Bioelectrochemical Systems (BES) for Biomethane Production—Review
Abstract
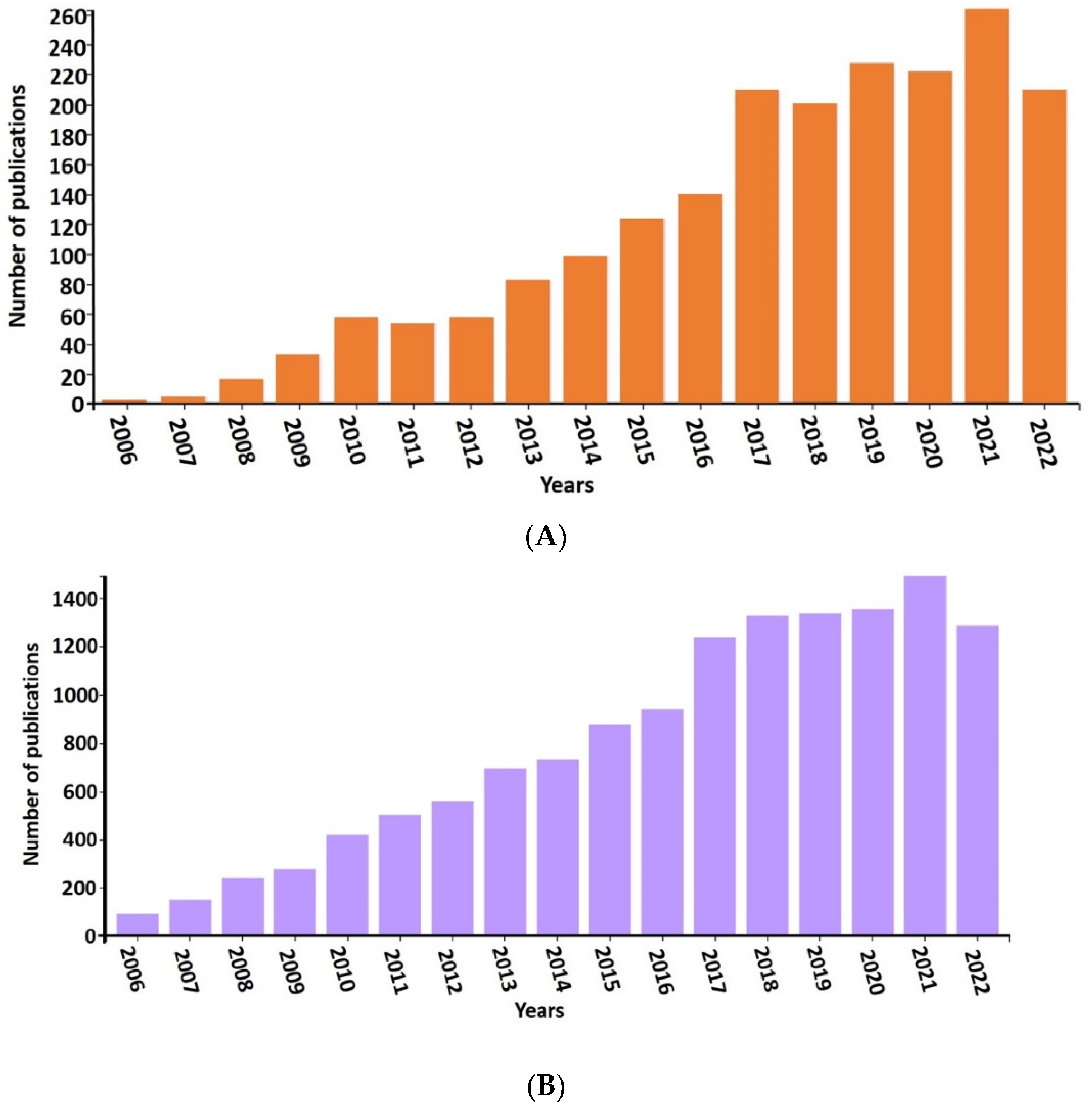
1. Introduction
2. How It Started?
3. Which Is What?
- Bioelectrochemical system (BES): BES consists of an anode, where oxidation takes place, and a cathode, where reduction occurs and at least one of the electrodes utilizes microorganisms to catalyse the redox reaction via interaction with the electrode directly or through mediators. The electrode and surrounding microbiota, usually organized in biofilm, is called bioelectrode. The anode and the cathode can be separated by a membrane, but the membrane is not an indispensable component of BES. Frequently used synonyms: microbial electrochemical technology (MET) or microbial electrochemical system (MES) [26,27,28,29,30,31,32,33].
- Biogas cleaning removes impurities, like water, hydrogen sulphides, etc., from the raw biogas by physicochemical means, such as adsorption, differential solubility, or membrane separation. Biogas cleaning can be divided into specific processes according to the target, for example, biogas desulphurization (removal of H2S) or biogas drying (removal of water moisture) [34,35].
- Biogas upgrading: Raw biogas contains predominantly methane (CH4), CO2, and other gasses, such as H2S. The non-CH4 gas components decrease the calorific value of biogas, can be harmful to live organisms, and some of them (for example, H2S) are extremely corrosive, so they have to be removed before injection into the natural gas grids or used as alternative engine/vehicle fuel. As per the definition, biogas upgrading refers to removing CO2 via transformation by catalytic conversion or separation of this major biogas component [34,35].
- Biohythane: Hythane is a balanced mixture of hydrogen (10–30 v/v%) and methane (70–90 v/v%), a promising alternative to the conventional fossil gaseous energy carriers. Hythane has a higher fuel and heat efficiency. It can reduce carbon emission, increases burning speed, extends flammability range, and enhances combustion efficiency. Biohytane is produced from renewable biomass [21,36,37].
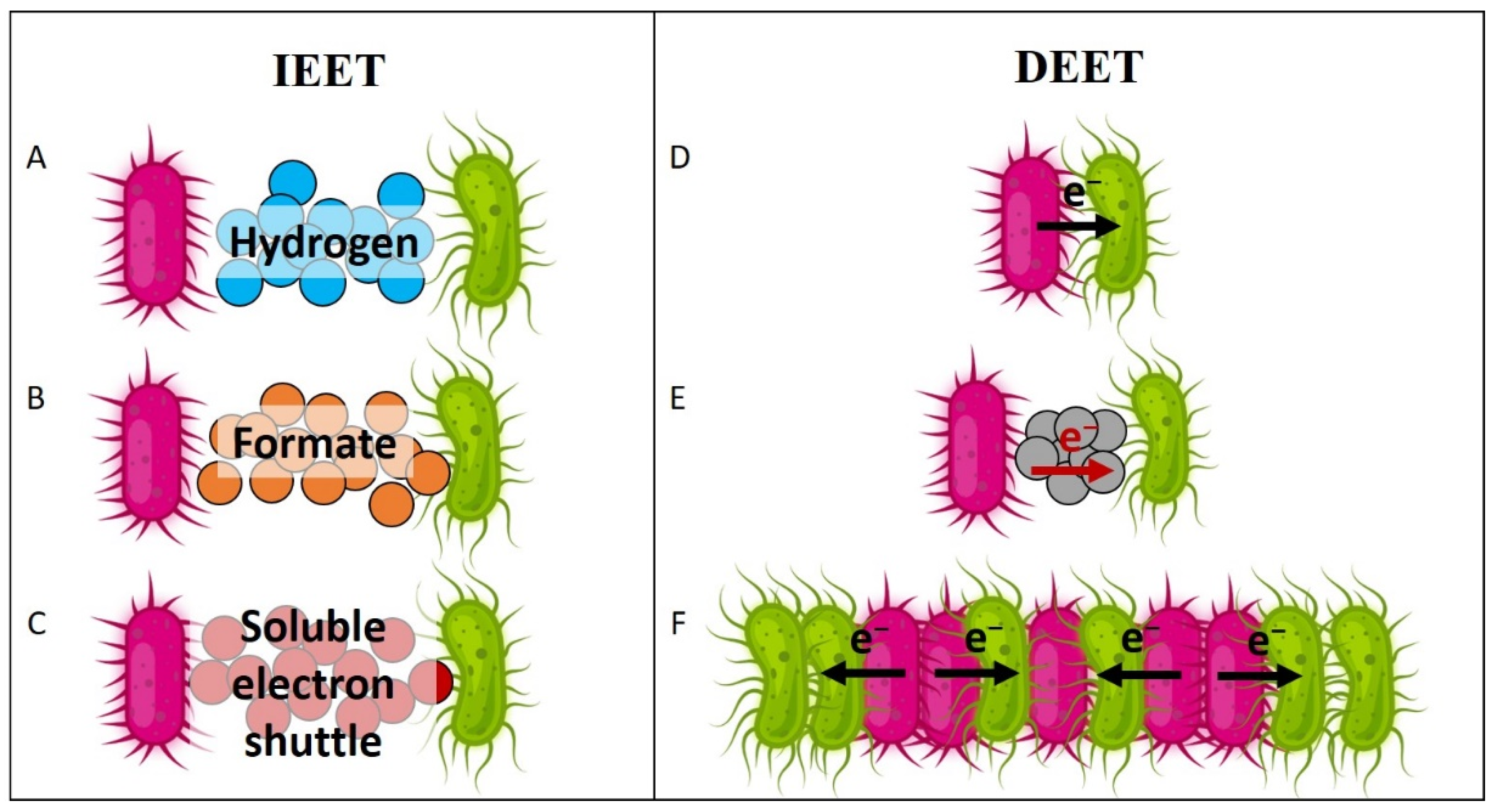
- Direct interspecies electron transfer (DIET) is a syntrophic microbial interaction where free electrons are transferred/exchanged between microorganisms [38].
- Electrohydrogenesis: During electrohydrogenesis, the protons and electrons generated on the anode are transferred to the cathode. The microbial catalyst components, driven by the applied potential, combine electrons and protons to H2, released from the cathode compartment [41].
- Electromethanogenesis: Electromethanogenesis produces methane via electroactive microbes using CO2 as the sole carbon source in an engineered system (biocathode) powered by electric current. Electromethanogenesis is a specific form of BES/MES when only CH4 is produced from CO2 with the additional input from electricity to provide the extra energy needed to recombine CO2 with electrons and protons [42]. Electromethanogenesis is thus a subset of BES/MES, the microbial electrosynthesis of various chemicals.
- Electrotrophic microorganisms: Electrotrophic microorganisms act as electron acceptors in electrogenic reactions. They are capable of taking up electrons from the environment and utilize in their metabolic reactions [43].
- Exoelectrogenic microorganisms: Exoelectrogenic microorganisms are capable of generating electrical energy via the transfer the electrons, produced by substrate oxidation, to extracellular electron acceptors [44].
