Obtaining and Study of Peptide Compositions Based on Hydrolysates of Collagen-Containing Fish Raw Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
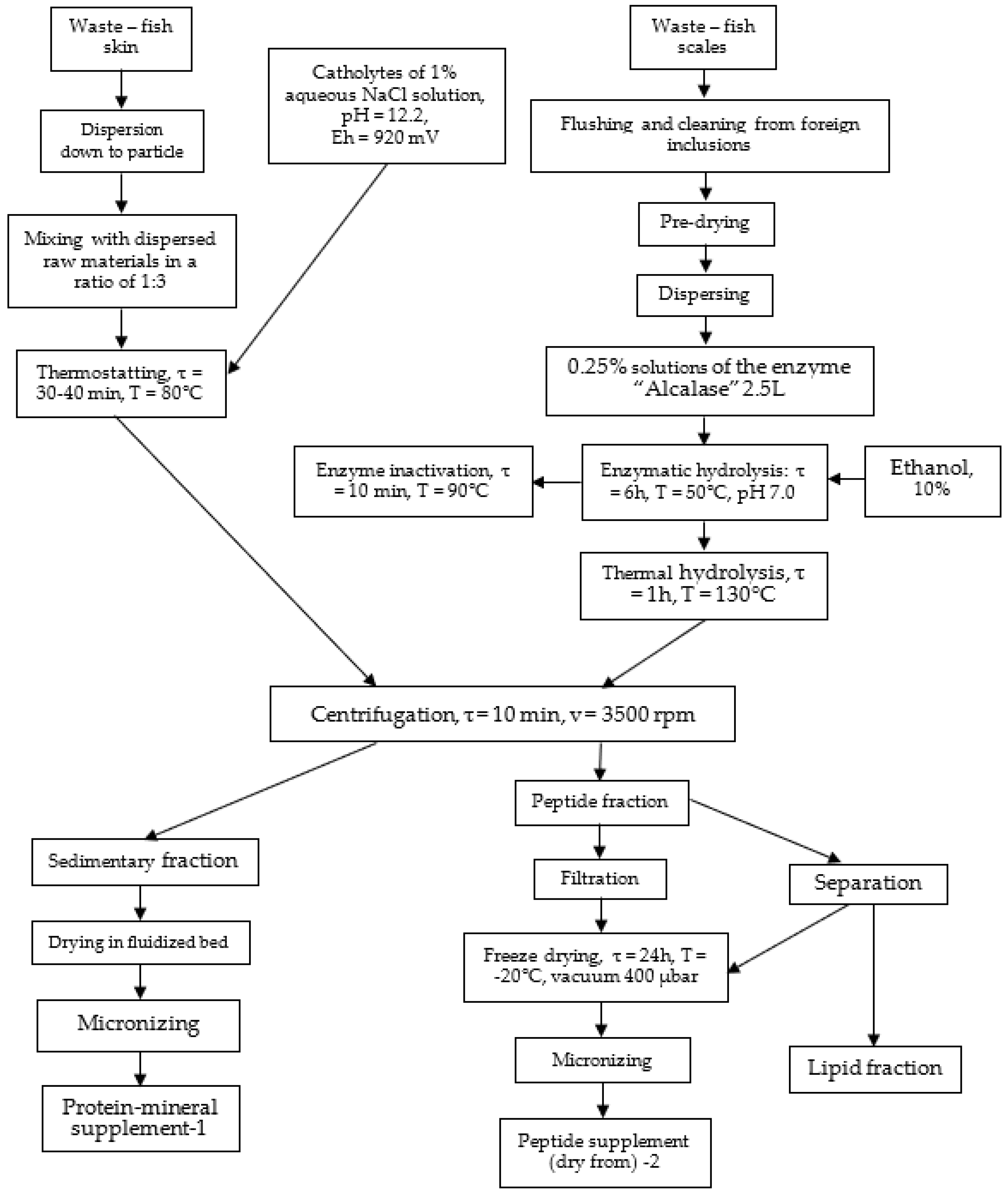
2.2.1. Electrochemical Method for Obtaining Hydrolysates
2.2.2. Electrochemical Method for Obtaining Hydrolysates
2.2.3. Physical and Chemical Research Methods
- ADPPH—optical density of the DPPH working solution
- Apep—optical density of the peptide solution
- 16 × 170 mm SepharoseCL-4B (Pharmacia Fine Chemicals Co., Stockholm, Sweden)—for molecular weights from 30 kDa to 5 MDa;
- 16 × 255 mm SephadexG-75 (Pharmacia Fine Chemicals Co., Sweden)—for molecular weights from 3 kDa to 70 kDa;
- 9 × 372 mm SephadexG-15 (Pharmacia Fine Chemicals Co., Sweden)—for molecular weights from 0 to 1500 Da.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mucci, A.; Schenetti, L.; Volpi, N. 1H and 13C nuclear magnetic resonance identification and characterization of components of chondroitin sulfates of various origin. Carbohydr. Polym. 2000, 41, 37–45. [Google Scholar] [CrossRef]
- Mezenova, N.Y.; Baidalinova, L.S.; Mezenova, O.Y.; Moersel, J.-T.; Hoeling, A. Active peptides of fish scales in gainers for sports nutrition. J. Int. Acad. Refrig. 2014, 2, 48–52. [Google Scholar]
- Mezenova, N.Y. Development of Technology for Bioproduct for Sports Nutrition Using Biomodified Collagen-Containing Fish Raw Materials. Ph.D. Thesis, KSTU, Kaliningrad, Russia, 2017; 24p. [Google Scholar]
- Sviridenko, Y.Y.; Myagkonosov, D.S.; Abramov, D.V.; Ovchinnikova, E.G. Scientific and methodological approaches to the development of technology of protein hydrolysates for special nutrition. Part 2. Production technology and technical characteristics of hydrolysates. Food Ind. 2017, 6, 18–51. [Google Scholar]
- Kim, S.K.; Ngo, D.H.; Vo, T.S. Marine fish-derived bioactive peptides as potential antihypertensive agents. Adv. Food Nutr. Res. 2012, 65, 249–260. [Google Scholar] [CrossRef]
- Cai, S.-Y.; Wang, Y.-M.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Cytoprotective Effect of Antioxidant Pentapeptides from the Protein Hydrolysate of Swim Bladders of Miiuy Croaker (Miichthysmiiuy) against H2O2-Mediated Human Umbilical Vein Endothelial Cell (HUVEC) Injury. Int. J. Mol. Sci. 2019, 20, 5425. [Google Scholar] [CrossRef] [PubMed]
