The Influence of Trctf1 Gene Knockout by CRISPR–Cas9 on Cellulase Synthesis by Trichoderma reesei with Various Soluble Inducers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Strains
2.2. Cellulase Production by Shake Culture
2.3. Expression of the Codon-Optimized Cas9 Gene in T. reesei Rut C30
2.4. Gene Deletion Using the CRISPR–Cas9 System
2.5. Cellulase Hydrolysis of Alkali-Treated Corn Stover (APCS)
2.6. Analysis Method
3. Results
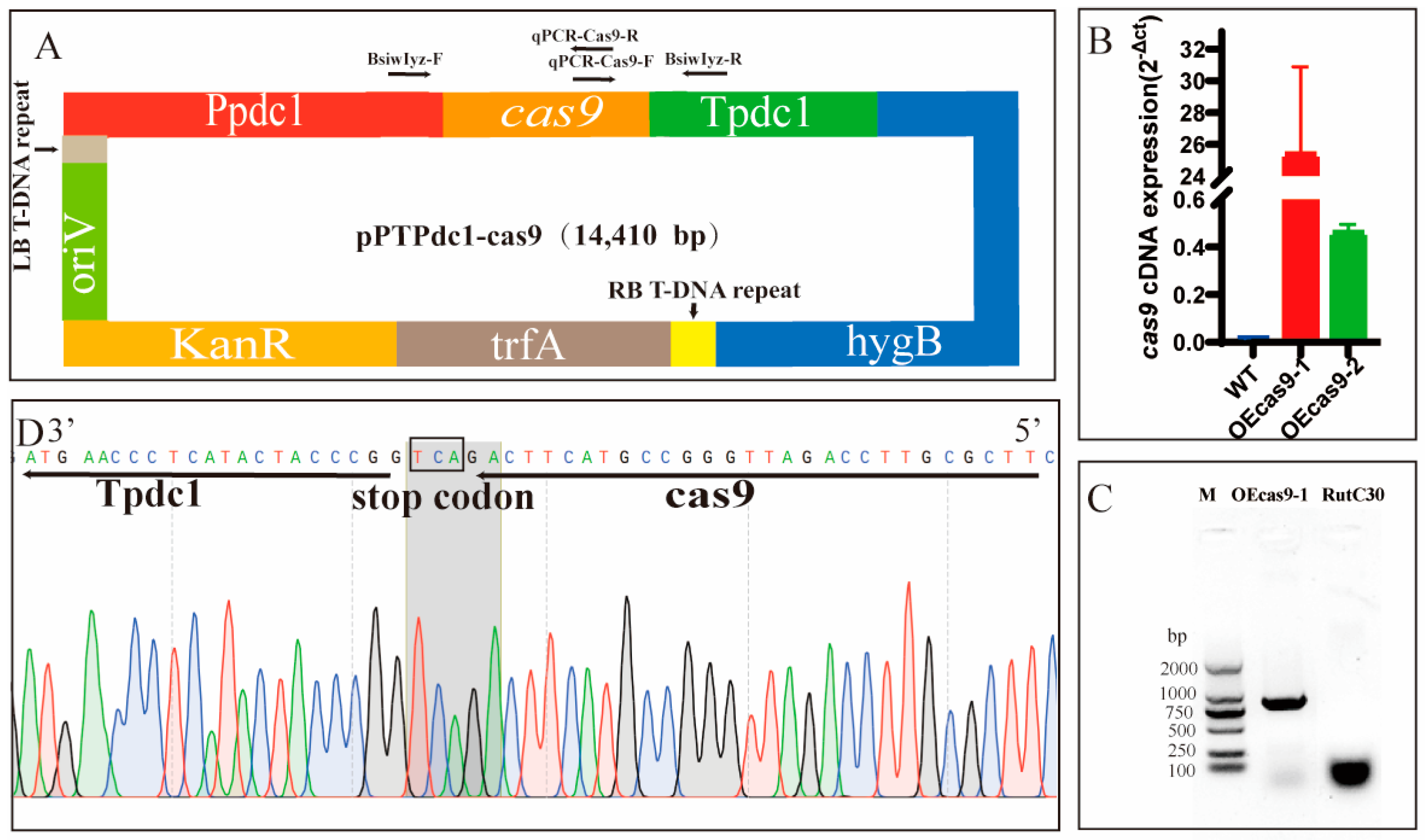
3.1. Development of a CRISPR–Cas9 Genome Editing System for Trichoderma reesei Rut C30
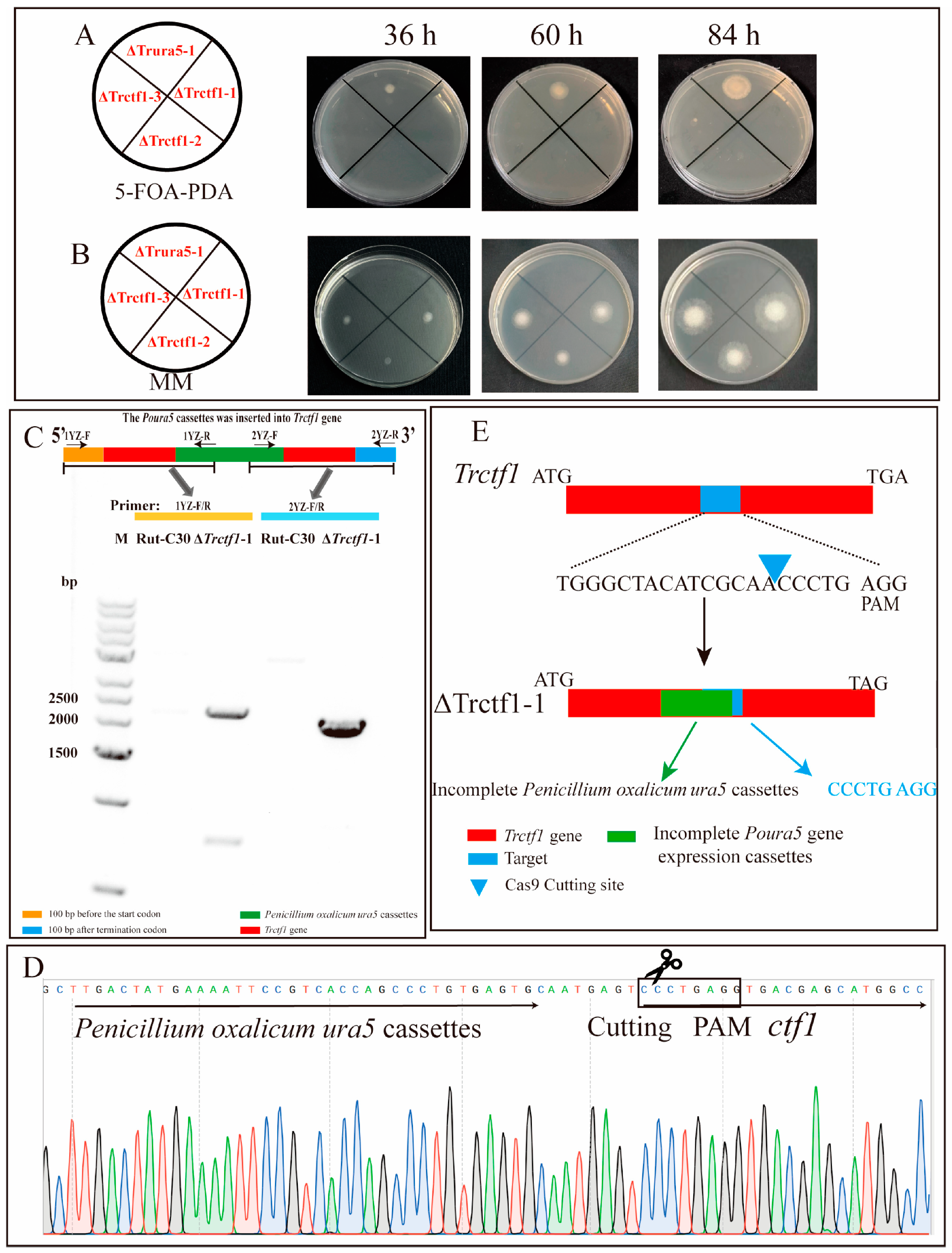
3.2. CRISPR–Cas9 System Directed Trctf1 Gene Mutagenesis in T. reesei
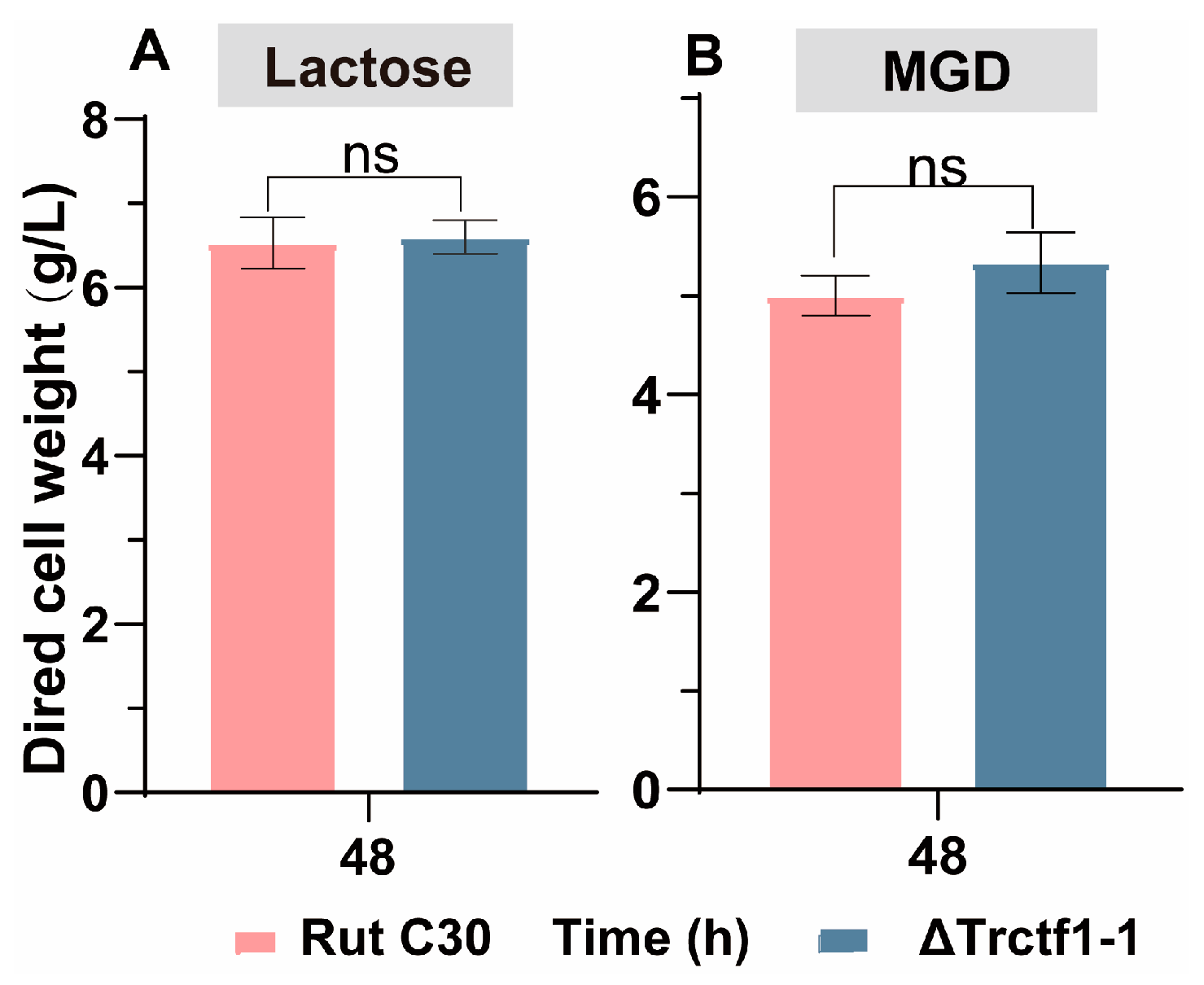
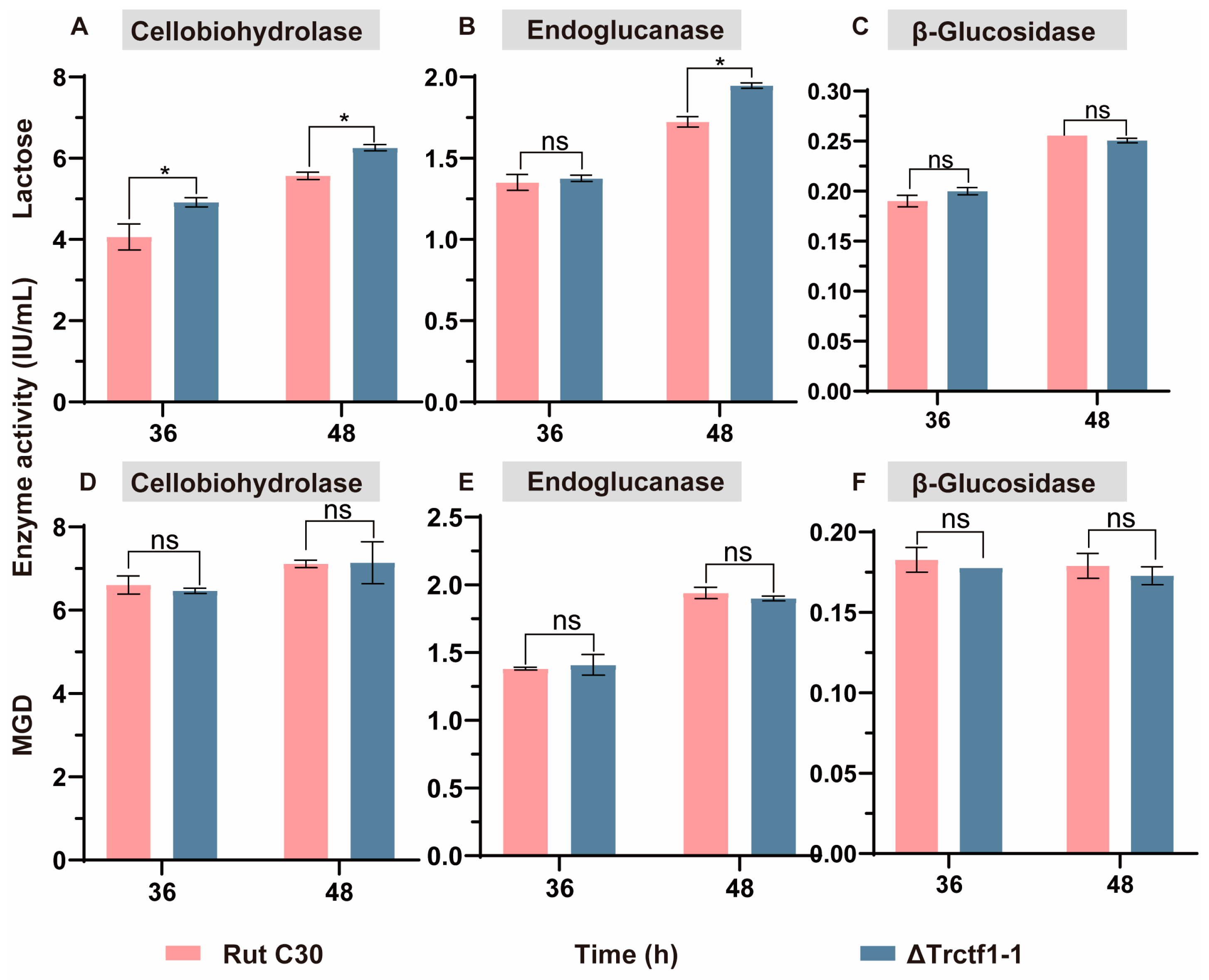
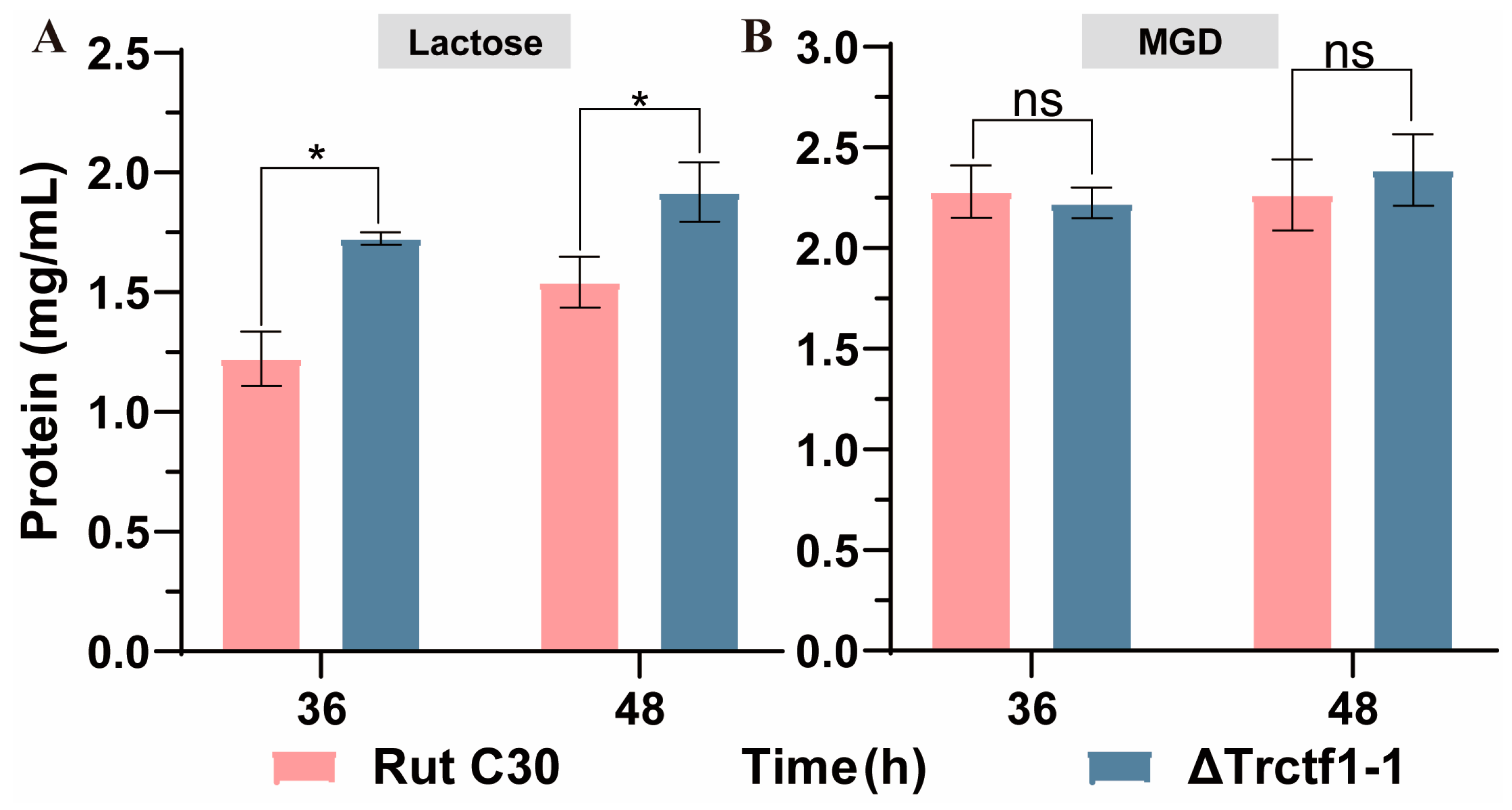
3.3. Impact of the Trctf1 Gene on Cellulase Production with Various Soluble Inducers
3.4. Hydrolysis of Corn Stover by Recombinant Cellulases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phillips, S.D.; Jones, S.B.; Meyer, P.A.; Snowden-Swan, L.J. Techno-economic analysis of cellulosic ethanol conversion to fuel and chemicals. Biofuels Bioprod. Biorefining 2022, 16, 640–652. [Google Scholar] [CrossRef]
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of bioethanol—A review of factors affecting ethanol yield. Fermentation 2021, 7, 268. [Google Scholar] [CrossRef]
- Sperandio, G.B.; Filho, E. An overview of Trichoderma reesei co-cultures for the production of lignocellulolytic enzymes. Appl. Microbiol. Biotechnol. 2021, 105, 3019–3025. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Peng, N.; Gao, Y.; Li, Q.; Wang, Z.; Yao, B.; Li, Y. Two-stage pretreatment of jerusalem artichoke stalks with wastewater recycling and lignin recovery for the biorefinery of lignocellulosic biomass. Processes 2023, 11, 127. [Google Scholar] [CrossRef]
- Fang, H.; Li, C.; Zhao, J.; Zhao, C. Biotechnological advances and trends in engineering Trichoderma reesei towards cellulase hyperproducer. Biotechnol. Bioprocess Eng. 2021, 26, 517–528. [Google Scholar] [CrossRef]
