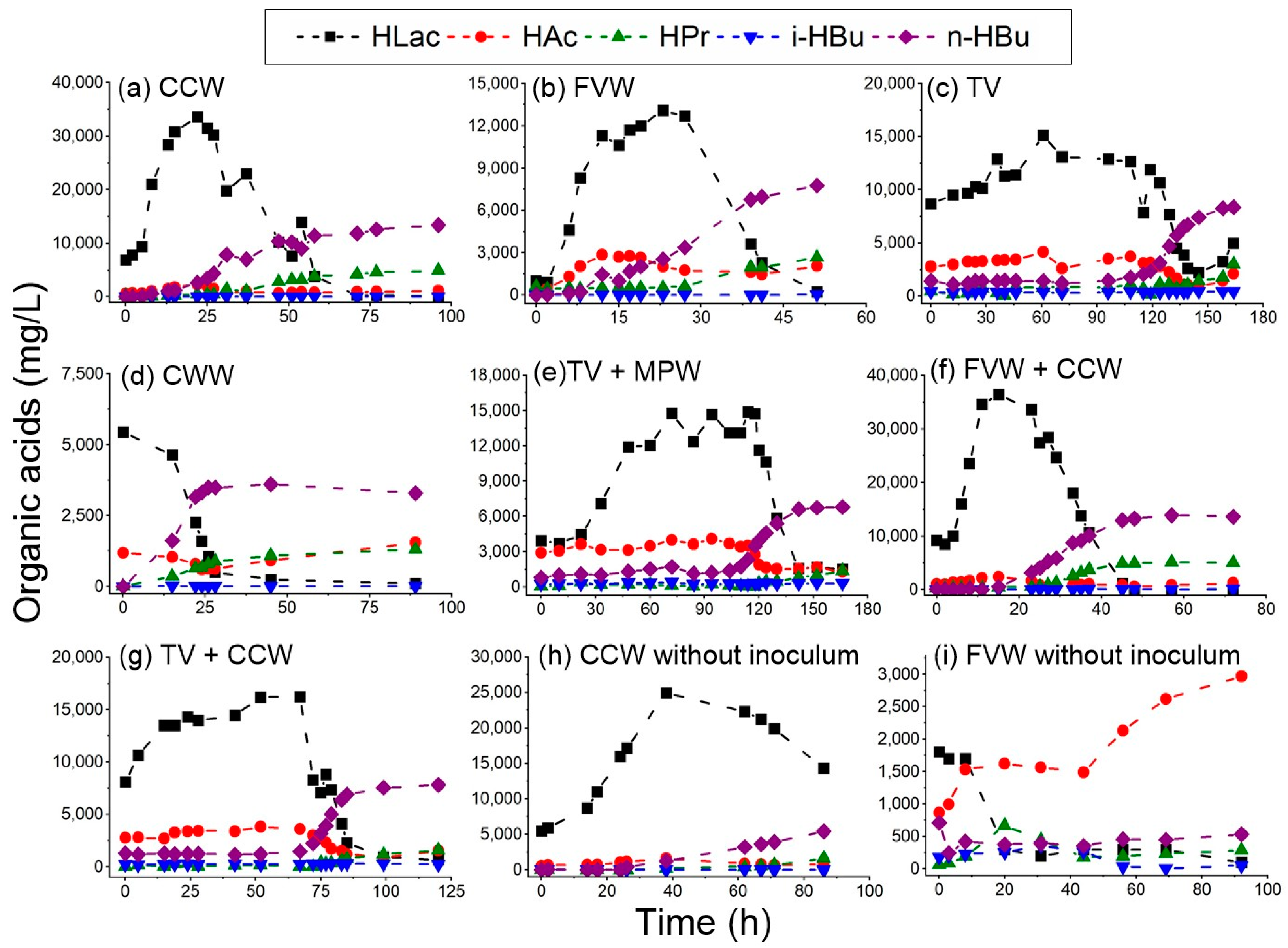
3.1. Hydrogen Production Performance
3.2. Oxygen Depletion during Activation and Inoculation Steps
3.3. Testing the Dark Self-Fermentation of Crude Cheese Whey and Fruit–Vegetable Waste
3.4. Microbial Community Structure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, S.K.; Das, D.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Lee, J.-K. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew. Sustain. Energy Rev. 2021, 150, 111491. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ghimire, A.; Ezeokoli, O.T.; Rao, S.; Ngan, W.Y.; Habimana, O.; Yao, Y.; Yang, P.; Fung, A.H.Y.; Yoro, K.O.; et al. Valorization of volatile fatty acids from the dark fermentation waste Streams-A promising pathway for a biorefinery concept. Renew. Sustain. Energy Rev. 2021, 143, 110971. [Google Scholar] [CrossRef]
- Garcia-Navarro, J.; Isaacs, M.A.; Favaro, M.; Ren, D.; Ong, W.J.; Grätzel, M.; Jiménez-Calvo, P. Updates on hydrogen value chain: A strategic roadmap. Glob. Chall. 2023, 2300073. [Google Scholar] [CrossRef]
- Balachandar, G.; Varanasi, J.L.; Singh, V.; Singh, H.; Das, D. Biological hydrogen production via dark fermentation: A holistic approach from lab-scale to pilot-scale. Int. J. Hydrogen Energy 2020, 45, 5202–5215. [Google Scholar] [CrossRef]
- Patel, S.K.; Gupta, R.K.; Das, D.; Lee, J.K.; Kalia, V.C. Continuous biohydrogen production from poplar biomass hydrolysate by a defined bacterial mixture immobilized on lignocellulosic materials under non-sterile conditions. J. Clean. Prod. 2021, 287, 125037. [Google Scholar] [CrossRef]
- Morya, R.; Raj, T.; Lee, Y.; Pandey, A.K.; Kumar, D.; Singhania, R.R.; Singh, S.; Verma, J.P.; Kim, S.-H. Recent updates in biohydrogen production strategies and life–cycle assessment for sustainable future. Bioresour. Technol. 2022, 366, 128159. [Google Scholar] [CrossRef]
- Shah, A.; Favaro, L.; Alibardi, L.; Cagnin, L.; Sandon, A.; Cossu, R.; Casella, S.; Basaglia, M. Bacillus sp. strains to produce bio-hydrogen from the organic fraction of municipal solid waste. Appl. Energy 2016, 176, 116–124. [Google Scholar] [CrossRef]