- Slizyte, R.; Rommi, K.; Mozuraityte, R.; Eck, P.; Five, K.; Rustad, T. Bioactivities of fish protein hydrolysates from defatted salmon backbones. Biotechnol. Rep. 2016, 11, 99–109. [Google Scholar] [CrossRef]
- Lin, Y.; Cai, X.; Wu, X.; Lin, S.; Wang, S. Fabrication of snapper fish scales protein hydrolysate-calcium complex and the promotion in calcium cellular uptake. J. Funct. Foods 2020, 65, 1037–1047. [Google Scholar] [CrossRef]
- Zhou, W.-J.; Wang, F.-X.; Yu, J.; Li, X.-H.; Liu, Y.-L. Cryoprotective Effects of Protein Hydrolysates Prepared from By-Products of Silver Carp (Hypophthalmichthys Molitrix) on Freeze-Thawed Surimi. Appl. Sci. 2019, 9, 563. [Google Scholar] [CrossRef]
- Agafonova, S.V.; Baidalinova, L.S.; Volkov, V.V.; Gorodnichenko, L.V. Method of Obtaining Food Additives from Secondary Fish Raw Materials Using Hydrolysis. RF Patent 2 681 352, 6 March 2019. [Google Scholar]
- Shan, A.Z. Antibacterial Peptide of Bone Collagen of Larimichthyspolyactis and Application of Antibacterial Peptide. Patent CN110547384, 26 June 2013. [Google Scholar]
- Kim, D.-U.; Chung, H.-C.; Choi, J.; Sakai, Y.; Lee, B.-Y. Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2018, 10, 826. [Google Scholar] [CrossRef]
- Hsin-Chieh, L.; Adeola, M.; Alashi, I.; Rotimi, E.; Aluko, I.; Bonnie, S.P.; Chang, Y.-W. Antihypertensive properties of tilapia (Oreochromis spp.) frame and skin enzymatic protein hydrolysates. Food Nutr. Res. 2017, 61, 1391666. [Google Scholar] [CrossRef]
- Lee, S.H.; Qian, Z.J.; Kim, S.K. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive. Food Chem. 2010, 118, 96–102. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Rodríguez-Amado, I.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Valcárcel Production, J. Characterization, and Bioactivity of Fish Protein Hydrolysates from Aquaculture Turbot (Scophthalmus maximus) Wastes. Biomolecules 2020, 10, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Chang-Feng, C.; Bin, W.; Yu-Mei, W.; Bin, Z.; Shang-Gui, D. Isolation and characterization of three antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) heads. J. Funct. Foods 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Heffernan, S.; Giblin, L.; O’Brien, N. Assessment of the biological activity of fish muscle protein hydrolysates using in vitro model systems. Food Chem. 2021, 359, 129852. [Google Scholar] [CrossRef]