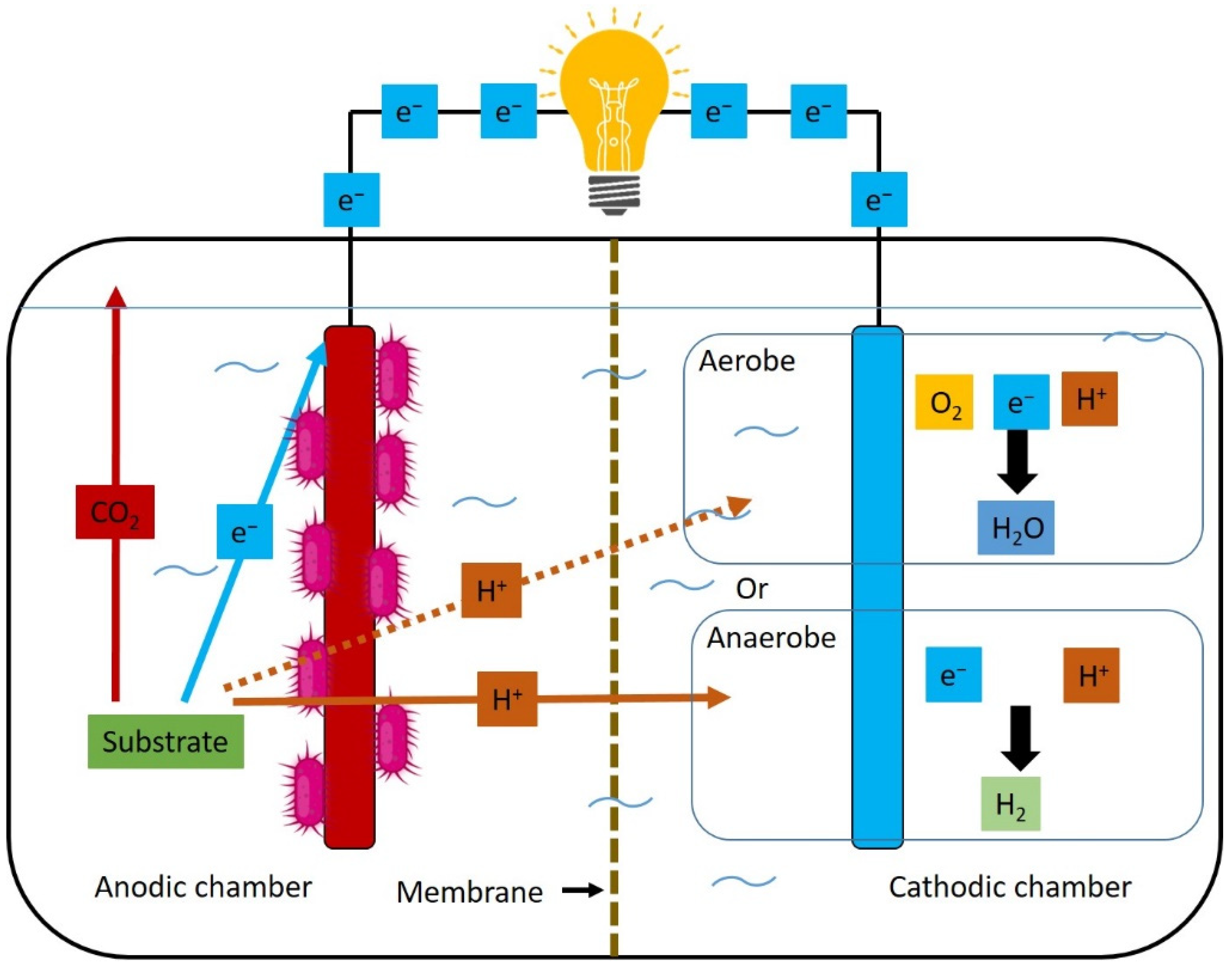
- Microbial electrolysis cell (MEC): MECs are a distinct BES construction in which an external power source supplements the energy generated at the bioanode via biomass fermentation. Valuable commodities are formed at the cathode by overcoming the thermodynamically unfavourable reduction reactions. MECs may also operate with abiotically evolved H2 in the cathodic chamber. Alternatively, the electrons are harvested from the cathode by electroactive microorganisms or soluble electron acceptors to produce H2, CH4, or other chemicals [15,45,46,47,48].
- Microbial electrosynthesis (MES): Microbial electrosynthesis (MES) is a cathode-related process when electroactive microorganisms convert electricity to chemicals through CO2 reduction. MES is a promising technology for renewable electricity storage, CO2 capture and valuable commodities production. Methane, various alcohols, volatile fatty acids, terpenoids, bioplastics etc., can be produced in an MES reactor [5,24,25,31,48,49,50,51]. “Electrofermentation” (EF) is used as a synonym for MES in some literature reports [5,52].
- Microbial fuel cell (MFC): MFC is a type of BES where organic matter is decomposed via exoelectrogenic microbes near the anode, which serves as a terminal electron acceptor. The spontaneous electron movement from the electronegative bioanodes to the electropositive cathode in a circuit generates electric current [14,26,27,53,54,55,56].
4. The BES Drivers
5. Bioelectrochemical System (BES) Concepts
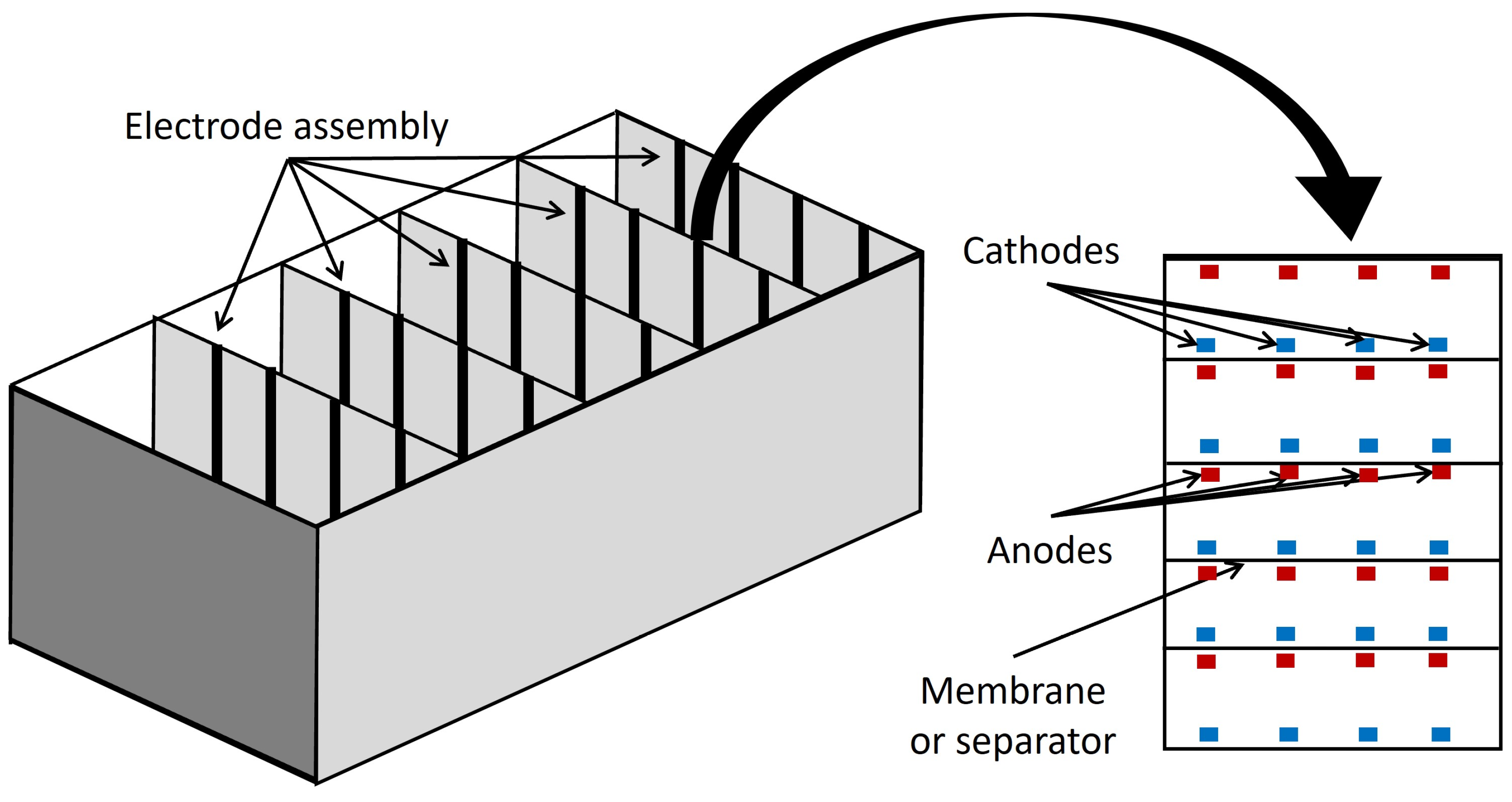
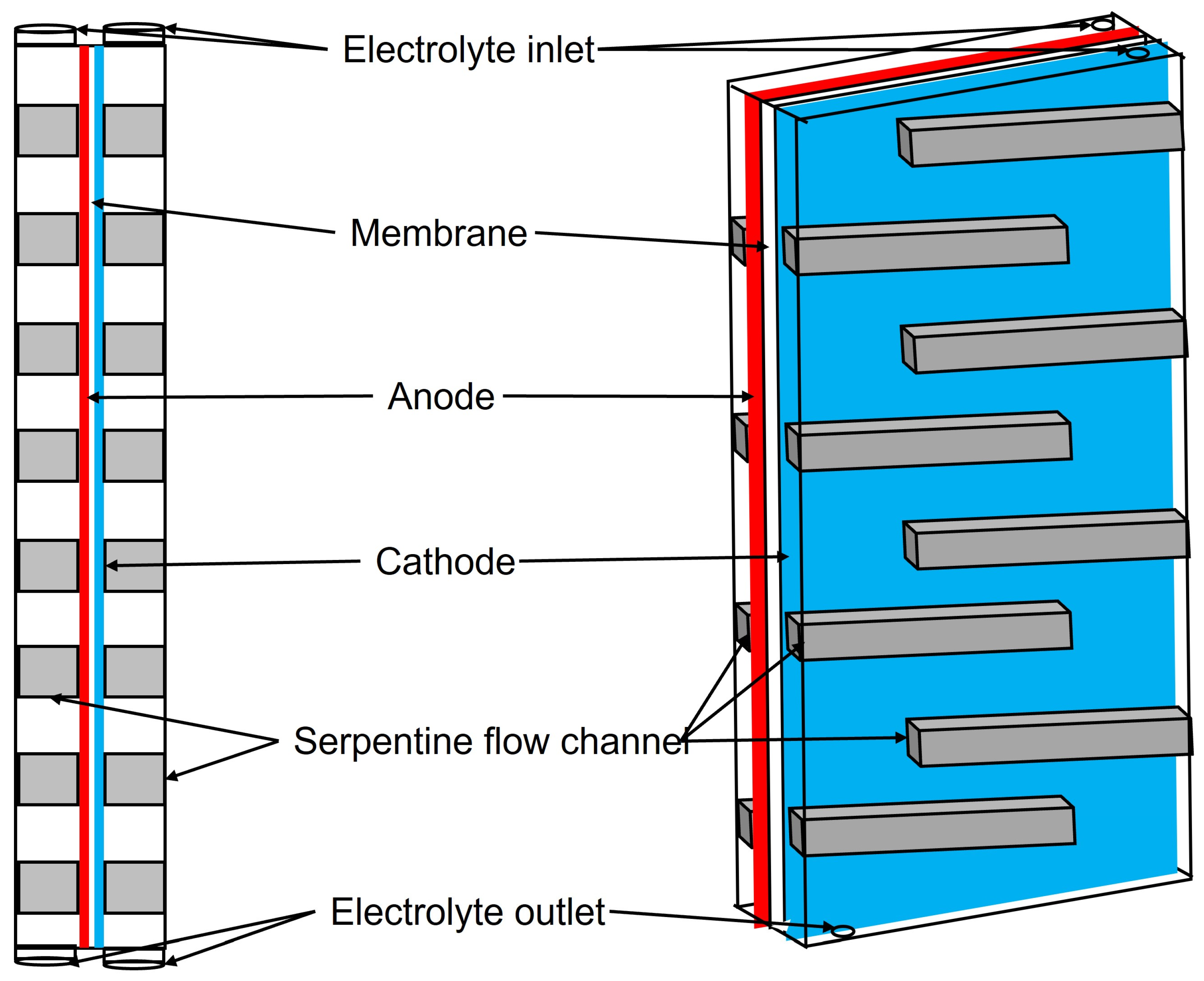
6. Trends in Reactor Design
6.1. Single Chamber Systems
6.2. Two Chamber Systems
6.3. Advanced Designs
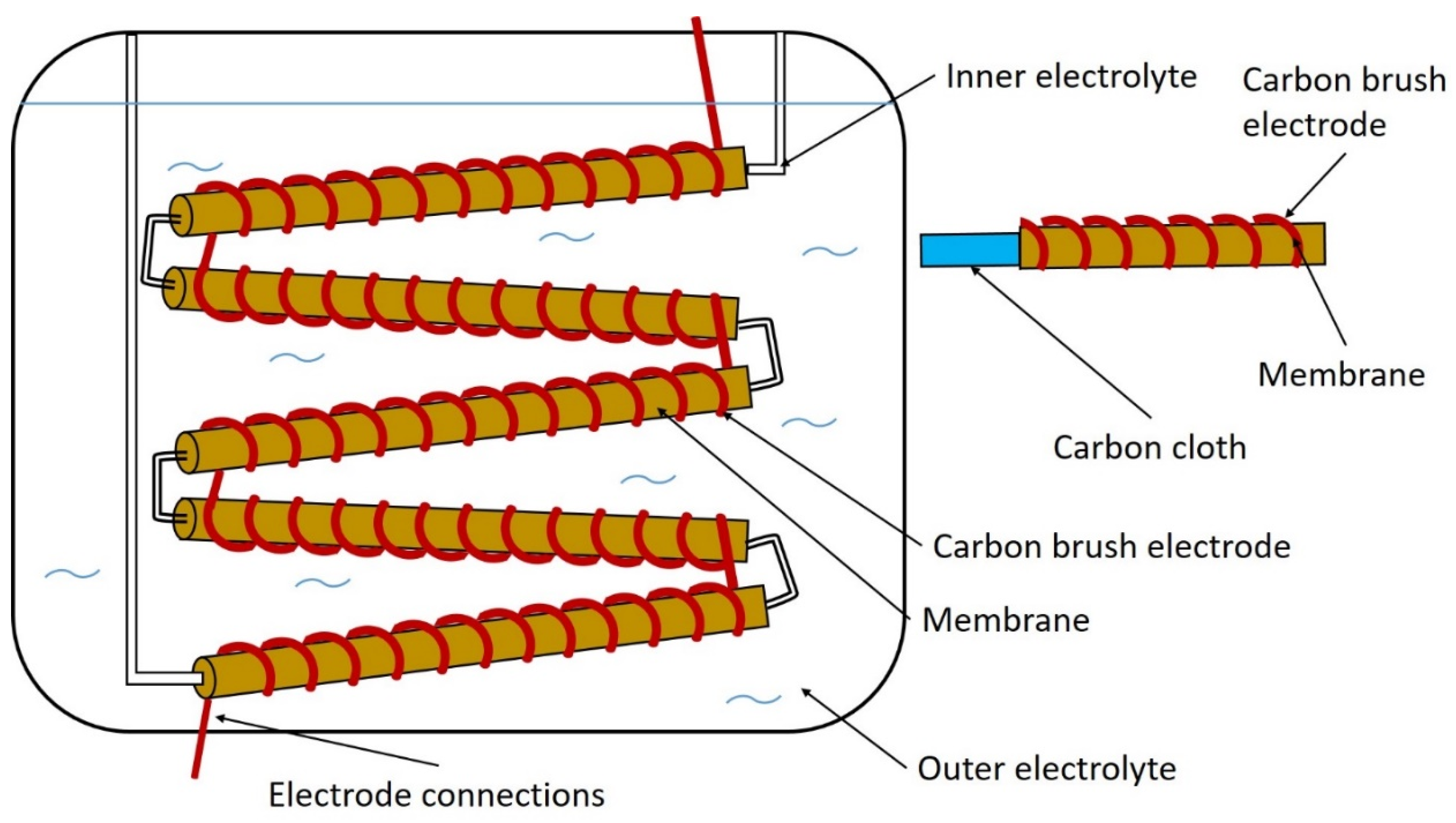
7. The Components of the BES Systems
7.1. Membranes
7.1.1. Proton Exchange Membranes (PEMs)
- Perfluorinated
- Partially fluorinated
- Non-fluorinated
- Acid-base blend
- Others
7.1.2. Ion Exchange Membranes (IEMs)
- High permselectivity
- Low electrical resistance
- Good mechanical plasticity
- High chemical stability
- Easy and cheap production
- Cation exchange membranes (CEMs)
- Anion exchange membranes (AEMs)
- Bipolar membranes (BPMs) and other composite membranes
- fast chemical kinetics at the interface
- high conductivity of the individual bulk layers
- high water permeability
- low parasitic (ion) crossover
- long lifetime under operational current densities
- distinct hydrated ionic radii
- different migration rate within the membrane
- the affinity of the ions to the membrane
7.2. Electrodes
7.2.1. Carbon-Based Electrodes
7.2.2. Metal-Based Electrodes
7.2.3. Composite Electrodes and Surface Modifications
8. BES Operational Parameters
8.1. Modified Gompertz Model
8.2. Coulombic Efficiency
8.3. Current Density
8.4. Methane Production Rate
8.5. CO2 Conversion Rate
8.6. Other Indicative Parameters
9. Microbial Background
10. Conclusions
- In this review, we compiled a cross-section of the ongoing research on bioelectrochemical systems (BES), emphasising electrochemical biomethane formation. In this endeavor, the first observation has been the large number and exponential growth of relevant scientific publications. This is not surprising in light of the recommenced interest towards renewable energy research and development.
- We note that the various BES systems developed in numerous laboratories worldwide comprise a very distinct and diverse collection of the infrastructure, i.e., reaction vessels and parts thereof. This reflects the inventive approaches of the scientists working in the field, and the pioneering efforts should be welcomed by the scientific community. This can also be rationalized when a multitude of reactor designs, electrodes, and membranes are selected to perform optimally in specific applications. Unfortunately, the almost chaotic infrastructural assortments make comparing the various BES systems difficult. Therefore, it is advised to specify a few “general or basic BES reactor systems” to be included in the related studies as built controls to compare to the new or novel system designs.
- This kind of standardization may help the development of BES systems beyond the curiosity-driven laboratory scale studies towards industrial applications, which is now hindered by the variety of diverse laboratory studies using several reactor designs and components’ selection.
- A consensus is needed regarding the indicator parameters in evaluating the various BES performances.
- An equally important aspect is the need to consider that all BES systems employ biological components, i.e., pure strains of specific microbes or mixed microbial communities. These microbes make fundamental contributions to the job accomplished and thus have a great share in the success of the BES electrobiomethanization systems. The complexity of the physiology and biochemistry of these microbial participants significantly alters the success of the electrochemical process. The associated tasks to optimize electrochemistry with microbial fermentation/conversion are largely beyond the scope of this review. Only a short sketch of this viewpoint is outlined here. The amalgamation of the electrochemistry and biotechnology issues will be the subject of an upcoming report and much-related research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritchie, H.; Roser, M.; Rosado, P. Energy—Our World in Data. Available online: https://ourworldindata.org/energy (accessed on 9 September 2020).
- Palanisamy, G.; Jung, H.Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.H. A Comprehensive Review on Microbial Fuel Cell Technologies: Processes, Utilization, and Advanced Developments in Electrodes and Membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Wang, J.; Ren, K.; Zhu, Y.; Huang, J.; Liu, S. A Review of Recent Advances in Microbial Fuel Cells: Preparation, Operation, and Application. BioTech 2022, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Lu, X.; Cai, T.; Niu, C.; Han, Y.; Zhang, Z.; Zhu, X.; Zhen, G. Magnetite-Enhanced Bioelectrochemical Stimulation for Biodegradation and Biomethane Production of Waste Activated Sludge. Sci. Total Environ. 2021, 789, 82–90. [Google Scholar] [CrossRef]
- Roy, M.; Aryal, N.; Zhang, Y.; Patil, S.A.; Pant, D. Technological Progress and Readiness Level of Microbial Electrosynthesis and Electrofermentation for Carbon Dioxide and Organic Wastes Valorization. Curr. Opin. Green Sustain. Chem. 2022, 35, 100605. [Google Scholar] [CrossRef]
- Naderi, A.; Kakavandi, B.; Giannakis, S.; Angelidaki, I.; Rezaei Kalantary, R. Putting the Electro-Bugs to Work: A Systematic Review of 22 Years of Advances in Bio-Electrochemical Systems and the Parameters Governing Their Performance. Environ. Res. 2023, 229, 115843. [Google Scholar] [CrossRef] [PubMed]
- Blasco-Gómez, R.; Batlle-Vilanova, P.; Villano, M.; Balaguer, M.D.; Colprim, J.; Puig, S. On the Edge of Research and Technological Application: A Critical Review of Electromethanogenesis. Int. J. Mol. Sci. 2017, 18, 874. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, Y.; Araki, K.; Tanaka, T.; Watanabe, T.; Kuroda, M. Denitrification and Meutralization with an Electrochemical and Biological Reactor. Water Sci. Technol. 1994, 30, 151–155. [Google Scholar] [CrossRef]
- Kuroda, M.; Watanabe, T. CO2 Reduction to Methane and Acetate Using a Bio-Electro Reactor with Immobilized Methanogens and Homoacetogens on Electrodes. Energy Convers. Manag. 1995, 36, 787–790. [Google Scholar] [CrossRef]
- Call, D.F.; Merrill, M.D.; Logan, B.E. High Surface Area Stainless Steel Brushes as Cathodes in Microbial Electrolysis Cells. Environ. Sci. Technol. 2009, 43, 2179–2183. [Google Scholar] [CrossRef]
- Parameswaran, P.; Torres, C.I.; Lee, H.S.; Krajmalnik-Brown, R.; Rittmann, B.E. Syntrophic Interactions among Anode Respiring Bacteria (ARB) and Non-ARB in a Biofilm Anode: Electron Balances. Biotechnol. Bioeng. 2009, 103, 513–523. [Google Scholar] [CrossRef]