- Yao, C.; Sun, N.; Gao, W.; Sun, Y.; Zhang, J.; Liu, H.; Zhong, Y. Overexpression of a Novel Vacuolar Serine Protease-Encoding Gene (spt1) to Enhance Cellulase Production in Trichoderma reesei. Fermentation 2023, 9, 191. [Google Scholar] [CrossRef]
- Knapp, J.S.; Legg, M. The effect of different cellulosic growth substrates and pH on the production of cellulolytic enzymes by Trichoderma reesei. J. Appl. Microbiol. 2010, 61, 319–329. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, C.; Shen, Y.; Ma, Y.; Wei, D.; Wang, W. N,N-dimethylformamide induces cellulase production in the filamentous fungus Trichoderma reesei. Biotechnol. Biofuels 2019, 12, 36. [Google Scholar] [CrossRef]
- Carle-Urioste, J.C.; Escobar-Vera, J.; El-Gogary, S.; Henrique-Silva, F.; Torigoi, E.; Crivellaro, O.; Herrera-Estrella, A.; El-Dorry, H. Cellulase induction in Trichoderma reesei by cellulose requires its own basal expression. J. Biol. Chem. 1997, 272, 10169–10174. [Google Scholar] [CrossRef]
- Gao, L.; Xia, C.; Xu, J.; Li, Z.; Yu, L.; Liu, G.; Song, X.; Qu, Y. Constitutive expression of chimeric transcription factors enables cellulase synthesis under non-Inducing conditions in Penicillium oxalicum. Biotechnol. J. 2017, 12, 1700297. [Google Scholar] [CrossRef]
- Yu, L.; Chao, Y.; Wensel, P.C.; Chen, S. Hydrodynamic and kinetic study of cellulase production by Trichoderma reesei with pellet morphology. Biotechnol. Bioeng. 2012, 109, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Adsul, M.G.; Dixit, P.; Saini, J.K.; Gupta, R.P.; Ramakumar, S.S.V.; Mathur, A.S. Morphologically favorable mutant of Trichoderma reesei for low viscosity cellulase production. Biotechnol. Bioeng. 2022, 119, 2167–2181. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, C.; BååTH, J.A.; Seiboth, B.; Kubicek, C.P. Systems analysis of lactose metabolism in Trichoderma reesei identifies a lactose permease that is essential for cellulase induction. PLoS ONE 2013, 8, e62631. [Google Scholar] [CrossRef] [PubMed]