- Takenawa, T.; Takahashi, K.; Sun, L.; Okazaki, E.; Osako, K. The effect of ovalbumin on the protease activity. KnE Life Sci. 2013, 1, 39–41. [Google Scholar] [CrossRef]
- Boutin, Y.; Paradis, M.E.; Couture, P. Lamarche Immunological effects of fish protein supplementation on healthy adults. J. Nat. Prod. 2012, 5, 37–44. [Google Scholar]
- Gu, R.Z.; Li, C.Y.; Liu, W.Y.; Yi, W.X.; Cai, M.Y. Angiotensin I-converting enzyme inhibitory activity of low-molecular-weight peptides from Atlantic salmon (Salmosalar L.) skin. Food Res. Int. 2011, 44, 1536–1540. [Google Scholar] [CrossRef]
- Hashim, P.; Ridzwan, M.M.; Bakar, J.; Hashim, M.D. Collagen in food and beverage industries. Int. Food Res. J. 2015, 22, 1–8. [Google Scholar]
- Idowu, A.T.; Igiehon, O.O.; Idowu, S.; Olatunde, O.O.; Benjakul, S. Bioactivity Potentials and General Applications of Fish Protein Hydrolysates. Int. J. Pept. Res. Ther. 2020, 27, 109–118. [Google Scholar] [CrossRef]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Suzuki, N. Isolation of collagen from fish waste material—Skin, bone and fins. Food Chem. 2000, 68, 277–281. [Google Scholar] [CrossRef]
- Nagai, T.; Yamashita, E.; Taniguchi, K.; Kanamori, N.; Suzuki, N. Isolation and characterisation of collagen from the outer skin waste material of cuttlefish (Sepia lycidas). Food Chem. 2001, 72, 425–429. [Google Scholar] [CrossRef]
- Noorzai, S.; Verbeek, C.J.R.; Lay, M.C.; Swan, J. Collagen extraction from various waste bovine hide sources. Waste Biomass Valorization 2020, 11, 5687–5698. [Google Scholar] [CrossRef]
- Mezenova, O.Y.; Volkov, V.V.; Grimm, T.; Lange, T. Study of Various Methods of Hydrolysis of Secondary Raw Materials of Pacific Salmonids Using the Example of Sockeye Salmon (Oncorhynchus nerka); Izvestiya KSTU; KSTU: Kaliningrad, Russia, 2017; pp. 136–147. [Google Scholar]
