New Online Monitoring Approaches to Describe and Understand the Kinetics of Acetaldehyde Concentration during Wine Alcoholic Fermentation: Access to Production Balances
Abstract
1. Introduction
2. Materials and Methods
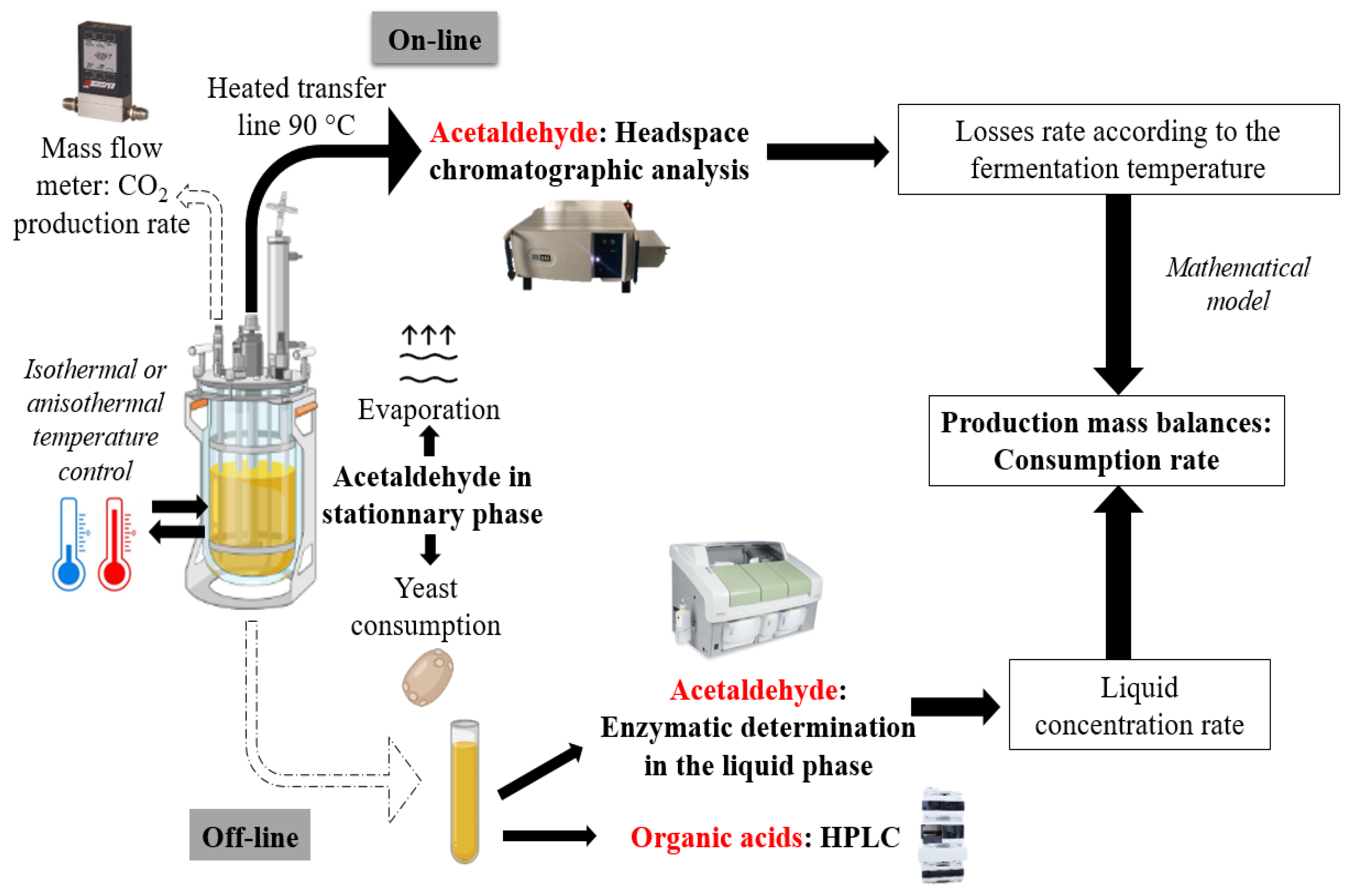
2.1. Schematic Overview of the Experimental Program
2.2. Yeast Strains
2.3. Natural Must
2.4. Fermentation Conditions
2.5. Quantification of Sterols and Fatty Acids in Grape Solids
2.5.1. Dry Matter
2.5.2. Lipid Composition
2.6. Quantification of Assimilable Nitrogen
2.7. Determination of Concentration of Metabolites of Central Carbon Metabolism
2.8. Online Acetaldehyde Monitoring
2.9. Determination of Acetaldehyde
2.9.1. Calibration in the Gas Phase
2.9.2. Losses during Fermentation
2.9.3. Production Mass Balances
2.10. Statistical Analysis
3. Results and Discussion
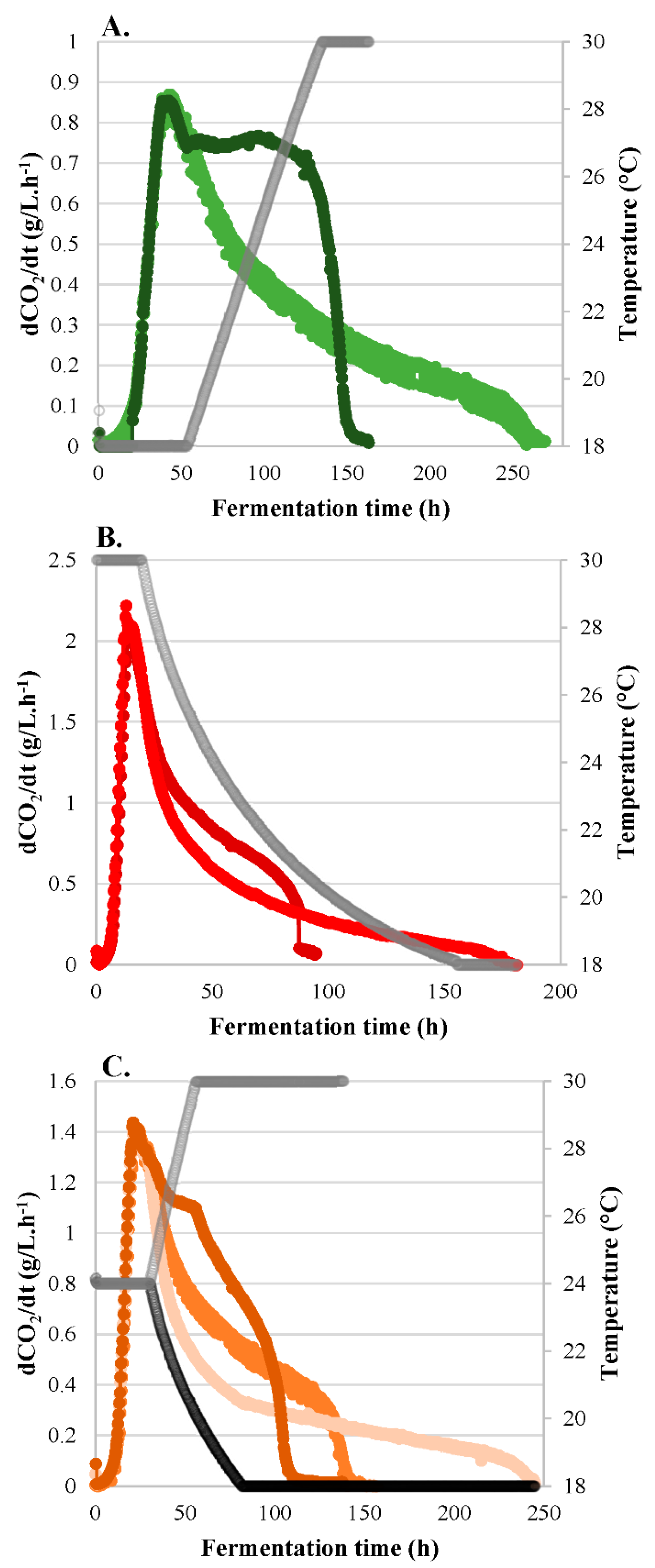
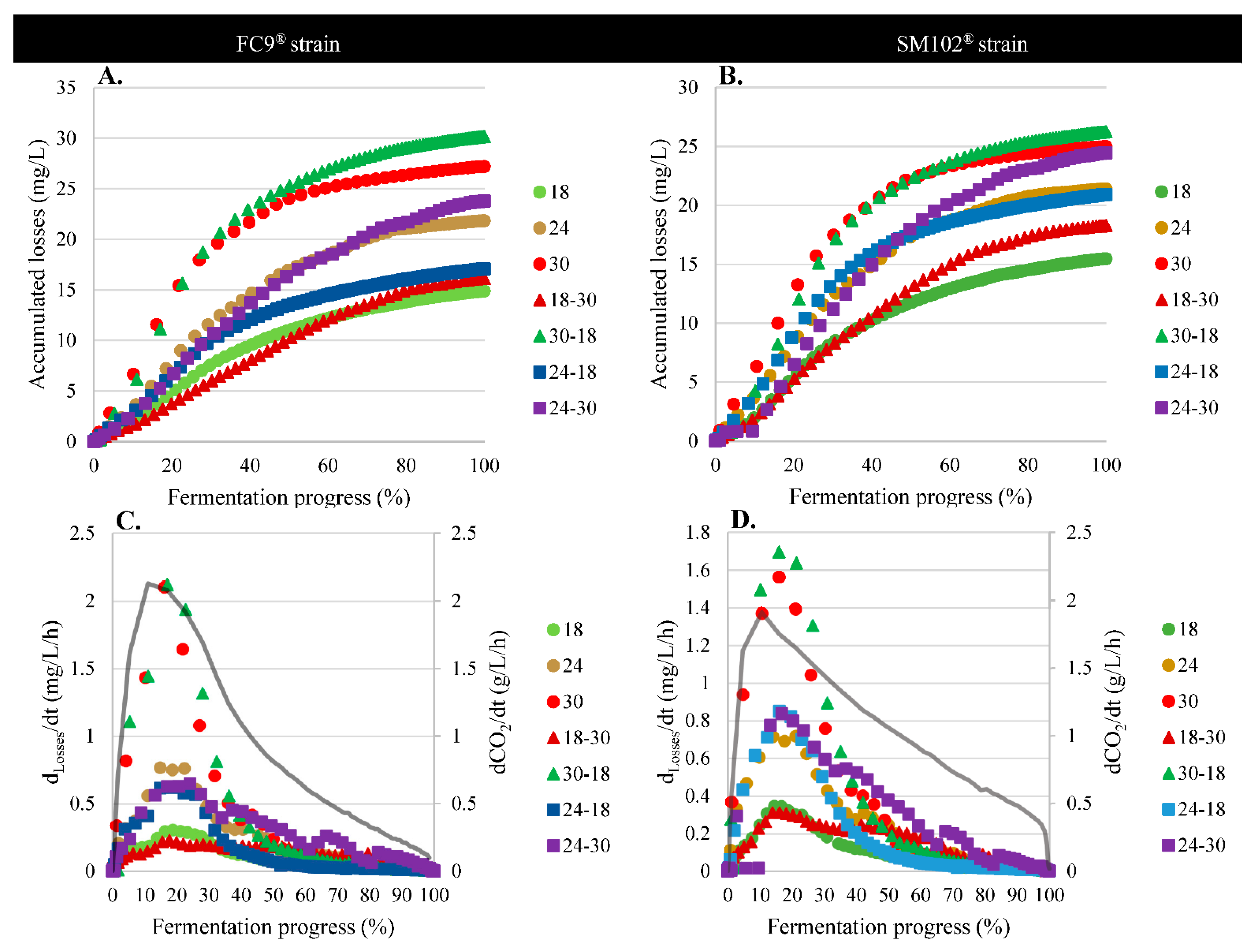
3.1. Fermentation Kinetics
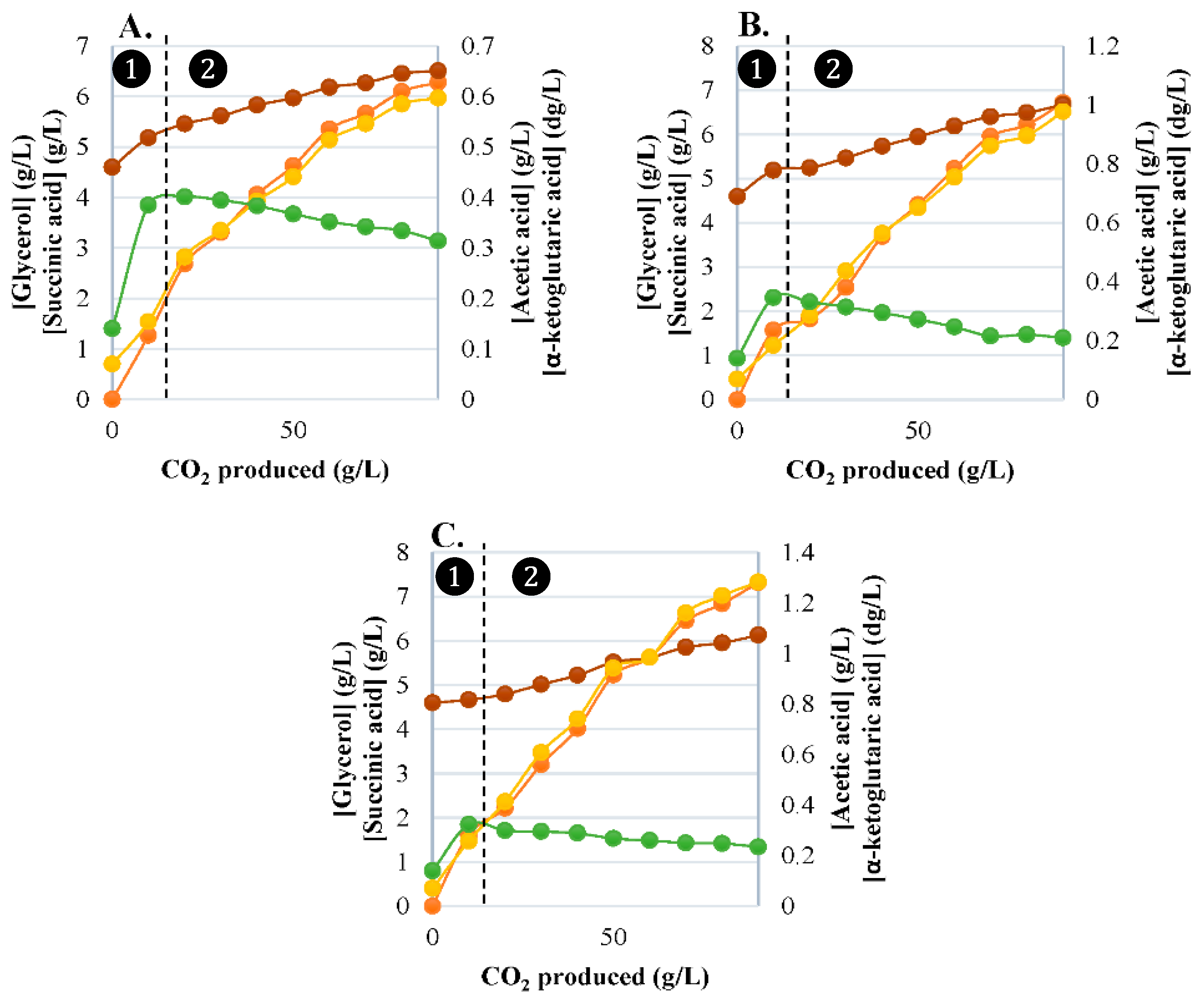
3.2. Synthesis of Primary Carbon Metabolites
3.2.1. Glycerol Production
3.2.2. α-Ketoglutaric Acid Production
3.2.3. Succinic Acid Production
3.2.4. Acetic Acid Production
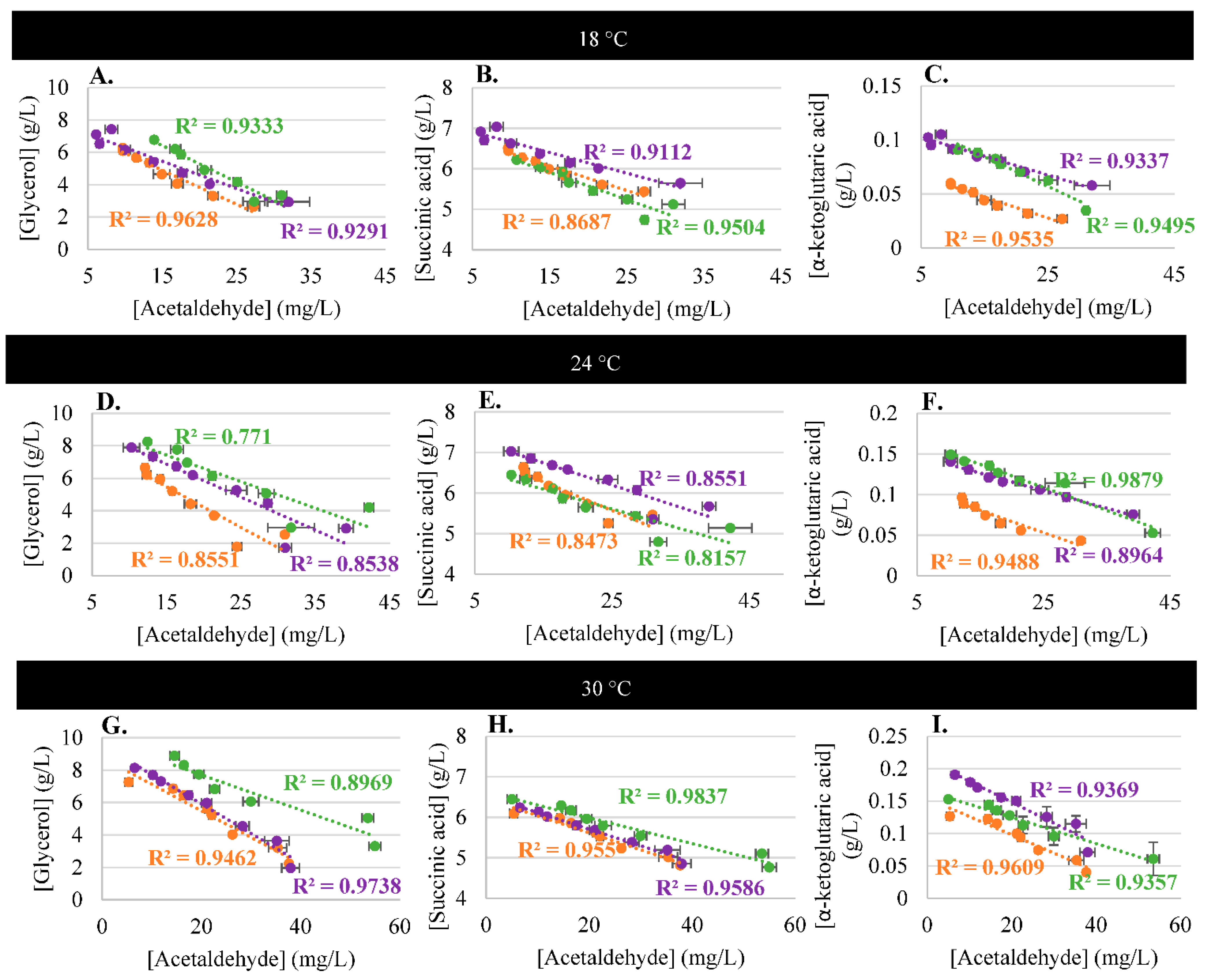
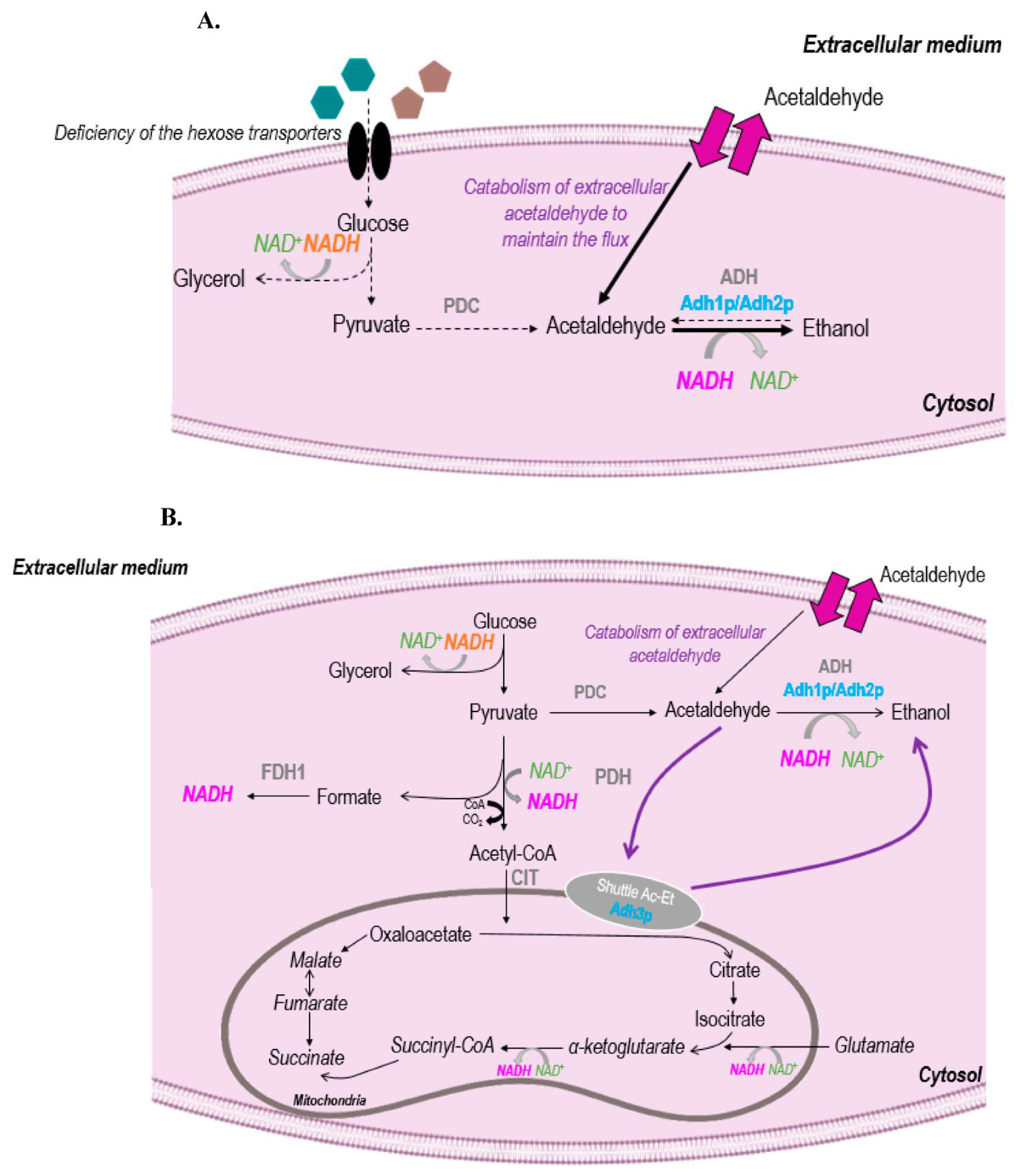
3.3. Acetaldehyde and Metabolism
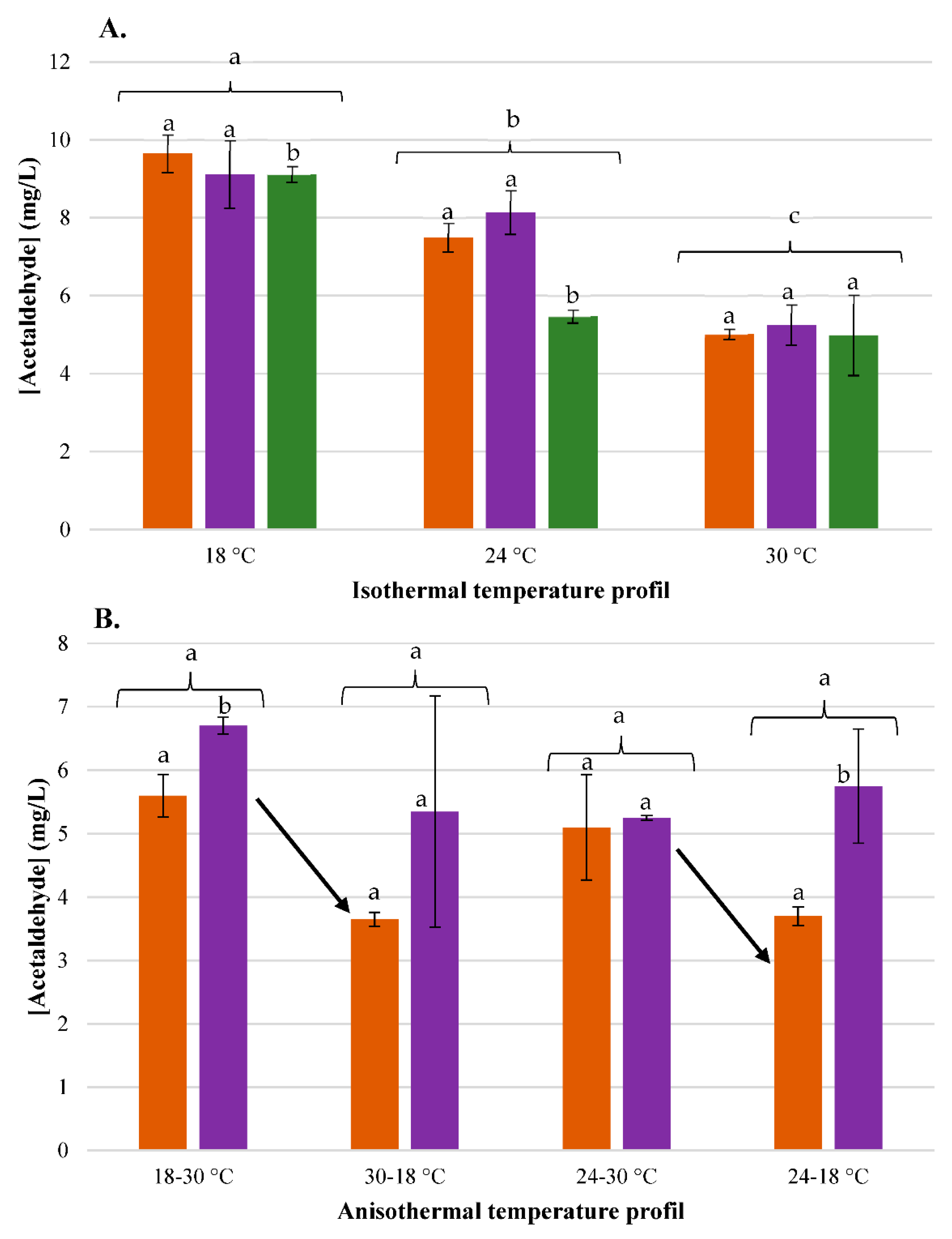
3.4. Impact of Temperature on Acetaldehyde
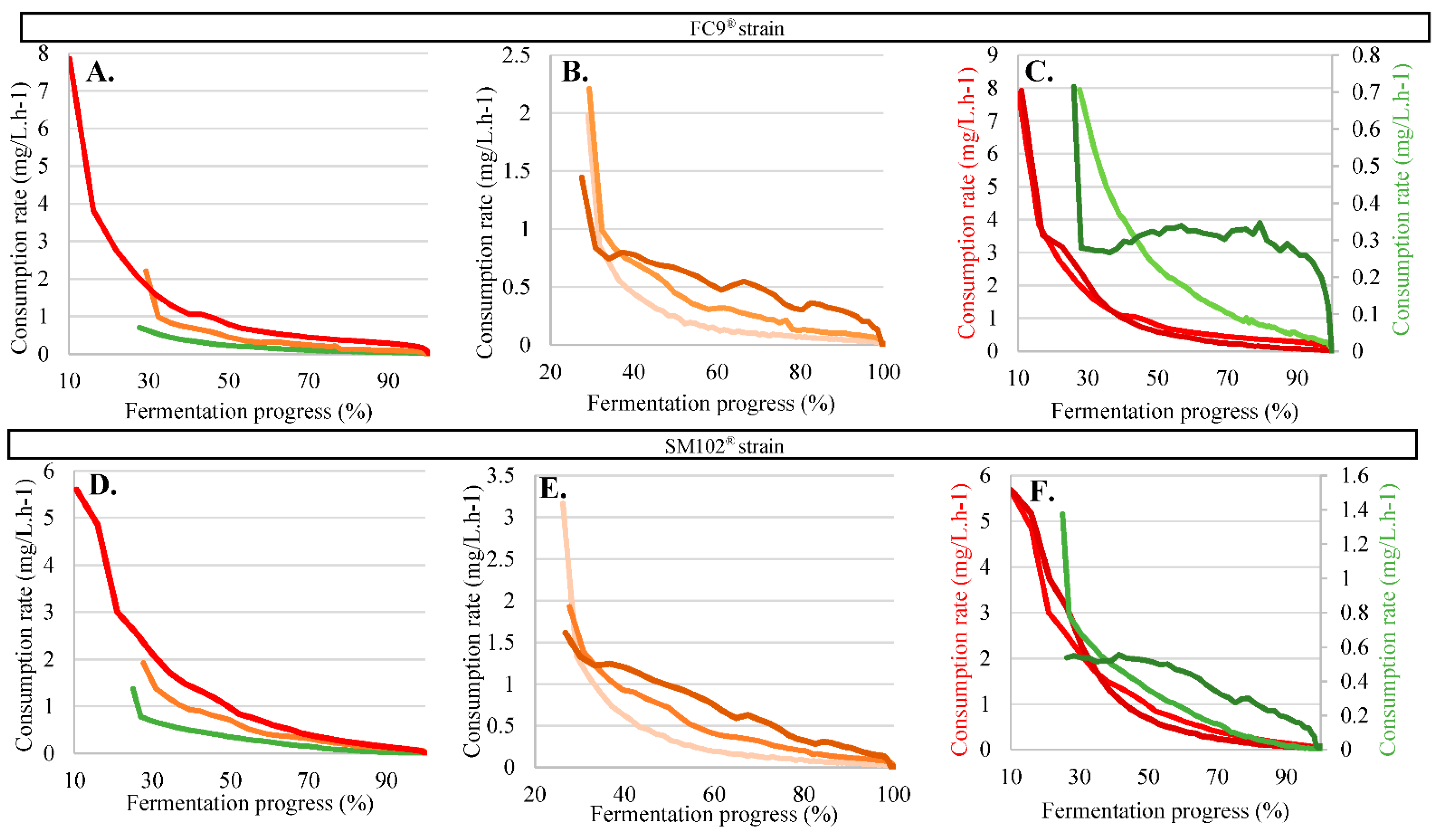
3.4.1. Physical Effect: Evaporation
3.4.2. Biological Effects: Consumption by Yeast
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.-Q.; Pilone, G.J. An Overview of Formation and Roles of Acetaldehyde in Winemaking with Emphasis on Microbiological Implications. Int. J. Food Sci. Technol. 2000, 35, 49–61. [Google Scholar] [CrossRef]
- Ochando, T.; Mouret, J.-R.; Humbert-Goffard, A.; Aguera, E.; Sablayrolles, J.-M.; Farines, V. Comprehensive Study of the Dynamic Interaction between SO2 and Acetaldehyde during Alcoholic Fermentation. Food Res. Int. 2020, 136, 109607. [Google Scholar] [CrossRef] [PubMed]