- Kalathinathan, P.; Sain, A.; Pulicherla, K.; Muthukaliannan, G.K. A review on the various sources of β-galactosidase and its lactose hydrolysis property. Curr. Microbiol. 2023, 80, 122. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Q.; Chen, Y.; Peng, N.; Liu, W.; Wang, X.; Li, Y. Induction of cellulase production in Trichoderma reesei by a glucose-sophorose mixture as an inducer prepared using stevioside. RSC Adv. 2022, 12, 17392–17400. [Google Scholar] [CrossRef]
- Mandels, M.; Parrish, F.W.; Reese, E.T. Sophprpse as an inducer of cellulase in Trichoderma viride. J. Bacteriol. 1962, 83, 400–408. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Bai, F.; Zhao, X. Overproduction of cellulase by Trichoderma reesei RUT C30 through batch-feeding of synthesized low-cost sugar mixture. Bioresour. Technol. 2016, 216, 503–510. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, F.; Peng, L.-C.; Zhang, D.-B.; Kondo, A.; Bai, F.-W.; Zhao, X.-Q. Optimization of cellulolytic enzyme components through engineering Trichoderma reesei and on-site fermentation using the soluble inducer for cellulosic ethanol production from corn stover. Biotechnol. Biofuels 2018, 11, 49. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Gao, Y.; Mo, Y.; Long, T.; Yao, B.; Li, Y. Engineering Trichoderma reesei for the hyperproduction of cellulose induced protein 1 (Cip1) on a sophorose-containing inducer to efficiently saccharify alkali-pretreated corn stover. Prep. Biochem. Biotechnol. 2022, 23, 880–890. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Zhu, D.; Yao, B.; Hasunuma, T.; Kondo, A.; Zhao, X. Efficient preparation of soluble inducer for cellulase production and saccharification of corn stover using in-house generated crude enzymes. Biochem. Eng. J. 2022, 178, 108296. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, L.; Xia, L. High-level production of a fungal β-glucosidase with application potentials in the cost-effective production of Trichoderma reesei cellulase. Process Biochem. 