Abstract
This paper aims to present the advances related to the biotechnological application of lipases Y. lipolytica, presenting their properties and more efficient ways to use them in different industrial applications. Waste treatment and bioremediation highlight recent studies and advances and the interest in large-scale applications in the food sector and biofuel production. The USA and China, two major world powers in industy, are of utmost importance in the search for the improvement in the development and properties of a controlled system for the large-scale production of a significant number of applications of lipase from Y. lipolytica.
1. Introduction
The production of enzymes with unique properties for each type of process or that have a broad set of industrial methods aiming at large-scale production has been a topic widely analyzed in recent years [,]. It is a field in which biotechnology can actively contribute when we consider the reduction of waste and the decreased use of solvents in chemical reactions. In engineering, the reduction of these environmental impacts is significant because the adoption of sustainable practices is increasingly demanded by companies, arising from the relationship between ESG (environmental, social, and governance) and its point of convergence with the environment [,,,,,,]. This paper aims to present and discuss the advances obtained by the study of lipase Y. lipolytica and the provisions of the future of its industrial applications.
Biocatalysis is a sustainable method that acts through a process that performs the insertion of enzymes into chemical reactions that act as catalysts, thus increasing the speed and restricting the level of excreta at the end of processes [,,,,]. Lipase is an enzyme that catalyzes the hydrolysis of fatty acid esters [,], and Y. lipolytica is a yeast capable of producing lipase (Figure 1). Y. lipolytica is used in different industrial applications, e.g., bioprocesses such as biodiesel production, ester synthesis, and bioremediation, as well as assisting in food and pharmaceutical product synthesis processes [,,,].
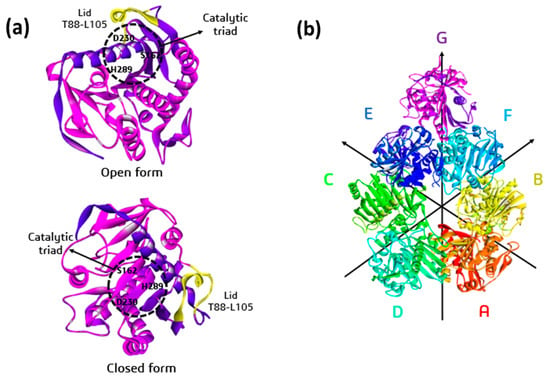
Figure 1.
(a) Ribbon representation of the structure of lipase lip2 from Y. lipolytica showing the open and closed form of LID (T88-L105) and indicating the catalytic triad of this enzyme, formed by S162, D230, and H289. (b) Packing of the asymmetric units that form the macromolecule, corresponding to the A–G units, highlighting an orientation plane for better visualization of these units that form the enzymatic complex.
The main characteristic of Y. lipolytica lipase is its ability to remain stable over an extensive pH and temperature range. In addition, it exhibits high activity in aqueous media, which makes it useful in applications involving the hydrolysis of fats in aqueous systems []. One property that draws attention is its specificity for medium- and long-chain fatty acid esters [,]. This makes the enzyme valuable in applications that require hydrolysis of fats, and it is used in the production of cleaning products because its action makes it more accessible to remove oils and fats present on surface areas.
Moreover, another point that makes the lipase Y. lipolytica an attractive option for industrial processes is that it is highly tolerant to organic solvents [], which makes it suitable for applications in industrial processes involving the hydrolysis of esters in organic solutions.
Figure 2 shows data from an advanced bibliometric analysis using the Web of Science Core Collection platform with a database of 524 articles related to Y. lipolytica. The database was built with the keywords Y. lipolytica and lipase. Articles published between 2000 and 2022 were analyzed. The free software R Studio with the extension “bibliometrix::biblioshiny()” was used [,]. Figure 2a shows that the terms Y lipolytica, lipase, and purification were the words that appeared the most during this search period. Figure 2b shows that 2015 had the highest number of articles published in the area, with 42 articles. However, Figure 2c shows that the highest number of citations in the area occurred in 2002, with about 12 citations, using the terms of this research.
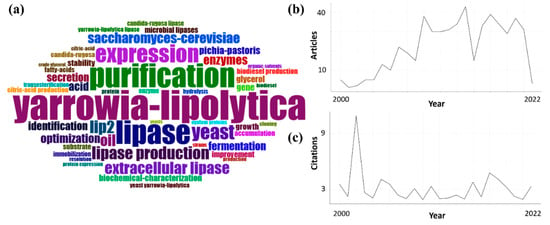
Figure 2.
(a) Map of keywords related to Y. lipolytica between 2000 and 2022. (b) The number of articles related to Y. lipolytica. (c) The number of citations related to Y. lipolytica.
Based on the above rationale, this research addressed an advanced review and bibliometric analysis of scientific research related to Y. lipolytica. This review pays attention to the analysis of this yeast’s research evolution, updates, and study trends. This review contributes to the description of available technologies and methodologies for biotechnological applications, enabling the evaluation of different areas of use and exploring innovative opportunities to use its by-products in sustainable processes.
2. Lipase Biosynthesis from Extreme Yeasts
Producing lipases from extreme yeasts is defined as biosynthesis []. Extreme yeasts are microorganisms that can thrive and survive in extreme conditions, e.g., Highalt temperatures and pH []. The lipases synthesized by yeasts in these environments acquire these characteristics intrinsically and thus develop similar resistances, thus becoming thermo, halo, and osmo-tolerant lipases [].
In general, lipases are enzymes with the potential to break ester bonds in fats and oils, resulting in fatty acids and glycerol []. Thus, applying these enzymes will be possible in different areas of industry, such as the production of detergents, biofuels, and even the food industry [,,,,,,,]. This diversity of applications is made possible due to the versatility of these biomolecules. Thus, the powerful yeast biosynthesis method becomes increasingly relevant and essential for these processes [].
The lipase biosynthesis process in extreme yeasts occurs with the gene expression that produces these enzymes []. Thus, the genes encoding lipases are transcribed into messenger RNA (mRNA), which undergoes translation into protein during translation []. Messenger RNA is produced from DNA by RNA polymerase, following the genetic code present in DNA []. Finally, these enzymes are processed and secreted by the yeast into the external environment. On the other hand, they can remain inside the cells [,]. Secretion of lipases depends on the organism in question and the growth conditions [].
However, the production of these lipases by extreme yeasts can be stimulated through various strategies such as selecting specific strains, optimization of cultivation, and even genetic engineering []. Ultimately, these strategies only increase the productivity, resistance, and activity of these lipases []. It is still important to highlight several species of extreme yeasts, such as Candida, Yarrowia, and Pichia [,,]. Each species has its specificities that produce lipase production with distinct characteristics [,].
Still, concerning yeast species and their lipases, it is possible to highlight the types of tolerances already mentioned here in addition to those that classify these lipases []. Where thermotolerant lipases are obtained to maintain their activity even at elevated temperatures [], these biomolecules have a specific structural stability that guarantees such resistance []. Among the enzymes synthesized by yeast, lipases from Candida antarctica type B and Rhizopus oryzae can be highlighted, which are used in the food, detergent, and biofuel industries [,].
Halotolerant lipases can maintain their activity in environments with high salt concentrations []. Changes in the structures of these lipases allow for improvements in their electrostatic and hydrophobic interactions, facilitating the transport of ions and favoring intracellular osmotic balance []. Examples of halotolerant lipases are those obtained from Y. lipolytica yeasts and Candida rugosa, which are applied in the food industry [,].
Finally, we highlight the osmotolerant lipases that can maintain activity in environments with high concentrations of solutes, sugars, and organic compounds []. This feature makes them able to remain unchanged in high osmotic conditions and resistant to the dehydrating effects of the environment, such as changes in electrostatic and hydrophobic interactions []. Examples of these species are lipases from Candida cylindracea and Debaryomyces hansenii, which are applied in industrial processes that require osmotic resistance and produce fermented foods, respectively [,].
3. Context of Y. lipolytica in a Bibliometric Review
An advanced bibliometric review is widely used to verify aspects of research that will contribute significantly to evaluating a particular field or subfield of study [,]. Several characteristics will be visualized from this type of analysis and used as a source of incremental knowledge for statistical analyses of the subject matter [,,,].
Figure 3 shows a bibliometric review of Y. lipolytica, which shows data obtained from the Web of Science Core Collection platform with a database of 524 articles related to Y. lipolytica (keywords: Y. lipolytica and Lipase, analysis period from 2000 to 2022). The software used for analysis was the free software R Studio with the extension “bibliometrix::biblioshiny()” and the software VOSviewer version 1.6.18 [,].
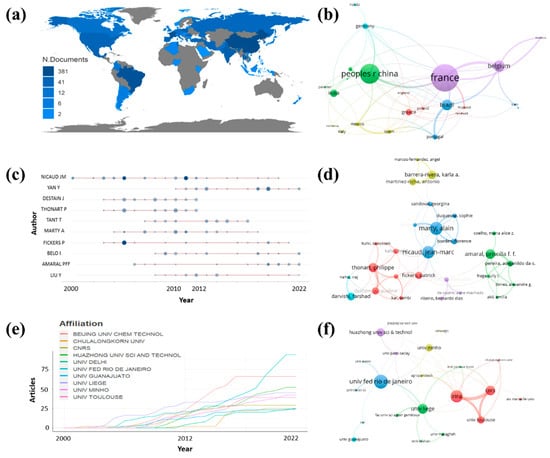
Figure 3.
Data were obtained between 2000 and 2022 with the keywords Y. lipolytica and lipase. (a) Several documents were published by each country and obtained by bibliometrics. (b) Co-authorship between countries in the publication, using a minimum of 5 articles per country, obtained by VOSviewer. (c) Production of authors over time, obtained by bibliometrics. (d) Co-authorship between the authors in the publication, using at least 5 articles per author, obtained by VOSviewer. (e) Production of affiliations over time, obtained by the bibliometrics. (f) Co-authorship between affiliations, using a minimum of 5 articles per affiliation, obtained by VOSviewer.
Figure 3a shows the publications of the countries of the globe in the related area. The bibliometrics showed that the countries that published the most were China, France, and Brazil, with 381, 236, and 213 publications, respectively. It is interesting to observe that Belgium, the country that appeared in fourth place in the number of publications, presented 90 publications, less than half of the country in third place. It was found that the first three countries mastered the theme in the analyzed period. Figure 3b shows co-authorship and research correlations between countries, forming well-defined clusters with different colors. China cooperates with India (73 articles) and has a robust connection with France. France has strong cooperation with Belgium, and Brazil has strong cooperation with Portugal (55 articles).
Figure 3c shows the production of authors over time. Nicaud J.M. was the author who presented the highest number of publications, with publications between 2000 and 2020. The year 2011 was the year in which this author presented the highest number of articles, with five articles with a total of 32 citations. Nicaud J.M. was also the author who presented the most significant number of articles, with 32 articles, followed by Yany and Destain J. with 24 and 23 articles, respectively. The authors with the most significant impact, and the highest H factor, were Nicaud J.M., Marty A., and Thonart P., with 24, 16, and 15 H factor indexes, respectively. Figure 3d shows the co-authorship between the authors. It is possible to observe six large distinct cooperation nuclei. It is possible to notice that the authors with the most significant impact are present in the blue core, while Thonart P. appears in greater cooperation with the authors in red. It is also possible to observe that, like the countries, all authors communicate with each other, showing that research related to Y. lipolytica is globalized.
Figure 3e shows the production of affiliations over time. The Univ. Fed. Rio de Janeiro, Beijing Univ. chem. Technol. and Huazhong Univ. Sci. and Technol. were the affiliations presenting the highest number of publications, with 94, 66, and 52, respectively. It can be seen that Beijing Univ. chem. Technol. has stagnated in the number of publications since 2017, while the other affiliations continue to grow constantly. Figure 3f shows co-authorship between affiliations. It is possible to observe five large distinct cooperation nuclei. The Univ. Fed. Rio de Janeiro stands out with the highest number of cooperation. However, the Univ. Fed. Rio de Janeiro is from Brazil, and that country is in third place in the number of publications. Therefore, we cannot necessarily relate the affiliation to the country that publishes the most since, as shown, publications in the area of Y. lipolytica have a global aspect in which authors of all countries cooperate.
Figure 4 shows the cross-country dependency wheel plot and the three-campus plot. The dependency wheel in Figure 4a shows that although China has the highest number of publications, countries such as France, Belgium, and Brazil generate a greater exchange of communication with other countries. In Figure 4b, the graph of three campuses reaffirms that research in the area of Y. lipolytica is globalized, where almost all authors communicate with each other, in a correlation between countries and affiliations. We highlight the authors who communicate the most from countries such as China, Brazil, Belgium, and France.
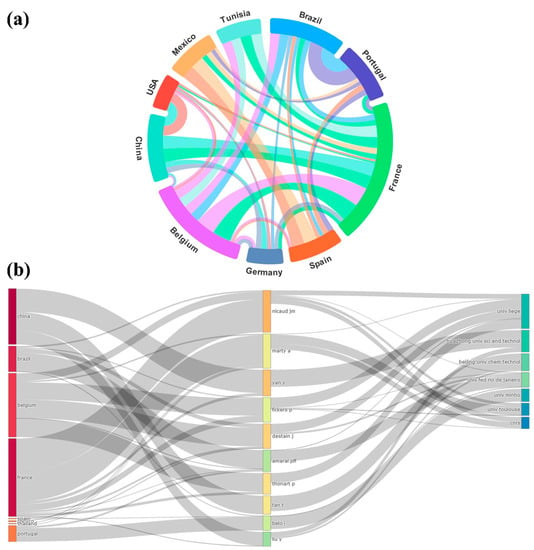
Figure 4.
Data were obtained between 2000 and 2022 with the keywords Y. lipolytica and lipase. (a) Country dependency wheel. (b) Three-campus graph correlating countries, authors, and affiliations.
Figure 5 shows the journals that published the most from 2000 to 2022 and the articles with the highest number of citations, both obtained by the bibliometrics. Figure 5a shows the journals with the most articles. Applied Microbiology and Biotechnology, Bioresource Technology, and Process Biochemistry had the highest number of articles, with 21, 16, and 16, respectively. Figure 5b shows the articles with the most citations. The article entitled “Hydrophobic substrate utilization by the yeast Y. lipolytica, and its potential applications”, published by Fickers P. in 2005 in the journal FEMS Yeast Research, obtained the highest number of citations in the period []. With 436 citations, the article reports that the alkane-assimilating yeast Y. lipolytica very efficiently degrades hydrophobic substrates such as n-alkanes, fatty acids, fats, and oils for which it has specific metabolic pathways. Oxidative degradation to alkanes and triglycerides in Y. lipolytica is provided, with new insights arising from recent genome sequencing of this yeast []. The second article that obtained the highest number of citations was entitled “Y. lipolytica as a potential producer of citric acid from raw glycerol” and was published by Papanikolaou S. in 2002 in the Journal of Applied Microbiology, where it obtained a total of 256 citations []. The article studies the biochemical response of Y. lipolytica LGAM S(7)1 during growth in crude glycerol (the main by-product of biodiesel production plants) to produce metabolic products of industrial importance []. The third article with the highest number of citations was published by Bilal M. in 2018 in the International Journal of Biological Macromolecules, totaling 237 citations. The article “Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review” comprehensively reviews the supportability of immobilizing enzymes where Y. lipolytica is present [].
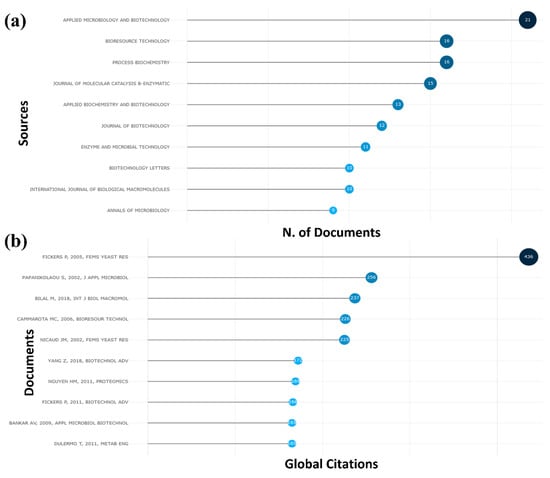
Figure 5.
Analysis of newspapers and articles obtained by bibliometrics. (a) Many articles were published in the 10 newspapers that published the most. (b) Most cited articles on the topic, showing the author, year, and journal in which it was published [,,,,,,,,,].
Table 1 shows the most cited articles, identifying the authors, year of publication, journal, and the reference for each article. It is possible to observe that the normalization of citations per year can be greater than that of the articles that have a more significant number of citations, as it is shown that the article by Bilal M. 2018 [] has a greater total of normalized citations than the article by Fickers P. 2005 [], which can be justified by being a more general article, which includes other enzymes in its text, not just Y. lipolytica.

Table 1.
Most cited articles related to Y. lipolytica.
4. Applications of the Lipases from Y. lipolytica
From the database that shows the applications of lipases from Y. lipolytica, it was possible to find 146 articles between the years 2000 to 2022 confirming the approach to be presented in the present work. The three most relevant articles have, respectively, 13 citations [], 8 citations [], and 165 citations []. The first two articles address the extracellular lipase from Y. lipolytica in different ways of application, in which they are similar in general aspects which portray the yeast and its strains. The third is a review that details the genetics, production, biochemical characterization, and especially, the biotechnological applications of Y. lipolytica regarding the production of lipases in several sectors destined for the environment and industries. Despite the difference in the number of citations of the articles, the relevancies are highlighted in a higher number of accesses to the article or the more significant number of recent works directly related to the theme to be analyzed. Several applications of Y. lipolytica are constantly featured in perfected studies, especially in biotechnological applications based on enzymatic engineering, in environmental methodologies that collaborate to minimize the impacts caused by pollution from ecologically correct processes that may come to be applied industrially. Thus, this will be the first study published in the literature that conducts a complete bibliometric analysis of Y. lipolytica and presents lipase from Y. lipolytica and its prospects as an industrial biocatalyst for biotechnological applications.
Y. lipolytica is a yeast belonging to the Ascomycota phylum that has been extensively studied for biotechnological applications in recent years []. Initially, the most significant interest in studies on Y. lipolytica was due to its particular physiological capabilities, such as its use of organic acids and long-chain hydrocarbons as substrates, in addition to having other characteristics, such as the production of high levels of lipolytic enzymes, the production of organic acids, such as pyruvate, from different carbon sources [], as well as its condition of dimorphism, as it presents filamentous forms or yeast cells []. Y. lipolytica is considered a very interesting yeast for the production of heterologous proteins because it has an efficient transformation yield, production capacity, and stability related to gene integration. One of its most significant characteristics is the fact that it can secrete high amounts of metabolites, unicellular oil, extracellular proteins, and aroma, among other aspects, that make it academically and scientifically attractive for research []. It is an aerobic or aerobic and non-pathogenic yeast. All processes involving this microorganism are generally recognized as safe (GRAS) according to the FDA—American Food and Drug Administration (https://www.fda.gov/food/food-ingredients-packaging/generallyrecognized-safe-gras) (accessed on 15 March 2023).
Y. lipolytica is valuable for industries, especially pharmaceutical and food industries, for secreting large amounts of lipases []. Lipases are biocatalysts considered ecologically correct. They perform the process of catalyzing the hydrolysis of carboxylic acid esters in an aqueous phase, but they also perform the same process in reactions in organic solvents, such as in esterification and transesterification reactions [,]. The production of enzymes by microorganisms (OMs) from mutation process creation has been widely explored in a scientific environment for some years []. In a more recent study, Y. lipolytica Wt-11 was isolated with the aim of producing extracellular lipase, in which it was subjected to the mutation process of implantation of low energy ions, in which Y. lipolytica Mut-96 was obtained after the process; then, after the purification process had taken place, the characteristics and properties of the lipase found were identified. Subsequently, the enzymatic immobilization process took place for application in the synthesis process of L-ascorbyl palmitate, in which it is an ascorbyl ester and an antioxidant used in some foods due to its ability to scavenge oxygen [], proposing a biotransformation route for the production of L-ascorbyl palmitate on an industrial scale [].
The most attractive characteristics of the Y. lipolytica yeast already have complete studies that detail their properties and mechanisms of processes of potential applications, such as the degradation of hydrophobic substrates [] and the production of natural derivatives []. Focusing on the enzymes produced by Y. lipolytica, it was shown that 16 paralogs of genes encode lipase, which was only possible to be discovered due to studies of the complete sequence of the genome of the haploid strain Y. lipolytica E150 (CLIB99) []. However, only a few extracellular lipase isozymes are known. They are synthesized by Y. lipolytica, which are Lip2p, Lip7p, and Lip8p, with Lip2p being the primary one [], and have broad substrate specificity, ranging from medium-chain fatty acids to long-chain fatty acids. The enzymatic immobilization from the adsorption of Lip2p on celite and silica gel already shows expressive results that benefit its high performance, such as the protection of the lipase enzyme in alkaline pH and benefits in its thermoresistance capacity, in addition to the recovery of enzymatic activity being possible [], which shows that the process of immobilization of extracellular lipase from Y. lipolytica is quite efficient and significantly conducive to reducing expenses involving this method.