- Cabrol, L.; Marone, A.; Tapia-Venegas, E.; Steyer, J.-P.; Ruiz-Filippi, G.; Trably, E. Microbial ecology of fermentative hydrogen producing bioprocesses: Useful insights for driving the ecosystem function. FEMS Microbiol. Rev. 2017, 41, 158–181. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrere, H.; Steyer, J.-P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- García-Depraect, O.; Castro-Muñoz, R.; Muñoz, R.; Rene, E.R.; León-Becerril, E.; Valdez-Vazquez, I.; Kumar, G.; Reyes-Alvarado, L.C.; Martínez-Mendoza, L.J.; Carrillo-Reyes, J.; et al. A review on the factors influencing biohydrogen production from lactate: The key to unlocking enhanced dark fermentative processes. Bioresour. Technol. 2021, 324, 124595. [Google Scholar] [CrossRef]
- Sikora, A.; Błaszczyk, M.; Jurkowski, M.; Zielenkiewicz, U. Lactic acid bacteria in hydrogen-producing consortia: On purpose or by coincidence? In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes, 1st ed.; Kongo, J.M., Ed.; InTech: London, UK, 2013. [Google Scholar]
- Martínez-Mendoza, L.J.; Lebrero, R.; Muñoz, R.; García-Depraect, O. Influence of key operational parameters on biohydrogen production from fruit and vegetable waste via lactate-driven dark fermentation. Bioresour. Technol. 2022, 364, 128070. [Google Scholar] [CrossRef] [PubMed]
- Regueira-Marcos, L.; García-Depraect, O.; Muñoz, R. Elucidating the role of pH and total solids content in the co-production of biohydrogen and carboxylic acids from food waste via lactate-driven dark fermentation. Fuel 2023, 338, 127238. [Google Scholar] [CrossRef]
- García-Depraect, O.; Rene, E.R.; Diaz-Cruces, V.F.; León-Becerril, E. Effect of process parameters on enhanced biohydrogen production from tequila vinasse via the lactate-acetate pathway. Bioresour. Technol. 2019, 273, 618–626. [Google Scholar] [CrossRef] [PubMed]
- García-Depraect, O.; Lebrero, R.; Rodriguez-Vega, S.; Börner, R.A.; Börner, T.; Muñoz, R. Production of volatile fatty acids (VFAs) from five commercial bioplastics via acidogenic fermentation. Bioresour. Technol. 2022, 360, 127655. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Lim, W.-T.; Lee, M.-K.; Kim, M.-S. Effect of temperature on continuous fermentative lactic acid (LA) production and bacterial community, and development of LA-producing UASB reactor. Bioresour. Technol. 2012, 119, 355–361. [Google Scholar] [CrossRef] [PubMed]
- García-Depraect, O.; Gómez-Romero, J.; León-Becerril, E.; López-López, A. A novel biohydrogen production process: Co-digestion of vinasse and Nejayote as complex raw substrates using a robust inoculum. Int. J. Hydrogen Energy 2017, 42, 5820–5831. [Google Scholar] [CrossRef]
- Gomez-Romero, J.; Gonzalez-Garcia, A.; Chairez, I.; Torres, L.; Garcia-Peña, E.I. Selective adaptation of an anaerobic microbial community: Biohydrogen production by co-digestion of cheese whey and vegetables fruit waste. Int. J. Hydrogen Energy 2014, 39, 12541–12550. [Google Scholar] [CrossRef]