- Sohrabvandi, S.; Mousavi, S.M.; Razavi, S.H.; Mortazavian, A.M.; Rezaei, K. Alcohol-Free Beer: Methods of Production, Sensorial Defects, and Healthful Effects. Food Rev. Int. 2010, 26, 335–352. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.; Guadagni, D.G. Food Volatiles. Volatilities of Aldehydes, Ketones, and Esters in Dilute Water Solution. J. Agric. Food Chem. 1969, 17, 385–389. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Bao, M.; Niu, C.; Liu, C.; Zheng, F.; Li, Y.; Li, Q. Reverse Metabolic Engineering in Lager Yeast: Impact of the NADH/NAD+ Ratio on Acetaldehyde Production during the Brewing Process. Appl. Microbiol. Biotechnol. 2019, 103, 869–880. [Google Scholar] [CrossRef]
- Cheraiti, N.; Guezenec, S.; Salmon, J.-M. Very Early Acetaldehyde Production by Industrial Saccharomyces Cerevisiae Strains: A New Intrinsic Character. Appl. Microbiol. Biotechnol. 2010, 86, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Aguera, E.; Sire, Y.; Mouret, J.-R.; Sablayrolles, J.-M.; Farines, V. Comprehensive Study of the Evolution of the Gas–Liquid Partitioning of Acetaldehyde during Wine Alcoholic Fermentation. J. Agric. Food Chem. 2018, 66, 6170–6178. [Google Scholar] [CrossRef]
- Jackowetz, J.N.; Dierschke, S.; Mira de Orduña, R. Multifactorial Analysis of Acetaldehyde Kinetics during Alcoholic Fermentation by Saccharomyces Cerevisiae. Food Res. Int. 2011, 44, 310–316. [Google Scholar] [CrossRef]
- Li, E.; Mira de Orduña Heidinger, R. Acetaldehyde Metabolism in Industrial Strains of Saccharomyces Cerevisiae Inhibited by SO2 and Cooling during Alcoholic Fermentation. OENO One 2020, 54, 351–358. [Google Scholar] [CrossRef]
- Li, E.; Mira de Orduña, R. Evaluation of the Acetaldehyde Production and Degradation Potential of 26 Enological Saccharomyces and Non-Saccharomyces Yeast Strains in a Resting Cell Model System. J. Ind. Microbiol. Biotechnol. 2011, 38, 1391–1398. [Google Scholar] [CrossRef]