- Selembo, P.A.; Merrill, M.D.; Logan, B.E. The Use of Stainless Steel and Nickel Alloys as Low-Cost Cathodes in Microbial Electrolysis Cells. J. Power Sources 2009, 190, 271–278. [Google Scholar] [CrossRef]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically Assisted Microbial Production of Hydrogen from Acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef] [PubMed]
- Call, D.; Logan, B.E. Hydrogen Production in a Single Chamber Microbial Electrolysis Cell Lacking a Membrane. Environ. Sci. Technol. 2008, 42, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, L.; Chen, Y.; Zhu, S.; Shen, S. High Yield Hydrogen Production in a Single-Chamber Membrane-Less Microbial Electrolysis Cell. Water Sci. Technol. 2010, 61, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Summers, Z.M.; Fogarty, H.E.; Leang, C.; Franks, A.E.; Malvankar, N.S.; Lovley, D.R. Direct Exchange of Electrons within Aggregates of an Evolved Syntrophic Coculture of Anaerobic Bacteria. Science 2010, 330, 1413–1415. [Google Scholar] [CrossRef]
- Hara, M.; Onaka, Y.; Kobayashi, H.; Fu, Q.; Kawaguchi, H.; Vilcaez, J.; Sato, K. Mechanism of Electromethanogenic Reduction of CO2 by a Thermophilic Methanogen. Energy Procedia 2013, 37, 7021–7028. [Google Scholar] [CrossRef]
- Siegert, M.; Yates, M.D.; Call, D.F.; Zhu, X.; Spormann, A.; Logan, B.E. Comparison of Nonprecious Metal Cathode Materials for Methane Production by Electromethanogenesis. ACS Sustain. Chem. Eng. 2014, 2, 910–917. [Google Scholar] [CrossRef]
- Jiang, Y.; Su, M.; Li, D. Removal of Sulfide and Production of Methane from Carbon Dioxide in Microbial Fuel Cells-Microbial Electrolysis Cell (MFCs-MEC) Coupled System. Appl. Biochem. Biotechnol. 2014, 172, 2720–2731. [Google Scholar] [CrossRef]
- Jiang, Y.; Su, M.; Zhang, Y.; Zhan, G.; Tao, Y.; Li, D. Bioelectrochemical Systems for Simultaneously Production of Methane and Acetate from Carbon Dioxide at Relatively High Rate. Int. J. Hydrogen Energy 2013, 38, 3497–3502. [Google Scholar] [CrossRef]
- Liu, W.; He, Z.; Yang, C.; Zhou, A.; Guo, Z.; Liang, B.; Varrone, C.; Wang, A.J. Microbial Network for Waste Activated Sludge Cascade Utilization in an Integrated System of Microbial Electrolysis and Anaerobic Fermentation. Biotechnol. Biofuels 2016, 9, 83. [Google Scholar] [CrossRef]
- Ma, X.; Li, Z.; Zhou, A.; Yue, X. Energy Recovery from Tubular Microbial Electrolysis Cell with Stainless Steel Mesh as Cathode. R. Soc. Open Sci. 2017, 4, 170967. [Google Scholar] [CrossRef]
- Shen, R.X.; Lu, J.W.; Zhu, Z.B.; Duan, N.; Lu, H.F.; Zhang, Y.H.; Liu, Z.D. Effects of Organic Strength on Performance of Microbial Electrolysis Cell Fed with Hydrothermal Liquefied Wastewater. Int. J. Agric. Biol. Eng. 2017, 10, 206–217. [Google Scholar] [CrossRef]
- Marshall, C.W.; Ross, D.E.; Fichot, E.B.; Norman, R.S.; May, H.D. Electrosynthesis of Commodity Chemicals by an Autotrophic Microbial Community. Appl. Environ. Microbiol. 2012, 78, 8412–8420. [Google Scholar] [CrossRef] [PubMed]
- Enzmann, F.; Holtmann, D. Rational Scale-Up of a Methane Producing Bioelectrochemical Reactor to 50 L Pilot Scale. Chem. Eng. Sci. 2019, 207, 1148–1158. [Google Scholar] [CrossRef]
- Villano, M.; Aulenta, F.; Ciucci, C.; Ferri, T.; Giuliano, A.; Majone, M. Bioelectrochemical Reduction of CO2 to CH4 via Direct and Indirect Extracellular Electron Transfer by a Hydrogenophilic Methanogenic Culture. Bioresour. Technol. 2010, 101, 3085–3090. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, Y.; Fu, Q.; Kobayashi, H.; Ikarashi, M.; Wakayama, T.; Kawaguchi, H.; Vilcaez, J.; Maeda, H.; Sato, K. Electromethanogenic CO2 Conversion by Subsurface-Reservoir Microorganisms. Energy Procedia 2013, 37, 7014–7020. [Google Scholar] [CrossRef]
- Geppert, F.; Liu, D.; van Eerten-Jansen, M.; Weidner, E.; Buisman, C.; ter Heijne, A. Bioelectrochemical Power-to-Gas: State of the Art and Future Perspectives. Trends Biotechnol. 2016, 34, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kim, S.; Kwon, O.S. Effect of Applied Voltage and Temperature on Methane Production and Microbial Community in Microbial Electrochemical Anaerobic Digestion Systems Treating Swine Manure. J. Ind. Microbiol. Biotechnol. 2019, 46, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Escalera, A.; Molognoni, D.; Bosch-Jimenez, P.; Shahparasti, M.; Bouchakour, S.; Luna, A.; Guisasola, A.; Borràs, E.; Della Pirriera, M. Bioelectrochemical Systems for Energy Storage: A Scaled-up Power-to-Gas Approach. Appl. Energy 2020, 260, 114138. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Pugazhendhi, A.; Zhen, G.; Kumar, G.; Kadier, A.; Sivagurunathan, P. Microbiome Involved in Microbial Electrochemical Systems (MESs): A Review. Chemosphere 2017, 177, 176–188. [Google Scholar] [CrossRef]
- Lee, M.; Nagendranatha Reddy, C.; Min, B. In Situ Integration of Microbial Electrochemical Systems into Anaerobic Digestion to Improve Methane Fermentation at Different Substrate Concentrations. Int. J. Hydrogen Energy 2019, 44, 2380–2389. [Google Scholar] [CrossRef]
- van Eerten-Jansen, M.C.A.A.; Jansen, N.C.; Plugge, C.M.; de Wilde, V.; Buisman, C.J.N.; ter Heijne, A. Analysis of the Mechanisms of Bioelectrochemical Methane Production by Mixed Cultures. J. Chem. Technol. Biotechnol. 2015, 90, 963–970. [Google Scholar] [CrossRef]
- Angelidaki, I.; Xie, L.; Luo, G.; Zhang, Y.; Oechsner, H.; Lemmer, A.; Munoz, R.; Kougias, P.G. Biogas Upgrading: Current and Emerging Technologies, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128168561. [Google Scholar]
- Batlle-Vilanova, P.; Puig, S.; Gonzalez-Olmos, R.; Vilajeliu-Pons, A.; Balaguer, M.D.; Colprim, J. Deciphering the Electron Transfer Mechanisms for Biogas Upgrading to Biomethane within a Mixed Culture Biocathode. RSC Adv. 2015, 5, 52243–52251. [Google Scholar] [CrossRef]
- Noori, M.T.; Min, B. Fundamentals and Recent Progress in Bioelectrochemical System-Assisted Biohythane Production. Bioresour. Technol. 2022, 361, 127641. [Google Scholar] [CrossRef]
- Luo, S.; Jain, A.; Aguilera, A.; He, Z. Effective Control of Biohythane Composition through Operational Strategies in an Innovative Microbial Electrolysis Cell. Appl. Energy 2017, 206, 879–886. [Google Scholar] [CrossRef]
- Dubé, C.D.; Guiot, S.R. Direct Interspecies Electron Transfer in Anaerobic Digestion: A Review. In Biogas Science and Technology; Springer International Publishing: Cham, Switzerland, 2015; pp. 101–115. ISBN 9783319219936. [Google Scholar]
- Sydow, A.; Krieg, T.; Mayer, F.; Schrader, J.; Holtmann, D. Electroactive Bacteria—Molecular Mechanisms and Genetic Tools. Appl. Microbiol. Biotechnol. 2014, 98, 8481–8495. [Google Scholar] [CrossRef]
- Holmes, D.E.; Zhou, J.; Smith, J.A.; Wang, C.; Liu, X.; Lovley, D.R.; Yang, Y. Different Outer Membrane c-type Cytochromes Are Involved in Direct Interspecies Electron Transfer to Geobacter or Methanosarcina Species. mLife 2022, 1, 272–286. [Google Scholar] [CrossRef]
- Cheng, S.; Xing, D.; Call, D.F.; Logan, B.E. Direct Biological Conversion of Electrical Current into Methane by Electromethanogenesis. Environ. Sci. Technol. 2009, 43, 3953–3958. [Google Scholar] [CrossRef]
- Cerrillo, M.; Burgos, L.; Bonmatí, A. Biogas Upgrading and Ammonia Recovery from Livestock Manure Digestates in a Combined Electromethanogenic Biocathode—Hydrophobic Membrane System. Energies 2021, 14, 503. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive Microorganisms in Bioelectrochemical Systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Guang, L.; Koomson, D.A.; Jingyu, H.; Ewusi-Mensah, D.; Miwornunyuie, N. Performance of Exoelectrogenic Bacteria Used in Microbial Desalination Cell Technology. Int. J. Environ. Res. Public Health 2020, 17, 1121. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.Y.; Ho, G.; Cord-Ruwisch, R. Novel Methanogenic Rotatable Bioelectrochemical System Operated with Polarity Inversion. Environ. Sci. Technol. 2011, 45, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Villano, M.; Scardala, S.; Aulenta, F.; Majone, M. Carbon and Nitrogen Removal and Enhanced Methane Production in a Microbial Electrolysis Cell. Bioresour. Technol. 2013, 130, 366–371. [Google Scholar] [CrossRef]
