Abstract
γ-Valerolactone (GVL) is a platform chemical for the synthesis of both biofuels and biochemicals. The LA production from depithed sugarcane bagasse (DSB) resulted in a 55% LA yield, and the resulting LA was used to produce GVL. The effect of process parameters, namely, temperature (25–200 °C), time (2–10 h), and catalyst loading (0.5–5 g) were investigated for the GVL production from LA. Thereafter, the optimized conditions were used to produce GVL from LA derived from depithed sugarcane bagasse (DSB) yielded a GVL of 77.6%. The hydrogen required for the reduction of LA to GVL was formed in situ by formic acid and triethylamine in the presence of methanesulfonic acid (MsOH). Different solvents (including water and alcohols) were also tested to determine their effect on GVL yield, and water yielded the highest GVL of 78.6%. Different types of catalysts, which included mineral acids and ionic liquids, were used to determine their effect on GVL yield, and to provide a benchmark against MsOH. The GVL yield from DSB-derived LA is 1.0% lower than the GVL yield from a commercial sample of LA. LA generated from DSB has the potential to replace fossil fuel-derived LA.
1. Introduction
The increase in global temperatures due to the release of greenhouse gases mainly due to carbon dioxide released by the combustion of fossil fuels has led to the development of renewable and sustainable sources of energy and chemicals from biomass [1]. Fossil fuels are converted to carbon-based chemicals and fuels and combusted to produce energy. Biomass-derived platform chemicals are nontoxic, biodegradable, and are used to produce energy, feedstock chemicals, and fine chemicals [2,3]. Many platform chemicals are derived from biomass, including succinic acid, furfural, 2,5-furandicarboxylic acid, 3-hydroxypropionic acid, aspartic acid, glucaric acid, glutamic acid, itaconic acid, levulinic acid, hydroxybutyrolactone, glycerol, sorbitol, and xylitol [4,5,6]. Levulinic acid (LA) is an important derivative that can be obtained from second-generation lignocellulosic biomass. It is a versatile platform chemical, which can be used for several applications, for example in the production of polymers, lubricants, fuels, coatings, or pharmaceuticals [7]. LA was identified as one of the 15 most promising carbohydrate-derived platform chemicals by the US Department of Energy [8,9]. One of the LA derivatives, γ-valerolactone (GVL) is also a platform chemical for the synthesis of both biofuels and biochemicals. Some of the chemicals produced from GVL are: butene, toluene, 1,4-pentanediol, 4-hydroxypentanamide, methyl pentanoate, 5-methyltetrahydrofolate, and α-methylene-γ-methyl-γ-butyrolactone [10].
GVL is produced by catalytic hydrogenation of LA [11], using homogeneous and heterogeneous catalysts, or metal-free catalysts [12,13,14] with no external hydrogen source. Figure 1 illustrates the reaction steps of producing GVL from depithed sugarcane bagasse (DSB); the first reaction includes the production of LA from DSB using 1-ethyl-3-methylimidazolium hydrogen sulfate [EMim][HSO4] as a catalyst and the second reaction includes the production of GVL from LA using methanesulfonic acid (MsOH) as a catalyst, and formic acid as the hydrogen donor with trimethylamine (Et3N) as a stabilizer [15].
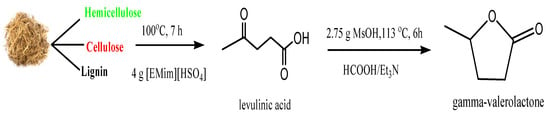
Figure 1.
GVL production from LA derived from DSB.
GVL can be used as a solvent [16] or “drop-in” fuel because of its physicochemical properties such as: inertness towards oxygen and water, high boiling and flash point, low melting point, and low vapor pressure [17,18]. GVL is mostly used as a solvent for lacquers, insecticides, liquid fuel, food additives and adhesives, and it is also used in cutting oil, brake fluid, and as a coupling agent in the dye bath [19,20]. Aromas is one of the main GVL manufacturers, accounting for roughly 62% of global output. Kunshan Qiandeng Baihua, Zhongyue Aroma, Soda Aromatic, Inoue Perfumery MFG, and others are also involved in GVL manufacture [21]. The Global Gamma-valerolactone (CAS 108-29-2) market is expected to grow at a CAGR of 5.02% from 2022 to 2030 [22]. GVL is a better alternative to ethanol as a fuel additive due to its lower vapor pressure, relatively higher energy content, safety in storage, and more importantly, it does not form an azeotrope with water [10,15].
