Evaluation of the Effect of Incorporating Olive Mill Wastewater on Nutrients, Quality, and Bacterial Flora in Fermented Total Mixed Ration
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented TMR
2.2. Analysis of Proximate Composition
2.3. Measurement of pH
2.4. Measurement of Lactic Acid and SCFA
2.5. Estimation of Volatile Basic Nitrogen (VBN) and V-SCORE
2.6. Measurement of Polyphenol Concentration
2.7. Microbiota Analysis in the Fermented TMR
2.8. Lactic Acid Bacteria Colony Count
2.9. Statistical Analysis
3. Results
3.1. Chemical Composition of OMW
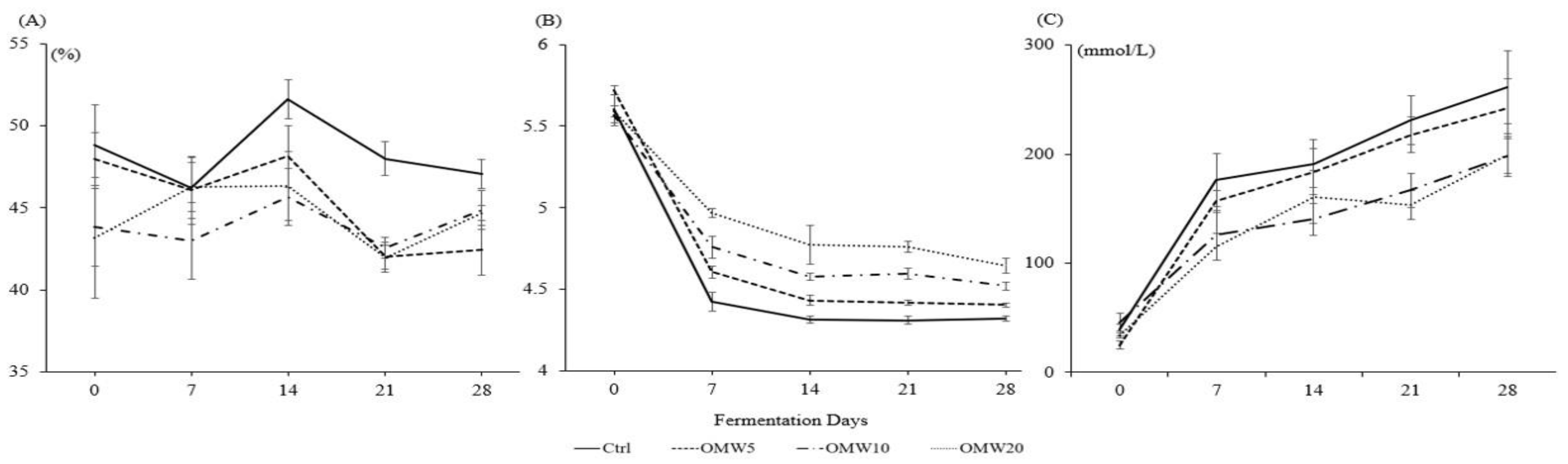
3.2. Dry Matter, pH, and Lactic Acid Concentration Changes in the Fermented TMR
3.3. Nutritional Compositions and Quality Parameters of the TMR after Four-Week Fermentation
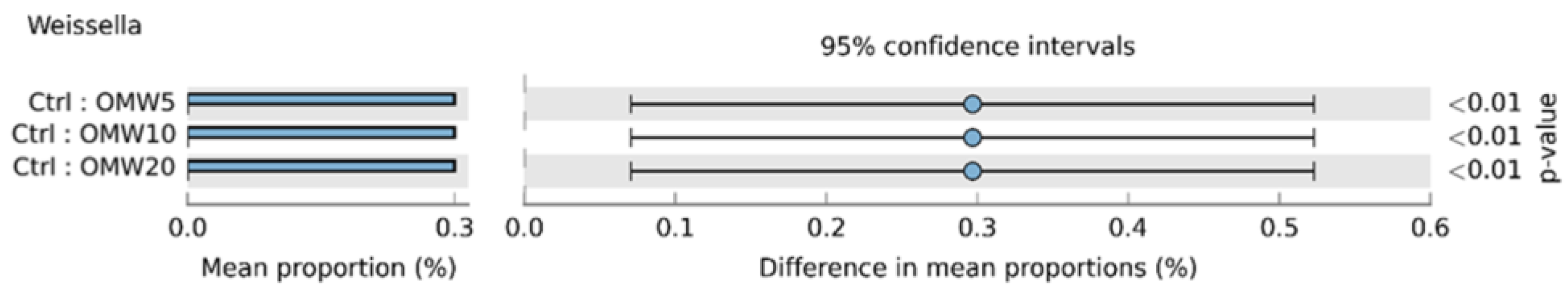
3.4. Microbiota Composition in Fermented TMR after Four-Week Fermentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IEA (International Energy Agency). Mobilization of Agricultural Residues for Bioenergy and Higher Value Bio-Products: Resources, Barriers and Sustainability; IEA Bioenergy: Paris, France, 2017. [Google Scholar]
- Iram, A.; Cekmecelioglu, D.; Demirci, A. Distillers’ dried grains with solubles (DDGS) and its potential as fermentation feedstock. Appl. Microbiol. Biotechnol. 2020, 104, 6115–6128. [Google Scholar] [CrossRef] [PubMed]
- Farràs, M.; Almanza-Aguilera, E.; Hernáez, Á.; Agustí, N.; Julve, J.; Fitó, M.; Castañer, O. Beneficial effects of olive oil and mediterranean diet on cancer physio-pathology and incidence. Semin. Cancer Biol. 2021, 73, 178–195. [Google Scholar] [CrossRef]
- International Olive Council. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/12/IOC-Olive-Oil-Dashboard-2.html#production-1 (accessed on 7 July 2023).
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R.Z.; Almalki, W.H. Treatment technologies for olive mill wastewater with impacts on plants. Environ. Res. 2023, 216, 114399. [Google Scholar] [CrossRef] [PubMed]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional compounds from olive pomace to obtain high-added value foods—A review. J. Sci. Food Agric. 2021, 101, 15–26. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Chiarello, E.; Caboni, M.F.; Capozzi, F.; Gianotti, A.; Bordoni, A. Olive oil by-product as functional ingredient in bakery products. Influence of processing and evaluation of biological effects. Food Res. Int. 2020, 131, 108940. [Google Scholar] [CrossRef]
- Hossain, M.Z.; von Fragstein und Niemsdorff, P.; Heß, J. Plant origin wastes as soil conditioner and organic fertilizer: A review. J. Agric. Food Environ. Sci. 2016, 16, 1362–1371. [Google Scholar] [CrossRef]
- Galanakis, C.M. Phenols recovered from olive mill wastewater as additives in meat products. Trends Food Sci. Technol. 2018, 79, 98–105. [Google Scholar] [CrossRef]