- Park, S.G.; Rhee, C.; Shin, S.G.; Shin, J.; Mohamed, H.O.; Choi, Y.J.; Chae, K.J. Methanogenesis Stimulation and Inhibition for the Production of Different Target Electrobiofuels in Microbial Electrolysis Cells through an On-Demand Control Strategy Using the Coenzyme M and 2-Bromoethanesulfonate. Environ. Int. 2019, 131, 105006. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Y.; Zheng, S.; Zhen, G.; Lu, X.; Takuro, K.; Xu, K.; Bakonyi, P. Electro-Conversion of Carbon Dioxide (CO2) to Low-Carbon Methane by Bioelectromethanogenesis Process in Microbial Electrolysis Cells: The Current Status and Future Perspective. Bioresour. Technol. 2019, 279, 339–349. [Google Scholar] [CrossRef]
- Giddings, C.G.S.; Nevin, K.P.; Woodward, T.; Lovley, D.R.; Butler, C.S. Simplifying Microbial Electrosynthesis Reactor Design. Front. Microbiol. 2015, 6, 468. [Google Scholar] [CrossRef]
- Cai, W.; Cui, K.; Liu, Z.; Jin, X.; Chen, Q.; Guo, K.; Wang, Y. An Electrolytic-Hydrogen-Fed Moving Bed Biofilm Reactor for Efficient Microbial Electrosynthesis of Methane from CO2. Chem. Eng. J. 2022, 428, 132093. [Google Scholar] [CrossRef]
- Jourdin, L.; Freguia, S.; Flexer, V.; Keller, J. Bringing High-Rate, CO2-Based Microbial Electrosynthesis Closer to Practical Implementation through Improved Electrode Design and Operating Conditions. Environ. Sci. Technol. 2016, 50, 1982–1989. [Google Scholar] [CrossRef]
- Li, X.; Liu, G.; He, Z. Flexible Control of Biohythane Composition and Production by Dual Cathodes in a Bioelectrochemical System. Bioresour. Technol. 2020, 295, 122270. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Aulenta, F.; Villano, M.; Angenent, L.T. Cathodes as Electron Donors for Microbial Metabolism: Which Extracellular Electron Transfer Mechanisms Are Involved? Bioresour. Technol. 2011, 102, 324–333. [Google Scholar] [CrossRef]
- Enzmann, F.; Mayer, F.; Stöckl, M.; Mangold, K.M.; Hommel, R.; Holtmann, D. Transferring Bioelectrochemical Processes from H-Cells to a Scalable Bubble Column Reactor. Chem. Eng. Sci. 2019, 193, 133–143. [Google Scholar] [CrossRef]
- Hassanein, A.; Witarsa, F.; Lansing, S.; Qiu, L.; Liang, Y. Bio-Electrochemical Enhancement of Hydrogen and Methane Production in a Combined Anaerobic Digester (AD) and Microbial Electrolysis Cell (MEC) from Dairy Manure. Sustainability 2020, 12, 8491. [Google Scholar] [CrossRef]
- Amrut Pawar, A.; Karthic, A.; Lee, S.; Pandit, S.; Jung, S.P. Microbial Electrolysis Cells for Electromethanogenesis: Materials, Configurations and Operations. Environ. Eng. Res. 2020, 27, 200484. [Google Scholar] [CrossRef]
- Geppert, F.; Liu, D.; Weidner, E.; ter Heijne, A. Redox-Flow Battery Design for a Methane-Producing Bioelectrochemical System. Int. J. Hydrogen Energy 2019, 44, 21464–21469. [Google Scholar] [CrossRef]
- Tanaka, K.; Yokoe, S.; Igarashi, K.; Takashino, M.; Ishikawa, M.; Hori, K.; Nakanishi, S.; Kato, S. Extracellular Electron Transfer via Outer Membrane Cytochromes in a Methanotrophic Bacterium Methylococcus capsulatus (Bath). Front. Microbiol. 2018, 9, 2905. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Vu, M.T.; Abu Hasan Johir, M.; Pernice, M.; Ngo, H.H.; Zdarta, J.; Jesionowski, T.; Nghiem, L.D. Promotion of Direct Interspecies Electron Transfer and Potential Impact of Conductive Materials in Anaerobic Digestion and Its Downstream Processing—A Critical Review. Bioresour. Technol. 2021, 341, 125847. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and Potential of Direct Interspecies Electron Transfer in Anaerobic Digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef]
- Umar, M.F.; Abbas, S.Z.; Mohamad Ibrahim, M.N.; Ismail, N.; Rafatullah, M. Insights into Advancements and Electrons Transfer Mechanisms of Electrogens in Benthic Microbial Fuel Cells. Membranes 2020, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Paquete, C.M.; Rosenbaum, M.A.; Bañeras, L.; Rotaru, A.E.; Puig, S. Let’s Chat: Communication between Electroactive Microorganisms. Bioresour. Technol. 2022, 347, 126705. [Google Scholar] [CrossRef]
- Thapa, B.S.; Pandit, S.; Patwardhan, S.B.; Tripathi, S.; Mathuriya, A.S.; Gupta, P.K.; Lal, R.B.; Tusher, T.R. Application of Microbial Fuel Cell (MFC) for Pharmaceutical Wastewater Treatment: An Overview and Future Perspectives. Sustainability 2022, 14, 8379. [Google Scholar] [CrossRef]
- Aryal, N.; Ammam, F.; Patil, S.A.; Pant, D. An Overview of Cathode Materials for Microbial Electrosynthesis of Chemicals from Carbon Dioxide. Green Chem. 2017, 19, 5748–5760. [Google Scholar] [CrossRef]
- Paritosh, K.; Yadav, M.; Chawade, A.; Sahoo, D.; Kesharwani, N.; Pareek, N.; Vivekanand, V. Additives as a Support Structure for Specific Biochemical Activity Boosts in Anaerobic Digestion: A Review. Front. Energy Res. 2020, 8, 88. [Google Scholar] [CrossRef]
- Electrical Effects Accompanying the Decomposition of Organic Compounds. Proc. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character 1911, 84, 260–276. [CrossRef]
- Daniels, L.; Belay, N.; Rajagopal, B.S.; Weimer, P.J. Bacterial Methanogenesis and Growth from CO2 with Elemental Iron as the Sole Source of Electrons. Science 1987, 237, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A New Model for Electron Flow during Anaerobic Digestion: Direct Interspecies Electron Transfer to Methanosaeta for the Reduction of Carbon Dioxide to Methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Shirkosh, M.; Hojjat, Y.; Mardanpour, M.M. Boosting Microfluidic Microbial Fuel Cells Performance via Investigating Electron Transfer Mechanisms, Metal-Based Electrodes, and Magnetic Field Effect. Sci. Rep. 2022, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; ul Haq, I.; Qaisar, K.; Gunes, B.; Raja, S.I.; Mohyuddin, K.; Amin, H. Microbial Fuel Cells: Insight into Simultaneous Wastewater Treatment and Bioelectricity Generation. Process Saf. Environ. Prot. 2022, 161, 357–373. [Google Scholar] [CrossRef]
- Baby, M.G.; Ahammed, M.M. Nutrient Removal and Recovery from Wastewater by Microbial Fuel Cell-Based Systems—A Review. Water Sci. Technol. 2022, 86, 29–55. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Islam, N.; Parisa, T.A.; Rafa, N.; Bokhari, A.; Klemeš, J.J.; Indra Mahlia, T.M. Insights into the Development of Microbial Fuel Cells for Generating Biohydrogen, Bioelectricity, and Treating Wastewater. Energy 2022, 254, 124163. [Google Scholar] [CrossRef]
- Van Eerten-Jansen, M.C.A.A.; Ter Heijne, A.; Buisman, C.J.N.; Hamelers, H.V.M. Microbial Electrolysis Cells for Production of Methane from CO2: Long-Term Performance and Perspectives. Int. J. Energy Res. 2012, 36, 809–819. [Google Scholar] [CrossRef]
- Rabaey, K.; Rozendal, R.A. Microbial Electrosynthesis—Revisiting the Electrical Route for Microbial Production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Schlager, S.; Haberbauer, M.; Fuchsbauer, A.; Hemmelmair, C.; Dumitru, L.M.; Hinterberger, G.; Neugebauer, H.; Sariciftci, N.S. Bio-Electrocatalytic Application of Microorganisms for Carbon Dioxide Reduction to Methane. ChemSusChem 2017, 10, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, R.; Ketep, S.F.; Etcheverry, L.; Délia, M.L.; Bergel, A. Microbial Electrolysis Cell (MEC): A Step Ahead towards Hydrogen-Evolving Cathode Operated at High Current Density. Bioresour. Technol. Rep. 2020, 9, 100399. [Google Scholar] [CrossRef]
- Yin, Q.; Zhu, X.; Zhan, G.; Bo, T.; Yang, Y.; Tao, Y.; He, X.; Li, D.; Yan, Z. Enhanced Methane Production in an Anaerobic Digestion and Microbial Electrolysis Cell Coupled System with Co-Cultivation of Geobacter and Methanosarcina. J. Environ. Sci. 2016, 42, 210–214. [Google Scholar] [CrossRef]
- Zhen, G.; Kobayashi, T.; Lu, X.; Xu, K. Understanding Methane Bioelectrosynthesis from Carbon Dioxide in a Two-Chamber Microbial Electrolysis Cells (MECs) Containing a Carbon Biocathode. Bioresour. Technol. 2015, 186, 141–148. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kobayashi, T.; Kumar, G.; Xu, K. Promoted Electromethanosynthesis in a Two-Chamber Microbial Electrolysis Cells (MECs) Containing a Hybrid Biocathode Covered with Graphite Felt (GF). Chem. Eng. J. 2016, 284, 1146–1155. [Google Scholar] [CrossRef]