In general, longer reaction times favor higher yields of GVL [2,15,19,20], which make it a more energy intensive process; therefore, a method needs to be developed that would have a shorter reaction time while producing a higher yield. Metal-based catalyst for GVL production can produce up to 96–100% of GVL yield [2,15,20,23] but the high cost of these metal catalysts means that they are not affordable for scale-up applications. Common noble metals for GVL production from LA are palladium (Pd) and ruthenium (Ru) [15].
Dutta et al. [24] showed that non-noble metals such as copper (Cu) and zirconium (Zr) catalysts contribute in a green GVL production because of their higher abundance and milder reaction conditions (e.g., low pressure and temperature without an external source of H2). Non-noble metals can replace noble metals such as Pd and Ru, with the latter having been extensively used as the catalyst for GVL production from LA [25].
Currently, there are numerous methods for producing GVL through hydrogenation of levulinic acid in the presence of molecular hydrogen, alcohol, or formic acid and a catalyst. Heterogeneous catalysts are more commonly used when compared to homogeneous catalysts [16]. To the authors’ best knowledge, no ionic liquids (ILs) have been used for the conversion of LA to GVL in the absence of a noble metal [21,26,27]. ILs are a class of salts that are liquid at 100 °C and can be employed as solvents or catalysts. ILs are sometimes known as “designer solvents” because their unique characteristics are tailored to a specific purpose by suitable cation or anion modification. When compared to traditional volatile organic solvents often used in industry, ILs have little vapor pressure. As a result, IL evaporation into the atmosphere is limited, and environmental pollution is negligible. This is one of the reasons why ILs are considered environmentally friendly solvents. Ionic liquids are not flammable, are thermally and chemically stable, are recoverable, and are recyclable [28]. Many ILs can dissolve biomass-related chemicals and are effective reaction solvents or catalysts; they are intensively investigated for converting biomass-related compounds into materials and second-generation biofuels [26], hence they will also be investigated in this work. Formic acid has the potential to be used as a hydrogen source in the production of GVL from LA [29,30]. In situ formation of formic acid and LA during biomass fractionation allows for the former to convert LA to GVL using the green chemistry principle of “atom economy” [10]. For the first time, the environmentally friendly catalyst methanesulfonic acid (MsOH) will be employed to catalyze the manufacture of GVL from LA in this work. LA will be generated from sugarcane bagasse. The impact of several catalysts, including two ionic liquids, will also be investigated. According to the literature, ionic liquids have never been utilized without noble metals.
In this work, GVL production from commercial LA was optimized using MsOH because sulfonic acids are strong, non-oxidizing, biodegradable compounds that are environmentally friendly, highly reactive catalysts, and less corrosive [31]. The investigated parameters were time, temperature, and catalyst loading. The optimized conditions for the GVL production from commercial LA were then used to produce GVL from DSB-derived LA. The optimum conditions were also used to investigate the effect of other catalysts and solvents on the yield of GVL from DSB-derived LA.
2. Materials and Methods
2.1. Materials
All the chemicals were purchased from Merck (Johannesburg, South Africa), and were all used without any further purification: levulinic acid (98%), methanesulfonic acid (95%), γ-valerolactone (98%), formic acid (95%), triethylamine (95%), sulfuric acid (95%), ethyl acetate (95%), 1-butyl-3-methylimidazolium hydrogen sulphate (≥95%), sulfuric acid (95%), tosylic acid (98.5%), 1-ethyl-3-methylimidazolium tosylate (≥98%), butanol (≥99.4%), ethanol (≥99.8%), and methanol (99.8%). Depithed sugarcane bagasse was supplied by a local sugar milling research institute (SMRI) of South Africa.