- Gao, Y.-T.; Zhang, Y.-S.; Wen, X.; Song, X.-W.; Meng, D.; Li, B.-J.; Wang, M.-Y.; Tao, Y.-Q.; Zhao, H.; Guan, W.-Q.; et al. The Glycerol and Ethanol Production Kinetics in Low-Temperature Wine Fermentation Using Saccharomyces Cerevisiae Yeast Strains. Int. J. Food Sci. Technol. 2019, 54, 102–110. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Traité D’oenologie, Tome 1: Microbiologie Du Vin, Vinifications; Dunod: Malakoff, France, 1998. [Google Scholar]
- Henschke, P.A.; Jiranek, V. Yeast-Metabolism of Nitrogen Compounds; Taylor & Francis Group: New York, NY, USA, 1993. [Google Scholar]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Springer: Boston, MA, USA, 1996; ISBN 978-1-4613-5718-6. [Google Scholar]
- Mouret, J.-R.; Aguera, E.; Perez, M.; Farines, V.; Sablayrolles, J.-M. Study of Oenological Fermentation: Which Strategy and Which Tools? Fermentation 2021, 7, 155. [Google Scholar] [CrossRef]
- Amerine, M.; Ough, C. Studies with Controlled Fermentation. VIII. Factors Affecting Aldehyde Accumulation. Am. J. Enol. Vitic. 1964, 15, 23–33. [Google Scholar]
- Bosso, A.; Guaita, M. Study of Some Factors Involved in Ethanal Production during Alcoholic Fermentation. Eur. Food Res. Technol. 2008, 227, 911–917. [Google Scholar] [CrossRef]
- Li, E.; Mira de Orduña, R. Acetaldehyde Kinetics of Enological Yeast during Alcoholic Fermentation in Grape Must. J. Ind. Microbiol. Biotechnol. 2017, 44, 229–236. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G.; Turbanti, L.; Polsinelli, M. Acetaldehyde Production in Saccharomyces Cerevisiae Wine Yeasts. FEMS Microbiol. Lett. 1994, 118, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Torija, M. Effects of Fermentation Temperature on the Strain Population of Saccharomyces Cerevisiae. Int. J. Food Microbiol. 2003, 80, 47–53. [Google Scholar] [CrossRef]
- Colombié, S.; Malherbe, S.; Sablayrolles, J.-M. Modeling of Heat Transfer in Tanks during Wine-Making Fermentation. Food Control 2007, 18, 953–960. [Google Scholar] [CrossRef]
- Casalta, E.; Salmon, J.-M.; Picou, C.; Sablayrolles, J.-M. Grape Solids: Lipid Composition and Role during Alcoholic Fermentation under Enological Conditions. Am. J. Enol Vitic. 2019, 70, 147–154. [Google Scholar] [CrossRef]