Mediterranean countries are responsible for over 95% of the world’s olive oil production. Wastewater from these olive oil processing industries can cause high pollution loads for these countries and the environment []. The waters of the oil mills have pectins, metals, and sugars in their composition, in addition to fats and triglycerides. The yeast Y. lipolytica ATCC20255, among the different yeasts studied, was the one that obtained the best performances in terms of adaptation to growth in oil mill water, reducing the chemical oxygen demand (COD) value by 80% in the bioreactor tank equipment for 24 h []. A recent study carried out a combination of the characteristics present in the oleaginous yeast Y. lipolytica associated with fungal lipases, precisely because they have high activity for the treatment of oily effluents. The functional domain of Saccharomyces cerevisiae resulted in a significant decrease in the removal of chemical oxygen demand (COD) by 91.8%, demonstrating its effectiveness []. In addition, Y. lipolytica has also been highlighted in studies in palm oil mills [,,], pineapple residues [], and fish residues in a solid state []. This is because this yeast has potential applications in different conditions and has a variety of wild-type, recombinant, or mutant strains [].
In this way, we observe the valuable contribution of Y. lipolytica in the ecological management of waste or effluents that cause pollution, minimizing the impacts caused by improper disposal in the environment and enabling alternative ways to reuse some of these wastes as new products, contributing as an essential factor in biotechnological processes based on “green” methods with a focus on reducing environmental impacts []. Several studies are being carried out in order to minimize the impacts caused, such as reducing toxicity and improving biodegradability [,]. Lipases are efficient in treating oily effluents, but they have variables that do not make them viable for large-scale applications, such as instability and solubility, which is why the enzymatic immobilization process is necessary. Y. lipolytica emerges as a suitable microorganism to be used, as it has advantages such as remaining alive in oily effluents for prolonged periods [,].
Y. lipolytica yeast is also present in scientific studies aimed at the possibility of application in treating hydrophobic industrial waste, especially waste from the food industry []. Although Y. lipolytica is not considered pathogenic, it can cause infections in patients with low immunity who have a disease, such as immunocompromised people []. As one of its characteristics, Y. lipolytica can degrade hydrophobic substrates, so for the scientific branch of the food area, it becomes attractive for applications in waste management. Some of its strains can be found in products such as in some types of cheese [] or environments with a significant amount of fats or hydrocarbons []. Because it has several characteristics favorable to Y. lipolytica, it can be used to purify palm oil effluents (POME), which have lignocellulosic residues and a mixture of carbohydrates and oil in their composition. Incomplete extraction of POME can directly affect the increase in COD values and biochemical oxygen demand (BOD). COD [] causes significant impacts when there is no proper treatment of effluents before they are released into nature.
It is verified that the use of Y. lipolytica in the biotechnology area has good future perspectives, with significant growth of applications in several areas, especially enzymatic engineering for improvements in industrial processes. In the area of industrial waste management, this yeast has also been applied, and this is due to the commitment to paper in different conditions to which it is subjected since it can also be applied in a wide range of pHs and at different temperatures, depending on the conditions under which they were obtained and cultivated. The function of yeast Y. lipolytica in environmental applications becomes increasingly necessary because it presents excellent environmental benefits, reducing the risks caused by pollution from various industrial applications. Figure 6 below is a diagram of the main applications of the lipases from Y. lipolytica.
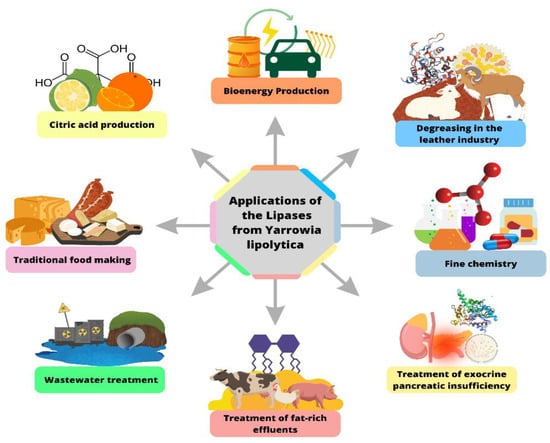
Figure 6.
Applications of the lipases from Y. lipolytica.
Table 2 shows 17 types of environmental and industrial applications of different Y. lipolytica strains that were selected, with results already consolidated and exemplified in the studies. The 17 articles were selected to demonstrate that over the years, as the studies are relatively current, there have been advances in the study of Y. lipolytica in several areas and with different application methodologies. Although most of these studies are still limited to the laboratory, they all have great future perspectives of promising results in large-scale environmental and industrial applications in an economically viable way.

Table 2.
Applications and results of Y. lipolytica.
Microbial biosynthesis has been gaining ground in the scientific community, especially in applications for the production of biofuels. Y. lipolytica is one of the microorganisms tested for these applications, as it consumes hydrophobic substrates and produces different lipases that help in the production of biofuels based on fatty acids []. The production of biofuels from OMs is widely accepted because their applications are considered favorable since they have vast metabolic diversity, which allows them to make use of different substrates, in addition to some being used as direct raw material for the production of biofuels, such as microalgae and [] the widely known yeast Saccharomyces cerevisiae, which is also used in the production of biofuels such as ethanol and biodiesel [,]. It is in this context that Y. lipolytica is being progressively more analyzed for its biotechnological applications due to its facile consumption of hydrocarbons [] and the ability of this yeast to generate different types of lipase that can be used as biocatalysts in biodiesel production, accumulating a significant amount of lipids.
It is valid to analyze that Y. lipolytica has many more environmental than industrial applications, especially in the bioremediation of areas contaminated by different types and sources of oils, whether aquatic or terrestrial [], as well as in applications as a detoxifier of heavy metals such as Cu (II) [] and Ni (II) [] in wastewater. In industrial applications, it can be demonstrated in the production of citric acid [] and in the production of γ-decalactone as peach flavoring []. Thus, recently, it has become one of the most studied yeasts. Due to massive demand in the search for alternative mechanisms for more sustainable, cheap, and safe production, as well as methodologies capable of being applied in large-scale production, and based on the results of recent studies, Y. lipolytica has become entirely viable.
5. Preparation of Immobilized Biocatalysts of Lipases from Y. lipolytica
The immobilization of Y. lipolytica lipase is a widely used strategy to produce biocatalysts with high stability and reuse capability, in addition to allowing their application in different industrial processes [,]. Below are the main steps for preparing immobilized lipase from Y. lipolytica biocatalysts, as shown in Figure 7.
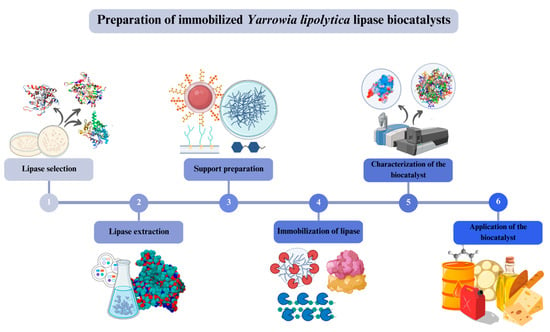
Figure 7.
Representation of the step-by-step procedure of preparing immobilized biocatalysts of lipases from Y. lipolytica.
Lipase selection: The first step is the selection of the suitable lipase for the process in question. Different strains of Y. lipolytica present lipases with different characteristics, such as thermal stability and activity in different pHs and organic solvents [,].
Lipase extraction: Lipase can be extracted from yeast cells under study using different methods, such as organic solvents or fermentation in a liquid medium. After extraction, the lipase is purified by different techniques, such as affinity chromatography or solvent precipitation [,,,,].
Support preparation: The support chosen for lipase immobilization must have the appropriate characteristics for the process in question, such as stability in different pHs and temperature conditions, representing chemical groups that can be used to fix the enzyme [,,]. Examples of supports include silica, cellulose, agarose, and polyacrylamide [,,,,,,,,,,,,,].
Immobilization of lipase: Lipase can be immobilized on the support by different methods, such as adsorption, covalent binding, or cross-linking [,,,,]. The method chosen depends on the lipase characteristics and the support [,,,,,,,].
Characterization of the biocatalyst: The immobilized biocatalyst must be characterized to determine its activity, stability, and reuse capability under different pHs, temperatures, and organic solvents [,]. Spectrophotometric or titrimetric methods can measure enzyme activity, while stability and reuse capability can be assessed through long-term assays [,,,,,].
Application of the biocatalyst: The immobilized biocatalyst can be used in different industrial processes, such as biodiesel production, synthesis of esters, and hydrolysis of fats and oils, among others [,,,,,,,,].
Finally, the preparation of immobilized lipase biocatalysts from Y. lipolytica involves the selection of a suitable lipase, the extraction and purification of the enzyme, the preparation of the support, the immobilization of the lipase, the characterization of the biocatalyst, and its application in industrial processes [,]. This strategy is a promising alternative to produce biocatalysts with high stability and reuse capability in different chemical processes [].
5.1. Lipases from Y. lipolytica Immobilization
Immobilization attaches an enzyme to a solid support such as a polymer, matrix, or resin []. Enzyme immobilization can improve their stability, activity, and reuse capability, making them more attractive for industrial use []. The immobilization of Y. lipolytica lipase is a widely used technique in the production of biocatalysts [].
Several lipase immobilization techniques include physical, chemical, and biological factors [,,]. In physical immobilization, the enzyme is adsorbed onto the surface of the solid support, while in chemical immobilization, the enzyme is covalently attached to the support [,,,]. In biological immobilization, the enzyme is encapsulated in a polymeric matrix, such as alginate []. Thus, immobilizing Y. lipolytica, lipase can improve its thermal and pH stability, increase its activity, and reduce inhibition by compounds in the reaction medium []. In addition, lipase immobilization can allow its reuse in several reaction cycles, reducing the process’s cost [].
Figure 8 shows a schematic that demonstrates some of the methods of enzyme immobilization, dividing them into chemical and physical techniques.
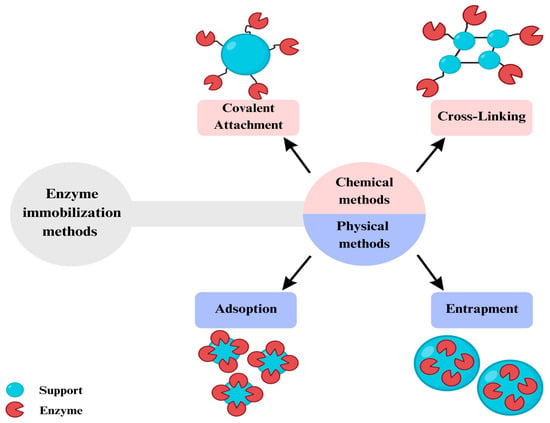
Figure 8.
Enzyme immobilization methods.
Y. lipolytica lipase immobilization depends on the characteristics of the industrial process to be applied [,]. Some of the most used supports are silica, cellulose, and chitosan [,,,,]. The type of support can influence the stability and activity of the immobilized enzyme, as well as the ease of separating the enzyme from the final product [].
5.1.1. Immobilization of Lipases from Y. lipolytica on Hydrophobic Supports
Hydrophobic supports are materials with a low affinity for water and a high affinity for hydrophobic compounds []. They are widely used in enzymatic immobilization, as they allow the enzyme to be fixed on its surface while reducing the inhibition of enzymatic activity by hydrophobic compounds present in the reaction medium [,,]. Below are some examples of hydrophobic supports for enzyme immobilization.
Styrene-divinylbenzene resins (SDB) are widely used to immobilize lipases and other hydrolytic enzymes in the oils and fats hydrolysis process []. SDB resins are characterized by having a porous and hydrophobic structure, which facilitates the adsorption of the enzyme on its surface []. Modified silicas are commonly applied in immobilizing hydrolytic enzymes in processes in the aqueous medium []. Modifying silica with hydrophobic groups such as methyl or propyl groups increases its affinity for hydrophobic compounds, reducing the inhibition of enzymatic activity [,].
Polyesters are also applied in the immobilization of hydrolytic enzymes in processes involving the hydrolysis of esters [,]. Polyesters are characterized by having a hydrophobic chain, which increases their affinity for hydrophobic compounds, enabling better enzyme–support interaction [,]. Octyl-agarose is widely used to immobilize hydrolytic enzymes, focusing primarily on lipases []. Octyl-agarose is characterized by having a well-defined hydrophobic chain, which enhances its affinity for hydrophobic compounds, avoiding problems that result in the reduction of enzymatic activity due to the influence of the support itself [,].
The immobilization of Y. lipolytica lipase on hydrophobic supports is used in several industrial processes [,]. This approach involves enzyme immobilization on supports with hydrophobic properties, such as styrene-divinylbenzene resins, modified silicas, and polyesters [,]. This immobilization can improve enzyme stability under extreme conditions, such as high temperatures and low water activity [,]. Furthermore, these supports can reduce enzyme inhibition by hydrophobic compounds present in the reaction medium [].
The use of hydrophobic supports can be carried out through physical and chemical immobilization techniques []. In physical immobilization, the enzyme is adsorbed on the surface of the support, while in chemical immobilization, the enzyme is covalently attached to the support [,]. The choice of hydrophobic support for the immobilization of Y. lipolytica lipase depends on the properties of the reaction medium and the conditions of the process to be applied [,]. For example, immobilization on styrene-divinylbenzene resins is more suitable for the hydrolysis of oils and fats under acidic conditions, while immobilization on modified silicas is more suitable for processes in aqueous media [,,].
5.1.2. Use of Heterobifunctional Supports to Prevent Enzyme Release
Heterofunctional supports are materials used in enzymatic immobilization with more than one chemical function on their surface [,]. These supports allow the enzyme to be fixed through covalent or non-covalent interactions with the different chemical functions present on its surface []. This results in more stable and long-lasting enzyme immobilization, allowing correct orientation to catalyze the desired reaction []. Below are some examples of heterofunctional supports for enzymatic immobilization.
Agarose activated with epoxy groups: Agarose activated with epoxy groups has epoxy groups on its surface that can fix the enzyme through nucleophilic addition reactions [,]. This matrix can immobilize nucleophilic enzymes such as amine or thiol groups [,].
Activated silica with amino groups: Activated silica with amino groups has amino groups on its surface that can be used to fix the enzyme through cross-linking reactions with bifunctional binding agents, such as Glutaraldehyde []. This matrix can be used to immobilize enzymes that have carboxylic or aldehyde groups [].
Cellulose activated with cyano groups: Cellulose with cyano groups has cyano groups on its surface that can fix the enzyme through cross-linking reactions with bifunctional linking agents, such as dicyanobenzene [,]. This matrix can be used to immobilize enzymes that have nucleophilic groups [].
Silica activated with thiol groups: Silica activated with thiol groups has thiol groups on its surface that can fix the enzyme through cross-linking reactions with bifunctional linking agents, such as N-succinimidyl 3-(2-pyridyldithio) propionate []. This matrix can be used to immobilize enzymes that have amine or maleimide groups [].
In summary, using heterofunctional supports in enzymatic immobilization is essential because it allows greater efficiency and specificity in enzyme fixation [,,], which results in more excellent stability and reuse capability, in addition to allowing the creation of multifunctional systems []. These systems lead to the performance of several consecutive reactions based on the possibility of immobilizing several different enzymes on the same support [].
5.1.3. Modulation of Lipases from Y. lipolytica Properties via Immobilization on Different Supports
Lipases can be modulated according to how they were immobilized and the wide range of possible supports used for immobilization [,,,]. These different supports generate benefits that allow enzymatic biocatalysts to be applied in several industrial operations [,,]. Y. lipolytica, although it has multiple biotechnological applications, its cell size and morphology require a thorough investigation to select suitable supports for immobilization and modulation []. In this sense, superficial adaptations, with activations and functionalizations of metallic supports and those based on silica, cellulose, and chitosan, have been showing promising results in the mutation of Y. lipolytica [,].
Akil et al. [] immobilized Y. lipolytica via physical adsorption on magnetic nanoparticles (MNPs) of Fe3O4 trapped in Ca alginate and obtained better stability results in temperature and pH ranges compared to the free enzyme. In addition, free lipase showed a strict profile of 1,3-regioselectivity in homogeneous and heterogeneous TAGs. In the immobilized form, the hydrolysis rate and regioselectivity sn-1,3 decreased []. Other researchers also showed better stability results for Y. lipolytica in temperature and pH when immobilized in MNPs, in addition to allowing rapid biocatalyst reuse when subjected to a magnetic field [,].
Mesoporous silica-based supports can imprison Y. lipolytica in an electrostatic immobilization that insignificantly decreases the activity of the lipase when compared to the original free lipase, as it enables immobilization that allows more active sites without being occupied in immobilization []. Rios et al. [] immobilized Y. lipolytica on a support based on silica SBA-15, which has a hexagonal structure and managed to obtain high immobilization yields (>90%) when compared to the immobilization of lipase on commercial supports (Celite 535 and Silica Gel 60) []. Wang et al. [] immobilized Y. lipolytica in silicatein, where it showed an aggregation of almost 100%, forming a biosilica–yeast hybrid material, where it can achieve greater efficiency in the removal of chromium and n-hexadecane ions (above 99%) compared to free lipases [].
Cellulose-based supports have high chemical purity and can bring unique modular benefits in the immobilization of enzymes [,]. Zywicka et al. [] increased citric acid yield by modulating Y. lipolytica with carriers based on bacterial cellulose (BC) []. Furthermore, it was shown that BC-modulated yeast utilized more glucose than free lipases. Bacterial cellulose as a modulator of Y. lipolytica is widely used in the food and cosmetics industries, linking benefits associated with low toxicity and high biocompatibility [,]. For example, Y. lipolytica was immobilized on bacterial cellulose to improve the production of natural lactone gamma-decalactone (GDL). The authors showed that the maximum concentration of GDL in a culture prepared with lipase biotransformed with cellulose was 3.7 times greater than the medium with an alginate-only carrier [].
Chitosan has also shown excellent results in lipase immobilization. It is a polyamine with amino and hydroxyl groups, which are reactive to be used in covalent binding with enzymes, providing adequate stabilities to improve the chemical properties of the transporter [,]. In this sense, several studies link this carrier to the modulation of Y. lipolytica [,]. Pereira et al. [] immobilized Y. lipolytica by ionotropic gelation with alginate and chitosan as encapsulating agents. It was shown that the optimum reaction temperature for microencapsulated lipase was higher than for free lipase and the optimum pH. A protective effect of the microcapsule was detected in the storage of the enzyme, where after 75 days, 35% of the activity was maintained, while no activity remained in the free enzyme [].
As shown, enzymatic modulation is vast and necessary, allowing Y. lipolytica to work in different ranges of temperature, pH, and medium, in addition to generating better stability in its storage when compared to free enzymes.
5.2. Physical or Chemical Modification of Y. lipolytica Lipases to Modulate Enzymatic Properties
As mentioned, different strains of Y. lipolytica have different lipases that must be immobilized in a particular way. The physical or chemical modifications necessary for immobilization are directly related to the industrial application that the biocatalyst will perform, working temperature and pH, storage time, operating cycles, and yield of biocatalysis [,].
Various supports capable of immobilizing enzymes often require activations, functionalizations, or modifications of their structure in order to carry out physical interactions such as adsorption or entrapment, chemical interactions such as covalent or cross-linked bonds, or heterobifunctional interactions that, in turn, carry out the two forms of immobilization, providing better stability of the biocatalyst [,,].
Supports that do not have a porous or reactive surface need to seek surface modifications, performing a physical modification, such as increasing their porosity, as is the case of mesoporous silica, for enzymatic imprisonment or carrying out chemical modifications, creating reactive groups [,,,,]. Among several possible materials capable of carrying out chemical modifications, the epoxy groups, polyethyleneimine (PEI) and glutaraldehyde, deserve to be highlighted. Among these, PEI and glutaraldehyde were successful in immobilizing Y. lipolytica lipases [].
In a work that aimed to modify surface surfaces, polyurethane foam (PUF) was used to immobilize Lip2 lipase to Y. lipolytica via polyethyleneimine coating (PEI) and glutaraldehyde coupling. It was found that the activity of the immobilized lipases depends on the size of the PEI polymers and the form of glutaraldehyde treatment, with better results obtained for the covalent binding enzyme in PEI-PUF activated by glutaraldehyde (MW 70,000 Da), which had activity 1.7 times higher compared to the same enzyme immobilized without PEI and glutaraldehyde [].
The physical and chemical modifications of the support for the more efficient immobilization of lipases have been growing more and more through the search for new reactive materials to bind the enzyme, ways to make the material more susceptible to carry out the imprisonment of the enzyme, or even to correlate these two forms of immobilization to obtain better stability of the carrier.