- Tao, Y.; Hu, X.; Zhu, X.; Jin, H.; Xu, Z.; Tang, Q.; Li, X. Production of butyrate from lactate by a newly isolated Clostridium sp. BPY5. Appl. Biochem. Biotechnol. 2016, 179, 361–374. [Google Scholar] [CrossRef]
- Juang, C.-P.; Whang, L.-M.; Cheng, H.-H. Evaluation of bioenergy recovery processes treating organic residues from ethanol fermentation process. Bioresour. Technol. 2011, 102, 5394–5399. [Google Scholar] [CrossRef]
- Sträuber, H.; Lucas, R.; Kleinsteuber, S. Metabolic and microbial community dynamics during the anaerobic digestion of maize silage in a two-phase process. Appl. Microbiol. Biotechnol. 2016, 100, 479–491. [Google Scholar] [CrossRef]
- Regueira-Marcos, L.; Muñoz, R.; García-Depraect, O. Continuous lactate-driven dark fermentation of restaurant food waste: Process characterization and new insights on transient feast/famine perturbations. Bioresour. Technol. 2023, 385, 129385. [Google Scholar] [CrossRef]
- García-Depraect, O.; Diaz-Cruces, V.F.; Rene, E.R.; Castro-Muñoz, R.; León-Becerril, E. Long-term preservation of hydrogenogenic biomass by refrigeration: Reactivation characteristics and microbial community structure. Bioresour. Technol. Rep. 2020, 12, 100587. [Google Scholar] [CrossRef]
- García-Depraect, O.; León-Becerril, E. Fermentative biohydrogen production from tequila vinasse via the lactate-acetate pathway: Operational performance, kinetic analysis and microbial ecology. Fuel 2018, 234, 151–160. [Google Scholar] [CrossRef]
- De Gioannis, G.; Friargiu, M.; Massi, E.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Biohydrogen production from dark fermentation of cheese whey: Influence of pH. Int. J. Hydrogen Energy 2014, 39, 20930–20941. [Google Scholar] [CrossRef]
- García-Depraect, O.; Rene, E.R.; Gómez-Romero, J.; López-López, A.; León-Becerril, E. Enhanced biohydrogen production from the dark co-fermentation of tequila vinasse and nixtamalization wastewater: Novel insights into ecological regulation by pH. Fuel 2019, 253, 159–166. [Google Scholar] [CrossRef]
- García-Depraect, O.; Martínez-Mendoza, L.J.; Diaz, I.; Muñoz, R. Two-stage anaerobic digestion of food waste: Enhanced bioenergy production rate by steering lactate-type fermentation during hydrolysis-acidogenesis. Bioresour. Technol. 2022, 358, 127358. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2005. [Google Scholar]