2018, 70, 55–60. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, C.; Fan, X.; Zhao, X.; Zhao, X.; Shen, T.; Wei, D.; Wang, W. Engineering of Trichoderma reesei for enhanced degradation of lignocellulosic biomass by truncation of the cellulase activator ACE3. Biotechnol. Biofuels 2020, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Daranagama, N.D.; Suzuki, Y.; Shida, Y.; Ogasawara, W. Involvement of xyr1 and are1 for Trichodermapepsin gene expression in response to cellulose and galactose in Trichoderma reesei. Curr. Microbiol. 2020, 77, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, T.; Margeot, A.; Seidl-Seiboth, V.; Le, C.S.; Ben, C.F.; Linke, R.; Seiboth, B.; Kubicek, C.P. Differential regulation of the cellulase transcription factors Xyr1, Ace2, and Ace1 in Trichoderma reesei strains producing high and low levels of cellulase. Eukaryot. Cell 2011, 10, 262–271. [Google Scholar] [CrossRef]
- Ivanova, C.; Ramoni, J.; Aouam, T.; Frischmann, A.; Seiboth, B.; Baker, S.E.; Le, C.S.; Lemoine, S.; Margeot, A.; Bidard, F. Genome sequencing and transcriptome analysis of Trichoderma reesei QM9978 strain reveals a distal chromosome translocation to be responsible for loss of vib1 expression and loss of cellulase induction. Biotechnol. Biofuels 2017, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, X.; Huang, H.; Wang, Y.; Tu, T.; Bai, Y.; Wang, Y.; Zhang, J.; Luo, H.; Yao, B.; et al. Engineering the cbh1 promoter of Trichoderma reesei for enhanced protein production by replacing the binding sites of a transcription repressor ace1 to those of the activators. J. Agric. Food Chem. 2020, 68, 1337–1346. [Google Scholar] [CrossRef]