- Mezenova, O.Y.; Volkov, V.V.; Merzel, T.; Grimm, T. Comparative evaluation of the methods of hydrolysis of collagen-containing fish raw materials when obtaining peptides and study of their amino acid balance. Izvestia universities. Appl. Chem. Biotechnol. 2018, 8, 83–94. [Google Scholar] [CrossRef]
- Grishin, D.V.; Podobed, O.V.; Gladilina, Y.A.; Pokrovskaya, M.V.; Aleksandrova, S.S.; Pokrovsky, V.S.; Sokolov, N.N. Bioactive proteins and peptides: Current state and new trends in practical application in the food industry and fodder production. Quest. Nutr. 2017, 86, 20–31. [Google Scholar]
- Chi, C.F.; Cao, Z.H.; Wang, B.; Hu, F.Y.; Li, Z.R.; Zhang, B. Antioxidant and Functional Properties of Collagen Hydrolysates from Spanish Mackerel Skin as Influenced by Average Molecular Weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef]
- Ahn, C.B.; Kim, J.G.; Je, J.Y. Purification and Antioxidant Properties of Octapeptide from Salmon Byproduct Protein Hydrolysate by Gastrointestinal Digestion. Food Chem. 2014, 147, 78–83. [Google Scholar] [CrossRef]
- Wisuthiphaet, N.; Kongruang, S.; Chamcheun, C. Production of fish protein hydrolysates by acid and enzymatic hydrolysis. J. Med. Bioeng. 2015, 4, 466–470. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Meduíña, A.; Durán, A.I.; Nogueira, M.; Fernández-Compás, A.; Pérez-Martín, R.I.; Rodríguez-Amado, I. Production of valuable compounds and bioactive metabolites from by-products of fish discards using chemical processing, enzymatic hydrolysis, and bacterial fermentation. Mar. Drugs 2019, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tu, D.; Shen, Q.; Dai, Z. Fish scale valorization by hydrothermal pretreatment followed by enzymatic hydrolysis for gelatin hydrolysate production. Molecules 2019, 24, 2998. [Google Scholar] [CrossRef] [PubMed]
- Nam, P.V.; Van Hoa, N.; Anh, T.T.L.; Trung, T.S. Towards zero-waste recovery of bioactive compounds from catfish (Pangasius hypophthalmus) by-products using an enzymatic method. Waste Biomass Valorization 2020, 11, 4195–4206. [Google Scholar] [CrossRef]