- Gorini, I.; Iorio, S.; Ciliberti, R.; Licata, M.; Armocida, G. Olive oil in pharmacological and cosmetic traditions. J. Cosmet. Dermatol. 2019, 18, 1575–1579. [Google Scholar] [CrossRef]
- Galloni, M.G.; Ferrara, E.; Falletta, E.; Bianchi, C.L. Olive mill wastewater remediation: From conventional approaches to photocatalytic processes by easily recoverable materials. Catalysts 2022, 12, 9233. [Google Scholar] [CrossRef]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; Lyamlouli, K. Olive mill waste sludge: From permanent pollution to a highly beneficial organic biofertilizer: A critical review and future perspectives. Ecotoxicol. Environ. Saf. 2023, 259, 114997. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, L.; Zhang, Q.; Zi, X.; Lv, R.; Tang, J.; Zhou, H. Impacts of citric acid and malic acid on fermentation quality and bacterial community of cassava foliage silage. Front. Microbiol. 2020, 11, 595622. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.; Romeo, F.V.; Russo, N.; Pino, A.; Vaccalluzzo, A.; Caggia, C.; Randazzo, C.L. Olive mill wastewater as renewable raw materials to generate high added-value ingredients for agro-food industries. Appl. Sci. 2021, 11, 7511. [Google Scholar] [CrossRef]
- Neofytou, M.C.; Miltiadou, D.; Sfakianaki, E.; Constantinou, C.; Symeou, S.; Sparaggis, D.; Hager-Theodorides, A.L.; Tzamaloukas, O. The use of ensiled olive cake in the diets of Friesian cows increases beneficial fatty acids in milk and Halloumi cheese and alters the expression of SREBF1 in adipose tissue. J. Dairy Sci. 2020, 103, 8998–9011. [Google Scholar] [CrossRef]
- El Otmani, S.; Chebli, Y.; Taminiau, B.; Chentouf, M.; Hornick, J.L.; Cabaraux, J.F. Effect of olive cake and cactus cladodes incorporation in goat kids’ diet on the rumen microbial community profile and meat fatty acid composition. Biology 2021, 10, 1237. [Google Scholar] [CrossRef]
- Roila, R.; Valiani, A.; Miraglia, D.; Ranucci, D.; Forte, C.; Trabalza-Marinucci, M.; Servili, M.; Codini, M.; Branciari, R. Olive mill wastewater phenolic concentrate as natural antioxidant against lipid-protein oxidative deterioration in chicken meat during storage. Ital. J. Food Saf. 2018, 7, 7342. [Google Scholar] [CrossRef]
- Schingoethe, D.J. A 100-year review: Total mixed ration feeding of dairy cows. J. Dairy Sci. 2017, 100, 10143–10150. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists, 18th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Furuhashi, T.; Sugitate, K.; Nakai, T.; Jikumaru, Y.; Ishihara, G. Rapid profiling method for mammalian feces short chain fatty acids by GC-MS. Anal. Biochem. 2018, 543, 51–54. [Google Scholar] [CrossRef]