- Crépin, L.; Nidelet, T.; Sanchez, I.; Dequin, S.; Camarasa, C. Sequential Use of Nitrogen Compounds by Saccharomyces Cerevisiae during Wine Fermentation: A Model Based on Kinetic and Regulation Characteristics of Nitrogen Permeases. Appl. Environ. Microbiol. 2012, 78, 8102–8111. [Google Scholar] [CrossRef]
- Sablayrolles, J.M. Control of Alcoholic Fermentation in Winemaking: Current Situation and Prospect. Food Res. Int. 2009, 42, 418–424. [Google Scholar] [CrossRef]
- Sablayrolles, J.M.; Ball, C.B. Fermentation Kinetics and the Production of Volatiles During Alcoholic Fermentation. J. Am. Soc. Brew. Chem. 1995, 53, 72–78. [Google Scholar] [CrossRef]
- Sablayrolles, J.-M.; Barre, P. Kinetics of Alcoholic Fermentation Under Anisothermal Enological Conditions. I. Influence of Temperature Evolution on the Instantaneous Rate of Fermentation. Am. J. Enol. Vitic. 1993, 44, 127–133. [Google Scholar] [CrossRef]
- Remize, F.; Roustan, J.L.; Sablayrolles, J.M.; Barre, P.; Dequin, S. Glycerol Overproduction by Engineered Saccharomyces Cerevisiae Wine Yeast Strains Leads to Substantial Changes in By-Product Formation and to a Stimulation of Fermentation Rate in Stationary Phase. Appl. Environ. Microbiol. 1999, 65, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Van Dijken, J.P.; Scheffers, W.A. Redox Balances in the Metabolism of Sugars by Yeasts. FEMS Microbiol Rev. 1986, 1, 199–224. [Google Scholar] [CrossRef]
- Roustan, J.L.; Sablayrolles, J.-M. Modification of the Acetaldehyde Concentration during Alcoholic Fermentation and Effects on Fermentation Kinetics. J. Biosci. Bioeng. 2002, 93, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Deroite, A.; Legras, J.-L.; Rigou, P.; Ortiz-Julien, A.; Dequin, S. Lipids Modulate Acetic Acid and Thiol Final Concentrations in Wine during Fermentation by Saccharomyces Cerevisiae × Saccharomyces Kudriavzevii Hybrids. AMB Expr. 2018, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Guittin, C.; Maçna, F.; Sanchez, I.; Barreau, A.; Poitou, X.; Sablayrolles, J.-M.; Mouret, J.-R.; Farines, V. The Impact of Must Nutrients and Yeast Strain on the Aromatic Quality of Wines for Cognac Distillation. Fermentation 2022, 8, 51. [Google Scholar] [CrossRef]