- Mohtar, N.F.; Perera, C.; Quek, S.Y. Optimisation of gelatine extraction from hoki (Macruronus novaezelandiae) skins and measurement of gel strength and SDS–PAGE. Food Chem. 2010, 122, 307–313. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Song, S.; Wang, X.; Qin, Y.; Si, S.; Guo, Y. Combined oral administration of bovine collagen peptides with calcium citrate inhibits bone loss in ovariectomized rats. PLoS ONE 2015, 10, e0135019. [Google Scholar] [CrossRef] [PubMed]
- Oesser, S.; Schulze, C.H.; Zdzieblik, D.; König, D. Efficacy of specific bioactive collagen peptides in the treatment of joint pain. Osteoarthr. Cartil. 2016, 24, S189. [Google Scholar] [CrossRef]
- O’Keeffe, M.B.; Norris, R.; Alashi, M.A.; Aluko, R.E.; FitzGerald, R.J. Peptide identification in a porcine gelatin prolyl endoproteinase hydrolysate with angiotensin converting enzyme (ACE) inhibitory and hypotensive activity. J. Funct. Foods 2017, 34, 77–88. [Google Scholar] [CrossRef]
- Igase, M.; Kohara, K.; Okada, Y.; Ochi, M.; Igase, K.; Inoue, N.; Kutsuna, T.; Miura, H.; Ohyagi, Y. A double-blind, placebo-controlled, randomised clinical study of the effect of pork collagen peptide supplementation on atherosclerosis in healthy older individuals. Biosci. Biotechnol. Biochem. 2018, 82, 893–895. [Google Scholar] [CrossRef]
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, biomedical and pharmaceutical applications of fish gelatin/hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef]
- Kim, S.-K.; Mendis, E. Bioactive compounds from marine processing byproducts-A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Dıaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical properties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Larsen, R.; Eilertsen, K.-E.; Elvevoll, E.O. Health benefits of marine foods and ingredients. Biotechnol. Adv. 2011, 29, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.F.; Li, G.Z.; Peng, H.B.; Zhang, F.; Chen, Y.; Li, Y. Treatment with marine collagen peptides modulates glucose and lipid metabolism in Chinese patients with type 2 diabetes mellitus. Appl. Physiol. Nutr. Metab. 2010, 35, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine collagen as a promising biomaterial for biomedical applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.K. Bioactive peptide of marine origin for the prevention and treatment of non-communicable diseases. Mar. Drugs 2017, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Ianora, A. Marine organisms with anti-diabetes properties. Mar. Drugs 2016, 14, 220. [Google Scholar] [CrossRef]