5.3. Co-Immobilization of Lipases from Y. lipolytica and Other Enzymes
Using co-immobilized enzymes creates a multi-enzyme biocatalyst design, producing several kinetic advantages making chemoenzymatic cascade reactions [,]. A cascade reaction is when the product of enzyme one serves as enzyme-substrate two and involves multiple sequentially active enzymes in a synthetic pathway [,]. Its main advantage is to reduce the reaction delay time; however, this can be critical when the product of the first enzyme is unstable [,]. In this sense, co-immobilizing enzymes can generate several technological problems, making them require careful economic analysis to be advantageous []. Figure 9 presents some multienzyme immobilization techniques.
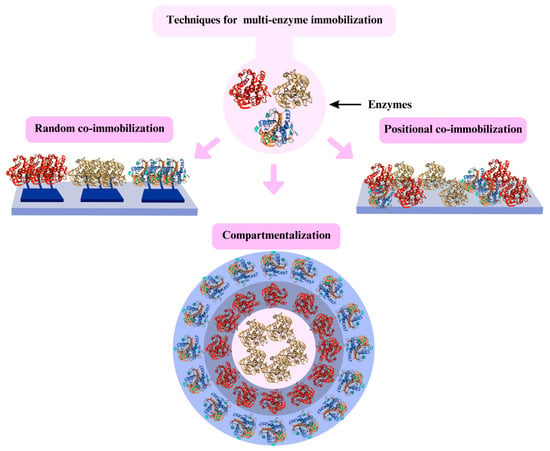
Figure 9.
Three techniques for multi-enzyme immobilization.
Only one study from 1995 was found in the literature associated with the co-immobilization of Y. lipolytica. Mansfeld et al. [] co-immobilized Investase and Y. lipolytica in complex polyelectrolyte microcapsules prepared from sodium cellulose sulfate and poly (dimethyldialylomonium chloride). The co-immobilized system was subjected to 800–1000 h of continuous fermentation; however, its productivity was not higher than encapsulated Y. lipolytica alone, without invertase. The co-immobilized system’s average citric acid production rate was 0.014 g L−1h−1, while the system without invertase achieved 0.125 and 0.25 g L−1h−1 [].
Y. lipolytica and other enzymes may present kinetic advantages as long aswhich second enzyme to use and how to carry out the co-immobilization procedure are investigated.
6. Bioreactor Designs for Uses of Lipases from Y. lipolytica
The lipases (E.C.3.1.1.3) produced by Y. lipolytica are of great industrial interest, as they are efficient biocatalysts used in esterification [], transesterification [], interesterification [], and even hydrolysis reactions [,]. Among the various applications, their use in obtaining glycerides, aromas, wastewater treatment, and optically active drugs from the kinetic resolution are widely used in organic synthesis []. The reuse of these enzymes is another parameter that makes their use even more favorable. The reaction conditions are mild, and for specific substrates, they present high efficiency, being necessary to control the reaction conditions and the excellent choice of the bioreactor [].
Generally, lipases are produced intracellularly, more attached explicitly to the yeast cell walls, and only then are they excreted into the culture medium when carbon amounts become scarce [,]. Y. lipolytica is considered one of the most promising sources due to its ability to metabolize the raw substrate, express genes at very high levels, and secrete proteins on a large scale, parameters that are influenced by the specifications of the bioreactor in question [].
Considering many favorable substrates for Y. lipolytica to act efficiently, a series of industrial processes emphasizing the application of these lipases have been explored []. Thus, an approach focused on developing bioreactors is necessary to maximize efficiency. For this, in addition to reaction parameters such as pH [], temperature [], concentration, and composition of the medium [], other dependent factors can also be evaluated, such as the oxygenation of the medium []; all of this is carried out to ensure that these biotechnological processes, in addition to being environmentally friendly, are also economically favorable []. In Figure 10, it is possible to highlight some of the main benefits that a well-chosen bioreactor for biocatalysis can present.
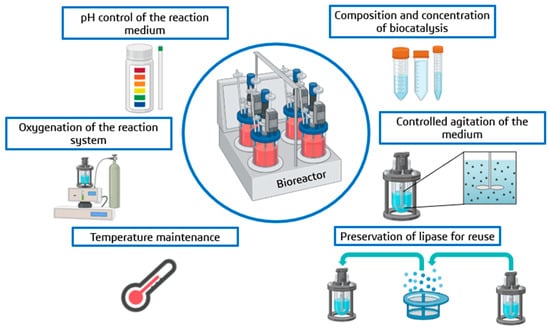
Figure 10.
Main benefits of a bioreactor in conventional enzymatic catalysis reactions, from its operational issues to the optimization of specific parameters.
The design of these bioreactors is designed to optimize the reaction conditions and increase the biocatalysis yield []. Due to the release of lipases occurring to a greater or lesser extent, an efficient bioreactor allows changes in the dependent parameters []. Some of these bioreactors can control the oxygenation conditions so that the excretion of these lipases is accentuated; agitation control is employed to increase the surface contact of the enzyme with the substrate in question and temperature control is used to reach the ideal enzymatic activity, and among others []. A practical issue is that most of these bioreactors allow the rescue of the enzyme if immobilized quickly, allowing its reuse in successive reactions [].
Table 3 shows some types of bioreactors and the main applications of lipases produced by Y. lipolytica, showing the versatility of these biocatalysts produced by this yeast and presenting different designs of bioreactors used in biocatalysis.

Table 3.
Bioreactors used in enzymatic reactions with lipases produced by the organism Y. lipolytica and their main chemical and biotechnological applications.
7. Future Trends
Given the presentation of all this academic and quantitative context exposed above, we observe the insertion and adaptation of Y. lipolytica in different industrial biotechnological applications so that we can question ourselves about future trends in the market for these lipases. An in-depth bibliometric analysis using the right keywords can also generate a reasonable forecast of future trends in the subject. With the bibliometric analysis, it is possible to analyze the keywords that appear the most on the subject, its evolution over time, the possibility of analyzing the most cited articles, and which subjects within the subject have been addressed in recent years. With that, Figure 11, used from the bibliometric analysis carried out on the topic “2. Y. lipolytica context in a bibliometric review”, demonstrates, through keywords and a thematic map, the future perspectives for the theme related to Y. lipolytica. Figure 11a shows the keywords obtained. It is possible to see the formation of five clusters, where the words Y. lipolytica, lipase, and enzymes appear as central themes, making connections with all other themes. Figure 11b shows the keywords obtained and their citation evolution over time. It is possible to observe that lipid production, carbon, and crude glycerol are the most recent themes related to Y. lipolytica. Figure 11c shows a thematic map relating the emerging or declining, essential, niche, and motor themes related to Y. lipolytica. We can see that Y. lipolytica and purification are basic themes related to each other. However, expression and yeast also show up as basic themes in a smaller number of articles. Candida rugosa and hydrolysis are niche topics, along with lipase production and glycerol. The oil and enzymes cluster stands out as the central theme. It generates a motor theme using essential, niche, and emerging themes. One of the future prospects for Y. lipolytica is finding increasingly better ways for it to be used as a free fatty acid catalyst for forming sustainable biofuels, whether used in its free form or immobilized by the various different supports obtained in materials engineering.
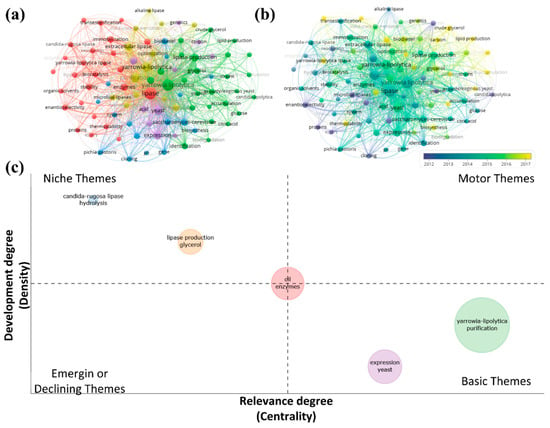
Figure 11.
Data were obtained between 2000 and 2022 with the keywords Y. lipolytica and lipase. (a) Network visualization with cluster-forming keywords; minimum occurrences of keywords 5, 2387, and 197 meet the threshold. (b) Overlay visualization with cluster-forming keywords; minimum occurrences of a keyword 5, 2387, and 197 meet the threshold. (c) Thematic map of the clusters formed from the keywords.
Table 4 summarizes the clusters formed with the database used, showing the centrality and density of each cluster and the number of times this cluster was mentioned.

Table 4.
Formation of clusters obtained by keywords related to Y. lipolytica.
Table 5 summarizes the articles that represent each cluster, with the most cited articles forming the most significant number of nodes and providing the scientific community with a new line of research related to the area. The numbers of each cluster (#oil, #yarrowia-lipolytica, #candida-rugosa lipase, #expression, #lipase production) in front of each article represent the article’s influence on the formation of that specific cluster. The #oil cluster presents itself as a future perspective of the Y. lipolytica theme and is represented by the articles entitled “Two-Step Synthesis of Fatty Acid Ethyl Ester From Soybean Oil Catalyzed By Y. lipolytica Lipase” and “Different Strategies for The Lipase Immobilization On The Chitosan Based Supports And Their Applications.” The first article shows the relationship between the esterification of a solvent-free system and without glycerol release for the production of biodiesel, developing two-step catalysis of soybean oil hydrolysis followed by esterification with Y. lipolytica lipase []. The second article deals with a new support for lipase immobilization, intending to generate better lipase stability and used for various purposes []. Other future trends regarding Y. lipolytica research can be predicted and correlated with the formation of clusters. For example, an important front is global climate change, which has required all nations to seek to reduce CO2 emissions [,]. Thus, there is a constant need to improve technology capable of assisting in the development of renewable energies and zero-emission biofuels, where biotechnological advances in metabolic engineering have already managed to expand, for example, the capacity of Y. lipolytica to degrade these carbon sources [,,]. In addition, this adaptive and receptive capacity brings a range of studies aimed at its genetic improvement.

Table 5.
Articles that promoted the formation of clusters obtained by keywords related to Y. lipolytica.
8. Patents with Lipases from Y. lipolytica
Notably, the volume of bibliographic material about Y. lipolytica is significant, and since 2013, more than 100 articles have been published annually. Subjects such as genetic sequencing, genomic scale modeling, and multi-omics approaches have gained notoriety in parallel with Y. lipolytica. In the academic scenario, research on this yeast significantly impacts natural science. In parallel with academia, industrial research has noted the valuable potential of Y. lipolytica for systemic applications within industrial production. In searches by research platforms or robust databases on patents such as the United States Patent and Trademark office—USPTO []; II. European-Patent Office—EPO [] found in 1951—2022 the volume of 7242 international patents containing Y. lipolytica.
It should be noted that the volume of patents granted or filed has increased in just over 30 years, with industries being the main ones responsible for this exacerbated increase, representing over 80% of patents filed or granted, while academia only represents about 16% of that total. This increase is due to the development of engineering or computational tools that allow greater depth in the analysis of this yeast. The graph in Figure 12 presents the above data with greater visibility, showing the growth in the volume of patents over the last 30 years.
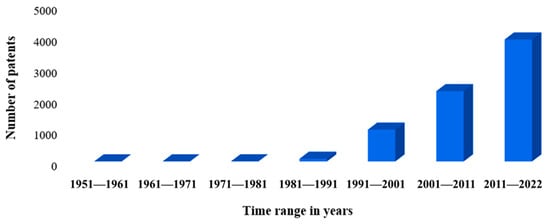
Figure 12.
Primary graphic: number of patents by interval in years.
It is also noteworthy that some global potentials stand out when these data are related to the countries where the patent was filed. United States, China, and Europe occupy 1st, 2nd, and 3rd place in the ranking of most patents deposited. With the Americans responsible for about 59.18% of the patents granted, the Chinese for 34.63%, and the Europeans responsible for about 5.06%. In Figure 13, the graph presents these data in a visual way and with greater clarity than the data collected from the patent search platforms. It is worth remembering that the fundamental language of the scientific writing of these patents is English, with 7242 patents granted, and 7125 in the other languages reported above. In summary, the patent scenario for Y. lipolytica is encouraging and has been growing vehemently, seeking more and more different applications of this yeast in the industrial field.
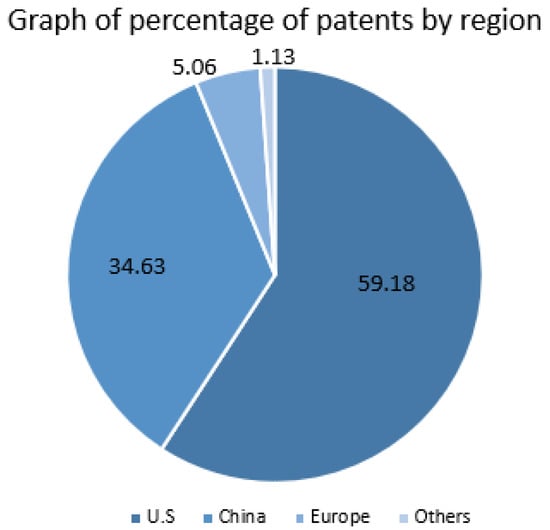
Figure 13.
Graphic Y- Percentage of patents by region.
9. Conclusions
This work shows that over the years, in academia, several studies have been conducted on using lipases derived from Y. lipolytica. Through these applications that aimed to identify their properties and characteristics in different processes, there was an improvement in the use and more efficient immobilization methods of these lipases in different industrial sectors, where through these advances, their applications are occupying even more space in the area of biotechnology applied in industry and in bioremediation of contaminated areas, which helps to reduce waste generation and treatment of damaged areas.
As we have seen, two of the major industrial powers in the world, the USA and China, currently occupy the rankings with the highest percentages of patents obtained over the past 30 years, so we can say that the search for improvement of the properties of Y. lipolytica should continue steadily. It is a matter of time before its rise happens in a controlled manner, resulting in large-scale production and allowing even more reduction of costs in the process. As we have seen, the role of engineering and computational tools against the discoveries made so far are fundamental and already allow the induction and manipulation of the functions of these lipases and their ability to adapt to different environments. We have left an opening for more and more researchers and students to seek ways to create a control system that can develop the production of Y. lipolytica and its lipases in a unified way for the most significant number of industrial applications.
Author Contributions
Conceptualization, J.L.d.S., M.B.S., F.S.N., V.d.C.B., M.M.R.N., A.K.d.S.B., A.M.d.F., A.L.G.C., P.d.S.S., R.L.F.M., P.G.D.S.J. and J.C.S.d.S.; methodology, J.L.d.S., M.B.S., F.S.N., V.d.C.B., M.M.R.N., A.M.d.F., A.L.G.C., P.d.S.S., R.L.F.M., P.G.D.S.J. and J.C.S.d.S.; software, J.L.d.S., M.B.S., F.S.N., V.d.C.B., M.M.R.N., A.M.d.F., A.L.G.C., P.d.S.S., R.L.F.M., P.G.D.S.J. and J.C.S.d.S.; validation, J.L.d.S., M.B.S., F.S.N., V.d.C.B., M.M.R.N., A.M.d.F., A.K.d.S.B., A.L.G.C., P.d.S.S., R.L.F.M., P.G.D.S.J. and J.C.S.d.S.; formal analysis, J.L.d.S., M.B.S., F.S.N., V.d.C.B., M.M.R.N., A.M.d.F., A.L.G.C., P.d.S.S., R.L.F.M., P.G.D.S.J. and J.C.S.d.S.; investigation, J.L.d.S., M.B.S., F.S.N., V.d.C.B., M.M.R.N., A.M.d.F., A.L.G.C., P.d.S.S., R.L.F.M., P.G.D.S.J. and J.C.S.d.S.; resources, J.L.d.S., M.B.S., F.S.N., V.d.C.B., M.M.R.N., A.M.d.F., A.L.G.C., P.d.S.S., R.L.F.M., P.G.D.S.J. and J.C.S.d.S.; data curation, J.L.d.S., M.B.S., F.S.N., V.d.C.B., M.M.R.N., A.M.d.F., A.L.G.C., P.d.S.S., R.L.F.M., P.G.D.S.J. and J.C.S.d.S.; writing—original draft preparation, F.S.N., M.B.S., A.K.d.S.B., V.d.C.B., F.S.N., J.C.S.d.S., J.L.d.S., M.B.S., F.S.N., V.d.C.B., M.M.R.N., A.M.d.F., A.L.G.C., P.d.S.S., R.L.F.M., P.G.D.S.J. and J.C.S.d.S.; writing—review and editing, J.L.d.S., M.B.S., F.S.N., V.d.C.B., M.M.R.N., A.M.d.F., A.L.G.C., P.d.S.S., R.L.F.M., P.G.D.S.J. and J.C.S.d.S.; visualization, J.L.d.S., M.B.S., F.S.N., V.d.C.B., M.M.R.N., A.M.d.F., A.L.G.C., P.d.S.S., R.L.F.M., P.G.D.S.J. and J.C.S.d.S.; supervision, J.C.S.d.S.; project administration, J.C.S.d.S.; funding acquisition, J.C.S.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We gratefully acknowledge the following Brazilian Agencies for Scientific and Technological Development: Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP) (PS1-0186-00216.01.00/21), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (307454/2022-3), and Coordenação de Aperfeiçoamento de Ensino Superior (CAPES) (finance code 001).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heux, S.; Meynial-Salles, I.; O’Donohue, M.J.; Dumon, C. White Biotechnology: State of the Art Strategies for the Development of Biocatalysts for Biorefining. Biotechnol. Adv. 2015, 33, 1653–1670. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef] [PubMed]
- Singhania, D.M.; Saini, D.N. Systems Approach to Environment, Social and Governance (ESG): Case of Reliance Industries. Sustain. Oper. Comput. 2022, 3, 103–117. [Google Scholar] [CrossRef]
- De Sousa, I.G.; Mota, G.F.; Cavalcante, A.L.G.; Rocha, T.G.; Da Silva Sousa, P.; Holanda Alexandre, J.Y.N.; Da Silva Souza, J.E.; Neto, F.S.; Cavalcante, F.T.T.; Lopes, A.A.S.; et al. Renewable Processes of Synthesis of Biolubricants Catalyzed by Lipases. J. Environ. Chem. Eng. 2023, 11, 109006. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Cavalcante, A.L.G.; de Sousa, I.G.; Neto, F.S.; dos Santos, J.C.S. Current Status and Future Perspectives of Supports and Protocols for Enzyme Immobilization. Catalysts 2021, 11, 1222. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; de A. Falcão, I.R.; da S. Souza, J.E.; Rocha, T.G.; de Sousa, I.G.; Cavalcante, A.L.G.; de Oliveira, A.L.B.; de Sousa, M.C.M.; dos Santos, J.C.S. Designing of Nanomaterials-Based Enzymatic Biosensors: Synthesis, Properties, and Applications. Electrochem 2021, 2, 149–184. [Google Scholar] [CrossRef]