- Antoniêto, A.C.; de Paula, R.G.; Castro, L.S.; Silva-Rocha, R.; Persinoti, G.F.; Silva, R.N. Trichoderma reesei CRE1-mediated carbon catabolite repression in re-sponse to sophorose through RNA sequencing analysis. Curr. Genom. 2016, 17, 119–131. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Liu, P.; Lin, A.; Fan, X.; Wu, C.; Li, N.; Wei, L.; Wei, D. The novel repressor Rce2 competes with Ace3 to regulate cellulase gene expression in the filamentous fungus Trichoderma reesei. Mol. Microbiol. 2021, 116, 1298–1314. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, F.; Liu, C.; Zhao, X.; Bai, F. Identification of a novel repressor encoded by the putative gene ctf1 for cellulase biosynthesis in Trichoderma reesei through artificial zinc finger engineering. Biotechnol. Bioeng. 2020, 117, 1747–1760. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, F.; Wang, L.; Zhao, G.; Chen, G.; Zhang, W.; Liu, W. Rce1, a novel transcriptional repressor, regulates cellulase gene expression by antagonizing the transactivator Xyr1 in Trichoderma reesei. Mol. Microbiol. 2017, 105, 65–83. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.; Zhang, P.; Long, T.; Mo, Y.; Li, J.; Li, Q. Comparative transcriptome analysis of Trichoderma reesei reveals different gene regulatory networks induced by synthetic mixtures of glucose and β-disaccharide. Bioresour. Bioprocess. 2021, 8, 57. [Google Scholar] [CrossRef]
- Dos Santos Castro, L.; de Paula, R.G.; Antoniêto, A.C.; Persinoti, G.F.; Silva-Rocha, R.; Silva, R.N. Understanding the Role of the Master Regulator XYR1 in Trichoderma reesei by Global Transcriptional Analysis. Front. Microbiol. 2016, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Castro, L.; Pedersoli, W.R.; Antoniêto, A.C.C.; Steindorff, A.S.; Silva-Rocha, R.; Martinez-Rossi, N.M.; Rossi, A.; Brown, N.A.; Goldman, G.H.; Faça, V.M.; et al. Comparative metabolism of cellulose, sophorose and glucose in Trichoderma reesei using high-throughput genomic and proteomic analyses. Biotechnol. Biofuels 2014, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Xiong, L.; Mehmood, M.A.; Zhao, X.; Bai, F. On-Site cellulase production and efficient saccharification of corn stover employing cbh2 overexpressing Trichoderma reesei with novel induction system. Bioresour. Technol. 