- García-Depraect, O.; Muñoz, R.; Rodríguez, E.; Rene, E.R.; León-Becerril, E. Microbial ecology of a lactate-driven dark fermentation process producing hydrogen under carbohydrate-limiting conditions. Int. J. Hydrogen Energy 2021, 46, 11284–11296. [Google Scholar] [CrossRef]
- Asunis, F.; Carucci, A.; De Gioannis, G.; Farru, G.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Spiga, D. Combined biohydrogen and polyhydroxyalkanoates production from sheep cheese whey by a mixed microbial culture. J. Environ. Manag. 2022, 322, 116149. [Google Scholar] [CrossRef]
- Aranda-Jaramillo, B.; León-Becerril, E.; Aguilar-Juárez, O.; Castro-Muñoz, R.; García-Depraect, O. Feasibility study of biohydrogen production from acid cheese whey via lactate-driven dark fermentation. Fermentation 2023, 9, 644. [Google Scholar] [CrossRef]
- Martínez-Mendoza, L.J.; García-Depraect, O.; Muñoz, R. Unlocking the high-rate continuous performance of fermentative hydrogen bioproduction from fruit and vegetable residues by modulating hydraulic retention time. Bioresour. Technol. 2023, 373, 128716. [Google Scholar] [CrossRef]
- Montoya, A.C.V.; da Silva Mazareli, R.C.; Delforno, T.P.; Centurion, V.B.; Sakamoto, I.K.; de Oliveira, V.M.; Silva, E.L.; Varesche, M.B.A. Hydrogen, alcohols and volatile fatty acids from the co-digestion of coffee waste (coffee pulp, husk, and processing wastewater) by applying autochthonous microorganisms. Int. J. Hydrogen Energy 2019, 44, 21434–21450. [Google Scholar] [CrossRef]
- Blanco, V.M.C.; Oliveira, G.H.D.D.; Zaiat, M. Dark fermentative biohydrogen production from synthetic cheese whey in an anaerobic structured-bed reactor: Performance evaluation and kinetic modeling. Renew. Energy 2019, 139, 1310–1319. [Google Scholar] [CrossRef]
- Asunis, F.; De Gioannis, G.; Isipato, M.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Spiga, D. Control of fermentation duration and pH to orient biochemicals and biofuels production from cheese whey. Bioresour. Technol. 2019, 289, 121722. [Google Scholar] [CrossRef] [PubMed]
- Dareioti, M.A.; Vavouraki, A.I.; Kornaros, M. Effect of pH on the anaerobic acidogenesis of agroindustrial wastewaters for maximization of bio-hydrogen production: A lab-scale evaluation using batch tests. Bioresour. Technol. 2014, 162, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Fuess, L.T.; Júnior, A.D.N.F.; Machado, C.B.; Zaiat, M. Temporal dynamics and metabolic correlation between lactate-producing and hydrogen-producing bacteria in sugarcane vinasse dark fermentation: The key role of lactate. Bioresour. Technol. 2018, 247, 426–433. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Park, J.-H.; Park, J.-H.; Park, H.-D.; Yoon, J.-J.; Kim, S.-H. Feasibility of enriched mixed cultures obtained by repeated batch transfer in continuous hydrogen fermentation. Int. J. Hydrogen Energy 2016, 41, 4393–4403. [Google Scholar] [CrossRef]