2.2. Hydrogenation of Commercial LA into GVL
2.2.1. Effect of Temperature, Time, and Catalyst Loading on GVL Production from Commercial LA Using MsOH
To determine the optimum conditions for GVL production from commercial LA, the minimum and maximum of the investigated reaction conditions were used (Table 1). After reviewing the literature [2,15,19,20,23] and considering the technique that was required to be created, the minimum and maximum reaction conditions in Table 1 were chosen.

Table 1.
Investigated levels for the three parameters: temperature, time, and catalyst loading using BBD.
A set of experiments (Table 2) were generated using the Box–Behnken design (BBD). A constant mass of 1.0 g of commercial LA was added to a 100 mL stainless steel reactor (Parr Instruments Company, Moline, IL, USA) with 10 mL of water, 700 µL of formic acid, and 220 µL of triethylamine [15]. A predetermined catalyst loading of MsOH (Table 2) was added to the reaction vessel. The mixture was stirred at 200 rpm and the duration of the reaction was measured when the set temperature was reached. At the end of the reaction the heat supply was removed, and the reactor vessel was inserted in a cold-water bath to cool the reaction to room temperature. The liquid component of the cooled reaction mixture was extracted with (10 mL × 4) of ethyl acetate; the solvent was removed by vacuum. The products were stored in a refrigerator at 4 °C before high performance liquid chromatography (HPLC) (Shimadzu, Japan) analysis. The procedure for the HPLC analysis is detailed later.

Table 2.
Investigated reaction parameters with responses for GVL, LA conversions, and GVL selectivity.
2.2.2. Effect of Catalysts on GVL Yield
The optimum condition for the GVL production from commercial LA using MsOH was used for the following catalysts: tosylic acid [TsOH], 1-butyl-3-methylimidazolium hydrogen sulphate [BMim][HSO4], 1-ethyl-3-methylimidazolium tosylate [EMim][OTs], and sulfuric acid [H2SO4].
2.2.3. Effect of Solvent on the Production of GVL
Solvents: water (H2O), methanol (CH3OH), ethanol (C2H5OH), ethanol and water (C2H5OH and H2O), and butanol (C4H10O) were used for GVL production from commercial LA in the solvent optimization reactions using the conditions optimized for the catalyst MsOH.
2.3. DSB Preparation
The DSB was dried in an oven (Scientific, South Africa) at 105 °C for 24 h, milled by Pulverisette 16 (Fritsch, Germany), and sieved to 40-mesh particle size [32].
2.4. DSB Conversion to GVL
2.4.1. Conversion of DSB to LA
The procedure for the production of LA from DSB is similar to the one used in our previous study [28], although for this study, the production was upscaled from the ratio (1:4) 1 g of bagasse and 4 g of 1-ethyl-3-methylimidazolium hydrogen sulfate [EMim][HSO4] to 100 g of bagasse and 400 g of [EMim][HSO4] at the CSIR’s Forestry and Forest Products Research Centre in Durban. The reaction was carried out in a round bottom flask with a stirrer immersed in an oil bath for 7 h at 100 °C. The product was analyzed using HPLC (Shimadzu, Japan).
2.4.2. Hydrogenation of LA Derived from DSB into GVL
The LA derived from DSB was used to produce GVL using the optimized conditions obtained from the optimization study where a commercial sample of LA was used.
2.5. Product Analysis Using High Performance Liquid Chromatography
The qualitative and quantitative analysis of LA and GVL were carried out using HPLC (Shimadzu, Japan) equipped with an ultraviolet detector at 210 nm fitted with a Aminex HPX 87 H column (Bio-Rad, Hercules, CA, USA) at a flow rate of 0.5 mL/min with a column temperature of 50 °C and mobile phase of aqueous sulfuric acid (0.005 M). Using a syringe, 1 mL samples and standards were filtered over a 0.45-micron filter to prevent any solids from entering the HPLC column. Standards were dissolved in distilled water. The concentration of LA and GVL was calculated using standard calibration curves. The retention time of LA and GVL was 16 and 35 min, respectively. The GVL yield (YGVL), actual yield (Yact), theoretical yield (Yth), LA conversion (XLA), and GVL selectivity (SGVL) were calculated according to Equations (1)–(5):
where nLA is the number of moles of LA and MrGVL is the molar mass of GVL. LA molar mass is 116.11 g·mol−1 and GVL molar mass is 100.12 g·mol−1.
where CLA,O is the initial concentration of LA and CLA is the concentration of LA.