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Genovese, L. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: Scientific background and clinical studies. Open Nutraceuticals J. 2015, 8, 29–42. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.; Li-Chan, E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Unnikrishnan, P.; Puthenveetil Kizhakkethil, B.; Anant Jadhav, M.; Sivam, V.; Ashraf, P.M.; Ninan, G.; Aliyamveetil Abubacker, Z. Protein hydrolysate from yellowfin tuna red meat as fortifying and stabilizing agent in mayonnaise. J. Food Sci. Technol. 2020, 57, 413–425. [Google Scholar] [CrossRef]
- Gaikwad, S.B.; More, P.R.; Sonawane, S.K.; Arya, S.S. Antioxidant and anti-hypertensive bioactive peptides from Indian mackerel fish waste. Int. J. Pept. Res. Ther. 2021, 27, 2671–2684. [Google Scholar] [CrossRef]
- Anal, A.K.; Noomhorm, A.; Vongsawasdi, P. Protein hydrolysates and bioactive peptides from seafood and crustacean waste: Their extraction, bioactive properties and industrial perspectives. In Marine Proteins and Peptides: Biological Activities and Applications; John Wiley & Sons, Ltd., Inc.: Hoboken, NJ, USA, 2013; pp. 709–735. [Google Scholar] [CrossRef]
- Padial-Domínguez, M.; Espejo-Carpio, F.J.; García-Moreno, P.J.; Jacobsen, C.; Guadix, E.M. Protein derived emulsifiers with antioxidant activity for stabilization of omega-3 emulsions. Food Chem. 2020, 329, 127148. [Google Scholar] [CrossRef] [PubMed]

| Object Name | Waste Name | Water | Fat | Protein N Total × 5.62 m | Ash | Total Nitrogen |
|---|---|---|---|---|---|---|
| Sardines | Scales | 72.9 ± 0.5 | 8.4 ± 0.2 | 15.3 ± 0.75 | 3.3 ± 2.25 | 2.7 ± 0.5 |
| Sardinella | Scales | 66.7 ± 0.5 | 9.5 ± 0.2 | 20.2 ± 0.75 | 3.6 ± 2.25 | 3.6 ± 0.5 |
| Lightly salted herring | Skin | 57.4 ± 0.6 | 19.6 ± 0.2 | 19.2 ± 1.0 | 5.4 ± 2.00 | 3.4 ± 0.5 |
| Cod | Skin | 69.3 ± 0.6 | 1.2 ± 0.2 | 20.0 ± 1.0 | 6.6 ± 2.00 | 3.5 ± 0.5 |
| Trout | Skin | 57.2 ± 0.6 | 15.2 ± 0.2 | 22.5 ± 1.0 | 5.1 ± 2.00 | 4.0 ± 0.5 |