- Valério, R.B.R.; da Silva, N.A.; Junior, J.R.P.; Chaves, A.V.; de Oliveira, B.P.; Souza, N.F.; de Morais, S.M.; dos Santos, J.C.S.; Abreu, F.O.M.d.S. Chitosan-Based Nanoparticles for Cardanol-Sustained Delivery System. Polymers 2022, 14, 4695. [Google Scholar] [CrossRef]
- dos Santos, J.C.S.; Dhenadhayalan, N.; Li, Y.; Pinilla, J.L. Editorial: Chemical Reactions and Catalysis for a Sustainable Future. Front. Chem. 2023, 11, 100248. [Google Scholar] [CrossRef]
- Nogueira, R.C.; Neto, F.S.; Junior, P.G.d.S.; Valério, R.B.R.; Serpa, J.d.F.; Lima, A.M.d.S.; de Souza, M.C.M.; de Lima, R.K.C.; Lopes, A.A.S.; Guimarães, A.P.; et al. Research Trends and Perspectives on Hydrothermal Gasification in Producing Biofuels. Energy Nexus 2023, 10, 100199. [Google Scholar] [CrossRef]
- Haque, S.; Singh, R.; Harakeh, S.; Teklemariam, A.D.; Alharthy, S.A.; Tripathi, S.C.; Singh, R.P.; Aly Hassan, A.; Srivastava, N.; Gupta, V.K. Enzyme-Based Biocatalysis for the Treatment of Organic Pollutants and Bioenergy Production. Curr. Opin. Green Sustain. Chem. 2022, 38, 100709. [Google Scholar] [CrossRef]
- Domínguez de María, P. Biocatalysis, Sustainability, and Industrial Applications: Show Me the Metrics. Curr. Opin. Green Sustain. Chem. 2021, 31, 100514. [Google Scholar] [CrossRef]
- Brandão Júnior, J.; Andrade do Nascimento, J.G.; França Silva, M.P.; Lima Brandão, E.d.A.; de Castro Bizerra, V.; dos Santos, K.M.; Serpa, J.d.F.; dos Santos, J.C.S.; da Fonseca, A.M.; Vasconcelos de Oliveira, D.L.; et al. Performance of Eversa Transform 2.0 Lipase in Ester Production Using Babassu Oil (Orbignya sp.) and Tucuman Oil (Astrocaryum vulgar): A Comparative Study between Liquid and Immobilized Forms in Fe3O4 Nanoparticles. Catalysts 2023, 13, 571. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Ortiz, C.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Lecitase Ultra: A Phospholipase with Great Potential in Biocatalysis. Mol. Catal. 2019, 473, 110405. [Google Scholar] [CrossRef]
- Moreira, K.S.; Moura Júnior, L.S.; Monteiro, R.R.C.; de Oliveira, A.L.B.; Valle, C.P.; Freire, T.M.; Fechine, P.B.A.; de Souza, M.C.M.; Fernandez-Lorente, G.; Guisan, J.M.; et al. Optimization of the Production of Enzymatic Biodiesel from Residual Babassu Oil (Orbignya sp.) via RSM. Catalysts 2020, 10, 414. [Google Scholar] [CrossRef]
- Fickers, P.; Marty, A.; Nicaud, J.M. The Lipases from Yarrowia lipolytica: Genetics, Production, Regulation, Biochemical Characterization and Biotechnological Applications. Technol. Biotechnol. Adv. 2011, 29, 632–644. [Google Scholar] [CrossRef]
- Akil, E.; Carvalho, T.; Bárea, B.; Finotelli, P.; Lecomte, J.; Torres, A.G.; Amaral, P.; Villeneuve, P. Accessing Regio-and Typo-Selectivity of Yarrowia lipolytica Lipase in Its Free Form and Immobilized onto Magnetic Nanoparticles. Biochem. Eng. J. 2016, 109, 101–111. [Google Scholar] [CrossRef]
- Barrera-Rivera, K.A.; Martínez-Richa, A. Yarrowia lipolytica Extracellular Lipase Lip2 as Biocatalyst for the Ring-Opening Polymerization of ε-Caprolactone. Molecules 2017, 22, 1917. [Google Scholar] [CrossRef]
- Bruder, S.; Moldenhauer, E.J.; Lemke, R.D.; Ledesma-Amaro, R.; Kabisch, J. Drop-in Biofuel Production Using Fatty Acid Photodecarboxylase from Chlorella Variabilis in the Oleaginous Yeast Yarrowia lipolytica. Technol. Biotechnol. Biofuels 2019, 12, 202. [Google Scholar] [CrossRef]
- Shimada, Y.; Watanabe, Y.; Sugihara, A.; Tominaga, Y. Enzymatic Alcoholysis for Biodiesel Fuel Production and Application of the Reaction to Oil Processing. J. Mol. Catal. B Enzym. 2002, 17, 133–142. [Google Scholar] [CrossRef]
- Mota, G.F.; de Sousa, I.G.; de Oliveira, A.L.B.; Cavalcante, A.L.G.; Moreira, K.d.S.; Cavalcante, F.T.T.; Souza, J.E.d.S.; Falcão, Í.R.d.A.; Rocha, T.G.; Valério, R.B.R.; et al. Biodiesel Production from Microalgae Using Lipase-Based Catalysts: Current Challenges and Prospects. Algal Res. 2022, 62, 102616. [Google Scholar] [CrossRef]
- Park, Y.K.; Ledesma-Amaro, R. What Makes Yarrowia lipolytica Well Suited for Industry? Trends Technol. Biotechnol. 2023, 41, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, J.; Xu, J.; Zhang, T.; Deng, Y.; He, J. Citric Acid Production in Yarrowia lipolytica SWJ-1b Yeast When Grown on Waste Cooking Oil. Appl. Biochem. Technol. Biotechnol. 2015, 175, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Finogenova, T.V.; Lunina, Y.N.; Perevoznikova, O.A.; Minachova, L.N.; Morgunov, I.G. Characteristics of the Growth on Rapeseed Oil and Synthesis of Citric and Isocitric Acids by Yarrowia lipolytica Yeasts. Microbiology 2007, 76, 20–24. [Google Scholar] [CrossRef]
- Walker, C.; Ryu, S.; Trinh, C.T. Exceptional Solvent Tolerance in Yarrowia lipolytica Is Enhanced by Sterols. Metab. Eng. 2019, 54, 83–95. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-Tool for Comprehensive Science Mapping Analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Omotehinwa, T.O. Examining the Developments in Scheduling Algorithms Research: A Bibliometric Approach. Heliyon 2022, 8, e09510. [Google Scholar] [CrossRef] [PubMed]
- Ramdass, A.C.; Rampersad, S.N. Detection and Diversity of the Mannosylerythritol Lipid (MEL) Gene Cluster and Lipase A and B Genes of Moesziomyces Antarcticus Isolated from Terrestrial Sites Chronically Contaminated with Crude Oil in Trinidad. BMC Microbiol. 2022, 22, 43. [Google Scholar] [CrossRef]
- Anantayanon, J.; Jeennor, S.; Panchanawaporn, S.; Chutrakul, C.; Laoteng, K. Significance of Two Intracellular Triacylglycerol Lipases of Aspergillus Oryzae in Lipid Mobilization: A Perspective in Industrial Implication for Microbial Lipid Production. Gene 2021, 793, 145745. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Chu, Y.; Zhang, Q.; Zhou, R.; Yu, D.; Wang, S.; Lyu, L.; Xu, G.; Zhao, Z.K. Genetic Manipulation of the Interconversion between Diacylglycerols and Triacylglycerols in Rhodosporidium Toruloides. Front. Bioeng. Technol. Biotechnol. 2022, 10, 2026. [Google Scholar] [CrossRef]
- Zan, X.; Cui, F.; Sun, J.; Zhou, S.; Song, Y. Novel Dual-Functional Enzyme Lip10 Catalyzes Lipase and Acyltransferase Activities in the Oleaginous Fungus Mucor Circinelloides. J. Agric. Food Chem. 2019, 67, 13176–13184. [Google Scholar] [CrossRef]
- Wierzchowska, K.; Zieniuk, B.; Fabiszewska, A. Use of Non-Conventional Yeast Yarrowia lipolytica in Treatment or Upgradation of Hydrophobic Industry Wastes. Waste Biomass Valorization 2021, 13, 757–779. [Google Scholar] [CrossRef]
- Verdasco-Martín, C.M.; Villalba, M.; dos Santos, J.C.S.; Tobajas, M.; Fernandez-Lafuente, R.; Otero, C. Effect of Chemical Modification of Novozym 435 on Its Performance in the Alcoholysis of Camelina Oil. Biochem. Eng. J. 2016, 111, 75–86. [Google Scholar] [CrossRef]
- Sales, M.B.; Borges, P.T.; Ribeiro Filho, M.N.; Miranda da Silva, L.R.; Castro, A.P.; Sanders Lopes, A.A.; Chaves de Lima, R.K.; de Sousa Rios, M.A.; dos Santos, J.C.S. Sustainable Feedstocks and Challenges in Biodiesel Production: An Advanced Bibliometric Analysis. Bioengineering 2022, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.Y.N.H.; Cavalcante, F.T.T.; Freitas, L.M.; Castro, A.P.; Borges, P.T.; de Sousa Junior, P.G.; Filho, M.N.R.; Lopes, A.A.S.; da Fonseca, A.M.; Lomonaco, D.; et al. A Theoretical and Experimental Study for Enzymatic Biodiesel Production from Babassu Oil (Orbignya sp.) Using Eversa Lipase. Catalysts 2022, 12, 1322. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; Rocha-Martin, J.; dos Santos, J.C.S. Editorial: Designing Carrier-Free Immobilized Enzymes for Biocatalysis. Front. Bioeng. Technol. Biotechnol. 2022, 10, 823. [Google Scholar] [CrossRef]
- Luthierre Gama Cavalcante, A.; Gama Cavalcante, C.; Paulo Colares, R.; Alves Ferreira, D.; Felipe Maia da Silva, F.; Yvay Almeida de Sousa, E.; Erick da Silva Souza, J.; Ramilton de Castro Monteiro, R.; Luiz Barros de Oliveira, A.; Cleiton Sousa dos Santos, J.; et al. Preparation, Characterization, and Enantioselectivity of Polyacrylate Microcapsules Entrapping Ananas Comosus Extract. Rev. Virtual Química 2021, 13, 1319–1329. [Google Scholar] [CrossRef]
- Carvalho, A.C.L.d.M.; de Oliveira, B.R.; Lima, G.V.; Negreiro, J.M.; Oliveira, M.C.F.; de Lemos, T.L.G.; da Silva, M.R.; Fonseca, T.d.S.; Bezerra, R.M.; dos Santos, J.C.S.; et al. Resolution of Racemic Aryloxy-Propan-2-Yl Acetates via Lipase-Catalyzed Hydrolysis: Preparation of Enantiomerically Pure/Enantioenriched Mexiletine Intermediates and Analogs. Catalysts 2022, 12, 1566. [Google Scholar] [CrossRef]
- Zinjanab, M.S.; Golmakani, M.T.; Eskandari, M.H.; Toh, M.; Liu, S.Q. Yeast-Lactobacillus Co-Cultures as in Situ Ethanol Producers for Flavor Ester Synthesis Using Lipase in Fermented Milks. Appl. Food Biotechnol. 2021, 8, 151–160. [Google Scholar] [CrossRef]
- Yang, K.; Qiao, Y.; Li, F.; Xu, Y.; Yan, Y.; Madzak, C.; Yan, J. Subcellular Engineering of Lipase Dependent Pathways Directed towards Lipid Related Organelles for Highly Effectively Compartmentalized Biosynthesis of Triacylglycerol Derived Products in Yarrowia lipolytica. Metab. Eng. 2019, 55, 231–238. [Google Scholar] [CrossRef]
- Snopek, P.; Nowak, D.; Zieniuk, B.; Fabiszewska, A. Aeration and Stirring in Yarrowia lipolytica Lipase Biosynthesis during Batch Cultures with Waste Fish Oil as a Carbon Source. Fermentation 2021, 7, 88. [Google Scholar] [CrossRef]
- Osuji, G.; Johnson, P. Structural Properties of the RNA Synthesized by Glutamate Dehydrogenase for the Degradation of Total RNA. Adv. Enzym. Res. 2018, 6, 29–52. [Google Scholar] [CrossRef]
- Nobusawa, T.; Yamakawa-Ayukawa, K.; Saito, F.; Nomura, S.; Takami, A.; Ohta, H. A Homolog of Arabidopsis SDP1 Lipase in Nannochloropsis Is Involved in Degradation of de Novo-Synthesized Triacylglycerols in the Endoplasmic Reticulum. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2019, 1864, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Wierzchowska, K.; Zieniuk, B.; Nowak, D.; Fabiszewska, A. Phosphorus and Nitrogen Limitation as a Part of the Strategy to Stimulate Microbial Lipid Biosynthesis. Appl. Sci. 2021, 11, 11819. [Google Scholar] [CrossRef]
- Marullo, P.; Trujillo, M.; Viannais, R.; Hercman, L.; Guillaumie, S.; Colonna-Ceccaldi, B.; Albertin, W.; Barbe, J.C. Metabolic, Organoleptic and Transcriptomic Impact of Saccharomyces cerevisiae Genes Involved in the Biosynthesis of Linear and Substituted Esters. Int. J. Mol. Sci. 2021, 22, 4026. [Google Scholar] [CrossRef]
- Nour, H.; Daoui, O.; Abchir, O.; ElKhattabi, S.; Belaidi, S.; Chtita, S. Combined Computational Approaches for Developing New Anti-Alzheimer Drug Candidates: 3D-QSAR, Molecular Docking and Molecular Dynamics Studies of Liquiritigenin Derivatives. Heliyon 2022, 8, e11991. [Google Scholar] [CrossRef] [PubMed]
- Soong, Y.H.V.; Liu, N.; Yoon, S.; Lawton, C.; Xie, D. Cellular and Metabolic Engineering of Oleaginous Yeast Yarrowia lipolytica for Bioconversion of Hydrophobic Substrates into High-Value Products. Eng. Life Sci. 2019, 19, 423–443. [Google Scholar] [CrossRef]
- Robert, J.M.; Betancur, M.O.; Machado, A.C.O.; Arruda, A.; Reis, V.C.B.; Almeida, R.V.; Torres, F.A.G.; Alegre, P.F.; Valero, F.; Freire, D.M.G. Increase of Candida Antarctica Lipase B Production under PGK Promoter in Pichia Pastoris: Effect of Multicopies. Braz. J. Microbiol. 2019, 50, 405–413. [Google Scholar] [CrossRef]
- Dervisi, I.; Valassakis, C.; Agalou, A.; Papandreou, N.; Podia, V.; Haralampidis, K.; Iconomidou, V.A.; Kouvelis, V.N.; Spaink, H.P.; Roussis, A. Investigation of the Interaction of DAD1-LIKE LIPASE 3 (DALL3) with Selenium Binding Protein 1 (SBP1) in Arabidopsis thaliana. Plant. Sci. 2020, 291, 110357. [Google Scholar] [CrossRef]
- Ali, S.S.; Al-Tohamy, R.; Koutra, E.; El-Naggar, A.H.; Kornaros, M.; Sun, J. Valorizing Lignin-like Dyes and Textile Dyeing Wastewater by a Newly Constructed Lipid-Producing and Lignin Modifying Oleaginous Yeast Consortium Valued for Biodiesel and Bioremediation. J. Hazard. Mater. 2021, 403, 123575. [Google Scholar] [CrossRef]
- Theron, C.W.; Vandermies, M.; Telek, S.; Steels, S.; Fickers, P. Comprehensive Comparison of Yarrowia lipolytica and Pichia Pastoris for Production of Candida Antarctica Lipase B. Sci. Rep. 2020, 10, 1741. [Google Scholar] [CrossRef]
- Martínez-Corona, R.; Carlos González-Hernández, J.; Biomédicas, C. Hongos Y Levaduras: Fábricas De Lipasas. Interciencia 2019, 44, 378–385. [Google Scholar]
- Mohamed, S.S.; Ahmed, H.M.; El-Bendary, M.A.; Moharam, M.E.; Amin, H.A. Response Surface Methodology for Optimization of Rhizopus Stolonifer 1aNRC11 Mutant F Whole-Cell Lipase Production as a Biocatalyst for Methanolysis of Waste Frying Oil. Biocatal. Biotransformation 2021, 39, 232–240. [Google Scholar] [CrossRef]
- Brito e Cunha, D.A.; Bartkevihi, L.; Robert, J.M.; Cipolatti, E.P.; Ferreira, A.T.S.; Oliveira, D.M.P.; Gomes-Neto, F.; Almeida, R.V.; Fernandez-Lafuente, R.; Freire, D.M.G.; et al. Structural Differences of Commercial and Recombinant Lipase B from Candida Antarctica: An Important Implication on Enzymes Thermostability. Int. J. Biol. Macromol. 2019, 140, 761–770. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, J.; Benaiges, M.D.; Valero, F. Constitutive Expression in Komagataella Phaffii of Mature Rhizopus oryzae Lipase Jointly with Its Truncated Prosequence Improves Production and the Biocatalyst Operational Stability. Catalysts 2021, 11, 1192. [Google Scholar] [CrossRef]
- Xu, L.; Xiao, X.; Wang, F.; He, Y.; Yang, X.; Hu, J.; Feng, Z.; Yan, Y. Surface-Displayed Thermostable Candida Rugosa Lipase 1 for Docosahexaenoic Acid Enrichment. Appl. Biochem. Technol. Biotechnol. 2020, 190, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Janek, T.; Mirończuk, A.M.; Rymowicz, W.; Dobrowolski, A. High-Yield Expression of Extracellular Lipase from Yarrowia lipolytica and Its Interactions with Lipopeptide Biosurfactants: A Biophysical Approach. Arch. Biochem. Biophys. 2020, 689, 108475. [Google Scholar] [CrossRef]
- Colacicco, M.; Ciliberti, C.; Agrimi, G.; Biundo, A.; Pisano, I. Towards the Physiological Understanding of Yarrowia lipolytica Growth and Lipase Production Using Waste Cooking Oils. Energies 2022, 15, 5217. [Google Scholar] [CrossRef]
- Hao, Y.; Zheng, X.; Zhang, X.; Zhang, K.; Lin, Y.; Liang, S. Combined Strategies for Engineering a Novel Whole-Cell Biocatalyst of Candida Rugosa Lipase with Improved Characteristics. Biochem. Eng. J. 2019, 151, 107337. [Google Scholar] [CrossRef]
- Wachowska, U.; Pluskota, W.; Jastrzębski, J.P.; Głowacka, K.; Szablewska-Stuper, K.; Balcerzak, M. A Method for Reducing the Concentrations of Fusarium Graminearum Trichothecenes in Durum Wheat Grain with the Use of Debaryomyces Hansenii. Int. J. Food Microbiol. 2023, 397, 110211. [Google Scholar] [CrossRef]
- Matten, K.J.; Hashikawa, S.; Harada, K. Preclinical Safety Evaluation of Lipase OF from Candida Cylindracea. J. Appl. Toxicol. 2023, 43, 517–533. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Liang, L.; Godana, E.A.; Zhang, X.; Yang, X.; Wu, M.; Song, Y.; Zhang, H. Changes in Quality and Microbiome Composition of Strawberry Fruits Following Postharvest Application of Debaryomyces hansenii, a Yeast Biocontrol Agent. Postharvest. Biol. Technol. 2023, 202, 112379. [Google Scholar] [CrossRef]
- Zieniuk, B.; Mazurczak-Zieniuk, P.; Fabiszewska, A. Exploring the Impact of Lipid-Rich Food Industry Waste Carbon Sources on the Growth of Candida Cylindracea DSM 2031. Fermentation 2020, 6, 122. [Google Scholar] [CrossRef]
- Catumba, B.D.; Sales, M.B.; Borges, P.T.; Ribeiro Filho, M.N.; Lopes, A.A.S.; de Sousa Rios, M.A.; Desai, A.S.; Bilal, M.; dos Santos, J.C.S. Sustainability and Challenges in Hydrogen Production: An Advanced Bibliometric Analysis. Int. J. Hydrog. Energy 2022, 22, 7975–7992. [Google Scholar] [CrossRef]
- Chen, C.; Chitose, A.; Kusadokoro, M.; Nie, H.; Xu, W.; Yang, F.; Yang, S. Sustainability and challenges in biodiesel production from waste cooking oil: An advanced bibliometric analysis. Energy Rep. 2021, 7, 4022–4034. [Google Scholar] [CrossRef]
- Souza, J.E.d.S.; de Oliveira, G.P.; Alexandre, J.Y.N.H.; Neto, J.G.L.; Sales, M.B.; Junior, P.G.d.S.; de Oliveira, A.L.B.; de Souza, M.C.M.; dos Santos, J.C.S. A Comprehensive Review on the Use of Metal–Organic Frameworks (MOFs) Coupled with Enzymes as Biosensors. Electrochem 2022, 3, 89–113. [Google Scholar] [CrossRef]
- Rodrigues, A.F.S.; da Silva, A.F.; da Silva, F.L.B.; dos Santos, K.M.; de Oliveira, M.P.; Nobre, M.M.R.; Catumba, B.D.; Sales, M.B.; Silva, A.R.M.; Braz, A.K.S.; et al. A Scientometric Analysis of Research Progress and Trends in the Design of Laccase Biocatalysts for the Decolorization of Synthetic Dyes. Process. Biochem. 2023, 126, 272–291. [Google Scholar] [CrossRef]
- Sales, M.B.; Neto, J.G.L.; De Sousa Braz, A.K.; De Sousa Junior, P.G.; Melo, R.L.F.; Valério, R.B.R.; Serpa, J.d.F.; Da Silva Lima, A.M.; De Lima, R.K.C.; Guimarães, A.P.; et al. Trends and Opportunities in Enzyme Biosensors Coupled to Metal-Organic Frameworks (MOFs): An Advanced Bibliometric Analysis. Electrochem 2023, 4, 181–211. [Google Scholar] [CrossRef]