- Kawasaki, K.; Wada, K.; Sato, A.; Zhao, J.; Takao, N.; Sato, M.; Ban, T.; Yano, K. Effects of dietary bamboo (Phyllostachys pubescens Mazel) culm powder on blood properties and intestinal environment of rabbits. Anim. Sci. J. 2022, 93, e13774. [Google Scholar] [CrossRef]
- JGFFSA. Japan Grassland Farming Forage Seed Association. Guide Book for Quality Evaluation of Forage; Japanese Society of Grassland Science: Tokyo, Japan, 1994; pp. 82–87. [Google Scholar]
- Hattori, A.; Matsuo, T.; Tsukamoto, Y. Determination of hydroxytyrosol and oleuropein derived from olive in a soft capsule by reversed phase HPLC. Bunseki Kagaku 2019, 68, 623–626. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.; Elkin, L.; Higgins, J.; Wobbrock, J. ARTool: Aligned Rank Transform for Nonparametric Factorial ANOVAs. In Proceedings of the 34th Annual Acm Symposium on User Interface Software and Technology (UIST ’21), Virtual Event, 10–14 October 2021; pp. 754–768. [Google Scholar]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Kapellakis, I.E.; Tsagarakis, K.P.; Avramaki, C. Olive mill wastewater management in river basins: A case study in Greece. Agric. Water Manag. 2006, 82, 354–370. [Google Scholar] [CrossRef]
- Aggoun, M.; Arhab, R.; Cornu, A.; Portelli, J.; Barkat, M.; Graulet, B. Olive mill wastewater microconstituents composition according to olive variety and extraction process. Food Chem. 2016, 209, 72–80. [Google Scholar] [CrossRef] [PubMed]
- El-Abbassi, A.; Hafidi, A.; Khayet, M.; García-Payo, M.C. Integrated direct contact membrane distillation for olive mill wastewater treatment. Desalination 2013, 323, 31–38. [Google Scholar] [CrossRef]
- Al-Shaweesh, M.; Matouq, M.; Al-Kabariti, D.; Khamash, D.; Al-Zawaidah, S.; Hindiyeh, M.; Omar, W. Olive mill wastewater (OMW) treatment by using ferric oxide dephenolization and chemical oxygen demand removal. Glob. Nest J. 2018, 20, 558–563. [Google Scholar] [CrossRef]
- Makri, S.; Kafantaris, I.; Savva, S.; Ntanou, P.; Stagos, D.; Argyroulis, I.; Kotsampasi, B.; Christodoulou, V.; Gerasopoulos, K.; Petrotos, K.; et al. Novel feed including olive oil mill wastewater bioactive compounds enhanced the redox status of lambs. In Vivo 2018, 32, 291–302. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Petrotos, K.; Kokkas, S.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with polyphenolic byproduct from olive mill wastewater processing improves the redox status in blood and tissues of piglets. Food Chem. Toxicol. 2015, 86, 319–327. [Google Scholar] [CrossRef]
- Ait Baddi, G.; Antonio Alburquerque, J.; Gonzálvez, J.; Cegarra, J.; Hafidi, M. Chemical and spectroscopic analyses of organic matter transformations during composting of olive mill wastes. Int. Biodeterior. Biodegrad. 2004, 54, 39–44. [Google Scholar] [CrossRef]