- García-Depraect, O.; Diaz-Cruces, V.F.; Rene, E.R.; León-Becerril, E. Changes in performance and bacterial communities in a continuous biohydrogen-producing reactor subjected to substrate-and pH-induced perturbations. Bioresour. Technol. Rep. 2020, 295, 122182. [Google Scholar] [CrossRef]
- Hung, C.-H.; Chang, Y.-T.; Chang, Y.-J. Roles of microorganisms other than Clostridium and Enterobacter in anaerobic fermentative biohydrogen production systems—A review. Bioresour. Technol. 2011, 102, 8437–8444. [Google Scholar] [CrossRef]
- Ohnishi, A.; Bando, Y.; Fujimoto, N.; Suzuki, M. Development of a simple bio-hydrogen production system through dark fermentation by using unique microflora. Int. J. Hydrogen Energy 2010, 35, 8544–8553. [Google Scholar] [CrossRef]
- Gadhamshetty, V.; Johnson, D.C.; Nirmalakhandan, N.; Smith, G.B.; Deng, S. Feasibility of biohydrogen production at low temperatures in unbuffered reactors. Int. J. Hydrogen Energy 2009, 34, 1233–1243. [Google Scholar] [CrossRef]
- Grause, G.; Igarashi, M.; Kameda, T.; Yoshioka, T. Lactic acid as a substrate for fermentative hydrogen production. Int. J. Hydrogen Energy 2012, 37, 16967–16973. [Google Scholar] [CrossRef]
- Marone, A.; Izzo, G.; Mentuccia, L.; Massini, G.; Paganin, P.; Rosa, S.; Varrone, C.; Signorini, A. Vegetable waste as substrate and source of suitable microflora for bio-hydrogen production. Renew. Energy 2014, 68, 6–13. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.-H.; Shin, H.-S. Hydrogen fermentation of food waste without inoculum addition. Enzym. Microb. Technol. 2009, 45, 181–187. [Google Scholar] [CrossRef]
- He, Y.; Cassarini, C.; Marciano, F.; Lens, P.N. Homoacetogenesis and solventogenesis from H2/CO2 by granular sludge at 25, 37 and 55 °C. Chemosphere 2021, 265, 128649. [Google Scholar] [CrossRef]