- Guittin, C.; Maçna, F.; Sanchez, I.; Poitou, X.; Sablayrolles, J.-M.; Mouret, J.-R.; Farines, V. Impact of High Lipid Contents on the Production of Fermentative Aromas during White Wine Fermentation. Appl. Microbiol. Biotechnol. 2021, 105, 6435–6449. [Google Scholar] [CrossRef] [PubMed]
- Scanes, K.T.; Hohrnann, S.; Prior, B.A. Glycerol Production by the Yeast Saccharomyces Cerevisiae and Its Relevance to Wine: A Review. SAJEV 2017, 19, 17–24. [Google Scholar] [CrossRef]
- Gancedo, J.M. The Early Steps of Glucose Signalling in Yeast. FEMS Microbiol. Rev. 2008, 32, 673–704. [Google Scholar] [CrossRef] [PubMed]
- Gombert, A.K.; dos Santos, M.M.; Christensen, B.; Nielsen, J. Network Identification and Flux Quantification in the Central Metabolism of Saccharomyces Cerevisiae under Different Conditions of Glucose Repression. J. Bacteriol. 2001, 183, 1441–1451. [Google Scholar] [CrossRef]
- Bely, M.; Sablayrolles, J.M.; Barre, P. Automatic Detection of Assimilable Nitrogen Deficiencies during Alcoholic Fermentation in Oenological Conditions. J. Ferment. Bioeng. 1990, 70, 246–252. [Google Scholar] [CrossRef]
- Malherbe, S.; Fromion, V.; Hilgert, N.; Sablayrolles, J.-M. Modeling the Effects of Assimilable Nitrogen and Temperature on Fermentation Kinetics in Enological Conditions. Biotechnol. Bioeng. 2004, 86, 261–272. [Google Scholar] [CrossRef]
- Rollero, S.; Bloem, A.; Camarasa, C.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.-M.; Dequin, S.; Mouret, J.-R. Combined Effects of Nutrients and Temperature on the Production of Fermentative Aromas by Saccharomyces Cerevisiae during Wine Fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 2291–2304. [Google Scholar] [CrossRef]
- Godillot, J.; Sanchez, I.; Perez, M.; Picou, C.; Galeote, V.; Sablayrolles, J.-M.; Farines, V.; Mouret, J.-R. The Timing of Nitrogen Addition Impacts Yeast Genes Expression and the Production of Aroma Compounds During Wine Fermentation. Front. Microbiol. 2022, 13, 829786. [Google Scholar] [CrossRef] [PubMed]