2.6. Experimental Design
To determine the optimum reaction conditions of GVL production from LA, response surface methodology (RSM) and BBD were used. BBD was used to design the set of experiments in in Table 2.
Five replicates in the central point (time: 6 h, temperature: 112.5 °C, and catalyst loading: 2.75 g) with a total of 17 experiments (Table 2) were performed for the optimization of GVL production from commercial LA. The independent variables were the time (A), temperature (B), and catalyst loading (C). The output variable Y was the yield of GVL (YGVL).
Design Expert Statistical 12 software (Stat Ease Inc., Minneapolis, USA) was used to regress and fit the data to a second order model, as well as to calculate the analysis of variance (ANOVA). A quadratic method was used to analyze the data. The terms of the model were tested at the 95% confidence level (p ≤ 0.05). Five replicates were performed in the central points to estimate random errors.
3. Results and Discussion
3.1. Effect of Temperature, Time, and Catalyst Loading for GVL Production from Commercial LA to GVL
The hydrogenation of commercial LA to GVL was optimized using data from Table 1 and the results are shown in Table 2. The optimum conditions are: a temperature of 112.5 °C, a reaction time of 6 h, and a catalyst loading of 2.75 g which yielded a maximum GVL of 78.6%. Table 2 also shows that the hydrogenation of commercial LA to GVL is reproducible since five runs were repeated and their response differed by <1.3%.
3.2. Response Surface Methodology (RSM) Analysis
The polynomial regression model for GVL production from commercial LA is given in Equation (6), which was obtained from Design Expert Statistical software. Where A is the temperature, B is the time, and C is the catalyst loading.
Y (GVL yield %) = +78.18 + 0.4062 A − 4.34 B + 4.75 C + 0.9625 AB − 1.60 AC − 0.1100 BC − 14.91 A2 − 21.30 B2 − 8.22 C2
The optimum experimental GVL yield (78.6%) is close to the theoretical GVL yield (78.2%) calculated from Equation (6).
Table 3 shows that the quadratic model is significant in determining the response (model p value ˂ 0.0001). The coefficient of determination (R2) value is 0.9998, also indicating that the variation around the average could be explained by the model, i.e., 99.98% of the variability in the responses can be explained by the model [33]. The model F-value of 4384.69 implies the model is significant. There is only a 0.01% chance that an F-value this large could occur due to noise in the data. p < 0.0500 indicates that the model terms are significant at the 95% confidence level. In this case, A, B, C, AB, AC, A2, B2, C2 are significant model terms. The lack of fit F-value of 1.57 implies the lack of fit is not significant relative to the pure error (random error). There is a 32.77% chance that a lack of fit F-value this large could occur due to noise. Non-significant lack of fit means that the model is fit to predict the response.

Table 3.
ANOVA for the response surface quadratic model for GVL production as a function of time (A), temperature (B), and catalyst loading (C).
The parity plot was used to determine the reliability of the model. The parity plot of GVL yield is shown in Figure 2 where the predicted GVL yield is compared with the experimental (observed) GVL yield. The points in the graph are in a straight line, indicating that the model is significant and can predict GVL yields accurately.
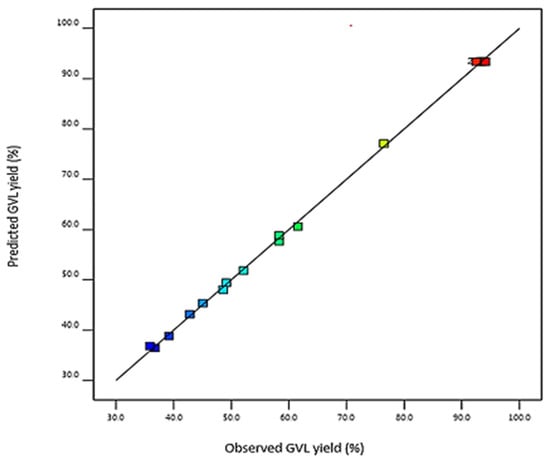
Figure 2.