- Neto, F.S.; Fernandes de Melo Neta, M.M.; Sales, M.B.; Silva de Oliveira, F.A.; de Castro Bizerra, V.; Sanders Lopes, A.A.; de Sousa Rios, M.A.; dos Santos, J.C.S. Research Progress and Trends on Utilization of Lignocellulosic Residues as Supports for Enzyme Immobilization via Advanced Bibliometric Analysis. Polymers 2023, 15, 2057. [Google Scholar] [CrossRef]
- Abdelwahab, S.I.; Taha, M.M.E.; Moni, S.S.; Alsayegh, A.A. Bibliometric Mapping of Solid Lipid Nanoparticles Research (2012–2022) Using VOSviewer. Med. Nov. Technol. Devices 2023, 17, 100217. [Google Scholar] [CrossRef]
- Almeida, F.L.C.; Castro, M.P.J.; Travália, B.M.; Forte, M.B.S. Trends in Lipase Immobilization: Bibliometric Review and Patent Analysis. Process. Biochem. 2021, 110, 37–51. [Google Scholar] [CrossRef]
- Fickers, P.; Benetti, P.H.; Waché, Y.; Marty, A.; Mauersberger, S.; Smit, M.S.; Nicaud, J.M. Hydrophobic Substrate Utilisation by the Yeast Yarrowia lipolytica, and Its Potential Applications. FEMS Yeast Res. 2005, 5, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Muniglia, L.; Chevalot, I.; Aggelis, G.; Marc, I. Yarrowia lipolytica as a Potential Producer of Citric Acid from Raw Glycerol. J. Appl. Microbiol. 2002, 92, 737–744. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic Nanoparticles as Versatile Carriers for Enzymes Immobilization: A Review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, M.C.; Freire, D.M.G. A Review on Hydrolytic Enzymes in the Treatment of Wastewater with High Oil and Grease Content. Bioresour. Technol. 2006, 97, 2195–2210. [Google Scholar] [CrossRef] [PubMed]
- Nicaud, J.M.; Madzak, C.; Van Den Broek, P.; Gysler, C.; Duboc, P.; Niederberger, P.; Gaillardin, C. Protein Expression and Secretion in the Yeast Yarrowia lipolytica. FEMS Yeast Res. 2002, 2, 371–379. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z. Engineering Strategies for Enhanced Production of Protein and Bio-Products in Pichia Pastoris: A Review. Technol. Biotechnol. Adv. 2018, 36, 182–195. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Baudet, M.; Cuiné, S.; Adriano, J.M.; Barthe, D.; Billon, E.; Bruley, C.; Beisson, F.; Peltier, G.; Ferro, M.; et al. Proteomic Profiling of Oil Bodies Isolated from the Unicellular Green Microalga Chlamydomonas Reinhardtii: With Focus on Proteins Involved in Lipid Metabolism. Proteomics 2011, 11, 4266–4273. [Google Scholar] [CrossRef]
- Bankar, A.V.; Kumar, A.R.; Zinjarde, S.S. Environmental and Industrial Applications of Yarrowia lipolytica. Appl. Microbiol. Technol. Biotechnol. 2009, 84, 847–865. [Google Scholar] [CrossRef]
- Dulermo, T.; Nicaud, J.M. Involvement of the G3P Shuttle and Β-Oxidation Pathway in the Control of TAG Synthesis and Lipid Accumulation in Yarrowia lipolytica. Metab. Eng. 2011, 13, 482–491. [Google Scholar] [CrossRef]
- Lee, G.-H.; Bae, J.-H.; Suh, M.-J.; Kim, I.-H.; T’ou, C.; Kim, H.-R. New Finding and Optimal Production of a Novel Extracellular Alkaline Lipase from Yarrowia lipolytica NRRL Y-2178. J. Microbiol. Biotechnol. 2007, 17, 1054–1057. [Google Scholar]
- Ping, L.; Yuan, X.; Zhang, M.; Chai, Y.; Shan, S. Improvement of Extracellular Lipase Production by a Newly Isolated Yarrowia lipolytica Mutant and Its Application in the Biosynthesis of L-Ascorbyl Palmitate. Int. J. Biol. Macromol. 2018, 106, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Heard, G.M.; Fleet, G.H. Yarrowia (Candida) Lipolytica. In Encyclopedia of Food Microbiology; Richard, K.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 360–365. [Google Scholar]
- Liu, H.H.; Ji, X.J.; Huang, H. Biotechnological Applications of Yarrowia lipolytica: Past, Present and Future. Technol. Biotechnol. Adv. 2015, 33, 1522–1546. [Google Scholar] [CrossRef] [PubMed]
- Madzak, C. Yarrowia Lipolytica: Recent Achievements in Heterologous Protein Expression and Pathway Engineering. Appl. Microbiol. Technol. Biotechnol. 2015, 99, 4559–4577. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.A.G.; Colen, G.; Takahashi, J.A. Yarrowia lipolytica and Its Multiple Applications in the Biotechnological Industry. Sci. World J. 2014, 2014, 476207. [Google Scholar] [CrossRef]
- Nagappan, A.; Park, K.I.; Park, H.S.; Kim, J.A.; Hong, G.E.; Kang, S.R.; Lee, D.H.; Kim, E.H.; Lee, W.S.; Won, C.K.; et al. Vitamin C Induces Apoptosis in AGS Cells by Down-Regulation of 14-3-3σ via a Mitochondrial Dependent Pathway. Food Chem. 2012, 135, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Zheng, X.B.; Zhang, S.P.; Zheng, Y.G. Cloning, Expression and Characterization of a Lipase Gene from the Candida Antarctica ZJB09193 and Its Application in Biosynthesis of Vitamin A Esters. Microbiol. Res. 2012, 167, 452–460. [Google Scholar] [CrossRef]
- Yu, C.; Zhixin, L.; Zuyao, Z.; Feng, Z.; Duo, L.; Xianghuai, L.; Jianzhong, T.; Weimin, Z.; Bo, H. High Yield Antibiotic Producing Mutants of Streptomyces Erythreus Induced by Low Energy Ion Implantation. Nucl. Instrum. Methods Phys. Res. B 1998, 140, 341–348. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Deng, C.; Zhong, H.; Gu, T.; Goh, K.L.; Han, Z.; Zheng, M.; Zhou, Y. Green and Efficient Synthesis of Highly Liposoluble and Antioxidant L-Ascorbyl Esters by Immobilized Lipases. J. Clean Prod. 2022, 379, 134772. [Google Scholar] [CrossRef]
- Beopoulos, A.; Cescut, J.; Haddouche, R.; Uribelarrea, J.L.; Molina-Jouve, C.; Nicaud, J.M. Yarrowia lipolytica as a Model for Bio-Oil Production. Prog. Lipid Res. 2009, 48, 375–387. [Google Scholar] [CrossRef]
- Casaregola, S.; Neuvéglise, C.; Lépingle, A.; Bon, E.; Feynerol, C.; Artiguenave, F.; Wincker, P.; Gaillardin, C. Genomic Exploration of the Hemiascomycetous Yeasts: 17. Yarrowia lipolytica. FEBS Lett. 2000, 487, 95–100. [Google Scholar] [CrossRef]
- Alloue, W.A.M.; Destain, J.; Ongena, M.; Blecker, C.; Thonart, P. Effect of Monopropylene Glycol and Gamma Irradiation on Yarrowia lipolytica Lipase Stabilization. Prep. Biochem. Technol. Biotechnol. 2008, 38, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Al-Malah, K.; Azzam, M.O.J.; Abu-Lail, N.I. Olive Mills Effluent (OME) Wastewater Post-Treatment Using Activated Clay. Sep. Purif. Technol. 2000, 20, 225–234. [Google Scholar] [CrossRef]
- Scioli, C.; Vollaro, L. The use of Yarrowia lipolytica to reduce pollution in olive mill wastewaters. Water Res. 1997, 31, 2520–2524. [Google Scholar] [CrossRef]
- Song, H.; Zhou, L.; Zhang, L.; Gao, B.; Wei, D.; Shen, Y.; Wang, R.; Madzak, C.; Jiang, Z. Construction of a Whole-Cell Catalyst Displaying a Fungal Lipase for Effective Treatment of Oily Wastewaters. J. Mol. Catal. B Enzym. 2011, 71, 166–170. [Google Scholar] [CrossRef]
- Lanciotti, R.; Gianotti, A.; Baldi, D.; Angrisani, R.; Suzzi, G.; Mastrocola, D.; Guerzoni, M.E. Use of Yarrowia lipolytica Strains for the Treatment of Olive Mill Wastewater. Bioresour. Technol. 2005, 96, 317–322. [Google Scholar] [CrossRef]
- Oswal, N.; Sarma, P.M.; Zinjarde, S.S.; Pant, A. Palm Oil Mill Effluent Treatment by a Tropical Marine Yeast. Bioresour. Technol. 2002, 85, 35–37. [Google Scholar] [CrossRef]
- Gonçalves, C.; Lopes, M.; Ferreira, J.P.; Belo, I. Biological Treatment of Olive Mill Wastewater by Non-Conventional Yeasts. Bioresour. Technol. 2009, 100, 3759–3763. [Google Scholar] [CrossRef]
- Imandi, S.B.; Bandaru, V.V.R.; Somalanka, S.R.; Bandaru, S.R.; Garapati, H.R. Application of Statistical Experimental Designs for the Optimization of Medium Constituents for the Production of Citric Acid from Pineapple Waste. Bioresour. Technol. 2008, 99, 4445–4450. [Google Scholar] [CrossRef]
- Yano, Y.; Oikawa, H.; Satomi, M. Reduction of Lipids in Fish Meal Prepared from Fish Waste by a Yeast Yarrowia lipolytica. Int. J. Food Microbiol. 2008, 121, 302–307. [Google Scholar] [CrossRef]
- Li, N.; Zong, M.H. Lipases from the Genus Penicillium: Production, Purification, Characterization and Applications. J. Mol. Catal. B Enzym. 2010, 66, 43–54. [Google Scholar] [CrossRef]
- Matsuoka, H.; Miura, A.; Hori, K. Symbiotic Effects of a Lipase-Secreting Bacterium, Burkholderia Arboris SL1B1, and a Glycerol-Assimilating Yeast, Candida Cylindracea SL1B2, on Triacylglycerol Degradation. J. Biosci. Bioeng. 2009, 107, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Mafakher, L.; Mirbagheri, M.; Darvishi, F.; Nahvi, I.; Zarkesh-Esfahani, H.; Emtiazi, G. Isolation of Lipase and Citric Acid Producing Yeasts from Agro-Industrial Wastewater. New Biotechnol. 2010, 27, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Jach, M.E.; Malm, A. Yarrowia lipolytica as an Alternative and Valuable Source of Nutritional and Bioactive Compounds for Humans. Molecules 2022, 27, 2300. [Google Scholar] [CrossRef]
- Roostita, R.; Fleet, G.H. The Occurrence and Growth of Yeasts in Camembert and Blue-Veined Cheeses. Int. J. Food Microbiol. 1996, 28, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahian, M.; Tebyanian, H.; Cappello, S. Isolation and Characterization of Two Crude Oil-Degrading Yeast Strains, Yarrowia lipolytica PG-20 and PG-32, from the Persian Gulf. Mar. Pollut Bull. 2012, 64, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ge, G.; Wan, J. Biodegradation of Oil Wastewater by Free and Immobilized Yarrowia lipolytica W29. J. Environ. Sci. 2009, 21, 237–242. [Google Scholar] [CrossRef]
- Martins, F.F.; Ferreira, T.F.; Azevedo, D.A.; Coelho, M.A.Z. Coelho Evaluation of Crude Oil Degradation by Yarrowia lipolytica. Chem. Eng. Trans. 2012, 27, 223–228. [Google Scholar] [CrossRef]
- da Silva, L.V.; Tavares, C.B.; Amaral, P.F.; Coelho, M.A.Z. Production of Citric Acid by Yarrowia lipolytica in Different Crude Glycerol Concentrations and in Different Nitrogen Sources. Chem. Eng. Trans. 2012, 27, 199–204. [Google Scholar] [CrossRef]
- Braun, A.; Geier, M.; Bühler, B.; Schmid, A.; Mauersberger, S.; Glieder, A. Steroid Biotransformations in Biphasic Systems with Yarrowia lipolytica Expressing Human Liver Cytochrome P450 Genes. Microb. Cell Fact. 2012, 11, 106. [Google Scholar] [CrossRef]
- Doruk Aracagök, Y.; cίhangίr, N. Decolorization of Reactive Black 5 by Yarrowia lipolytica NBRC 1658. Am. J. Microbiol. Res. 2013, 1, 16–20. [Google Scholar] [CrossRef]
- Moradi, H.; Asadollahi, M.A.; Nahvi, I. Improved γ-Decalactone Production from Castor Oil by Fed-Batch Cultivation of Yarrowia lipolytica. Biocatal. Agric. Technol. Biotechnol. 2013, 2, 64–68. [Google Scholar] [CrossRef]
- Casas-Godoy, L.; Meunchan, M.; Cot, M.; Duquesne, S.; Bordes, F.; Marty, A. Yarrowia lipolytica Lipase Lip2: An Efficient Enzyme for the Production of Concentrates of Docosahexaenoic Acid Ethyl Ester. J. Technol. Biotechnol. 2014, 180, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, M.R.; López-Núñez, J.C.; Jones, M.A.; Cox, E.J.; Pinkelman, R.J.; Bang, S.S.; Moser, B.R.; Jackson, M.A.; Iten, L.B.; Kurtzman, C.P.; et al. Irradiation of Yarrowia lipolytica NRRL YB-567 Creating Novel Strains with Enhanced Ammonia and Oil Production on Protein and Carbohydrate Substrates. Appl. Microbiol. Technol. Biotechnol. 2015, 99, 9723–9743. [Google Scholar] [CrossRef] [PubMed]
- Louhasakul, Y.; Cheirsilp, B.; Prasertsan, P. Valorization of Palm Oil Mill Effluent into Lipid and Cell-Bound Lipase by Marine Yeast Yarrowia lipolytica and Their Application in Biodiesel Production. Waste Biomass Valorization 2016, 7, 417–426. [Google Scholar] [CrossRef]
- Cui, C.; Guan, N.; Xing, C.; Chen, B.; Tan, T. Immobilization of Yarrowia lipolytica Lipase Ylip2 for the Biocatalytic Synthesis of Phytosterol Ester in a Water Activity Controlled Reactor. Colloids Surf. B Biointerfaces 2016, 146, 490–497. [Google Scholar] [CrossRef]
- Horincar, G.; Horincar, V.B.; Gottardi, D.; Bahrim, G. Tailoring the Potential of Yarrowia lipolytica for Bioconversion of Raw Palm Fat for Antimicrobials Production. LWT 2017, 80, 335–340. [Google Scholar] [CrossRef]
- Cybulski, K.; Tomaszewska-Hetman, L.; Rymowicz, W.; Rakicka, M.; Rywińska, A. Yarrowia lipolytica Application as a Prospective Approach for Biosynthesis of Pyruvic Acid from Glycerol. Chem. Pap. 2018, 72, 3077–3083. [Google Scholar] [CrossRef]
- de Souza, C.E.C.; Ribeiro, B.D.; Coelho, M.A.Z. Characterization and Application of Yarrowia lipolytica Lipase Obtained by Solid-State Fermentation in the Synthesis of Different Esters Used in the Food Industry. Appl. Biochem. Technol. Biotechnol. 2019, 189, 933–959. [Google Scholar] [CrossRef]
- Dunoyer, A.T.; Cuello, R.E.G.; Salinas, R.P. Biodegradation of Dairy Wastes Using Crude Enzymatic Extract of Yarrowia lipolytica ATCC 9773. Ambiente. E Agua.—Interdiscip. J. Appl. Sci. 2020, 15, 1. [Google Scholar] [CrossRef]
- Hamimed, S.; Barkaoui, T.; Trabelsi, I.; Landoulsi, A.; Chatti, A. High-Performance Biological Treatment of Tuna Wash Processing Wastewater Using Yarrowia lipolytica. Environ. Sci. Pollut. Res. 2021, 28, 1545–1554. [Google Scholar] [CrossRef]
- Darvishi, F.; Fathi, Z.; Ariana, M.; Moradi, H. Yarrowia lipolytica as a Workhorse for Biofuel Production. Biochem. Eng. J. 2017, 127, 87–96. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C. Microalgae as a Raw Material for Biofuels Production. J. Ind. Microbiol. Technol. Biotechnol. 2009, 36, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Matsushika, A.; Inoue, H.; Kodaki, T.; Sawayama, S. Ethanol Production from Xylose in Engineered Saccharomyces cerevisiae Strains: Current State and Perspectives. Appl. Microbiol. BioTechnol. 2009, 84, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Valle-Rodríguez, J.O.; Khoomrung, S.; Siewers, V.; Nielsen, J. Functional Expression and Characterization of Five Wax Ester Synthases in Saccharomyces cerevisiae and Their Utility for Biodiesel Production. Technol. Biotechnol. Biofuels 2012, 5, 7. [Google Scholar] [CrossRef]
- Xie, D. Integrating Cellular and Bioprocess Engineering in the Non-Conventional Yeast Yarrowia lipolytica for Biodiesel Production: A Review. Front. Bioeng. Technol. Biotechnol. 2017, 5, 65. [Google Scholar] [CrossRef]
- Akil, E.; Pereira, A.d.S.; El-Bacha, T.; Amaral, P.F.F.; Torres, A.G. Efficient Production of Bioactive Structured Lipids by Fast Acidolysis Catalyzed by Yarrowia lipolytica Lipase, Free and Immobilized in Chitosan-Alginate Beads, in Solvent-Free Medium. Int. J. Biol. Macromol. 2020, 163, 910–918. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Z.P.; Liu, S.; Wang, Y.L.; Zhang, Z.F.; Liu, X.M.; Du, Y.M.; Yuan, X.L. Overexpression of Secreted Sucrose Isomerase in Yarrowia lipolytica and Its Application in Isomaltulose Production after Immobilization. Int. J. Biol. Macromol. 2019, 121, 97–103. [Google Scholar] [CrossRef]
- Matran, R.M.; Galaction, A.I.; Blaga, A.C.; Turnea, M.; Caşcaval, D. Distribution of Mixing Efficiency in a Split-Cylinder Gas-Lift Bioreactor with Immobilized Yarrowia lipolytica Cells Used for Olive Oil Mill Wastewater Treatment. Chem. Eng. Commun. 2016, 203, 666–675. [Google Scholar] [CrossRef]
- Feng, W.; Sun, X.; Ji, P. Activation Mechanism of Yarrowia lipolytica Lipase Immobilized on Carbon Nanotubes. Soft Matter 2012, 8, 7143–7150. [Google Scholar] [CrossRef]
- Mupa, M.; Kubara, R.; Gere, J. Extraction, Growth and Immobilization of Yarrowia lipolytica Yeast Cells for Dye Effluent Treatment. Arch. Environ. Prot. 2018, 44, 48–54. [Google Scholar] [CrossRef]
- Casas-Godoy, L.; Marty, A.; Sandoval, G.; Ferreira-Dias, S. Optimization of Medium Chain Length Fatty Acid Incorporation into Olive Oil Catalyzed by Immobilized Lip2 from Yarrowia lipolytica. Biochem. Eng. J. 2013, 77, 20–27. [Google Scholar] [CrossRef]
- Synthesis, Biological Activity, and In Silico Study of Bioesters Derived from Bixin by the CALB Enzyme. Biointerface Res. Appl. Chem. 2021, 12, 5901–5917. [CrossRef]
- Understanding the Biocatalytic Potential of Lipase from Rhizopus Chinensis. Biointerface Res. Appl. Chem. 2021, 12, 4230–4260. [CrossRef]
- de Carvalho Selvati Rezende, D.; das Graças Cardoso, M.; Souza, R.; Teixeira, M.; Brandão, R.; Ferreira, V.; Nogueira, J.; Magalhães, M.; Marcussi, S.; Nelson, D. Essential Oils from Mentha piperita, Cymbopogon citratus, Rosmarinus officinalis, Peumus boldus and Foeniculum vulgare: Inhibition of Phospholipase A2 and Cytotoxicity to Human Erythrocytes. Am. J. Plant Sci. 2017, 8, 2196–2207. [Google Scholar] [CrossRef]
- de Oliveira, L.B.; Cavalcante, T.T.; Moreira, S.; Monteiro, R.C.; Rocha, G.; Souza, E.S.; da Fonseca, M.; Lopes, A.S.; Guimarães, P.; de Lima, K.C.; et al. Lipases Immobilized onto Nanomaterials as Biocatalysts in Biodiesel Production: Scientific Context, Challenges, and Opportunities. Rev. Virtual Química. 2021, 13, 875–891. [Google Scholar] [CrossRef]
- Moreira, K.d.S.; de Oliveira, A.L.B.; Júnior, L.S.d.M.; Monteiro, R.R.C.; da Rocha, T.N.; Menezes, F.L.; Fechine, L.M.U.D.; Denardin, J.C.; Michea, S.; Freire, R.M.; et al. Lipase From Rhizomucor Miehei Immobilized on Magnetic Nanoparticles: Performance in Fatty Acid Ethyl Ester (FAEE) Optimized Production by the Taguchi Method. Front. Bioeng. Technol. Biotechnol. 2020, 8, 693. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, I.G.; Chaves, A.V.; de Oliveira, A.L.B.; Moreira, K.d.S.; de Sousa Junior, P.G.; Simão Neto, F.; de Carvalho, S.C.F.; Valério, R.B.R.; Lima, G.V.; Lopes, A.A.S.; et al. A Novel Hybrid Biocatalyst from Immobilized Eversa ® Transform 2.0 Lipase and Its Application in Biolubricant Synthesis. Biocatal. Biotransformation 2022, 41, 1–22. [Google Scholar] [CrossRef]