- Fu, Q.; Kuramochi, Y.; Fukushima, N.; Maeda, H.; Sato, K.; Kobayashi, H. Bioelectrochemical Analyses of the Development of a Thermophilic Biocathode Catalyzing Electromethanogenesis. Environ. Sci. Technol. 2015, 49, 1225–1232. [Google Scholar] [CrossRef]
- Liu, S.Y.; Charles, W.; Ho, G.; Cord-Ruwisch, R.; Cheng, K.Y. Bioelectrochemical Enhancement of Anaerobic Digestion: Comparing Single- and Two-Chamber Reactor Configurations at Thermophilic Conditions. Bioresour. Technol. 2017, 245, 1168–1175. [Google Scholar] [CrossRef]
- Liu, Q.; Ren, Z.J.; Huang, C.; Liu, B.; Ren, N.; Xing, D. Multiple Syntrophic Interactions Drive Biohythane Production from Waste Sludge in Microbial Electrolysis Cells. Biotechnol. Biofuels 2016, 9, 162. [Google Scholar] [CrossRef]
- Dou, Z.; Dykstra, C.M.; Pavlostathis, S.G. Bioelectrochemically Assisted Anaerobic Digestion System for Biogas Upgrading and Enhanced Methane Production. Sci. Total Environ. 2018, 633, 1012–1021. [Google Scholar] [CrossRef]
- Giang, H.; Zhang, J.; Zhu, Z.; Suni, I.I.; Liang, Y. Single-Chamber Microbial Electrochemical Cell for CH4 Production from CO2 Utilizing a Microbial Consortium. Int. J. Energy Res. 2018, 42, 1308–1315. [Google Scholar] [CrossRef]
- Park, J.; Lee, B.; Tian, D.; Jun, H. Bioelectrochemical Enhancement of Methane Production from Highly Concentrated Food Waste in a Combined Anaerobic Digester and Microbial Electrolysis Cell. Bioresour. Technol. 2018, 247, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Tartakovsky, B.; Mehta, P.; Bourque, J.S.; Guiot, S.R. Electrolysis-Enhanced Anaerobic Digestion of Wastewater. Bioresour. Technol. 2011, 102, 5685–5691. [Google Scholar] [CrossRef]
- Bo, T.; Zhu, X.; Zhang, L.; Tao, Y.; He, X.; Li, D.; Yan, Z. A New Upgraded Biogas Production Process: Coupling Microbial Electrolysis Cell and Anaerobic Digestion in Single-Chamber, Barrel-Shape Stainless Steel Reactor. Electrochem. Commun. 2014, 45, 67–70. [Google Scholar] [CrossRef]
- Cusick, R.D.; Bryan, B.; Parker, D.S.; Merrill, M.D.; Mehanna, M.; Kiely, P.D.; Liu, G.; Logan, B.E. Performance of a Pilot-Scale Continuous Flow Microbial Electrolysis Cell Fed Winery Wastewater. Appl. Microbiol. Biotechnol. 2011, 89, 2053–2063. [Google Scholar] [CrossRef]
- Van Eerten-Jansen, M.C.A.A.; Veldhoen, A.B.; Plugge, C.M.; Stams, A.J.M.; Buisman, C.J.N.; Ter Heijne, A. Microbial Community Analysis of a Methane-Producing Biocathode in a Bioelectrochemical System. Archaea 2013, 2013, 481784. [Google Scholar] [CrossRef] [PubMed]
- Zeppilli, M.; Simoni, M.; Paiano, P.; Majone, M. Two-Side Cathode Microbial Electrolysis Cell for Nutrients Recovery and Biogas Upgrading. Chem. Eng. J. 2019, 370, 466–476. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, X.; Gu, Y.; Chen, H.; Sun, D.; Li, P.; Li, M.; Dang, Y.; Smith, J.A.; Holmes, D.E. Enhancement of Bioelectrochemical CO2 Reduction with a Carbon Brush Electrode via Direct Electron Transfer. ACS Sustain. Chem. Eng. 2020, 8, 11368–11375. [Google Scholar] [CrossRef]
- Liu, C.; Sun, D.; Zhao, Z.; Dang, Y.; Holmes, D.E. Methanothrix Enhances Biogas Upgrading in Microbial Electrolysis Cell via Direct Electron Transfer. Bioresour. Technol. 2019, 291, 121877. [Google Scholar] [CrossRef]
- Fu, X.Z.; Li, J.; Pan, X.R.; Huang, L.; Li, C.X.; Cui, S.; Liu, H.Q.; Tan, Z.L.; Li, W.W. A Single Microbial Electrochemical System for CO2 Reduction and Simultaneous Biogas Purification, Upgrading and Sulfur Recovery. Bioresour. Technol. 2020, 297, 122448. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, F.; Liu, J.; Zhang, X.; Huang, X.; Logan, B.E. Methane Production in Microbial Reverse-Electrodialysis Methanogenesis Cells (MRMCs) Using Thermolytic Solutions. Environ. Sci. Technol. 2014, 48, 8911–8918. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Li, Z.; Fu, Q.; Li, Y.; Li, J.; Zhang, L.; Liao, Q.; Zhu, X. Hybrid Microbial Photoelectrochemical System Reduces CO2 to CH4 with 1.28% Solar Energy Conversion Efficiency. Chem. Eng. J. 2020, 390, 124530. [Google Scholar] [CrossRef]
- Martín, A.J.; Larrazábal, G.O.; Pérez-Ramírez, J. Towards Sustainable Fuels and Chemicals through the Electrochemical Reduction of CO2: Lessons from Water Electrolysis. Green Chem. 2015, 17, 5114–5130. [Google Scholar] [CrossRef]
- Clauwaert, P.; Tolêdo, R.; van der Ha, D.; Crab, R.; Verstraete, W.; Hu, H.; Udert, K.M.; Rabaey, K. Combining Biocatalyzed Electrolysis with Anaerobic Digestion. Water Sci. Technol. 2008, 57, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Villano, M.; Monaco, G.; Aulenta, F.; Majone, M. Electrochemically Assisted Methane Production in a Biofilm Reactor. J. Power Sources 2011, 196, 9467–9472. [Google Scholar] [CrossRef]
- Call, D.F.; Logan, B.E. A Method for High Throughput Bioelectrochemical Research Based on Small Scale Microbial Electrolysis Cells. Biosens. Bioelectron. 2011, 26, 4526–4531. [Google Scholar] [CrossRef]
- Batlle-Vilanova, P.; Rovira-Alsina, L.; Puig, S.; Balaguer, M.D.; Icaran, P.; Monsalvo, V.M.; Rogalla, F.; Colprim, J. Biogas Upgrading, CO2 Valorisation and Economic Revaluation of Bioelectrochemical Systems through Anodic Chlorine Production in the Framework of Wastewater Treatment Plants. Sci. Total Environ. 2019, 690, 352–360. [Google Scholar] [CrossRef]
- Zeppilli, M.; Mattia, A.; Villano, M.; Majone, M. Three-Chamber Bioelectrochemical System for Biogas Upgrading and Nutrient Recovery. Fuel Cells 2017, 17, 593–600. [Google Scholar] [CrossRef]
- Clauwaert, P.; Verstraete, W. Methanogenesis in Membraneless Microbial Electrolysis Cells. Appl. Microbiol. Biotechnol. 2009, 82, 829–836. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, R.; Luo, H.; Liu, G.; Kim, Y.; Yu, S.; Zeng, J. Microbial Electrolysis Cell with Spiral Wound Electrode for Wastewater Treatment and Methane Production. Process Biochem. 2015, 50, 1103–1109. [Google Scholar] [CrossRef]
- Ditzig, J.; Liu, H.; Logan, B.E. Production of Hydrogen from Domestic Wastewater Using a Bioelectrochemically Assisted Microbial Reactor (BEAMR). Int. J. Hydrogen Energy 2007, 32, 2296–2304. [Google Scholar] [CrossRef]
- Guo, K.; Tang, X.; Du, Z.; Li, H. Hydrogen Production from Acetate in a Cathode-on-Top Single-Chamber Microbial Electrolysis Cell with a Mipor Cathode. Biochem. Eng. J. 2010, 51, 48–52. [Google Scholar] [CrossRef]
- Butler, C.S.; Lovley, D.R. How to Sustainably Feed a Microbe: Strategies for Biological Production of Carbon-Based Commodities with Renewable Electricity. Front. Microbiol. 2016, 7, 1879. [Google Scholar] [CrossRef]
- Allen, M.J. Symposium on Bioelectrochemistry of Microorganisms. II. Electrochemical Aspects of Metabolism. Bacteriol. Rev. 1966, 30, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Hongo, M.; Iwahara, M. Application of Electro-Energizing Method to L-Glutamic Acid Fermentation. Agric. Biol. Chem. 1979, 43, 2075–2081. [Google Scholar] [CrossRef]
- Nevin, K.P.; Woodard, T.L.; Franks, A.E.; Summers, Z.M.; Lovley, D.R. Microbial Electrosynthesis: Feeding Microbes Electricity To Convert Carbon Dioxide and Water to Multicarbon Extracellular Organic Compounds. MBio 2010, 1, e00103-10. [Google Scholar] [CrossRef]
- Zhou, H.; Xing, D.; Xu, M.; Su, Y.; Ma, J.; Angelidaki, I.; Zhang, Y. Optimization of a Newly Developed Electromethanogenesis for the Highest Record of Methane Production. J. Hazard. Mater. 2020, 407, 124363. [Google Scholar] [CrossRef]
- Krieg, T.; Madjarov, J.; Rosa, L.F.M.; Enzmann, F.; Harnisch, F.; Holtmann, D.; Rabaey, K. Reactors for Microbial Electrobiotechnology. Adv. Biochem. Eng. Biotechnol. 2019, 167, 231–271. [Google Scholar] [CrossRef]
- Kokkoli, A.; Zhang, Y.; Angelidaki, I. Microbial Electrochemical Separation of CO2 for Biogas Upgrading. Bioresour. Technol. 2018, 247, 380–386. [Google Scholar] [CrossRef]
- Aryal, N.; Zhang, Y.; Bajracharya, S.; Pant, D.; Chen, X. Microbial Electrochemical Approaches of Carbon Dioxide Utilization for Biogas Upgrading. Chemosphere 2022, 291, 132843. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhang, H.; Xu, X.; Teng, J. Integrating Microbial Electrolysis Cell Based on Electrochemical Carbon Dioxide Reduction into Anaerobic Osmosis Membrane Reactor for Biogas Upgrading. Water Res. 2021, 190, 116679. [Google Scholar] [CrossRef] [PubMed]