2017, 238, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Peláez, R.D.R.; Wischral, D.; Cunha, J.R.B.; Mendes, T.D.; Pacheco, T.F.; Siqueira, F.G.D.; Almeida, J.R.M.D. Production of enzymatic extract with high cellulolytic and oxidative activities by co-culture of Trichoderma reesei and Panus lecomtei. Fermentation 2022, 8, 522. [Google Scholar] [CrossRef]
- Liu, R.; Chen, L.; Jiang, Y.; Zhou, Z.; Zou, G. Efficient genome editing in filamentous fungus Trichoderma reesei using the CRISPR/Cas9 system. Cell Discov. 2015, 1, 15007. [Google Scholar] [CrossRef]
- Zou, G.; Xiao, M.; Chai, S.; Zhu, Z.; Wang, Y.; Zhou, Z. Efficient genome editing in filamentous fungi via an improved CRISPR-Cas9 ribonucleoprotein method facilitated by chemical reagents. Microb. Biotechnol. 2021, 14, 2343–2355. [Google Scholar] [CrossRef]
- Zou, G.; Zhou, Z. CRISPR/Cas9-Mediated Genome editing of Trichoderma reesei. Methods Mol. Biol. 2021, 2234, 87–98. [Google Scholar] [CrossRef]
- Chai, S.; Zhu, Z.; Tian, E.; Xiao, M.; Wang, Y.; Zou, G.; Zhou, Z. Building a versatile protein production platform using engineered Trichoderma reesei. ACS Synth. Biol. 2022, 11, 486–496. [Google Scholar] [CrossRef]
- Dai, P.; Liang, X.; Zhang, R.; Sun, G. Construction of Trichothecium roseum GFP tagged strains based on PEG-mediated protoplast transformation. Mycosystema 2019, 38, 90–97. [Google Scholar] [CrossRef]
- McIntosh, S.; Vancov, T. Enhanced enzyme saccharification of sorghum bicolor straw using dilute alkali pretreatment. Bioresour. Technol. 2010, 101, 6718–6727. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, X.; Bai, F. Improvement of cellulase production in Trichoderma reesei Rut-C30 by overexpression of a novel regulatory gene Trvib-1. Bioresour. Technol. 2018, 247, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Jalak, J.; Väljamäe, P. Mechanism of initial rapid rate retardation in cellobiohydrolase catalyzed cellulose hydrolysis. Biotechnol. Bioeng. 