| Amino Acid | Average Mass Fraction of AA on Dry Matter | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sardines Scales | Sardinella Scales | Herring Skin | Cod Skin | Rainbow Trout Skin | ||||||
| g/100 g Protein | g/100 g Scales | g/100 g Protein | g/100 g Scales | g/100 g Protein | g/100 g Skin | g/100 g Protein Skin | g/100 g | g/100 g Protein | g/100 g Skin | |
| Aalanine | 5.84 | 3.31 | 11.20 | 5.60 | 11.4 | 5.91 | 12.4 | 3.84 | 12.10 | 6.41 |
| Arginine | 7.23 | 4.10 | 7.90 | 4.00 | 8.00 | 3.58 | 9.20 | 2.85 | 11.20 | 5.93 |
| Asparagine | 0.00 | 0.00 | 0.10 | 0.10 | 6.9 | 3.00 | 1.56 | 0.48 | 6.22 | 3.22 |
| Aspartic acid | 6.05 | 3.43 | 4.90 | 2.50 | 2.10 | 1.09 | 2.23 | 0.69 | 2.14 | 1.13 |
| Carnosine | 0.00 | 0.00 | 0.10 | 0.01 | - | - | - | |||
| Citrulline | 0.02 | 0.01 | 0.00 | 0.00 | - | - | - | |||
| Cystine | 0.21 | 0.12 | 0.00 | 0.00 | - | - | - | |||
| Glutamine | 0.04 | 0.02 | 0.80 | 0.40 | 3.31 | 1.71 | 3.80 | 1.18 | 4.73 | 2.50 |
| Glutamic acid | 9.29 | 5.27 | 8.50 | 4.30 | 11.5 | 5.98 | 10.63 | 3.29 | 10.24 | 5.43 |
| Glycine | 12.57 | 7.13 | 26.00 | 13.1 | 6.50 | 3.38 | 6.20 | 1.92 | 5.90 | 3.12 |
| Histidine | 1.66 | 0.94 | 1.20 | 0.60 | 2.82 | 1.45 | 2.29 | 0.71 | 2.33 | 1.20 |
| Hydroxyproline | 9.45 | 4.99 | 10.70 | 4.40 | 4.40 | 2.28 | 3.72 | 1.15 | 3.90 | 2.07 |
| Leucine (n) | 3.65 | 2.07 | 2.70 | 1.30 | 1.85 | 0.96 | 2.10 | 0.65 | 1.90 | 1.01 |
| Isoleucine (n) | 2.12 | 1.21 | 1.00 | 0.50 | - | - | - | |||
| Lysine (n) | 2.72 | 1.54 | 4.00 | 2.00 | 0.70 | 0.36 | 0.35 | 0.11 | 0.39 | 0.21 |
| Methionine (n) | 2.84 | 1.61 | 0.01 | 0.01 | 5.32 | 2.70 | 5.10 | 1.58 | 5.60 | 2.97 |
| Ornithine | 0.07 | 0.04 | 0.00 | 0.00 | 3.80 | 1.98 | 3.11 | 0.96 | 3.34 | 1.77 |
| Phenylalanine (n) | 2.75 | 1.56 | 2.20 | 1.10 | - | - | - | |||
| Proline | 9.42 | 5.34 | 11.70 | 5.90 | 1.61 | 0.83 | 1.62 | 0.50 | 1.50 | 0.79 |
| Serine | 4.00 | 2.27 | 2.90 | 1.50 | 2.51 | 1.30 | 2.30 | 0.71 | 2.11 | 1.12 |
| Taurine | 0.10 | 0.06 | 0.00 | 0.00 | 3.90 | 2.03 | 3.40 | 1.05 | 3.42 | 1.81 |
| Threonine (n) | 1.22 | 1.69 | 2.10 | 1.10 | 6.50 | 3.38 | 6.20 | 1.92 | 5.90 | 3.12 |
| Tyrosine | 1.53 | 0.87 | 0.60 | 0.30 | 2.82 | 1.45 | 2.29 | 0.71 | 2.33 | 1.20 |
| Valine (n) | 2.05 | 1.16 | 1.50 | 0.80 | 4.40 | 2.28 | 3.72 | 1.15 | 3.90 | 2.07 |