- Califano, V.; Costantini, A. Immobilization of Cellulolytic Enzymes in Mesostructured Silica Materials. Catalysts 2020, 10, 706. [Google Scholar] [CrossRef]
- Sun, T.; Dong, Z.; Wang, J.; Huang, F.-H.H.; Zheng, M.-M.M.; Sun, T.; Dong, Z.; Wang, J.; Huang, F.-H.H. Ultrasound-Assisted Interfacial Immobilization of Lipase on Hollow Mesoporous Silica Spheres in a Pickering Emulsion System: A Hyperactive and Sustainable Biocatalyst. ACS Sustain. Chem. Eng. 2020, 8, 17280–17290. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Liang, C.; Qiao, L.; Du, K. High-strength and low-crystallinity cellulose/agarose composite microspheres: Fabrication, characterization and protein adsorption. Biochem. Eng. J. 2021, 166, 107826. [Google Scholar] [CrossRef]
- Ying, W.; Shi, Z.; Yang, H.; Xu, G.; Zheng, Z.; Yang, J. Effect of Alkaline Lignin Modification on Cellulase–Lignin Interactions and Enzymatic Saccharification Yield. Technol. Biotechnol. Biofuels 2018, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Rueda, N.; dos Santos, J.C.S.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R.; Torres, R. Chemical Amination of Lipases Improves Their Immobilization on Octyl-Glyoxyl Agarose Beads. Catal. Today 2016, 259, 107–118. [Google Scholar] [CrossRef]
- Cabrera, M.P.; da Fonseca, T.F.; de Souza, R.V.B.; de Assis, C.R.D.; Quispe Marcatoma, J.; Maciel, J.d.C.; Neri, D.F.M.; Soria, F.; de Carvalho, L.B. Polyaniline-Coated Magnetic Diatomite Nanoparticles as a Matrix for Immobilizing Enzymes. Appl. Surf. Sci. 2018, 457, 21–29. [Google Scholar] [CrossRef]
- dos Santos, J.C.S.; Garcia-Galan, C.; Rodrigues, R.C.; de Sant’Ana, H.B.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Stabilizing Hyperactivated Lecitase Structures through Physical Treatment with Ionic Polymers. Process. Biochem. 2014, 49, 1511–1515. [Google Scholar] [CrossRef]
- dos Santos, J.C.S.; Garcia-Galan, C.; Rodrigues, R.C.; de Sant’ Ana, H.B.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Improving the Catalytic Properties of Immobilized Lecitase via Physical Coating with Ionic Polymers. Enzym. Microb. Technol. 2014, 60, 1–8. [Google Scholar] [CrossRef]
- Bonazza, H.L.; Manzo, R.M.; dos Santos, J.C.S.; Mammarella, E.J. Operational and Thermal Stability Analysis of Thermomyces Lanuginosus Lipase Covalently Immobilized onto Modified Chitosan Supports. Appl. Biochem. Technol. Biotechnol. 2018, 184, 182–196. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Barbosa, O.; Hernandez, K.; Santos, J.; Rodrigues, R.; Fernandez-Lafuente, R. Evaluation of Styrene-Divinylbenzene Beads as a Support to Immobilize Lipases. Molecules 2014, 19, 7629–7645. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Hernandez, K.; Barbosa, O.; Rueda, N.; Garcia-Galan, C.; C. S. dos Santos, J.; Berenguer-Murcia, A.; Fernandez-Lafuente, R. Immobilization of Proteins in Poly-Styrene-Divinylbenzene Matrices: Functional Properties and Applications. Curr. Org. Chem. 2015, 19, 1707–1718. [Google Scholar] [CrossRef]
- Ferrarezi, A.; Pivetta, D.; Bonilla-Rodriguez, G.; Silva, R.; Guisan, J.; Gomes, E.; Pessela, B. Partial purification, immobilization and preliminary biochemical characterization of lipases from Rhizomucor pusillus. Adv. Enzym. Res. 2013, 1, 79–90. [Google Scholar] [CrossRef]
- Melo, A.; Silva, F.; dos Santos, J.; Fernández-Lafuente, R.; Lemos, T.; Dias Filho, F. Synthesis of Benzyl Acetate Catalyzed by Lipase Immobilized in Nontoxic Chitosan-Polyphosphate Beads. Molecules 2017, 22, 2165. [Google Scholar] [CrossRef]
- dos Santos, J.C.S.; Rueda, N.; Barbosa, O.; Fernández-Sánchez, J.F.; Medina-Castillo, A.L.; Ramón-Márquez, T.; Arias-Martos, M.C.; Millán-Linares, M.C.; Pedroche, J.; Yust, M.d.M.; et al. Characterization of Supports Activated with Divinyl Sulfone as a Tool to Immobilize and Stabilize Enzymes via Multipoint Covalent Attachment. Application to Chymotrypsin. RSC Adv. 2015, 5, 20639–20649. [Google Scholar] [CrossRef]
- Kharrat, N.; Ali, Y.B.; Marzouk, S.; Gargouri, Y.T.; Karra-Châabouni, M. Immobilization of Rhizopus oryzae Lipase on Silica Aerogels by Adsorption: Comparison with the Free Enzyme. Process. Biochem. 2011, 46, 1083–1089. [Google Scholar] [CrossRef]
- Gao, Z.; Chu, J.; Jiang, T.; Xu, T.; Wu, B.; He, B. Lipase Immobilization on Functionalized Mesoporous TiO2: Specific Adsorption, Hyperactivation and Application in Cinnamyl Acetate Synthesis. Process. Biochem. 2018, 64, 152–159. [Google Scholar] [CrossRef]
- Pacheco, S.; Júnior, A.; Morgado, A.; Júnior, A.; Amadi, O.; Guisán, J.; e Pessela, B. Isolamento e Triagem de Fungos Filamentosos Produtores de Lipase Extracelular com Potencial em Biodiesel Produção. Adv. Enzym. Res. 2015, 3, 101–114. [Google Scholar] [CrossRef]
- Kashefi, S.; Borghei, S.M.; Mahmoodi, N.M. Covalently Immobilized Laccase onto Graphene Oxide Nanosheets: Preparation, Characterization, and Biodegradation of Azo Dyes in Colored Wastewater. J. Mol. Liq. 2019, 276, 153–162. [Google Scholar] [CrossRef]
- Chi, M.C.; Huang, Y.F.; Lu, B.Y.; Lin, M.G.; Wang, T.F.; Lin, L.L. Magnetic Cross-Linked Enzyme Aggregates of a Transpeptidase-Specialized Variant (N450d) of Bacillus Licheniformis γ-Glutamyl Transpeptidase: An Efficient and Stable Biocatalyst for l-Theanine Synthesis. Catalysts 2021, 11, 243. [Google Scholar] [CrossRef]
- da Fonseca, A.M.; Cleiton Sousa dos Santos, J.; de Souza, M.C.M.; de Oliveira, M.M.; Colares, R.P.; de Lemos, T.L.G.; Braz-Filho, R. The Use of New Hydrogel Microcapsules in Coconut Juice as Biocatalyst System for the Reaction of Quinine. Ind. Crop. Prod. 2020, 145, 111890. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Immobilization of Lipases on Heterofunctional Octyl–Glyoxyl Agarose Supports. Methods Enzymol. 2016, 571, 73–85. [Google Scholar]
- Monteiro, R.R.C.; dos Santos, J.C.S.; Alcántara, A.R.; Fernandez-Lafuente, R. Enzyme-Coated Micro-Crystals: An Almost Forgotten but Very Simple and Elegant Immobilization Strategy. Catalysts 2020, 10, 891. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Torres, R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Reactivation of Lipases by the Unfolding and Refolding of Covalently Immobilized Biocatalysts. RSC Adv. 2015, 5, 55588–55594. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Neto, D.M.A.; Fechine, P.B.A.; Lopes, A.A.S.; Gonçalves, L.R.B.; dos Santos, J.C.S.; de Souza, M.C.M.; Fernandez-Lafuente, R. Ethyl Butyrate Synthesis Catalyzed by Lipases A and B from Candida Antarctica Immobilized onto Magnetic Nanoparticles. Improvement of Biocatalysts’ Performance under Ultrasonic Irradiation. Int. J. Mol. Sci. 2019, 20, 5807. [Google Scholar] [CrossRef] [PubMed]
- da S. Moreira, K.; Barros de Oliveira, A.L.; Saraiva de Moura Júnior, L.; Germano de Sousa, I.; Luthierre Gama Cavalcante, A.; Simão Neto, F.; Bussons Rodrigues Valério, R.; Valério Chaves, A.; de Sousa Fonseca, T.; Morais Vieira Cruz, D.; et al. Taguchi Design-Assisted Co-Immobilization of Lipase A and B from Candida Antarctica onto Chitosan: Characterization, Kinetic Resolution Application, and Docking Studies. Chem. Eng. Res. Des. 2022, 177, 223–244. [Google Scholar] [CrossRef]
- de Oliveira, U.M.F.; Lima de Matos, L.J.B.; de Souza, M.C.M.; Pinheiro, B.B.; dos Santos, J.C.S.; Gonçalves, L.R.B. Effect of the Presence of Surfactants and Immobilization Conditions on Catalysts’ Properties of Rhizomucor Miehei Lipase onto Chitosan. Appl. Biochem. Technol. Biotechnol. 2018, 184, 1263–1285. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; dos Santos, J.C.S.; Barbosa, O.; Torres, R.; Pereira, E.B.; Corberan, V.C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Tuning of Lecitase Features via Solid-Phase Chemical Modification: Effect of the Immobilization Protocol. Process. Biochem. 2014, 49, 604–616. [Google Scholar] [CrossRef]
- Pinheiro, M.P.; Monteiro, R.R.C.; Silva, F.F.M.; Lemos, T.L.G.; Fernandez-Lafuente, R.; Gonçalves, L.R.B.; dos Santos, J.C.S. Modulation of Lecitase Properties via Immobilization on Differently Activated Immobead-350: Stabilization and Inversion of Enantiospecificity. Process. Biochem. 2019, 87, 128–137. [Google Scholar] [CrossRef]
- Secundo, F. Conformational Changes of Enzymes upon Immobilisation. Chem. Soc. Rev. 2013, 42, 6250. [Google Scholar] [CrossRef] [PubMed]
- Morato, N.M.; Holden, D.T.; Cooks, R.G. High-Throughput Label-Free Enzymatic Assays Using Desorption Electrospray-Ionization Mass Spectrometry. Angew. Chem. Int. Ed. 2020, 59, 20459–20464. [Google Scholar] [CrossRef]
- Guo, J.; Gan, C.; Cheng, B.; Cui, B.; Yi, F. Exploration of Binding Mechanism of Apigenin to Pepsin: Spectroscopic Analysis, Molecular Docking, Enzyme Activity and Antioxidant Assays. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 290, 122281. [Google Scholar] [CrossRef]
- Villalba, M.; Verdasco-Martín, C.M.; dos Santos, J.C.S.; Fernandez-Lafuente, R.; Otero, C. Operational Stabilities of Different Chemical Derivatives of Novozym 435 in an Alcoholysis Reaction. Enzym. Microb. Technol. 2016, 90, 35–44. [Google Scholar] [CrossRef]
- Cavalcante, A.L.G.; Chaves, A.V.; Fechine, P.B.A.; Holanda Alexandre, J.Y.N.; Freire, T.M.; Davi, D.M.B.; Neto, F.S.; de Sousa, I.G.; da Silva Moreira, K.; de Oliveira, A.L.B.; et al. Chemical Modification of Clay Nanocomposites for the Improvement of the Catalytic Properties of Lipase A from Candida Antarctica. Process. Biochem. 2022, 120, 1–14. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; de Oliveira, A.L.B.; de Menezes, F.L.; de Souza, M.C.M.; Fechine, P.B.A.; dos Santos, J.C.S. Improvement of Enzymatic Activity and Stability of Lipase A from Candida Antartica onto Halloysite Nanotubes with Taguchi Method for Optimized Immobilization. Appl. Clay Sci. 2022, 228, 106634. [Google Scholar] [CrossRef]
- Silva, A.; Alexandre, J.; Souza, J.; Neto, J.; de Sousa Júnior, P.; Rocha, M.; dos Santos, J. The Chemistry and Applications of Metal–Organic Frameworks (MOFs) as Industrial Enzyme Immobilization Systems. Molecules 2022, 27, 4529. [Google Scholar] [CrossRef] [PubMed]
- Bedő, Z.; Bélafi-Bakó, K.; Nemestóthy, N.; Gubicza, L. Production of A Biolubricant by Enzymatic Esterification: Possible Synergism Between Ionic Liquid and Enzyme. Hung. J. Ind. Chem. 2018, 46, 27–31. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Z.; Shi, J.; Tang, H.; Xiang, X.; Huang, F.; Zheng, M. Carbon Nanoparticle-Stabilized Pickering Emulsion as a Sustainable and High-Performance Interfacial Catalysis Platform for Enzymatic Esterification/Transesterification. ACS Sustain. Chem. Eng. 2019, 7, 7619–7629. [Google Scholar] [CrossRef]
- Chowdhury, A.; Mitra, D.; Biswas, D. Biolubricant Synthesis from Waste Cooking Oil via Enzymatic Hydrolysis Followed by Chemical Esterification. J. Chem. Technol. Biotechnol. 2013, 88, 139–144. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Virgen-Ortiz, J.J.; Berenguer-Murcia, Á.; da Rocha, T.N.; dos Santos, J.C.S.; Alcántara, A.R.; Fernandez-Lafuente, R. Biotechnological Relevance of the Lipase A from Candida Antarctica. Catal. Today 2021, 362, 141–154. [Google Scholar] [CrossRef]
- Lima, P.J.M.; da Silva, R.M.; Neto, C.A.C.G.; Gomes e Silva, N.C.; Souza, J.E.d.S.; Nunes, Y.L.; Sousa dos Santos, J.C. An Overview on the Conversion of Glycerol to Value-added Industrial Products via Chemical and Biochemical Routes. Technol. Biotechnol. Appl. Biochem. 2022, 69, 2794–2818. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; da Fonseca, A.M.; Holanda Alexandre, J.Y.N.; dos Santos, J.C.S. A Stepwise Docking and Molecular Dynamics Approach for Enzymatic Biolubricant Production Using Lipase Eversa® Transform as a Biocatalyst. Ind. Crop. Prod. 2022, 187, 115450. [Google Scholar] [CrossRef]
- Rocha, T.G.; de L. Gomes, P.H.; de Souza, M.C.M.; Monteiro, R.R.C.; dos Santos, J.C.S. Lipase Cocktail for Optimized Biodiesel Production of Free Fatty Acids from Residual Chicken Oil. Catal. Lett. 2021, 151, 1155–1166. [Google Scholar] [CrossRef]
- Souza, J.E.S.; Monteiro, R.R.C.; Rocha, T.G.; Moreira, K.S.; Cavalcante, F.T.T.; de Sousa Braz, A.K.; de Souza, M.C.M.; dos Santos, J.C.S. Sonohydrolysis Using an Enzymatic Cocktail in the Preparation of Free Fatty Acid. 3 Biotech 2020, 10, 254. [Google Scholar] [CrossRef]
- Vandermies, M.; Kar, T.; Carly, F.; Nicaud, J.M.; Delvigne, F.; Fickers, P. Yarrowia lipolytica Morphological Mutant Enables Lasting in Situ Immobilization in Bioreactor. Appl. Microbiol. Technol. Biotechnol. 2018, 102, 5473–5482. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, Q.; Feng, W.; Pan, K. Immobilization of Yarrowia lipolytica Lipase on Bamboo Charcoal to Resolve (R,S)-Phenylethanol in Organic Medium. Chem. Eng. Technol. 2013, 36, 1249–1254. [Google Scholar] [CrossRef]
- Botelho, A.; Penha, A.; Fraga, J.; Barros-Timmons, A.; Coelho, M.A.; Lehocky, M.; Štepánková, K.; Amaral, P. Yarrowia lipolytica Adhesion and Immobilization onto Residual Plastics. Polymers 2020, 12, 649. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, Y.; Sheng, J.; Sun, M. Immobilization of Yarrowia lipolytica Lipase on Macroporous Resin Using Different Methods: Characterization of the Biocatalysts in Hydrolysis Reaction. Biomed. Res. Int. 2015, 2015, 139179. [Google Scholar] [CrossRef] [PubMed]
- Nunes, Y.L.; de Menezes, F.L.; de Sousa, I.G.; Cavalcante, A.L.G.; Cavalcante, F.T.T.; da Silva Moreira, K.; de Oliveira, A.L.B.; Mota, G.F.; da Silva Souza, J.E.; de Aguiar Falcão, I.R.; et al. Chemical and Physical Chitosan Modification for Designing Enzymatic Industrial Biocatalysts: How to Choose the Best Strategy? Int. J. Biol. Macromol. 2021, 181, 1124–1170. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, P. Immobilisation and Application of Lipases in Organic Media. Chem. Soc. Rev. 2013, 42, 6406. [Google Scholar] [CrossRef]
- Farhan, L.O.; Mehdi, W.A.; Taha, E.M.; Farhan, A.M.; Mehde, A.A.; Özacar, M. Various Type Immobilizations of Isocitrate Dehydrogenases Enzyme on Hyaluronic Acid Modified Magnetic Nanoparticles as Stable Biocatalysts. Int. J. Biol. Macromol. 2021, 182, 217–227. [Google Scholar] [CrossRef]
- Jambulingam, R.; Shalma, M.; Shankar, V. Biodiesel Production Using Lipase Immobilised Functionalized Magnetic Nanocatalyst from Oleaginous Fungal Lipid. J. Clean Prod. 2019, 215, 245–258. [Google Scholar] [CrossRef]
- Alamsyah, G.; Albels, V.A.; Sahlan, M.; Hermansyah, H. Effect of Chitosan’s Amino Group in Adsorption-Crosslinking Immobilization of Lipase Enzyme on Resin to Catalyze Biodiesel Synthesis. Energy Procedia 2017, 136, 47–52. [Google Scholar] [CrossRef]
- Mokhtar, N.F.; Raja Noor Zaliha, R.N.Z.R.; Muhd Noor, N.D.; Mohd Shariff, F.; Ali, M.S.M.; Abd. Rahman, R.N.Z.R.; Muhd Noor, N.D.; Mohd Shariff, F.; Mohamad Ali, M.S. The Immobilization of Lipases on Porous Support by Adsorption and Hydrophobic Interaction Method. Catalysts 2020, 10, 744. [Google Scholar] [CrossRef]
- Nagy, F.; Gyujto, I.; Tasnádi, G.; Barna, B.; Balogh-Weiser, D.; Faber, K.; Poppe, L.; Hall, M. Design and application of a bi-functional redox biocatalyst through covalent co-immobilization of ene-reductase and glucose dehydrogenase. J. Biotechnol. 2020, 323, 246–253. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.L.; da Silva, M.F.; da Silva, S.P.; de Araújo, A.C.V.; Cavalcanti, J.V.F.L.; Converti, A.; Porto, T.S. Fructo-Oligosaccharides Production by an Aspergillus aculeatus Commercial Enzyme Preparation with Fructosyltransferase Activity Covalently Immobilized on Fe3O4–Chitosan-Magnetic Nanoparticles. Int. J. Biol. Macromol. 2020, 150, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.d.S.; Fraga, J.L.; Diniz, M.M.; Fontes-Sant’ana, G.C.; Amaral, P.F.F. High Catalytic Activity of Lipase from Yarrowia lipolytica Immobilized by Microencapsulation. Int. J. Mol. Sci. 2018, 19, 3393. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Y.; Jiang, C. Efficient Biosynthesis of γ-Decalactone in Ionic Liquids by Immobilized Whole Cells of Yarrowia lipolytica G3-3.21 on Attapulgite. Bioprocess. Biosyst. Eng. 2015, 38, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Fraga, J.L.; Penha, A.C.B.; Pereira, A.d.S.; Silva, K.A.; Akil, E.; Torres, A.G.; Amaral, P.F.F. Use of Yarrowia lipolytica Lipase Immobilized in Cell Debris for the Production of Lipolyzed Milk Fat (Lmf). Int. J. Mol. Sci. 2018, 19, 3413. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, C.; Liu, C.Z. Efficient Kinetic Resolution of (R,S)-2-Octanol Catalyzed by Magnetite-Immobilized Yarrowia lipolytica Lipase in Mixed Ionic Liquids. Catal. Lett. 2014, 144, 1552–1556. [Google Scholar] [CrossRef]
- Fopase, R.; Paramasivam, S.; Kale, P.; Paramasivan, B. Strategies, Challenges and Opportunities of Enzyme Immobilization on Porous Silicon for Biosensing Applications. J. Environ. Chem. Eng. 2020, 8, 104266. [Google Scholar] [CrossRef]
- Gülay, S.; Şanlı-Mohamed, G. Immobilization of Thermoalkalophilic Recombinant Esterase Enzyme by Entrapment in Silicate Coated Ca-Alginate Beads and Its Hydrolytic Properties. Int. J. Biol. Macromol. 2012, 50, 545–551. [Google Scholar] [CrossRef]