2010, 106, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Bischof, R.; Fourtis, L.; Limbeck, A.; Gamauf, C.; Seiboth, B.; Kubicek, C.P. Comparative analysis of the Trichoderma reesei transcriptome during growth on the cellulase inducing substrates wheat straw and lactose. Biotechnol. Biofuels. 2013, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Xia, L. High activity cellulase production by recombinant Trichoderma reesei ZU-02 with the enhanced cellobiohydrolase production. Bioresour. Technol. 2013, 144, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Wang, Y.; Liu, G.; Zuo, B.; Zhang, Y.; Wang, Y.; Liu, W.; Liu, X.; Zhong, Y. The effects of deletion of cellobiohydrolase genes on carbon source-dependent growth and enzymatic lignocellulose hydrolysis in Trichoderma reesei. J. Microbiol. 2020, 58, 687–695. [Google Scholar] [CrossRef]
- Xiong, L.; Kameshwar, A.K.; Chen, X.; Guo, Z.; Mao, C.; Chen, S.; Qin, W. The ACEII recombinant Trichoderma reesei QM9414 strains with enhanced xylanase production and its applications in production of xylitol from tree barks. Microb. Cell Factories 2016, 15, 215. [Google Scholar] [CrossRef][Green Version]
- Liu, G.; Qu, Y. Integrated engineering of enzymes and microorganisms for improving the efficiency of industrial lignocellulose deconstruction. Eng. Microbiol. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Zheng, F.; Yang, R.; Cao, Y.; Zhang, W.; Lv, X.; Meng, X.; Zhong, Y.; Chen, G.; Zhou, Q.; Liu, W. Engineering Trichoderma reesei for hyperproduction of cellulases on glucose to efficiently saccharify pretreated corncobs. J. Agric. Food Chem. 2020, 68, 12671–12682. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Parreiras, L.S.; Murakami, M.T. Rational engineering of the Trichoderma reesei RUT-C30 strain into an industrially relevant platform for cellulase production. Biotechnol. Biofuels 2020, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, X.; Gao, J.; Li, X. Xylo-oligosaccharides inhibit enzymatic hydrolysis by influencing enzymatic activity of cellulase from Penicillium oxalicum. Energy Fuels 2018, 32, 9427–9437. [Google Scholar] [CrossRef]