- Ljungdahl, P.O.; Daignan-Fornier, B. Regulation of Amino Acid, Nucleotide, and Phosphate Metabolism in Saccharomyces Cerevisiae. Genetics 2012, 190, 885–929. [Google Scholar] [CrossRef] [PubMed]
- Whiting, G.C. Organic acid metabolism of yeasts during fermentation of alcoholic beverages-a review. J. Inst. Brew. 1976, 82, 84–92. [Google Scholar] [CrossRef]
- Thoukis, G.; Ueda, M.; Wright, D. The Formation of Succinic Acid during Alcoholic Fermentation. Am. J. Enol. Vitic. 1965, 16, 1–8. [Google Scholar]
- Camarasa, C.; Grivet, J.-P.; Dequin, S. Investigation by 13C-NMR and Tricarboxylic Acid (TCA) Deletion Mutant Analysis of Pathways for Succinate Formation in Saccharomyces Cerevisiae during Anaerobic Fermentation. Microbiology 2003, 149, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Pronk, J.T. Pyruvate Metabolism in Saccharomyces Cerevisiae. Yeast 1996, 12, 1607–1633. [Google Scholar] [CrossRef]
- Saint-Prix, F.; Bönquist, L.; Dequin, S. Functional Analysis of the ALD Gene Family of Saccharomyces Cerevisiae during Anaerobic Growth on Glucose: The NADP+-Dependent Ald6p and Ald5p Isoforms Play a Major Role in Acetate Formation. Microbiology 2004, 150, 2209–2220. [Google Scholar] [CrossRef]
- Beltran, G.; Novo, M.; Guillamón, J.M.; Mas, A.; Rozès, N. Effect of Fermentation Temperature and Culture Media on the Yeast Lipid Composition and Wine Volatile Compounds. Int. J. Food Microbiol. 2008, 121, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Llaurado, J.; Rozes, N.; Bobet, R.; Mas, A.; Constanti, M. Low Temperature Alcoholic Fermentations in High Sugar Concentration Grape Musts. J. Food Sci. 2002, 67, 268–273. [Google Scholar] [CrossRef]
- Neuberg, C.; Hirsch, J. Uber Den Verlauf Der Alkoholischen Garung Bei Alkalischer Reaktion: II. Garung Mit Lebender Hefe in Alkalischen Lösungen. Biochem. Z 1919, 96, 175–202. [Google Scholar]
- Salmon, J.M. Effect of Sugar Transport Inactivation in Saccharomyces Cerevisiae on Sluggish and Stuck Enological Fermentations. Appl. Environ. Microbiol. 1989, 55, 953–958. [Google Scholar] [CrossRef]