- Fideles, T.; Santos, J.; Tomás, H.; Furtado, G.; Lima, D.; Borges, S.; Fook, M. Characterization of Chitosan Membranes Crosslinked by Sulfuric Acid. Open Access Libr. J. 2018, 5, 1–13. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Lima, P.J.M.; Pinheiro, B.B.; Freire, T.M.; Dutra, L.M.U.; Fechine, P.B.A.; Gonçalves, L.R.B.; de Souza, M.C.M.; dos Santos, J.C.S.; Fernandez-Lafuente, R. Immobilization of Lipase A from Candida Antarctica onto Chitosan-Coated Magnetic Nanoparticles. Int. J. Mol. Sci. 2019, 20, 4018. [Google Scholar] [CrossRef]
- Stolarzewicz, I.A.; Zaborniak, P.; Fabiszewska, A.U.; Biaecka-Florjañczyk, E. Study on the Properties of Immobilized Biocatalysts with Lipase Activity Produced by Yarrowia lipolytica in Batch Culture. Chem. Biochem. Eng. Q 2017, 31, 251–259. [Google Scholar] [CrossRef]
- Molina-Fernández, C.; Péters, A.; Debecker, D.P.; Luis, P. Immobilization of Carbonic Anhydrase in a Hydrophobic Poly(Ionic Liquid): A New Functional Solid for CO2 Capture. Biochem. Eng. J. 2022, 187, 108639. [Google Scholar] [CrossRef]
- Yang, X.G.; Zhang, J.R.; Tian, X.K.; Qin, J.H.; Zhang, X.Y.; Ma, L.F. Enhanced Activity of Enzyme Immobilized on Hydrophobic ZIF-8 Modified by Ni2+ Ions. Angew. Chem.—Int. Ed. 2022, 62, e202216699. [Google Scholar] [CrossRef]
- Vishwakarma, N.K.; Kumar Mahto, S. An Universal Approach of Catalyst Immobilization inside Hydrophobic PFA Tubing under Well Dispersed Manner for Continuous-Flow Applications. Chem. Eng. J. 2023, 452, 139347. [Google Scholar] [CrossRef]
- Silva, J.M.F.; dos Santos, K.P.; dos Santos, E.S.; Rios, N.S.; Gonçalves, L.R.B. Immobilization of Thermomyces Lanuginosus Lipase on a New Hydrophobic Support (Streamline PhenylTM): Strategies to Improve Stability and Reusability. Enzym. Microb. Technol. 2023, 163, 110166. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.J.; Silva, M.R.L.; Ferreira, E.E.A.; Carvalho, A.K.F.; Basso, R.C.; Pereira, E.B.; de Castro, H.F.; Mendes, A.A.; Hirata, D.B. Enzymatic biodiesel production by hydroesterification using waste cooking oil as feedstock. Chem. Eng. Process. -Process Intensif. 2020, 157, 108131. [Google Scholar] [CrossRef]
- Qiu, Z.; Han, H.; Wang, T.; Dai, R.; Wang, Z. Nanofoaming by surfactant tunes morphology and performance of polyamide nanofiltration membrane. Desalination 2020, 552, 116457. [Google Scholar] [CrossRef]
- Chong, S.L.; Cardoso, V.; Brás, J.L.A.; Gomes, M.Z.d.V.; Fontes, C.M.G.A.; Olsson, L. Immobilization of Bacterial Feruloyl Esterase on Mesoporous Silica Particles and Enhancement of Synthetic Activity by Hydrophobic-Modified Surface. Bioresour. Technol. 2019, 293, 122009. [Google Scholar] [CrossRef]
- Urata, C.; Tamura, Y.; Yamauchi, Y.; Kuroda, K. Preparation of Mesostructured Silica-Micelle Hybrids and Their Conversion to Mesoporous Silica Modified Controllably with Immobilized Hydrophobic Blocks by Using Triethoxysilyl-Terminated PEO-PPO-PEO Triblock Copolymer. J. Mater. Chem. 2011, 21, 3711–3717. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Pettinari, R.; Belousov, Y.A.; Taydakov, I.V.; Krasnobrov, V.D.; Petukhov, D.I.; Drozdov, A.A. Synthesis of Novel Lanthanide Acylpyrazolonato Ligands with Long Aliphatic Chains and Immobilization of the Tb Complex on the Surface of Silica Pre-Modified via Hydrophobic Interactions. Dalton Trans. 2015, 44, 14887–14895. [Google Scholar] [CrossRef]
- Gao, Y.; Truong, Y.B.; Cacioli, P.; Butler, P.; Kyratzis, I.L. Bioremediation of Pesticide Contaminated Water Using an Organophosphate Degrading Enzyme Immobilized on Nonwoven Polyester Textiles. Enzym. Microb. Technol. 2014, 54, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Nemeshwaree, B.; Brigitte, M.; Anne, P.; Kalim, B.; Pascal, D.; Anne-Sophie, M.; Rénato, F. Activity of Enzymes Immobilized on Plasma Treated Polyester. J. Mol. Catal. B Enzym. 2016, 134, 261–272. [Google Scholar] [CrossRef]
- Morshed, M.N.; Behary, N.; Bouazizi, N.; Guan, J.; Chen, G.; Nierstrasz, V. Surface Modification of Polyester Fabric Using Plasma-Dendrimer for Robust Immobilization of Glucose Oxidase Enzyme. Sci. Rep. 2019, 9, 15730. [Google Scholar] [CrossRef] [PubMed]
- Shim, E.J.; Lee, S.H.; Song, W.S.; Kim, H.R. Development of an Enzyme-Immobilized Support Using a Polyester Woven Fabric. Text. Res. J. 2017, 87, 3–14. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Rios, N.S.; Carballares, D.; Mendez-Sanchez, C.; Lokha, Y.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Effects of Enzyme Loading and Immobilization Conditions on the Catalytic Features of Lipase From Pseudomonas Fluorescens Immobilized on Octyl-Agarose Beads. Front. Bioeng. Technol. Biotechnol. 2020, 28, 36. [Google Scholar] [CrossRef] [PubMed]
- Lokha, Y.; Arana-Peña, S.; Rios, N.S.; Mendez-Sanchez, C.; Gonçalves, L.R.B.; Lopez-Gallego, F.; Fernandez-Lafuente, R. Modulating the Properties of the Lipase from Thermomyces Lanuginosus Immobilized on Octyl Agarose Beads by Altering the Immobilization Conditions. Enzym. Microb. Technol. 2020, 133, 109461. [Google Scholar] [CrossRef] [PubMed]
- Arana-Peña, S.; Rios, N.S.; Mendez-Sanchez, C.; Lokha, Y.; Gonçalves, L.R.B.; Fernández-Lafuente, R. Use of Polyethylenimine to Produce Immobilized Lipase Multilayers Biocatalysts with Very High Volumetric Activity Using Octyl-Agarose Beads: Avoiding Enzyme Release during Multilayer Production. Enzym. Microb. Technol. 2020, 137, 109535. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Chen, D. Enhanced Catalysis of Yarrowia lipolytica Lipase LIP2 Immobilized on Macroporous Resin and Its Application in Enrichment of Polyunsaturated Fatty Acids. Bioresour. Technol. 2013, 131, 179–187. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wang, C.H.; Chang, W.R.; Li, J.W.; Hsu, M.F.; Sun, Y.S.; Liu, T.Y.; Chiu, C.W. Hydrophilic-Hydrophobic Nanohybrids of AuNP-Immobilized Two-Dimensional Nanomica Platelets as Flexible Substrates for High-Efficiency and High-Selectivity Surface-Enhanced Raman Scattering Microbe Detection. ACS Appl. Bio. Mater. 2022, 5, 1073–1083. [Google Scholar] [CrossRef]
- Liu, X.; Lv, J.; Zhang, T.; Deng, Y. Direct Conversion of Pretreated Straw Cellulose into Citric Acid by Co-Cultures of Yarrowia lipolytica SWJ-1b and Immobilized Trichoderma Reesei Mycelium. Appl. Biochem. Technol. Biotechnol. 2014, 173, 501–509. [Google Scholar] [CrossRef]
- Gokhale, D.; Chen, I.; Doyle, P.S. Coarse-Grained Molecular Dynamics Simulations of Immobilized Micelle Systems and Their Interactions with Hydrophobic Molecules. Soft Matter 2022, 18, 4625–4637. [Google Scholar] [CrossRef] [PubMed]
- Rostamabadi, H.; Falsafi, S.R.; Rostamabadi, M.M.; Assadpour, E.; Jafari, S.M. Electrospraying as a Novel Process for the Synthesis of Particles/Nanoparticles Loaded with Poorly Water-Soluble Bioactive Molecules. Adv. Colloid Interface Sci. 2021, 290, 102384. [Google Scholar] [CrossRef] [PubMed]
- Veeralingam, S.; Badhulika, S. Enzyme Immobilized Multi-Walled Carbon Nanotubes on Paper-Based Biosensor Fabricated via Mask-Less Hydrophilic and Hydrophobic Microchannels for Cholesterol Detection. J. Ind. Eng. Chem. 2022, 113, 401–410. [Google Scholar] [CrossRef]
- Ogata, M.; Sakamoto, M.; Yamauchi, N.; Nakazawa, M.; Koizumi, A.; Anazawa, R.; Kurumada, K.; Hidari, K.I.P.J.; Kono, H. Optimization of the Conditions for the Immobilization of Glycopolypeptides on Hydrophobic Silica Particulates and Simple Purification of Lectin Using Glycopolypeptide-Immobilized Particulates. Carbohydr Res. 2022, 519, 108624. [Google Scholar] [CrossRef]
- Pauli, O.; Ecker, A.; Cruz-Izquierdo, A.; Basso, A.; Serban, S. Visualizing Hydrophobic and Hydrophilic Enzyme Interactions during Immobilization by Means of Infrared Microscopy. Catalysts 2022, 12, 989. [Google Scholar] [CrossRef]
- Kaur, P.; Jana, A.K. Amino Functionalization of Magnetic Multiwalled Carbon Nanotubes with Flexible Hydrophobic Spacer for Immobilization of Candida Rugosa Lipase and Application in Biocatalytic Production of Fruit Flavour Esters Ethyl Butyrate and Butyl Butyrate. Appl. Nanosci. 2023, 13, 4291–4311. [Google Scholar] [CrossRef]
- de Menezes, L.H.S.; do Espírito Santo, E.L.; dos Santos, M.M.O.; de Carvalho Tavares, I.M.; Mendes, A.A.; Franco, M.; de Oliveira, J.R. Candida Rugosa Lipase Immobilized on Hydrophobic Support Accurel MP 1000 in the Synthesis of Emollient Esters. Technol. Biotechnol. Lett. 2022, 44, 89–99. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, C.; Sun, X.T.; Liu, C.Z. Improved Performance of Yarrowia lipolytica Lipase-Catalyzed Kinetic Resolution of (R,S)-2-Octanol by an Integrated Strategy of Interfacial Activation, Bioimprinting and Immobilization. Bioresour. Technol. 2013, 142, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Mól, P.C.G.; Veríssimo, L.A.A.; Minim, L.A.; da Silva, R. Adsorption and Immobilization of β-Glucosidase from Thermoascus Aurantiacus on Macroporous Cryogel by Hydrophobic Interaction. Prep. Biochem. Technol. Biotechnol. 2023, 53, 297–307. [Google Scholar] [CrossRef]
- Braga, A.; Belo, I. Immobilization of Yarrowia lipolytica for Aroma Production from Castor Oil. Appl. Biochem. Technol. Biotechnol. 2013, 169, 2202–2211. [Google Scholar] [CrossRef]
- Meng, Y.H.; Chen, B.Q.; Yang, N.; Wang, G.L.; Li, Y.; Tan, T.W. Oleic Acid Esterification in Solvent-Free Medium by Yarrowia lipolytica Lipase Immobilized on Fabric Membranes. J. Biobased Mater. Bioenergy 2010, 4, 73–78. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, B.; Lv, K.; Kumissay, L.; Wu, B.; Chu, J.; He, B. Specific Immobilization of Lipase on Functionalized 3D Printing Scaffolds via Enhanced Hydrophobic Interaction for Efficient Resolution of Racemic 1-Indanol. Biochem. Biophys. Res. Commun. 2021, 546, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, B.B.; Rios, N.S.; Rodríguez Aguado, E.; Fernandez-Lafuente, R.; Freire, T.M.; Fechine, P.B.A.; dos Santos, J.C.S.; Gonçalves, L.R.B. Chitosan Activated with Divinyl Sulfone: A New Heterofunctional Support for Enzyme Immobilization. Application in the Immobilization of Lipase B from Candida Antarctica. Int. J. Biol. Macromol. 2019, 130, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, R.M.; Monteiro, R.R.C.; Neto, D.M.A.; da Silva, F.F.M.; de Paula, R.C.M.; de Lemos, T.L.G.; Fechine, P.B.A.; Correa, M.A.; Bohn, F.; Gonçalves, L.R.B.; et al. A New Heterofunctional Support for Enzyme Immobilization: PEI Functionalized Fe3O4 MNPs Activated with Divinyl Sulfone. Application in the Immobilization of Lipase from Thermomyces Lanuginosus. Enzym. Microb. Technol. 2020, 138, 109560. [Google Scholar] [CrossRef]
- Okura, N.S.; Sabi, G.J.; Crivellenti, M.C.; Gomes, R.A.B.; Fernandez-Lafuente, R.; Mendes, A.A. Improved Immobilization of Lipase from Thermomyces Lanuginosus on a New Chitosan-Based Heterofunctional Support: Mixed Ion Exchange plus Hydrophobic Interactions. Int. J. Biol. Macromol. 2020, 163, 550–561. [Google Scholar] [CrossRef]
- Khozeymeh Nezhad, M.; Aghaei, H. Tosylated Cloisite as a New Heterofunctional Carrier for Covalent Immobilization of Lipase and Its Utilization for Production of Biodiesel from Waste Frying Oil. Renew. Energy 2021, 164, 876–888. [Google Scholar] [CrossRef]
- Yang, J.; Sun, L.; Guo, R.; Yang, H.; Feng, X.; Zhang, X. A Facile Route for Oriented Covalent Immobilization of Recombinant Protein A on Epoxy Agarose Gels: In Situ Generation of Heterofunctional Amino-Epoxy Supports. ChemistrySelect 2018, 3, 10320–10324. [Google Scholar] [CrossRef]
- Aghaei, H.; Yasinian, A.; Taghizadeh, A. Covalent Immobilization of Lipase from Candida Rugosa on Epoxy-Activated Cloisite 30B as a New Heterofunctional Carrier and Its Application in the Synthesis of Banana Flavor and Production of Biodiesel. Int. J. Biol. Macromol. 2021, 178, 569–579. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zhong, T.; Feng, X. Optimal Spacer Arm Microenvironment for the Immobilization of Recombinant Protein A on Heterofunctional Amino-Epoxy Agarose Supports. Process. Biochem. 2020, 91, 90–98. [Google Scholar] [CrossRef]
- Veljković, M.; Simović, M.; Banjanac, K.; Ćorović, M.; Milivojević, A.; Milivojević, M.; Bezbradica, D. Heterofunctional Epoxy Support Development for Immobilization of Fructosyltransferase from Pectinex® Ultra SP-L: Batch and Continuous Production of Fructo-Oligosaccharides. React. Chem. Eng. 2022, 7, 2518–2526. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, Y.; Han, N.; Wu, Y. Increase in IgG-Binding Capacity of Recombinant Protein a Immobilized on Heterofunctional Amino and Epoxy Agarose. IOP Conf Ser. Mater. Sci. Eng. 2018, 381, 012042. [Google Scholar] [CrossRef]
- da Rocha, T.N.; Morellon-Sterling, R.; Gonçalves, L.R.B.; Bolivar, J.M.; Alcántara, A.R.; Rocha-Martin, J.; Fernández-Lafuente, R. Synergy of Ion Exchange and Covalent Reaction: Immobilization of Penicillin G Acylase on Heterofunctional Amino-Vinyl Sulfone Agarose. Catalysts 2023, 13, 151. [Google Scholar] [CrossRef]
- Żywicka, A.; Junka, A.; Ciecholewska-Juśko, D.; Migdał, P.; Czajkowska, J.; Fijałkowski, K. Significant Enhancement of Citric Acid Production by Yarrowia lipolytica Immobilized in Bacterial Cellulose-Based Carrier. J. Technol. Biotechnol. 2020, 321, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.F.; Cunha, A.P.; Ricardo, N.M.P.S.; Freire, R.S.; Vieira, L.d.A.P.; Brígida, A.I.S.; Borges, M.d.F.; Rosa, M.d.F.; Vieira, R.S.; Andrade, F.K. Papain Immobilization on Heterofunctional Membrane Bacterial Cellulose as a Potential Strategy for the Debridement of Skin Wounds. Int. J. Biol. Macromol. 2020, 165, 3065–3077. [Google Scholar] [CrossRef]
- Żywicka, A.; Wenelska, K.; Junka, A.; Czajkowska, J.; Fijałkowski, K. An Efficient Method of Yarrowia lipolytica Immobilization Using Oil- and Emulsion-Modified Bacterial Cellulose Carriers. Electron. J. Biotechnol. 2019, 41, 30–36. [Google Scholar] [CrossRef]
- Lim, C.Y.; Owens, N.A.; Wampler, R.D.; Ying, Y.; Granger, J.H.; Porter, M.D.; Takahashi, M.; Shimazu, K. Succinimidyl Ester Surface Chemistry: Implications of the Competition between Aminolysis and Hydrolysis on Covalent Protein Immobilization. Langmuir 2014, 30, 12868–12878. [Google Scholar] [CrossRef] [PubMed]
- Bernal, C.; Illanes, A.; Wilson, L. Heterofunctional Hydrophilic-Hydrophobic Porous Silica as Support for Multipoint Covalent Immobilization of Lipases: Application to Lactulose Palmitate Synthesis. Langmuir 2014, 30, 3557–3566. [Google Scholar] [CrossRef]
- Xing, X.; Han, Y.; Jiang, Q.; Sun, Y.; Wang, X.; Qu, G.; Sun, G.; Li, Y. Immobilization of Laccases onto Cellulose Nanocrystals Derived from Waste Newspaper: Relationship between Immobilized Laccase Activity and Dialdehyde Content. Cellulose 2021, 28, 4793–4805. [Google Scholar] [CrossRef]
- de Melo Brites, M.; Cerón, A.A.; Costa, S.M.S.A.; Oliveira, R.C.; Ferraz, H.G.; Catalani, L.H.; Costa, S.M.S.A. Bromelain Immobilization in Cellulose Triacetate Nanofiber Membranes from Sugarcane Bagasse by Electrospinning Technique. Enzym. Microb. Technol. 2020, 132, 109384. [Google Scholar] [CrossRef]
- Suo, H.; Xu, L.; Xue, Y.; Qiu, X.; Huang, H.; Hu, Y. Ionic Liquids-Modified Cellulose Coated Magnetic Nanoparticles for Enzyme Immobilization: Improvement of Catalytic Performance. Carbohydr. Polym. 2020, 234, 115914. [Google Scholar] [CrossRef]
- Carballares, D.; Rocha-Martin, J.; Fernandez-Lafuente, R. Chemical Amination of Immobilized Enzymes for Enzyme Coimmobilization: Reuse of the Most Stable Immobilized and Modified Enzyme. Int. J. Biol. Macromol. 2022, 208, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Rios, N.S.; Honorato, T.L.; Cecilia, J.A.; Rodríguez-Castellón, E.; Coelho, M.A.Z.; da Silva Júnior, I.J.; Gonçalves, L.R.B. Applicability of Mesoporous Silica Type SBA-15 as Feasible Support for the Immobilization of Yarrowia lipolytica Lipase and Candida Antarctica Lipase B. Braz. J. Chem. Eng. 2022, 39, 1013–1021. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and Use of Immobilized Lipases in/on Nanomaterials: A Review from the Waste to Biodiesel Production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef]
- Ismail, A.R.; Baek, K. Lipase Immobilization with Support Materials, Preparation Techniques, and Applications: Present and Future Aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef] [PubMed]
- Remonatto, D.; Miotti, R.H., Jr.; Monti, R.; Bassan, J.C.; De Paula, A.V. Applications of Immobilized Lipases in Enzymatic Reactors: A Review. Process. Biochem. 2022, 114, 1–20. [Google Scholar] [CrossRef]
- Pereira, E.; Andrade, E.; Fernandez-lafuente, R.; Maria, D.; Freire, G. Support Engineering: Relation between Development of New Supports for Immobilization of Lipases and Their Applications. Biotechnol. Res. Innov. 2017, 1, 26–34. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, F.; Yuan, C.; Du, W.; Liu, D. Lipase-Catalyzed Process for Biodiesel Production: Enzyme Immobilization, Process Simulation and Optimization. Renew. Sustain. Energy Rev. 2015, 44, 182–197. [Google Scholar] [CrossRef]
- Tan, T.; Lu, J.; Nie, K.; Deng, L.; Wang, F. Biodiesel Production with Immobilized Lipase: A Review. Technol. Biotechnol. Adv. 2010, 28, 628–634. [Google Scholar] [CrossRef]
- Kolhe, N.; Damle, E.; Pradhan, A.; Zinjarde, S. A Comprehensive Assessment of Yarrowia lipolytica and Its Interactions with Metals: Current Updates and Future Prospective. Technol. Biotechnol. Adv. 2022, 59, 107967. [Google Scholar] [CrossRef]