- Long, T.; Zhang, P.; Yu, J.; Gao, Y.; Ran, X.; Li, Y. Regulation of β-Disaccharide accumulation by β-Glucosidase inhibitors to enhance cellulase production in Trichoderma reesei. Fermentation 2022, 8, 232. [Google Scholar] [CrossRef]
- She, L.; Su, Y.; Sun, Y.; Wang, G.; Chen, H.; Yu, X.; Zhang, S.; Chen, G. Establishment of a highly efficient and low cost mixed cellulase system for bioconversion of corn stover by Trichoderma reesei and Aspergillus niger. Biocatal. Agric. Biotechnol. 2021, 32, 101849. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, B.; Chen, H.; Wu, W.; Wu, S.; Jin, Y.; Xiao, H. Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels 2021, 14, 205. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Gao, Y.; Wang, Z.; Peng, N.; Ran, X.; Chen, T.; Liu, L.; Li, Y. The Influence of Trctf1 Gene Knockout by CRISPR–Cas9 on Cellulase Synthesis by Trichoderma reesei with Various Soluble Inducers. Fermentation 2023, 9, 746. https://doi.org/10.3390/fermentation9080746
Chen Y, Gao Y, Wang Z, Peng N, Ran X, Chen T, Liu L, Li Y. The Influence of Trctf1 Gene Knockout by CRISPR–Cas9 on Cellulase Synthesis by Trichoderma reesei with Various Soluble Inducers. Fermentation. 2023; 9(8):746. https://doi.org/10.3390/fermentation9080746
Chicago/Turabian StyleChen, Yudian, Yushan Gao, Zancheng Wang, Nian Peng, Xiaoqin Ran, Tingting Chen, Lulu Liu, and Yonghao Li. 2023. "The Influence of Trctf1 Gene Knockout by CRISPR–Cas9 on Cellulase Synthesis by Trichoderma reesei with Various Soluble Inducers" Fermentation 9, no. 8: 746. https://doi.org/10.3390/fermentation9080746
APA StyleChen, Y., Gao, Y., Wang, Z., Peng, N., Ran, X., Chen, T., Liu, L., & Li, Y. (2023). The Influence of Trctf1 Gene Knockout by CRISPR–Cas9 on Cellulase Synthesis by Trichoderma reesei with Various Soluble Inducers. Fermentation, 9(8), 746. https://doi.org/10.3390/fermentation9080746