- Xu, T. Ion Exchange Membranes: State of Their Development and Perspective. J. Memb. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Frilette, V.J. Preparation and characterization of bipolar ion-exchange membranes. J. Phys. Chem. 1955, 60, 435–439. [Google Scholar] [CrossRef]
- Parekh, A. Recent Developments of Proton Exchange Membranes for PEMFC: A Review. Front. Energy Res. 2022, 10, 956132. [Google Scholar] [CrossRef]
- Ogungbemi, E.; Ijaodola, O.; Khatib, F.N.; Wilberforce, T.; El Hassan, Z.; Thompson, J.; Ramadan, M.; Olabi, A.G. Fuel Cell Membranes—Pros and Cons. Energy 2019, 172, 155–172. [Google Scholar] [CrossRef]
- Zuo, Z.; Fu, Y.; Manthiram, A. Novel Blend Membranes Based on Acid-Base Interactions for Fuel Cells. Polymers 2012, 4, 1627–1644. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the Proton Exchange Membranes for Fuel Cell Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; Volume 35, ISBN 2177491223. [Google Scholar]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next Generation of Proton-Exchange Membrane Fuel Cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef]
- Atifi, A.; Mounir, H.; El Marjani, A. Effect of Internal Current, Fuel Crossover, and Membrane Thickness on a PEMFC Performance. In Proceedings of the 2014 International Renewable and Sustainable Energy Conference (IRSEC), Ouarzazate, Morocco, 17–19 October 2014; pp. 907–912. [Google Scholar] [CrossRef]
- Peterson, D.S. Encyclopedia of Microfluidics and Nanofluidics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–7. [Google Scholar] [CrossRef]
- Belafi-Bako, K.; Bakonyi, P. Integration of Membranes and Bioreactors. In Biotechnology and Bioengineering; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Strathmann, H.; Grabowski, A.; Eigenberger, G. Ion-Exchange Membranes in the Chemical Process Industry. Ind. Eng. Chem. Res. 2013, 52, 10364–10379. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion Exchange Membranes: New Developments and Applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Babiak, P.; Schaffer-Harris, G.; Kainuma, M.; Fedorovich, V.; Goryanin, I. Development of a New Hydrogel Anion Exchange Membrane for Swine Wastewater Treatment. Membranes 2022, 12, 984. [Google Scholar] [CrossRef]
- Takamuku, S.; Wohlfarth, A.; Manhart, A.; Räder, P.; Jannasch, P. Hypersulfonated Polyelectrolytes: Preparation, Stability and Conductivity. Polym. Chem. 2015, 6, 1267–1274. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, B.; Fan, Y.; Chen, Y. Mechanism Exploration of Ion Transport in Nanocomposite Cation Exchange Membranes. ACS Appl. Mater. Interfaces 2017, 9, 13491–13499. [Google Scholar] [CrossRef] [PubMed]
- Vengatesan, S.; Santhi, S.; Sozhan, G.; Ravichandran, S.; Davidson, D.J.; Vasudevan, S. Novel Cross-Linked Anion Exchange Membrane Based on Hexaminium Functionalized Poly(Vinylbenzyl Chloride). RSC Adv. 2015, 5, 27365–27371. [Google Scholar] [CrossRef]
- Das, G.; Choi, J.H.; Nguyen, P.K.T.; Kim, D.J.; Yoon, Y.S. Anion Exchange Membranes for Fuel Cell Application: A Review. Polymers 2022, 14, 1197. [Google Scholar] [CrossRef] [PubMed]
- Blommaert, M.A.; Aili, D.; Tufa, R.A.; Li, Q.; Smith, W.A.; Vermaas, D.A. Insights and Challenges for Applying Bipolar Membranes in Advanced Electrochemical Energy Systems. ACS Energy Lett. 2021, 6, 2539–2548. [Google Scholar] [CrossRef]
- Liu, L.; Wang, C.; He, Z.; Das, R.; Dong, B.; Xie, X.; Guo, Z. An Overview of Amphoteric Ion Exchange Membranes for Vanadium Redox Flow Batteries. J. Mater. Sci. Technol. 2021, 69, 212–227. [Google Scholar] [CrossRef]
- Guo, K.; Prévoteau, A.; Patil, S.A.; Rabaey, K. Engineering Electrodes for Microbial Electrocatalysis. Curr. Opin. Biotechnol. 2015, 33, 149–156. [Google Scholar] [CrossRef]
- Friess, K. Mosaic Membranes. In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2014; Volume 47, pp. 1–2. [Google Scholar]
- Besha, A.T.; Tsehaye, M.T.; Aili, D.; Zhang, W.; Tufa, R.A. Design of Monovalent Ion Selective Membranes for Reducing the Impacts of Multivalent Ions in Reverse Electrodialysis. Membranes 2020, 10, 7. [Google Scholar] [CrossRef]
- Das, P.; Banerjee, S.; Das, N.C. Polymer-Graphene Composite in Aerospace Engineering. In Polymer Nanocomposites Containing Graphene; Elsevier: Amsterdam, The Netherlands, 2022; Volume 13, pp. 683–711. ISBN 9780128216392. [Google Scholar]
- Mahmoud, R.H.; Gomaa, O.M.; Hassan, R.Y.A. Bio-Electrochemical Frameworks Governing Microbial Fuel Cell Performance: Technical Bottlenecks and Proposed Solutions. RSC Adv. 2022, 12, 5749–5764. [Google Scholar] [CrossRef]
- Pierson, H.O. Graphite Structure and Properties. Handb. Carbon Graph. Diam. Fuller. 1993, 43–69. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, J.; Li, H.; Chen, Q.; Sun, D.; Cheng, X.; Li, P.; Dang, Y.; Smith, J.A.; Holmes, D.E. High Efficiency In-Situ Biogas Upgrading in a Bioelectrochemical System with Low Energy Input. Water Res. 2021, 197, 117055. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Song, Y.C.; Ahn, Y. Electroactive Microorganisms in Bulk Solution Contribute Significantly to Methane Production in Bioelectrochemical Anaerobic Reactor. Bioresour. Technol. 2018, 259, 119–127. [Google Scholar] [CrossRef]
- Bharati, R.; Sundaramurthy, S.; Thakur, C. Nanomaterials and Food-Processing Wastewater; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128043004. [Google Scholar]
- Gomez-Gualdrón, D.A.; Burgos, J.C.; Yu, J.; Balbuena, P.B. Carbon Nanotubes: Engineering Biomedical Applications; Academic Press: Cambridge, MA, USA, 2011; Volume 104, ISBN 9780124160200. [Google Scholar]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific Surface Area of Carbon Nanotubes and Bundles of Carbon Nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Kang, S.; Mauter, M.S.; Elimelech, M. Physicochemical Determinants of Multiwalled Carbon Nanotube Bacterial Cytotoxicity. Environ. Sci. Technol. 2008, 42, 7528–7534. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Han, S.; Yun, Y.M.; Kang, S. Stimulation of Biomethane Productivity in Anaerobic Digestion Using Electro-Conductive Carbon-Nanotube Hollow-Fiber Media. Minerals 2021, 11, 179. [Google Scholar] [CrossRef]
- Deng, F.; Sun, J.; Hu, Y.; Chen, J.; Li, S.; Chen, J.; Zhang, Y. Biofilm Evolution and Viability during: In Situ Preparation of a Graphene/Exoelectrogen Composite Biofilm Electrode for a High-Performance Microbial Fuel Cell. RSC Adv. 2017, 7, 42172–42179. [Google Scholar] [CrossRef]
- Zhou, H.; Xing, D.; Xu, M.; Su, Y.; Zhang, Y. Biogas Upgrading and Energy Storage via Electromethanogenesis Using Intact Anaerobic Granular Sludge as Biocathode. Appl. Energy 2020, 269, 115101. [Google Scholar] [CrossRef]
- Kim, K.R.; Kang, J.; Chae, K.J. Improvement in Methanogenesis by Incorporating Transition Metal Nanoparticles and Granular Activated Carbon Composites in Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2017, 42, 27623–27629. [Google Scholar] [CrossRef]
- Sangeetha, T.; Guo, Z.; Liu, W.; Cui, M.; Yang, C.; Wang, L.; Wang, A. Cathode Material as an Influencing Factor on Beer Wastewater Treatment and Methane Production in a Novel Integrated Upflow Microbial Electrolysis Cell (Upflow-MEC). Int. J. Hydrogen Energy 2016, 41, 2189–2196. [Google Scholar] [CrossRef]
- Tartakovsky, B.; Lebrun, F.; Guiot, S.R.; Bock, C. A Comparison of Microbial and Bioelectrochemical Approaches for Biogas Upgrade through Carbon Dioxide Conversion to Methane. Sustain. Energy Technol. Assess. 2021, 45, 101158. [Google Scholar] [CrossRef]
- Matula, R.A. Electrical Resistivity of Copper, Gold, Palladium, and Silver. J. Phys. Chem. Ref. Data 1979, 8, 1147–1298. [Google Scholar] [CrossRef]
- Dumas, C.; Mollica, A.; Féron, D.; Basséguy, R.; Etcheverry, L.; Bergel, A. Marine Microbial Fuel Cell: Use of Stainless Steel Electrodes as Anode and Cathode Materials. Electrochim. Acta 2007, 53, 468–473. [Google Scholar] [CrossRef]