- Liu, X.; Dong, X.; Zhong, S.; Xia, J.; He, J.; Deng, Y.; Xu, J. Industrial Crops & Products One-Step Solid-State Fermentation for Efficient Erythritol Production from the Simultaneous Saccharified Crop Wastes by Incorporating Immobilized Cellulase. Ind. Crop. Prod. 2022, 176, 114351. [Google Scholar] [CrossRef]
- Carvalho, T.; Pereira, S.; Bonomo, R.C.F.; Franco, M.; Finotelli, P.V.; Amaral, P.F.F. Simple Physical Adsorption Technique to Immobilize Yarrowia lipolytica Lipase Puri Fi Ed by Different Methods on Magnetic Nanoparticles: Adsorption Isotherms and Thermodynamic Approach. Int. J. Biol. Macromol. 2020, 160, 889–902. [Google Scholar] [CrossRef]
- Rao, A.; Bankar, A.; Ravi, A.; Gosavi, S.; Zinjarde, S. Removal of Hexavalent Chromium Ions by Yarrowia lipolytica Cells Modi Fi Ed with Phyto-Inspired Fe0/Fe3O4 Nanoparticles. J. Contam Hydrol 2013, 146, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Fathi, Z.; Doustkhah, E.; Ebrahimipour, G.; Darvishi, F. Noncovalent Immobilization of Yarrowia lipolytica Lipase on Dendritic-Like Amino Acid-Functionalized Silica Nanoparticles. Biomolecules 2019, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Z.; Liu, G.; Cheng, X.; Chi, Z.; Madzak, C.; Liu, C.; Chi, Z. Genetical Surface Display of Silicatein on Yarrowia lipolytica Confers Living and Renewable Biosilica—Yeast Hybrid Materials. ACS Omega 2020, 5, 7555–7566. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Qin, X.; Dai, Y.; Liu, X.; Wang, W.; Zhong, J. Improved Catalytic Properties of Candida Antarctica Lipase B Immobilized on Cetyl Chloroformate-Modified Cellulose Nanocrystals. Int. J. Biol. Macromol. 2022, 220, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, N.; Najafpour-darzi, G. Preparation of Immobilized Lipase on Co 2 + -Chelated Carboxymethyl Cellulose Based MnFe 2 O 4 Magnetic Nanocomposite Particles. Mol. Catal. 2022, 519, 112118. [Google Scholar] [CrossRef]
- Han, Z.; Park, A.; Su, W.W. Valorization of Papaya Fruit Waste through Low-Cost Fractionation and Microbial Conversion of Both Juice and Seed Lipids. RSC Adv. 2018, 8, 27963–27972. [Google Scholar] [CrossRef] [PubMed]
- Anna, Ż.; Banach, A.; Junka, A.F.; Fija, K. Bacterial Cellulose as a Support for Yeast Immobilization—Correlation between Carrier Properties and Process e Ffi Ciency. J. Biotechnol. 2019, 291, 1–6. [Google Scholar] [CrossRef]
- Production, L.; Zhang, S.; He, H.; Guan, S.; Cai, B.; Li, Q.; Rong, S. Bacterial Cellulose-Alginate Composite Beads as Yarrowia lipolytica Cell Carriers For lactone production. Molecules 2020, 25, 928. [Google Scholar]
- Xie, M.; Hu, B.; Wang, Y.; Zeng, X. Grafting of Gallic Acid onto Chitosan Enhances Antioxidant Activities. J. Agric. Food Chem. 2014, 62, 9128–9136. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, W.; Zhao, L.; Gao, S.; Liu, T.; Yu, D. Immobilization of Lipase in Chitosan-Mesoporous Silica Material and Pore Size Adjustment. Int. J. Biol. Macromol. 2023, 235, 123789. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Men, X.; Yue, T.; Madzak, C.; Xiang, X.; Xiang, H.; Zhang, H. Enzyme and Microbial Technology Construction of Arming Yarrowia lipolytica Surface-Displaying Soybean Seed Coat Peroxidase for Use as Whole-Cell Biocatalyst. Enzym. Microb. Technol. 2020, 135, 109498. [Google Scholar] [CrossRef] [PubMed]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. ScienceDirect Cell Immobilization Strategies for Biotransformations. Curr. Opin. Green Sustain. Chem. 2022, 33, 100565. [Google Scholar] [CrossRef]
- Pereira, S.; Diniz, M.M.; De Jong, G.; Gama, H.S.; Marcelino, J.; Finotelli, P.V.; Ana, G.C.F.; Amaral, P.F.F. Chitosan-Alginate Beads as Encapsulating Agents for Yarrowia lipolytica Lipase: Morphological, Physico-Chemical and Kinetic Characteristics. Int. J. Biol. Macromol. 2019, 139, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.; Smith, F.; Dias, B.; Alice, M.; Coelho, Z. Extractive Fermentation for the Production and Partitioning of Lipase and Citric Acid by Yarrowia lipolytica. Process. Biochem. 2022, 122, 374–385. [Google Scholar] [CrossRef]
- He, Y.; Li, K.; Bo, G.; Wang, J.; Xu, L.; Yan, J.; Yang, M.; Yan, Y. Enhancing Biodiesel Production via Liquid Yarrowia lipolytica Lipase 2 in Deep Eutectic Solvents. Fuel 2022, 316, 123342. [Google Scholar] [CrossRef]
- Bilal, M.; Dourado, C.; Mehmood, T.; Nadeem, F.; Tabassam, Q.; Fernando, L.; Ferreira, R. Immobilized Lipases-Based Nano-Biocatalytic Systems—A Versatile Platform with Incredible Biotechnological Potential. Int. J. Biol. Macromol. 2021, 175, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Savina, A.A.; Zaitsev, I.S. Biochemical Aspects of Lipase Immobilization at Polysaccharides for Biotechnology. Adv. Colloid Interface Sci. 2019, 272, 102016. [Google Scholar] [CrossRef]
- Paiva, A.L.; Balca, V.M.; Malcata, F.X. Kinetics and Mechanisms of Reactions Catalyzed by Immobilized Lipases. Enzym. Microb. Technol. 2000, 27, 187–204. [Google Scholar] [CrossRef]
- Song, M.; Chang, J. Thermally Stable and Reusable Ceramic Encapsulated and Cross-Linked CalB Enzyme Particles for Rapid Hydrolysis and Esterification. Int. J. Mol. Sci. 2022, 23, 2459. [Google Scholar] [CrossRef]
- Saraiva, N.; Gomes, E.; Galvão, S.; Andrade, D.M.; Cleiton, J.; Bohn, F.; Correa, M.A.; Basílio, P.; Fechine, A.; Fernandez-lafuente, R.; et al. Further Stabilization of Lipase from Pseudomonas Fl Uorescens Immobilized on Octyl Coated Nanoparticles via Chemical Modi Fi Cation with Bifunctional Agents. Int. J. Biol. Macromol. 2019, 141, 313–324. [Google Scholar] [CrossRef]
- Awad, A.; Adel, W.; Özacar, M.; Ziyade, Z. Evaluation of Different Saccharides and Chitin as Eco-Friendly Additive to Improve the Magnetic Cross-Linked Enzyme Aggregates (CLEAs) Activities. Int. J. Biol. Macromol. 2018, 118, 2040–2050. [Google Scholar] [CrossRef]
- Villeneuve, P.; Muderhwa, J.M.; Graille, J.; Haas, M.J. Customizing Lipases for Biocatalysis: A Survey of Chemical, Physical and Molecular Biological Approaches. J. Mol. Catal. B Enzym. 2000, 9, 113–148. [Google Scholar] [CrossRef]
- Cui, C.; Tao, Y.; Li, L.; Chen, B.; Tan, T. Improving the Activity and Stability of Yarrowia lipolytica Lipase Lip2 by Immobilization on Polyethyleneimine-Coated Polyurethane Foam. J. Mol. Catal. B Enzym. 2013, 91, 59–66. [Google Scholar] [CrossRef]
- Hwang, E.T.; Lee, S. Multienzymatic Cascade Reactions via Enzyme Complex by Immobilization. Acs Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Carballares, D.; Rocha-martin, J.; Fernandez-lafuente, R. Coimmobilization of Lipases Exhibiting Three Very Different Stability Ranges. Reuse of the Active Enzymes and Selective Discarding of the Inactivated Ones. Int. J. Biol. Macromol. 2022, 206, 580–590. [Google Scholar] [CrossRef]
- Sri, D.; Venkataraman, S.; Kumar, P.S.; Rangasamy, G.; Bhattacharya, T.; Vo, D.N.; Kumar, V. Coimmobilized Enzymes as Versatile Biocatalytic Tools for Biomass Valorization and Remediation of Environmental Contaminants—A Review. Environ. Res. 2022, 214, 114012. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, K.; Kuthiala, T.; Singh, G.; Kumar, S. Employment of Polysaccharides in Enzyme Immobilization. React. Funct Polym. 2021, 167, 105005. [Google Scholar] [CrossRef]
- Ferna, R.; Guisa, J.M. The coimmobilization of D-amino acid oxidase and catalase enables the quantitative transformation of D-amino acids (D-phenylalanine) into α-keto acids (phenylpyruvic acid). Enzym. Microb. Technol. 1998, 23, 28–33. [Google Scholar]
- Kornecki, J.F.; Carballares, D.; Tardioli, P.W.; Rodrigues, R.C.; Berenguer-murcia, Á.; Alcántara, A.R.; Fernandez-lafuente, R. Enzyme Production of D -Gluconic Acid and Glucose Oxidase: Successful Tales of Cascade Reactions. Catal. Sci. Technol. 2020, 10, 5740–5771. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Carballares, D.; Morellon-sterlling, R.; Berenguer-Murcia, Á.; Alcántara, A.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Enzyme co-immobilization: Always the biocatalyst designers’ choice…or not? Biotechnol. Adv. 2021, 51, 107584. [Google Scholar] [CrossRef]
- Mansfeld, J.; Fiirster, M.; Hoffmann, T.; Dautzenberg, H. Coimmobilization of Yarrowia Zipolytica Cells and Invertase in Polyelectrolyte Complex Microcapsules. Enzym. Microb. Technol. 1995, 0229, 11–17. [Google Scholar] [CrossRef]
- da Silva, J.R.; de Souza, C.E.C.; Valoni, E.; de Castro, A.M.; Coelho, M.A.Z.; Ribeiro, B.D.; Henriques, C.A.; Langone, M.A.P. Biocatalytic Esterification of Fatty Acids Using a Low-Cost Fermented Solid from Solid-State Fermentation with Yarrowia lipolytica. 3 Biotech 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Louhasakul, Y.; Cheirsilp, B. Potential Use of Industrial By-Products as Promising Feedstock for Microbial Lipid and Lipase Production and Direct Transesterification of Wet Yeast into Biodiesel by Lipase and Acid Catalysts. Bioresour. Technol. 2022, 348, 126742. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.; Braga, A.; Teixeira, J.A.; Belo, I. Impact of Lipase-Mediated Hydrolysis of Castor Oil on γ-Decalactone Production by Yarrowia lipolytica. J. Am. Oil Chem. Soc. 2013, 90, 1131–1137. [Google Scholar] [CrossRef]
- Braga, A.; Gomes, N.; Belo, I. Lipase Induction in Yarrowia lipolytica for Castor Oil Hydrolysis and Its Effect on γ-Decalactone Production. J. Am. Oil Chem. Soc. 2012, 89, 1041–1047. [Google Scholar] [CrossRef]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.-H. State of the Art and Prospective of Lipase-Catalyzed Transesterification Reaction for Biodiesel Production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Neto, F.S.; Rafael de Aguiar Falcão, I.; Erick da Silva Souza, J.; de Moura Junior, L.S.; da Silva Sousa, P.; Rocha, T.G.; de Sousa, I.G.; de Lima Gomes, P.H.; de Souza, M.C.M.; et al. Opportunities for Improving Biodiesel Production via Lipase Catalysis. Fuel 2021, 288, 119577. [Google Scholar] [CrossRef]
- Destain, J.; Roblain, D.; Thonart, P. Improvement of Lipase Production from Yarrowia lipolytica. Technol. Biotechnol. Lett. 1997, 19, 105–108. [Google Scholar] [CrossRef]
- Corzo, G.; Revah, S. Production and Characteristics of the Lipase from Yarrowia lipolytica. Bioresour. Technol. 1999, 70, 173–180. [Google Scholar] [CrossRef]
- Vandermies, M.; Fickers, P. Bioreactor-Scale Strategies for the Production of Recombinant Protein in the Yeast Yarrowia lipolytica. Microorganisms 2019, 7, 40. [Google Scholar] [CrossRef]
- Aloulou, A.; Puccinelli, D.; De Caro, A.; Leblond, Y.; Carrière, F. A Comparative Study on Two Fungal Lipases from Thermomyces Lanuginosus and Yarrowia lipolytica Shows the Combined Effects of Detergents and PH on Lipase Adsorption and Activity. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2007, 1771, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.; Feihrmann, A.C.; Dariva, C.; Cunha, A.G.; Bevilaqua, J.V.; Destain, J.; Oliveira, J.V.; Freire, D.M.G. Influence of Compressed Fluids Treatment on the Activity of Yarrowia lipolytica Lipase. J. Mol. Catal. B Enzym. 2006, 39, 117–123. [Google Scholar] [CrossRef]
- Pignède, G.; Wang, H.; Fudalej, F.; Gaillardin, C.; Seman, M.; Nicaud, J.-M. Characterization of an Extracellular Lipase Encoded by LIP2 in Yarrowia lipolytica. J. Bacteriol. 2000, 182, 2802–2810. [Google Scholar] [CrossRef] [PubMed]
- García, E.E.; Belin, J.-M.; Waché, Y. Use of a Doehlert Factorial Design to Investigate the Effects of PH and Aeration on the Accumulation of Lactones by Yarrowia lipolytica. J. Appl. Microbiol. 2007, 103, 1508–1515. [Google Scholar] [CrossRef]
- Pyo, S.; Hayes, D.G. Designs of Bioreactor Systems for Solvent-Free Lipase-Catalyzed Synthesis of Fructose–Oleic Acid Esters. J. Am. Oil Chem. Soc. 2009, 86, 521–529. [Google Scholar] [CrossRef]
- Deive, F.J.; Sanromán, M.A.; Longo, M.A. A Comprehensive Study of Lipase Production by Yarrowia lipolytica CECT 1240 (ATCC 18942): From Shake Flask to Continuous Bioreactor. J. Chem. Technol. Biotechnol. 2010, 85, 258–266. [Google Scholar] [CrossRef]
- Mou, J.-H.; Tahar, I.B.; Wang, Z.-Y.; Ong, K.L.; Li, C.; Qin, Z.-H.; Wang, X.; Lin, C.S.K.; Fickers, P. Enhancing the Recombinant Protein Productivity of Yarrowia lipolytica Using Insitu Fibrous Bed Bioreactor. Bioresour. Technol. 2021, 340, 125672. [Google Scholar] [CrossRef]
- Fickers, P.; Destain, J.; Thonart, P. Improvement of Yarrowia lipolytica Lipase Production by Fed-Batch Fermentation. J. Basic Microbiol. 2009, 49, 212–215. [Google Scholar] [CrossRef]
- Rywińska, A.; Rymowicz, W. High-Yield Production of Citric Acid by Yarrowia lipolytica on Glycerol in Repeated-Batch Bioreactors. J. Ind. Microbiol. Technol. Biotechnol. 2010, 37, 431–435. [Google Scholar] [CrossRef]
- Braga, A.; Mesquita, D.P.; Amaral, A.L.; Ferreira, E.C.; Belo, I. Aroma Production by Yarrowia lipolytica in Airlift and Stirred Tank Bioreactors: Differences in Yeast Metabolism and Morphology. Biochem. Eng. J. 2015, 93, 55–62. [Google Scholar] [CrossRef]
- Li, C.; Gao, S.; Yang, X.; Lin, C.S.K. Green and Sustainable Succinic Acid Production from Crude Glycerol by Engineered Yarrowia lipolytica via Agricultural Residue Based in Situ Fibrous Bed Bioreactor. Bioresour. Technol. 2018, 249, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, J.-L.; Zhao, X.-R.; Liu, S.-C.; Liu, Z.-J.; Wei, L.-J.; Hua, Q. Yarrowia lipolytica as a Metabolic Engineering Platform for the Production of Very-Long-Chain Wax Esters. J. Agric. Food Chem. 2020, 68, 10730–10740. [Google Scholar] [CrossRef] [PubMed]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Aseptic Production of Citric and Isocitric Acid from Crude Glycerol by Genetically Modified Yarrowia lipolytica. Bioresour. Technol. 2019, 271, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wang, G.; Yang, N.; Zhou, Z.; Li, Y.; Liang, X.; Chen, J.; Li, Y.; Li, J. Two-Step Synthesis of Fatty Acid Ethyl Ester from Soybean Oil Catalyzed by Yarrowia lipolytica Lipase. Technol. Biotechnol. Biofuels 2011, 4, 6. [Google Scholar] [CrossRef]
- Rafiee, F.; Rezaee, M. Different Strategies for the Lipase Immobilization on the Chitosan Based Supports and Their Applications. Int. J. Biol. Macromol. 2021, 179, 170–195. [Google Scholar] [CrossRef]
- Soong, Y.-H.V.; Coleman, S.M.; Liu, N.; Qin, J.; Lawton, C.; Alper, H.S.; Xie, D. Using Oils and Fats to Replace Sugars as Feedstocks for Biomanufacturing: Challenges and Opportunities for the Yeast Yarrowia lipolytica. Technol. Biotechnol. Adv. 2023, 65, 108128. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Borodina, I. Engineering of Yarrowia lipolytica for Terpenoid Production. Metab. Eng. Commun. 2022, 15, e00213. [Google Scholar] [CrossRef] [PubMed]
- Caporusso, A.; De Bari, I.; Liuzzi, F.; Albergo, R.; Valerio, V.; Viola, E.; Pietrafesa, R.; Siesto, G.; Capece, A. Optimized Conversion of Wheat Straw into Single Cell Oils by Yarrowia lipolytica and Lipomyces Tetrasporus and Synthesis of Advanced Biofuels. Renew. Energy 2023, 202, 184–195. [Google Scholar] [CrossRef]
- Jach, M.E.; Sajnaga, E.; Janeczko, M.; Juda, M.; Kochanowicz, E.; Baj, T.; Malm, A. Production of Enriched in B Vitamins Biomass of Yarrowia lipolytica Grown in Biofuel Waste. Saudi J. Biol. Sci. 2021, 28, 2925–2932. [Google Scholar] [CrossRef]
- Lu, R.; Cao, L.; Wang, K.; Ledesma-Amaro, R.; Ji, X.J. Engineering Yarrowia lipolytica to Produce Advanced Biofuels: Current Status and Perspectives. Bioresour. Technol. 2021, 341, 125877. [Google Scholar] [CrossRef]
- Vakhlu, J.; Kour, A. Yeast Lipases: Enzyme Purification, Biochemical Properties and Gene Cloning. Electron. J. Biotechnol. 2006, 9, 69–85. [Google Scholar] [CrossRef]
- Gupta, R.; Kumari, A.; Syal, P.; Singh, Y. Molecular and Functional Diversity of Yeast and Fungal Lipases: Their Role in Biotechnology and Cellular Physiology. Prog. Lipid Res. 2015, 57, 40–54. [Google Scholar] [CrossRef]
- Bordes, F.; Barbe, S.; Escalier, P.; Mourey, L.; André, I.; Marty, A.; Tranier, S. Exploring the Conformational States and Rearrangements of Yarrowia lipolytica Lipase. Biophys. J. 2010, 99, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.Y.; Jiang, H.; Lin, R.F.; Jiang, Y.M.; Lin, L.; Huang, J.Z. Technical Methods to Improve Yield, Activity and Stability in the Development of Microbial Lipases. J. Mol. Catal. B Enzym. 2010, 62, 1–8. [Google Scholar] [CrossRef]
- Bordes, F.; Cambon, E.; Dossat-Létisse, V.; An Dré, I.; Croux, C.; Nicaud, J.M.; Marty, A. Improvement of Yarrowia lipolytica Lipase Enantioselectivity by Using Mutagenesis Targeted to the Substrate Binding Site. ChemBioChem 2009, 10, 1705–1713. [Google Scholar] [CrossRef]
- Guieysse, D.; Sandoval, G.; Faure, L.; Nicaud, J.M.; Monsan, P.; Marty, A. New Efficient Lipase from Yarrowia lipolytica for the Resolution of 2-Bromo-Arylacetic Acid Esters. Tetrahedron Asymmetry 2004, 15, 3539–3543. [Google Scholar] [CrossRef]
- Wipo—Search International and National Patent Collections. Available online: http://www.wipo.int/patentscope/search/en/search.jsf (accessed on 16 April 2023).
- European Patent Office: Espacenet—Advanced Search. Available online: http://worldwide.espacenet.com/advancedSearch?locale=en_EP (accessed on 16 April 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).