- Mikucka, W.; Zielinska, M. Individual phenolic acids in distillery stillage inhibit its biomethanization. Energies 2022, 15, 5377. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de-las-Rivas, B.; López-de-Felipe, F.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Ishler, V.; Heinrichs, J.; Varga, G. From Feed to Milk: Understanding Rumen Function; Pennstate Extension: State College, PA, USA, 2016. [Google Scholar]
- Cappelli, K.; Ferlisi, F.; Mecocci, S.; Maranesi, M.; Trabalza-Marinucci, M.; Zerani, M.; Dal Bosco, A.; Acuti, G. Dietary supplementation of olive mill waste water polyphenols in rabbits: Evaluation of the potential effects on hepatic apoptosis, inflammation and metabolism through RT-qPCR approach. Animals 2021, 11, 2932. [Google Scholar] [CrossRef] [PubMed]
- Sabino, M.; Cappelli, K.; Capomaccio, S.; Pascucci, L.; Biasato, I.; Verini-Supplizi, A.; Valiani, A.; Trabalza-Marinucci, M. Dietary supplementation with olive mill wastewaters induces modifications on chicken jejunum epithelial cell transcriptome and modulates jejunum morphology. BMC Genom. 2018, 19, 576. [Google Scholar] [CrossRef]
- Terramoccia, S.; Bartocci, S.; Taticchi, A.; Di Giovanni, S.; Pauselli, M.; Mourvaki, E.; Urbani, S.; Servili, M. Use of dried stoned olive pomace in the feeding of lactating buffaloes: Effect on the quantity and quality of the milk produced. Asian-Aust. J. Anim. Sci. 2013, 26, 971–980. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Why are Weissella spp. not used as commercial starter cultures for food fermentation? Fermentation 2017, 3, 38. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactobacillus spp. from an inoculant and of weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microbiol. 1998, 64, 2982–2987. [Google Scholar] [CrossRef]
- Kahn, S.K.; Ali, A.; Mobashar, M.; Inam, M.; Ahmed, I.; Khan, N.; Ali, M.; Khan, H. Effect of different levels of organic acids supplementation on feed intake, milk yield and milk composition of dairy cows during thermal stress. Gr. J. Agric. Sci. 2013, 3, 762–768. [Google Scholar]

| Ingredients | Ctrl | OMW5 | OMW10 | OMW20 |
|---|---|---|---|---|
| Sorghum silage | 22.0 | 22.0 | 22.0 | 22.0 |
| Concentrate feed 1 | 35.0 | 35.0 | 35.0 | 35.0 |
| Lucerne hay | 4.5 | 4.5 | 4.5 | 4.5 |
| Timothy hay | 9.0 | 9.0 | 9.0 | 9.0 |
| Klein grass | 9.0 | 9.0 | 9.0 | 9.0 |
| Calcium phosphate | 0.5 | 0.5 | 0.5 | 0.5 |
| Water | 20.0 | 15.0 | 10.0 | 0.0 |
| Olive mill wastewater | 0.0 | 5.0 | 10.0 | 20.0 |
| Proximate Nutrients (% in Dry Matter) | OMW |
|---|---|
| DM | 13.07 |
| CA | 14.70 |
| CP | 9.80 |
| EE | 23.28 |
| CF | 2.90 |
| ADF | 4.46 |
| NDF | 10.01 |
| Polyphenolic concentration in the fresh matter (mg/mL) | |
| Oleuropein | 0.79 |
| Hydroxytyrosol | 0.17 |
| Tyrosol | 0.09 |
| 0 to 4 wk | Groups | p-Values of Effects | |||||
|---|---|---|---|---|---|---|---|