- Noori, M.T.; Vu, M.T.; Ali, R.B.; Min, B. Recent Advances in Cathode Materials and Configurations for Upgrading Methane in Bioelectrochemical Systems Integrated with Anaerobic Digestion. Chem. Eng. J. 2020, 392, 123689. [Google Scholar] [CrossRef]
- Guo, K.; Donose, B.C.; Soeriyadi, A.H.; Prévoteau, A.; Patil, S.A.; Freguia, S.; Gooding, J.J.; Rabaey, K. Flame Oxidation of Stainless Steel Felt Enhances Anodic Biofilm Formation and Current Output in Bioelectrochemical Systems. Environ. Sci. Technol. 2014, 48, 7151–7156. [Google Scholar] [CrossRef]
- Prajapati, K.B.; Singh, R. Bio-Electrochemically Hydrogen and Methane Production from Co-Digestion of Wastes. Energy 2020, 198, 117259. [Google Scholar] [CrossRef]
- Wilhelm, M.J.; Sharifian Gh., M.; Wu, T.; Li, Y.; Chang, C.M.; Ma, J.; Dai, H.L. Determination of Bacterial Surface Charge Density via Saturation of Adsorbed Ions. Biophys. J. 2021, 120, 2461–2470. [Google Scholar] [CrossRef]
- Zhang, T.; Nie, H.; Bain, T.S.; Lu, H.; Cui, M.; Snoeyenbos-West, O.L.; Franks, A.E.; Nevin, K.P.; Russell, T.P.; Lovley, D.R. Improved Cathode Materials for Microbial Electrosynthesis. Energy Environ. Sci. 2013, 6, 217–224. [Google Scholar] [CrossRef]
- Tjørve, K.M.C.; Tjørve, E. The Use of Gompertz Models in Growth Analyses, and New Gompertz-Model Approach: An Addition to the Unified-Richards Family. PLoS ONE 2017, 12, e0178691. [Google Scholar] [CrossRef]
- Shanthi Sravan, J.; Butti, S.K.; Sarkar, O.; Vamshi Krishna, K.; Venkata Mohan, S. Electrofermentation of Food Waste—Regulating Acidogenesis towards Enhanced Volatile Fatty Acids Production. Chem. Eng. J. 2018, 334, 1709–1718. [Google Scholar] [CrossRef]
- Rosa, L.F.M.; Hunger, S.; Gimkiewicz, C.; Zehnsdorf, A.; Harnisch, F. Paving the Way for Bioelectrotechnology: Integrating Electrochemistry into Bioreactors. Eng. Life Sci. 2017, 17, 77–85. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H. Anaerobic Treatment of Apple Waste with Swine Manure for Biogas Production: Batch and Continuous Operation. Appl. Energy 2013, 103, 61–72. [Google Scholar] [CrossRef]
- Ren, G.; Hu, A.; Huang, S.; Ye, J.; Tang, J.; Zhou, S. Graphite-Assisted Electro-Fermentation Methanogenesis: Spectroelectrochemical and Microbial Community Analyses of Cathode Biofilms. Bioresour. Technol. 2018, 269, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fan, Y.; Stroe, D.-I.; Fernandez, C.; Yu, C.; Cao, W.; Chen, Z. Battery State Estimation Methods. In Battery System Modeling; Elsevier: Amsterdam, The Netherlands, 2021; Volume 11, pp. 125–156. [Google Scholar]
- Liu, D.; Roca-Puigros, M.; Geppert, F.; Caizán-Juanarena, L.; Na Ayudthaya, S.P.; Buisman, C.; Heijne, A. Granular Carbon-Based Electrodes as Cathodes in Methane-Producing Bioelectrochemical Systems. Front. Bioeng. Biotechnol. 2018, 9, 78. [Google Scholar] [CrossRef]
- Lee, H.S.; Rittmann, B.E. Significance of Biological Hydrogen Oxidation in a Continuous Single-Chamber Microbial Electrolysis Cell. Environ. Sci. Technol. 2010, 44, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Rader, G.K.; Logan, B.E. Multi-Electrode Continuous Flow Microbial Electrolysis Cell for Biogas Production from Acetate. Int. J. Hydrogen Energy 2010, 35, 8848–8854. [Google Scholar] [CrossRef]
- Walker, J.; Halliday, D.; Resnick, R. Fundamentals of Physics/Volume Two, 10th ed.; Wiley: Hoboken, NJ, USA, 2014; Volume 2, ISBN 9781118230732. [Google Scholar]
- Jin, X.; Zhang, Y.; Li, X.; Zhao, N.; Angelidaki, I. Microbial Electrolytic Capture, Separation and Regeneration of CO2 for Biogas Upgrading. Environ. Sci. Technol. 2017, 51, 9371–9378. [Google Scholar] [CrossRef]
- Namal, O.O. Investigation of the Effects of Different Conductive Materials on the Anaerobic Digestion. Int. J. Environ. Sci. Technol. 2020, 17, 473–482. [Google Scholar] [CrossRef]
- Buitrón, G.; Martínez-Valdez, F.J.; Ojeda, F. Evaluation of the Methane Production Rate from an Acidogenic Effluent Generated in a Two-Stage Process Treating Winery Wastewater. Biomass Convers. Biorefinery 2020, 10, 987–995. [Google Scholar] [CrossRef]
- Shah, Y.T.; Kelkar, B.G.; Godbole, S.P.; Deckwer, W.D. Design Parameters Estimations for Bubble Column Reactors. AIChE J. 1982, 28, 353–379. [Google Scholar] [CrossRef]
- Shah, Y.T. Design Parameters for Mechanically Agitated Reactors. In Advances in Chemical Engineering; Wei, J., Anderson, J.L., Bischoff, K.B., Eds.; Academic Press: Cambridge, MA, USA, 1992; Volume 17, ISBN 0-12-008517-8. [Google Scholar]
- Lovley, D.R. Live Wires: Direct Extracellular Electron Exchange for Bioenergy and the Bioremediation of Energy-Related Contamination. Energy Environ. Sci. 2011, 4, 4896–4906. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E. Electromicrobiology: The Ecophysiology of Phylogenetically Diverse Electroactive Microorganisms. Nat. Rev. Microbiol. 2022, 20, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Kim, J.; Lee, C. A Long-Term Study on the Effect of Magnetite Supplementation in Continuous Anaerobic Digestion of Dairy Effluent—Enhancement in Process Performance and Stability. Bioresour. Technol. 2016, 222, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Lu, Y. Putative Extracellular Electron Transfer in Methanogenic Archaea. Front. Microbiol. 2021, 12, 611739. [Google Scholar] [CrossRef] [PubMed]
- Beese-Vasbender, P.F.; Grote, J.P.; Garrelfs, J.; Stratmann, M.; Mayrhofer, K.J.J. Selective Microbial Electrosynthesis of Methane by a Pure Culture of a Marine Lithoautotrophic Archaeon. Bioelectrochemistry 2015, 102, 50–55. [Google Scholar] [CrossRef]
- Koch, C.; Harnisch, F. Is There a Specific Ecological Niche for Electroactive Microorganisms? ChemElectroChem 2016, 3, 1282–1295. [Google Scholar] [CrossRef]
- Hirano, S.; Matsumoto, N.; Morita, M.; Sasaki, K.; Ohmura, N. Electrochemical Control of Redox Potential Affects Methanogenesis of the Hydrogenotrophic Methanogen Methanothermobacter Thermautotrophicus. Lett. Appl. Microbiol. 2013, 56, 315–321. [Google Scholar] [CrossRef]
- Xu, H.; Wang, K.; Holmes, D.E. Bioelectrochemical Removal of Carbon Dioxide (CO2): An Innovative Method for Biogas Upgrading. Bioresour. Technol. 2014, 173, 392–398. [Google Scholar] [CrossRef]




| One Chamber Reactors | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Carbon Based Cathode | |||||||||
| Methane Production Rate (L/L/d) | Voltage (V) | Cathode | Anode | Anode Surface (cm2) | Cathode Surface (cm2) | Membrane | Temperature | Reactor Volume | Reference |
| 29.7 | 0.7 | Carbon cloth | Carbon cloth | 40.0 | 40.0 | No | 55 °C | 250 mL | [80] |
| 1.6 | 0.75 | Carbon felt | Carbon felt | 40.0 | 40.0 | No | 55 °C | 250 mL | [27] |
| 1 | −0.8–−1.2 vs. Ag/AgCl | Carbon felt | Graphite electrode | 11.9 | 132.0 | No | 55 °C | 350 mL | [81] |
| 0.7 | 1 | Coated carbon paper | Carbon paper | 3.0 | 3.0 | No | 60 °C | 10 mL | [17] |
| 0.1 | 0.6 | Carbon cloth | Carbon fiber brush | No | 30 °C | 40 mL | [82] | ||
| 0.1 | 0.9 | Graphite felt | Graphite felt | 36.0 | 36.0 | No | 25 °C | 500 mL | [29] |
| 0.1 | 0.8 | Graphite felt | Graphite felt | 36.0 | 36.0 | No | 25 °C | 500 mL | [29] |
| 0.1 | 0.7 | Thermally activated carbon felt | Thermally activated carbon felt | 77.0 | 77.0 | No | 32 °C | 32 L | [30] |
| 0.1 | 2.0 vs. Ag/AgCl | Carbon felt | Carbon felt | 388.0 | 388.0 | No | 22 °C | 2.8 L | [83] |
| 0.1 | 0.7 | Graphite felt | Graphite felt | 36.0 | 36.0 | No | 25 °C | 500 mL | [29] |
| 0.01 | 0.6 | Graphite rod + graphite granules bed (10 g) | Graphite rod | 2.1 | 4.0 | No | 41 °C | 50 mL | [84] |
| One Chamber Reactors | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Metal-Based and Composite Cathode | |||||||||
| Methane Production Rate (L/L/d) | Voltage (V) | Cathode | Anode | Anode Surface (cm2) | Cathode Surface (cm2) | Membrane | Temperature | Reactor Volume | Reference |
| 1.8 | 0.24 | Stainless steel pipe | Graphite felt sandwiched between cylindrical Ti collector | 800.0 | 220.0 | No | 40 °C | 6 L | [76] |
| 0.9 | 1.0 | Stainless steel | Carbon felt | 25.0 | 76.0 | No | 25 °C | 250 mL | [77] |
| 0.9 | 0.3 | Graphite carbon mesh coated with Ni, Cu, Fe | Graphite carbon mesh coated with Ni | 2700.0 | 2700.0 | No, nonwoven fabric separator | 35 °C | 20 L | [85] |