- De Vrieze, J.; Verstraete, W. Perspectives for microbial community composition in anaerobic digestion: From abundance and activity to connectivity. Environ. Microbiol. 2016, 18, 2797–2809. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, R.; McGarvey, J.A.; Benemann, J.R. Biohydrogen production from cheese processing wastewater by anaerobic fermentation using mixed microbial communities. Int. J. Hydrogen Energy 2007, 32, 4761–4771. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Sahoo, P.C.; Kumar, M.; Gupta, R.P.; Bhattacharyya, D.; Ramakumar, S.S.V. Unrevealing the role of metal oxide nanoparticles on biohydrogen production by Lactobacillus delbrueckii. Bioresour. Technol. 2023, 367, 128260. [Google Scholar] [CrossRef]
- Castelló, E.; Santos, C.G.; Iglesias, T.; Paolino, G.; Wenzel, J.; Borzacconi, L.; Etchebehere, C. Feasibility of biohydrogen production from cheese whey using a UASB reactor: Links between microbial community and reactor performance. Int. J. Hydrogen Energy 2009, 34, 5674–5682. [Google Scholar] [CrossRef]
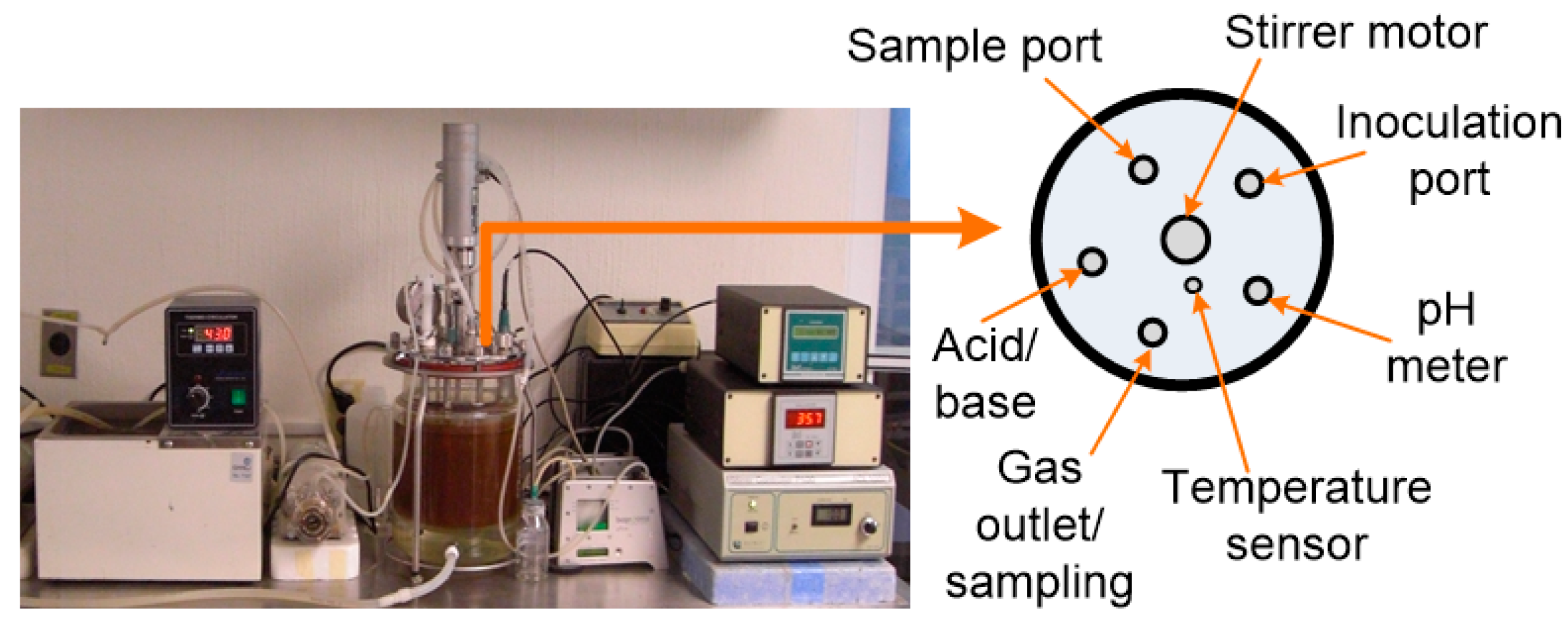




| Parameter | TV | FVW | CWW | CCW | TV-MPW | FVW-CCW | TV-CCW |
|---|---|---|---|---|---|---|---|
| Total COD (g/L) | 72.3 ± 0.1 | 100.2 ± 0.3 | 42.6 ± 0.6 | 89.4 ± 0.3 | 66.5 ± 0.1 | 110.3 ± 0.4 | 72.6 ± 3.2 |
| Soluble COD (g/L) | 58.6 ± 0.3 | 70.7 ± 0.1 | 26.9 ± 0.1 | 61.6 ± 0.0 | 42.9 ± 0.3 | 65.7 ± 0.1 | 56.9 ± 1.2 |
| BOD (g/L) | 32.1 ± 1.0 | 62.9 ± 1.7 | 17.1 ± 1.2 | 55.5 ± 0.4 | 32.9 ± 1.2 | 61.9 ± 5.6 | 39.1 ± 4.5 |
| TOC (g/L) | 21.7 ± 0.1 | 24.5 ± 0.2 | 11.0 ± 0.1 | 20.5 ± 0.1 | 21.7 ± 0.1 | 28.7 ± 0.1 | 22.2 ± 0.5 |
| Total nitrogen (mg/L) | 201.0 ± 1.4 | 850.0 ± 0.0 | 26.5 ± 0.7 | 1405.0 ± 7.1 | 225.0 ± 7.1 | 870.0 ± 14.1 | 515.0 ± 7.1 |
| Total phosphorous (mg/L) | 465.0 ± 2.8 | 490.5 ± 5.0 | 169.5 ± 0.7 | 573.5 ± 3.5 | 354.5 ± 3.5 | 599.5 ± 0.7 | 467.5 ± 24.8 |
| TS (g/L) | 52.2 ± 1.1 | 102.2 ± 0.2 | 19.9 ± 0.2 | 66.3 ± 2.1 | 44.0 ± 0.1 | 78.5 ± 3.2 | 52.6 ± 0.8 |
| TVS (g/L) | 48.5 ± 1.0 | 97.6 ± 0.5 | 18.5 ± 0.3 | 61.1 ± 2.1 | 40.1 ± 0.2 | 73.3 ± 3.0 | 48.5 ± 0.6 |
| TDS (g/L) | 27.5 ± 3.0 | 69.4 ± 3.1 | 9.7 ± 0.9 | 50.9 ± 2.2 | 23.1 ± 4.6 | 51.9 ± 9.0 | 24.8 ± 2.8 |
| TSS (g/L) | 24.7 ± 2.3 | 32.7 ± 3.3 | 10.5 ± 0.8 | 15.4 ± 0.2 | 20.9 ± 4.5 | 26.6 ± 6.4 | 27.8 ± 2.1 |
| pH | 3.6 ± 0.0 | 4.2 ± 0.1 | 3.7 ± 0.0 | 4.2 ± 0.1 | 3.9 ± 0.0 | 4.2 ± 0.0 | 3.8 ± 0.1 |
| Total alkalinity (mg CaCO3 eq./L) | - | - | - | - | 670.0 ± 1.3 | - | - |
| Total acidity (g CaCO3 eq./L) | 4.9 ± 0.0 | 1.7 ± 0.0 | 2.4 ± 0.0 | 2.3 ± 0.0 | 3.6 ± 0.3 | 2.6 ± 0.0 | 5.4 ± 0.2 |
| Reducing sugars (g/L) | 12.7 ± 0.4 | 35.3 ± 0.5 | 2.9 ± 0.0 | 34.4 ± 0.0 | 11.6 ± 0.9 | 31.3 ± 0.2 | 16.5 ± 0.1 |
| Total carbohydrates (g/L) | 25.2 ± 3.2 | 63.0 ± 0.7 | 2.9 ± 0.4 | 44.8 ± 0.4 | 19.3 ± 1.2 | 44.0 ± 2.2 | 24.5 ± 0.6 |