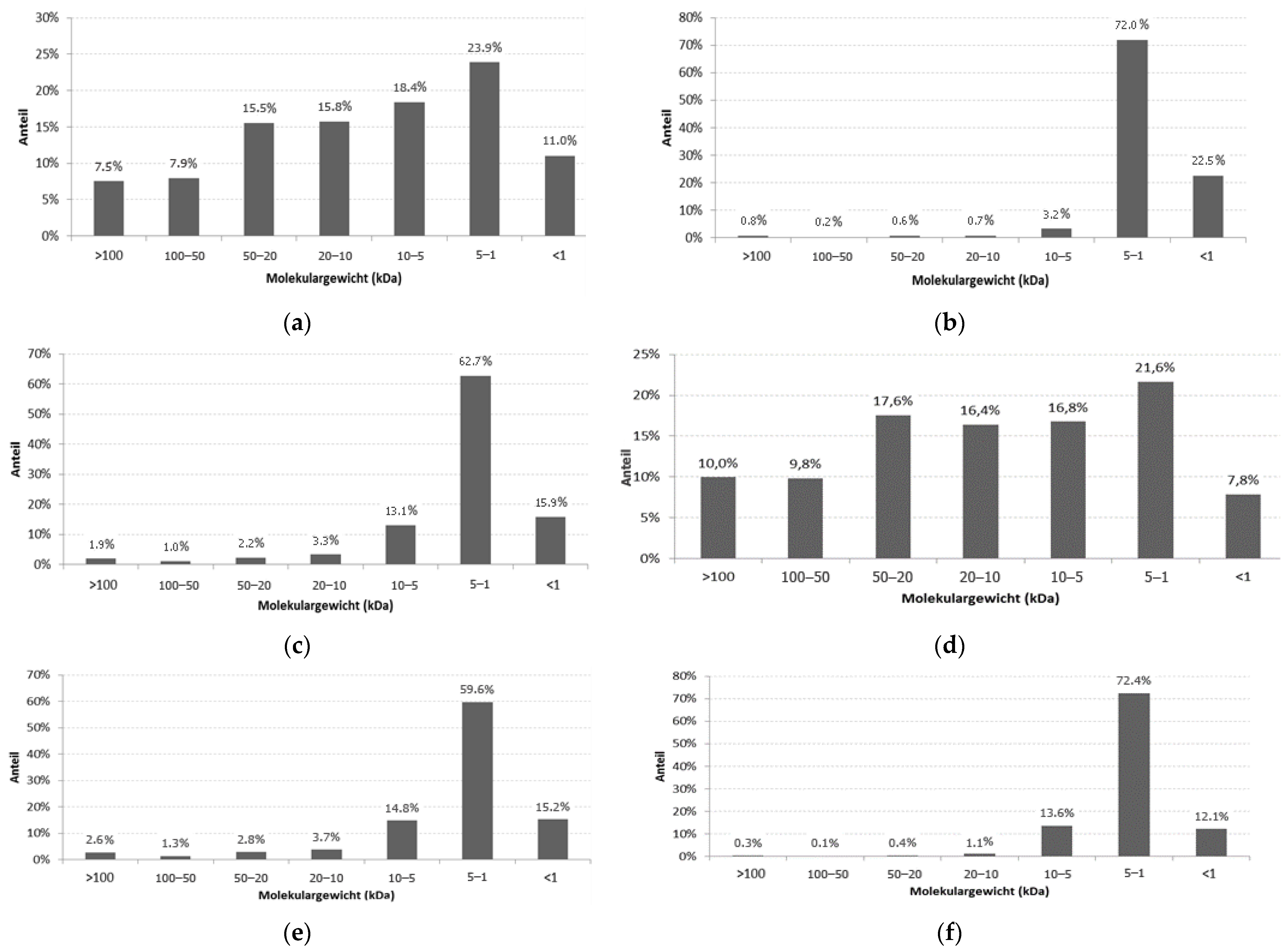
| Hydrolysis Methods | The Content of Protein Substances in the Sublimated Protein Fraction, % 1 | The Content of Peptides in the Protein Fraction with MW ≤ 10 kDa 2 |
|---|---|---|
| Sardine scales | ||
| Thermal | 27.5 | 53.3 |
| Enzymatic | 65.0 | 98.1 |
| Enzymatic-thermal | 83.9 | 91.7 |
| Sardinella scales | ||
| Thermal | 27.2 | 46.2 |
| Enzymatic | 55.9 | 97.7 |
| Enzymatic-thermal | 85.2 | 89.6 |
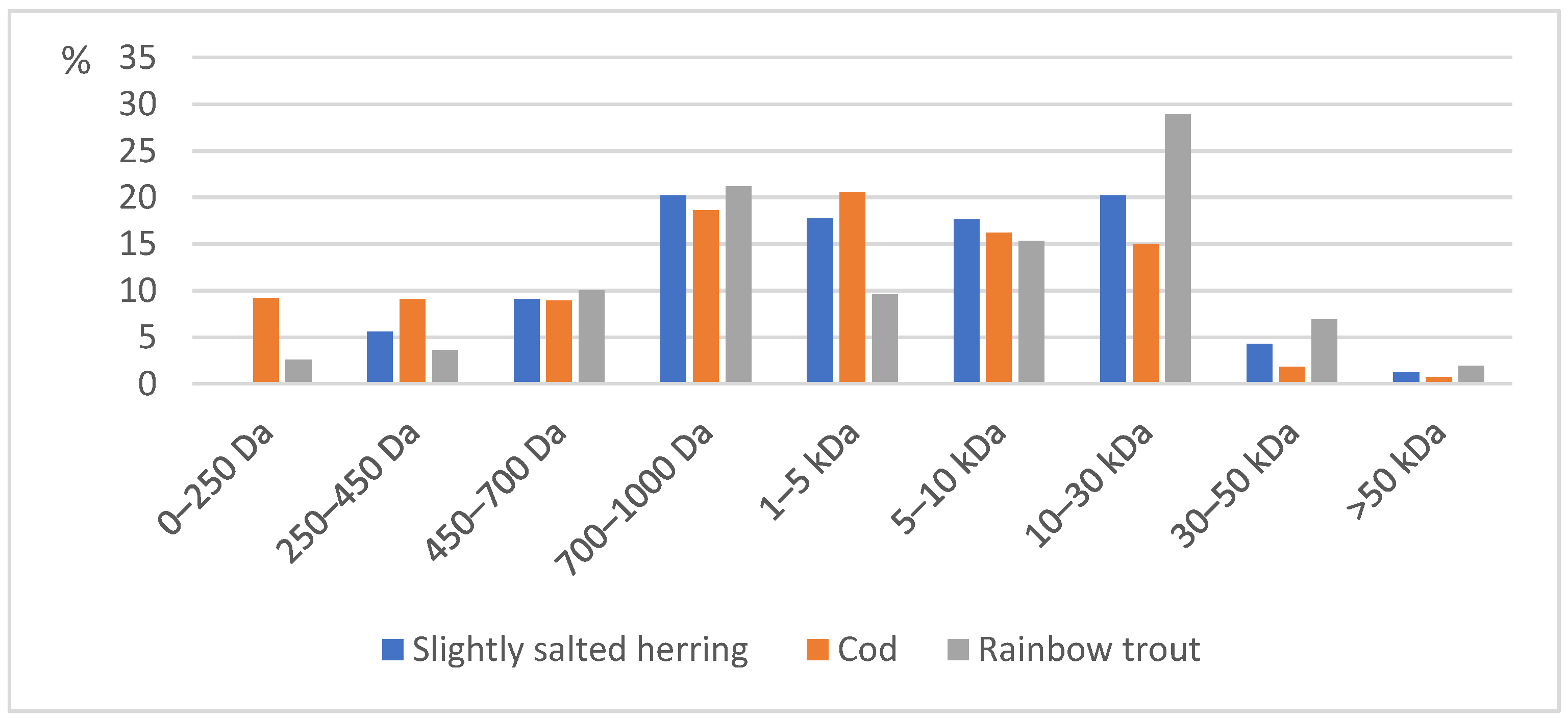
| Molecular Weight (MW) | 0–250 Da | 250–450 Da | 450–700 Da | 700–1000 Da | 1–5 kDa | 5–10 kDa | 10–30 kDa | 30–50 kDa | >50 kDa |
|---|---|---|---|---|---|---|---|---|---|
| Lightly salted herring skin | 4.0 | 5.6 | 9.1 | 20.2 | 17.8 | 17.6 | 20.2 | 4.3 | 1.2 |
| Cod skin | 9.2 | 9.1 | 8.9 | 18.6 | 20.5 | 16.2 | 15 | 1.8 | 0.7 |
| Trout skin | 2.6 | 3.6 | 10 | 21.2 | 9.6 | 15.3 | 28.9 | 6.9 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuprina, E.E.; Kiprushkina, E.I.; Abramzon, V.V.; Rogozina, E.A.; Romanenko, N.Y.; Mezenova, O.Y.; Grimm, T.; Mörsel, T. Obtaining and Study of Peptide Compositions Based on Hydrolysates of Collagen-Containing Fish Raw Materials. Fermentation 2023, 9, 458. https://doi.org/10.3390/fermentation9050458
Kuprina EE, Kiprushkina EI, Abramzon VV, Rogozina EA, Romanenko NY, Mezenova OY, Grimm T, Mörsel T. Obtaining and Study of Peptide Compositions Based on Hydrolysates of Collagen-Containing Fish Raw Materials. Fermentation. 2023; 9(5):458. https://doi.org/10.3390/fermentation9050458
Chicago/Turabian StyleKuprina, E. E., E. I. Kiprushkina, V. V. Abramzon, E. A. Rogozina, N. Y. Romanenko, O. Y. Mezenova, T. Grimm, and T. Mörsel. 2023. "Obtaining and Study of Peptide Compositions Based on Hydrolysates of Collagen-Containing Fish Raw Materials" Fermentation 9, no. 5: 458. https://doi.org/10.3390/fermentation9050458
APA StyleKuprina, E. E., Kiprushkina, E. I., Abramzon, V. V., Rogozina, E. A., Romanenko, N. Y., Mezenova, O. Y., Grimm, T., & Mörsel, T. (2023). Obtaining and Study of Peptide Compositions Based on Hydrolysates of Collagen-Containing Fish Raw Materials. Fermentation, 9(5), 458. https://doi.org/10.3390/fermentation9050458