| 0.8 | 3–3.5 | Stainless steel mesh | Ti mesh + Ir mixed metal oxides coating | 20.0 | 20.0 | No | 35 °C | 500 mL | [86] |
| 0.6 | −1.0 vs. Ag/AgCl | Stainless steel | Carbon felt | 10.0 | 183.7 | No | 31 °C | 180 mL | [87] |
| 0.5 | −0.4 vs. Ag/AgCl | Stainless steel | Carbon felt | 10.0 | 183.7 | No | 30 °C | 180 mL | [87] |
| 0.3 | 1.2 | Stainless steel cylinder | 11 graphite plates inserted into a Stainless-steel cylinder | 247.5 | 294.0 | No | 16 °C–35 °C | 153 mL | [55] |
| 0.2 | 0.9 | Stainless steel | Graphite fiber brush | No | 31 °C | 1000 L | [88] | ||
| Two or More Chamber Reactors | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Carbon-Based Cathode | |||||||||
| Methane Production Rate (L/L/d) | Voltage (V) | Cathode | Anode | Anode Surface (cm2) | Cathode Surface (cm2) | Membrane | Temperature | Reactor Volume | Reference |
| 12.5 | 0.85 | Graphite felt | Ti mesh, Ir oxide coated (12 g Ir/m2) | 0.1 | 0.4 m2/g | Nafion 117 proton exchange | 30 °C | 2 × 85 mL | [57] |
| 5.2 | −0.7 vs. SHE | Graphite felt | Ti mesh, Pt coated (50 g/m2) | 250.0 | 250.0 | Fumasep FKB cathion exchange | 31 °C | 2 × 250 mL | [33] |
| 2.4 | −0.7 vs. SHE | Graphite felt | Graphite felt | 290.0 | 290.0 | Fumasep FKB cahtion exchange | 30 °C | 2 × 620 mL | [89] |
| 1.8 | −0.5 | Carbon cloth | Carbon cloth | 40.0 | 40.0 | Nafion 117 proton exchange | 55 °C | 2 × 250 mL | [80] |
| 1.4 | −0.6 V | Graphite felt | Graphite felt | 290.0 | 290.0 | Fumasep FKB cathion exchange | 30 °C | 2 × 620 mL | [89] |
| 1 | −0.8–−1.2 vs. Ag/AgCl | Carbon felt | Graphite electrode | 11.9 | 132.0 | AS2S Cathion exchange | 55 °C | 2 × 350 mL | [81] |
| 0.8 | 1 | Carbon fiber felt | Carbon nanotubes | PEM | 25 °C | 2 × 290 mL | [23] | ||
| 0.5 | −0.85–−1.15 | Carbon felt | Carbon felt | 49.0 | 49.0 | AMI 7001 cathion exchange | 30 °C | 2 × 245 mL | [20] |
| 0.5 | 0.8 | Carbon cloth coated with activated carbon (5 mg/cm2) + Pt (0.1 mg/cm2) | Carbon brush | 1705.0 | AEM anion exchange tubes | room tp | A: 18 L C: 1 L | [37] | |
| 0.2 | 0.1 | Graphite granule bed (2–6 mm) | Graphite granule bed (2–6 mm) | Fumasep FAD anion exchange + Fumasep FKE cathion exchange | 25 °C | 3 × 860 mL | [90] | ||
| 0.2 | −0.5 vs. Ag/AgCl | Carbon brush | Graphite rod | 4.8 | 13,700.0 | CMI 7000 cathion exchange | 37 °C | 800 mL | [91] |
| 0.1 | −0.5 vs. Ag/AgCl | Graphite plate | Graphite rod | 4.8 | 40.3 | CMI 7000 cathion exchange | 37 °C | 800 mL | [91] |
| 0.1 | −0.5 vs. SHE | Graphite plate | Graphite rod | 15.6 | 15.0 | CMI 7000 cathion exchange | 37 °C | 850 mL | [92] |
| 0.1 | 0,7 | Carbon paper | Carbon paper | 10.0 | 10.0 | Nafion 117 proton exchange | 37 °C | 2 × 150 mL | [93] |
| 0.1 | −1.4 vs. Ag/AgCl | Carbon stick with graphite felt layer | Pt | 23 cm | 11.0 | Nafion 117 proton exchange | 35 °C | 200 mL | [79] |
| 0.1 | −0.4 vs. Ag/AgCl | Activated carbon fabric | Carbon fabric | 150.0 | 138.0 | Nafion 117 proton exchange | 30 °C | C:1 L | [54] |
| 0.1 | −0.8 vs. Ag/AgCl | Granular graphite bed | Carbon felt | 168.0 | CMI 7000 cathion exchange | 23 °C | 2 × 500 mL | [42] | |
| 0.1 | −0.9 vs. Ag/AgCl | Graphite rod | Carbon fabric | 150.0 | 69.0 | Nafion 117 proton exchange | 35 °C | C: 1 L | [54] |
| 0.03 | −1.04 vs. Ag/AgCl | Carbon cloth + carbon black | Graphite fiber brush | 1.0 | 7.0 | Nafion 117 proton exchange | 30 °C | 2 × 152 mL | [94] |
| 0.01 | −1.02 vs. Ag/AgCl | Graphite fiber brush | Graphite fiber brush | 1.0 | 6.3 | Nafion 117 proton exchange | 30 °C | 2 × 152 | [94] |
| 0.01 | 0.7 | Carbon felt | Carbon felt + Pt | 49.0 | 49.0 | CMI 7000 cathion exchange | 30 °C | 2 × 240 mL | [19] |
| 0.01 | 0.55 | Graphite felt | Ti mesh, Pt coated (50 g/m2) | 250.0 | 250.0 | Ralex CM cathion exchange | 30 °C | 2 × 280 mL | [73] |
| 0.01 | −1.1 vs. Ag/AgCl | Carbon laying | Carbon fabric | 15,900.0 | 30,000.0 | FKS-PET-130 cathion exchange | 35 °C | A:145 L C: 50 L | [25] |
| 0.003 | −0.55–−0.65 vs. Ag/AgCl | Carbon fiber brush | Carbon fiber brush | 7,400,000.0 | 7,400,000.0 | Nafion | 34 °C | 2 × 100 mL | [18] |
| Two or More Chamber reactors | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Metal-Based and Composite Chatode | |||||||||
| Methane Production Rate (L/L/d) | Voltage (V) | Cathode | Anode | Anode Surface (cm2) | Cathode Surface (cm2) | Membrane | Temperature | Reactor Volume | Reference |
| 1.4 | 1 | Stainless steel mesh | Ti mesh, IrO2 coated | 72.0 | 450.0 | CEM | 37 °C | A: 1 L C: 4.5 L | [50] |
| 0.01 | 0.8 | Wet proof carbon cloth + Pt (0.5 g/cm2) | Non-wet-proof carbon brush (pretreated) | 2 CEM | 21 °C | A:150 mL C: 80 mL | [52] | ||
| 0.1 | −0.86 vs. Ag/AgCl | Stainless steel mesh + Pt | Graphite fiber brush | 1.0 | 7.0 | Nafion 117 proton exchange | 30 °C | 2 × 152 mL | [94] |
| 0.02 | −0.7 vs. Ag/AgCl | Pt sheet | TiO2/CdS photoanode | 3.0 | 4.0 | Ultrex CMI 7000 cathion exchange membrane | 31 °C | 2 × 350 mL | [95] |
| 0.01 | −0.55–−0.65 | Graphite bloch + carbon black + metals (Pt, Ni, Stainless steel) | Carbon fiber brush | 7,400,000.0 | 10.6 | Nafion | 32 °C | 2 × 100 mL | [18] |
| Material | Conductivity (S/m) at 20 °C |
|---|---|
| Silver | 6.30 × 107 |
| Copper | 5.96 × 107 |
| Gold | 4.10 × 107 |
| Nickle | 1.43 × 107 |
| Platinum | 9.43 × 106 |
| Titanium | 2.38 × 106 |
| Stainless steel | 1.45 × 106 |
| Carbon (graphite) | 2–3 × 105 |
| Taxon | Chamber/Electrode | Possible Role | References |
|---|---|---|---|
| Desulfovibrio sp. | cathode | Catalyses BES H2 production at cathode potentials ≤−0.44 V versus NHE | [89] |
| Acetobacterium spp. | cathode | Most prevalent and active bacteria on the electrode in acetate production | [24] |
| Clostridium sp. | Bulk solution | Transferred electrons directly to an outside electron acceptor | [141] |
| Geobacter sp. | cathode | Well-known DIET partner | [82,163] |
| Hydrogenophaga sp. | cathode | Electroactive bacterium. Its role in electromethanogenesis is unclear | [163] |
| Azoarcus sp. | cathode | The facultative electroactive, role in BES needs further investigation | [148] |
| Tangfeifania sp. | cathode | It is detected frequently in BES reactors. They probably facilitate methanogenesis | [91] |
| Aminomonas sp. | cathode | Syntrophic methanogen partner electron transfer has not been documented | [91] |
| Desulfuromonas sp. | anode | Electroactive microbe | [77] |
| Bacteroidia sp. | Bulk solution | Hydrolyzes proteins and transforms the amino acids generated in the process into acetate | [85] |
| Azonexus sp. | cathode | Acetate oxidising bacterium, capable of DIET and DEET, it can be found frequently on anode as well | [92] |
| Archea | References |
|---|---|
| Methanobacterium palustre | [89] |
| Methanobacterium aarhusense | [89] |
| Methanothermobacter thermoautotrophicus | [80,180] |
| Methanothrix concillii | [29,91,92,181] |
| Methanospirillum hungatei | [29] |
| Methanosarcina flavescens | [29] |
| Methanoculleus bourgensis | [29] |
| Methanosphaera cuniculi | [29] |
| Methanobacterium formicicum | [83,85] |
| Methanobacterium petrolearium | [181] |
| Methanobacterium subterraneum | [35,181] |
| Methanosarcina thermophile | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horváth-Gönczi, N.N.; Bagi, Z.; Szuhaj, M.; Rákhely, G.; Kovács, K.L. Bioelectrochemical Systems (BES) for Biomethane Production—Review. Fermentation 2023, 9, 610. https://doi.org/10.3390/fermentation9070610
Horváth-Gönczi NN, Bagi Z, Szuhaj M, Rákhely G, Kovács KL. Bioelectrochemical Systems (BES) for Biomethane Production—Review. Fermentation. 2023; 9(7):610. https://doi.org/10.3390/fermentation9070610
Chicago/Turabian StyleHorváth-Gönczi, Noémi N., Zoltán Bagi, Márk Szuhaj, Gábor Rákhely, and Kornél L. Kovács. 2023. "Bioelectrochemical Systems (BES) for Biomethane Production—Review" Fermentation 9, no. 7: 610. https://doi.org/10.3390/fermentation9070610
APA StyleHorváth-Gönczi, N. N., Bagi, Z., Szuhaj, M., Rákhely, G., & Kovács, K. L. (2023). Bioelectrochemical Systems (BES) for Biomethane Production—Review. Fermentation, 9(7), 610. https://doi.org/10.3390/fermentation9070610