| Items | Ctrl | OMW5 | OMW10 | OMW20 | Groups | Period (wk) | Groups × Period |
| Dry matter | 48.33 ± 0.75 a | 45.31 ± 0.98 bcd | 43.99 ± 0.71 cd | 44.49 ± 0.80 d | <0.001 | <0.001 | 0.43 |
| pH | 4.60 ± 0.10 d | 4.72 ± 0.09 c | 4.80 ± 0.07 b | 4.95 ± 0.07 a | <0.001 | <0.001 | <0.001 |
| Lactic acid | 179.91 ± 17.21 a | 165.14 ± 15.87 b | 135.50 ± 11.66 cd | 132.58 ± 12.39 d | <0.001 | <0.001 | 0.35 |
| Item | Ctrl | OMW5 | OMW10 | OMW20 |
|---|---|---|---|---|
| DM | 47.07 ± 0.88 | 42.43 ± 0.46 | 44.89 ± 1.51 | 44.68 ± 1.18 |
| CA | 8.16 ± 0.16 | 9.04 ± 0.32 | 8.87 ± 0.24 | 8.90 ± 0.43 |
| CP | 17.42 ± 0.54 | 16.67 ± 0.50 | 19.15 ± 0.29 | 17.45 ± 0.90 |
| EE | 2.14 ± 0.10 cd | 2.56 ± 0.06 bc | 3.23 ± 0.22 b | 3.79 ± 0.12 a |
| CF | 14.42 ± 1.77 | 17.76 ± 1.03 | 14.01 ± 1.34 | 16.24 ± 1.55 |
| ADF | 23.92 ± 2.02 | 21.56 ± 1.90 | 21.04 ± 0.68 | 22.58 ± 2.93 |
| NDF | 48.48 ± 1.93 | 51.51 ± 1.09 | 56.14 ± 2.73 | 53.19 ± 3.13 |
| pH | 4.32 ± 0.02 cd | 4.41 ± 0.01 c | 4.52 ± 0.02 b | 4.65 ± 0.05 a |
| Lactobacilli (Log CFU/g) | 10.25 ± 0.19 | 10.51 ± 0.08 | 10.35 ± 0.16 | 10.77 ± 0.08 |
| Lactic acid (% of VFA) | 99.14 ± 0.10 | 98.49 ± 0.61 | 98.25 ± 0.59 | 99.19 ± 0.15 |
| Acetic acid (% of VFA) | 0.86 ± 0.10 | 1.51 ± 0.61 | 1.75 ± 0.59 | 0.81 ± 0.15 |
| Propionic acid (% of VFA) | ND | ND | ND | ND |
| Butyric acid (% of VFA) | ND | ND | ND | ND |
| VBN/T-N (%) | 0.72 ± 0.07 | 0.92 ± 0.06 | 0.78 ± 0.02 | 0.84 ± 0.03 |
| V-SCORE | 91 | 92 | 90 | 92 |
| Oleuropein (mg/kg of DM) | ND | ND | 315.6 ± 103.2 | 236.4 ± 106.7 |
| Hydroxytyrosol (mg/kg of DM) | ND | 152.6 ± 8.9 | 171.9 ± 12.5 | 166.1 ± 35.3 |
| Tyrosol (mg/kg of DM) | ND | ND | 15.4 ± 7.3 | 12.9 ± 8.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Kagami, M.; Yano, K.; Kawasaki, K. Evaluation of the Effect of Incorporating Olive Mill Wastewater on Nutrients, Quality, and Bacterial Flora in Fermented Total Mixed Ration. Fermentation 2023, 9, 665. https://doi.org/10.3390/fermentation9070665
Zhao J, Kagami M, Yano K, Kawasaki K. Evaluation of the Effect of Incorporating Olive Mill Wastewater on Nutrients, Quality, and Bacterial Flora in Fermented Total Mixed Ration. Fermentation. 2023; 9(7):665. https://doi.org/10.3390/fermentation9070665
Chicago/Turabian StyleZhao, Junliang, Masanori Kagami, Kiminobu Yano, and Kiyonori Kawasaki. 2023. "Evaluation of the Effect of Incorporating Olive Mill Wastewater on Nutrients, Quality, and Bacterial Flora in Fermented Total Mixed Ration" Fermentation 9, no. 7: 665. https://doi.org/10.3390/fermentation9070665
APA StyleZhao, J., Kagami, M., Yano, K., & Kawasaki, K. (2023). Evaluation of the Effect of Incorporating Olive Mill Wastewater on Nutrients, Quality, and Bacterial Flora in Fermented Total Mixed Ration. Fermentation, 9(7), 665. https://doi.org/10.3390/fermentation9070665