- Salmon, J.M.; Vincent, O.; Mauricio, J.C.; Bely, M.; Barre, P. Sugar Transport Inhibition and Apparent Loss of Activity in Saccharomyces Cerevisiae as a Major Limiting Factor of Enological Fermentations. Am. J. Enol. Vitic. 1993, 44, 56–64. [Google Scholar] [CrossRef]
- Roustan, J.L.; Sablayrolles, J.M. Impact of the Addition of Electron Acceptors on the By-Products of Alcoholic Fermentation. Enzym. Microb. Technol. 2002, 31, 142–152. [Google Scholar] [CrossRef]
- Bakker, B.M.; Bro, C.; Kötter, P.; Luttik, M.A.H.; van Dijken, J.P.; Pronk, J.T. The Mitochondrial Alcohol Dehydrogenase Adh3p Is Involved in a Redox Shuttle in Saccharomyces Cerevisiae. J. Bacteriol. 2000, 182, 4730–4737. [Google Scholar] [CrossRef]
- Ciriacy, M. Genetics of Alcohol Dehydrogenase InSaccharomyces Cerevisiac. Molec. Gen. Genet. 1975, 138, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Niu, C.; Liu, C.; Li, Q. Unraveling the Mechanisms for Low-Level Acetaldehyde Production during Alcoholic Fermentation in Saccharomyces Pastorianus Lager Yeast. J. Agric. Food Chem. 2019, 67, 2020–2027. [Google Scholar] [CrossRef] [PubMed]
- Lutstorf, U.; Megnet, R. Multiple Forms of Alcohol Dehydrogenase in S. Cerevisiae. I. Physiological Control of ADH-2 and Properties of ADH-2 and ADH-4. Arch. Biochem. Biophys. 1968, 126, 933–944. [Google Scholar] [CrossRef] [PubMed]

| Acetaldehyde Concentration (mg/L) | ||||
|---|---|---|---|---|
| FC9® | SM102® | 7013® | ||
| Isothermal temperature profiles | 18 °C | 27.3 ± 0.7 | 32.0 ± 0.1 | 31.0 ± 0.7 |
| 24 °C | 30.9 ± 0.2 | 39.1 ± 0.5 | 37.2 ± 7.7 | |
| 30 °C | 35.8 ± 0.8 | 40.0 ± 1.4 | 53.5 ± 1.2 | |
| Anisothermal temperature profiles | 18–30 °C | 28.2 ± 0.5 | 37.9 ± 1.0 | |
| 30–18 °C | 36.7 ± 0.6 | 40.3 ± 0.3 | ||
| 24–18 °C | 29.1 ± 2.6 | 39.5 ± 2.1 | ||
| 24–30 °C | 30.1 ± 0.5 | 39.4 ± 1.0 | ||
| Temperature effect ANOVA (p-value) | *** | * | *** | |
| Strain effect ANOVA (p-value) | *** | |||
| Vmax of Consumption (mg/L·h−1) | ||||
|---|---|---|---|---|
| FC9® | SM102® | 7013® | ||