| Sulphate (g/L) | 1.0 ± 0.0 | 0.1 ± 0.0 | 1.0 ± 0.0 | 0.7 ± 0.0 | 0.8 ± 0.0 | 0.4 ± 0.0 | 0.9 ± 0.0 |
| Total phenols (g GAE/L) | 0.4 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.4 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 |
| Feedstock | Nutrient Supplementation (g/L) | Fed Volume (L) |
|---|---|---|
| FVW | NH4Cl, 2.4; K2HPO4, 2.4; MgSO4·7H2O, 1.5; KH2PO4, 0.6; CaCl2, 0.11; FeSO4·7H2O, 0.05 | 1.2 a |
| CCW | Without nutrient supplementation | 2.7 |
| CWW | NH4Cl, 2.4 and FeSO4·7H2O, 0.05 | 2.7 |
| TV | NH4Cl, 2.4 and FeSO4·7H2O, 0.05 | 2.7 |
| TV–MPW | Without nutrient supplementation | 2.7 |
| TV–CCW | FeSO4·7H2O, 0.05 | 2.7 |
| CCW–FVW | Without nutrient supplementation | 2.7 |
| DF Type | Feedstock | P, NmL | VHPRmax c, NmL H2/L-h | λ, h | R2 |
|---|---|---|---|---|---|
| Inoculum-aided mono-fermentation | FVW | 4556.0 a, 9157.7 b | 84.8 a, 393.3 b | 7.7 a, 31.4 b | 0.9885 |
| CCW | 19,673.0 | 199.8 | 18.8 | 0.9991 | |
| TV | 3537.3 | 151.8 | 63.5 | 0.9984 | |
| CWW | 534.2 | 24.2 | 22.0 | 0.9899 | |
| Inoculum-aided co-fermentation | TV-MPW | 3281.7 | 94.7 | 95.5 | 0.9991 |
| TV-CCW | 5163.9 | 138.5 | 71.6 | 0.9947 | |
| FVW-CCW | 19,066.1 | 342.6 | 21.7 | 0.9975 | |
| Self-fermentation | CCW | 1257.0 a, 5263.5 b | 32.9 a, 92.7 b | 23.6 a, 53.46 b | 0.9982 |
| FVW | 59.3 a, 188.7 b | 2.2 a, 1.8 b | 9.9 a, 37.9 b | 0.9964 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Depraect, O.; León-Becerril, E. Use of a Highly Specialized Biocatalyst to Produce Lactate or Biohydrogen and Butyrate from Agro-Industrial Resources in a Dual-Phase Dark Fermentation. Fermentation 2023, 9, 787. https://doi.org/10.3390/fermentation9090787
García-Depraect O, León-Becerril E. Use of a Highly Specialized Biocatalyst to Produce Lactate or Biohydrogen and Butyrate from Agro-Industrial Resources in a Dual-Phase Dark Fermentation. Fermentation. 2023; 9(9):787. https://doi.org/10.3390/fermentation9090787
Chicago/Turabian StyleGarcía-Depraect, Octavio, and Elizabeth León-Becerril. 2023. "Use of a Highly Specialized Biocatalyst to Produce Lactate or Biohydrogen and Butyrate from Agro-Industrial Resources in a Dual-Phase Dark Fermentation" Fermentation 9, no. 9: 787. https://doi.org/10.3390/fermentation9090787
APA StyleGarcía-Depraect, O., & León-Becerril, E. (2023). Use of a Highly Specialized Biocatalyst to Produce Lactate or Biohydrogen and Butyrate from Agro-Industrial Resources in a Dual-Phase Dark Fermentation. Fermentation, 9(9), 787. https://doi.org/10.3390/fermentation9090787