| Isothermal temperature profiles | 18 °C | 0.68 ± 0.04 | 0.78 ± 0.01 | 0.99 ± 0.04 |
| 24 °C | 2.14 ± 0.09 | 2.00 ± 0.09 | 2.09 ± 0.06 | |
| 30 °C | 7.60 ± 0.35 | 5.38 ± 0.32 | 5.64 ± 0.07 | |
| Anisothermal temperature profiles | 18–30 °C | 0.79 ± 0.11 | 0.54 ± 0.005 | |
| 30–18 °C | 7.58 ± 0.49 | 5.36 ± 0.45 | ||
| 24–18 °C | 1.94 ± 0.05 | 2.90 ± 0.37 | ||
| 24–30 °C | 1.42 ± 0.05 | 1.63 ± 0.03 | ||
| Temperature effect ANOVA (p-value) | *** | *** | *** | |
| Strain effect ANOVA (p-value) | ** | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guittin, C.; Maçna, F.; Picou, C.; Perez, M.; Barreau, A.; Poitou, X.; Sablayrolles, J.-M.; Mouret, J.-R.; Farines, V. New Online Monitoring Approaches to Describe and Understand the Kinetics of Acetaldehyde Concentration during Wine Alcoholic Fermentation: Access to Production Balances. Fermentation 2023, 9, 299. https://doi.org/10.3390/fermentation9030299
Guittin C, Maçna F, Picou C, Perez M, Barreau A, Poitou X, Sablayrolles J-M, Mouret J-R, Farines V. New Online Monitoring Approaches to Describe and Understand the Kinetics of Acetaldehyde Concentration during Wine Alcoholic Fermentation: Access to Production Balances. Fermentation. 2023; 9(3):299. https://doi.org/10.3390/fermentation9030299
Chicago/Turabian StyleGuittin, Charlie, Faïza Maçna, Christian Picou, Marc Perez, Adeline Barreau, Xavier Poitou, Jean-Marie Sablayrolles, Jean-Roch Mouret, and Vincent Farines. 2023. "New Online Monitoring Approaches to Describe and Understand the Kinetics of Acetaldehyde Concentration during Wine Alcoholic Fermentation: Access to Production Balances" Fermentation 9, no. 3: 299. https://doi.org/10.3390/fermentation9030299
APA StyleGuittin, C., Maçna, F., Picou, C., Perez, M., Barreau, A., Poitou, X., Sablayrolles, J.-M., Mouret, J.-R., & Farines, V. (2023). New Online Monitoring Approaches to Describe and Understand the Kinetics of Acetaldehyde Concentration during Wine Alcoholic Fermentation: Access to Production Balances. Fermentation, 9(3), 299. https://doi.org/10.3390/fermentation9030299