GVL parity plot that compares the predicted GVL yield with experimental GVL yield.
The pareto chart illustrates which of the investigated parameters have a greater influence on GVL yield. Figure 3 shows that temperature (B) had the greatest effect, compared to time (A) and catalyst loading (C). The p values in Table 3 show that all the investigated parameters are significant, but the interaction of temperature and catalyst loading (BC) has minimal effect on GVL yield.
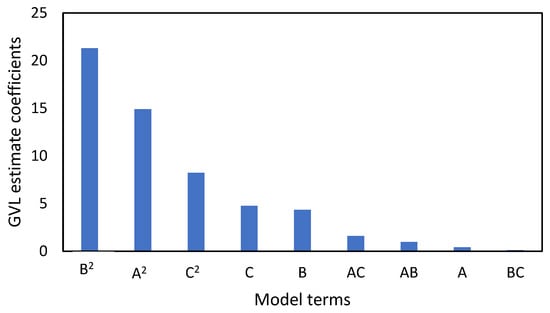
Figure 3.
GVL pareto chart of the model terms which illustrate the terms that have more effect on the GVL yield. Investigated model terms are temperature (A), time (B), and catalyst loading (C).
The response surface and contour plots (Figure 4a–c) illustrate the interaction of the investigated factors (time, temperature, and catalyst loading) to produce GVL from commercial LA. Figure 4a indicates that both temperature and time are significant to GVL yield. The minimum temperature (25 °C) and time (2 h) yielded a low GVL yield (46.7%). Increasing the temperature to 112.5 °C and time to 6 h resulted in maximum GVL yield (78.6%); however, increasing the temperature and reaction time to 200 °C and 10 h, respectively, resulted in a marked decrease in GVL yield (39.2%). Therefore, a temperature of 112.5 °C and time to 6 h are the optimum conditions for GVL production from LA using MsOH as the catalyst.
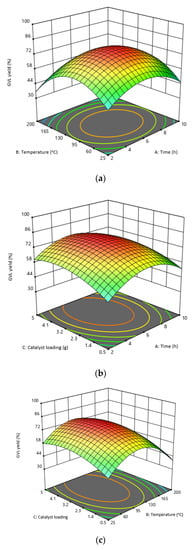
Figure 4.
Response surface and contour plots for the interaction of the investigated factors: (a) temperature vs. time; (b) catalyst loading vs. time; (c) catalyst loading vs. temperature.
Figure 4b illustrates the interaction of catalyst loading and time on GVL yield. Increasing the catalyst loading and time from 0.5 to 2.75 g and 2 to 6 h increases the GVL yield. A catalyst loading of 2.75 g and time of 6 h yielded higher GVL yields ranging from 77.9 to 78.6%. Increasing the time to 10 h with a catalyst loading of 2.75 g results in lower GVL yields (39.2 to 45.9%), whereas increasing the catalyst loading from 2.75 to 5 g for a reaction time of 10 h results in increased GVL (49.1 to 58.3%) yield. In general, increasing the catalyst loading and time is supposed to increase the rate of reaction thereby increasing the product yield, but that is not the case in this work. This may be due to the production of by-products (1,4-pentanediol and 4-hydroxypentanoic acid (4-HPA)) [34]. Even if the maximum reaction conditions produced the highest yield, increasing both time and catalyst loading means the production process of GVL will be expensive because it will require more energy and more catalyst.
The relationship of catalyst loading and temperature on the reaction is shown in Figure 4c, where a catalyst loading of 2.75 g and a temperature of 112.5 °C produced the maximum GVL yield (78.6%). Figure 5 indicates that moderate reaction conditions favor a high yield of GVL whereas the minimum and maximum factors lower the GVL yield. All the factors play an important role in the production of GVL, which was confirmed by ANOVA (Table 3). The optimized conditions are: temperature of 112.5 °C, time of 6 h, and catalyst loading of 2.75 g yielding 78.6% GVL.
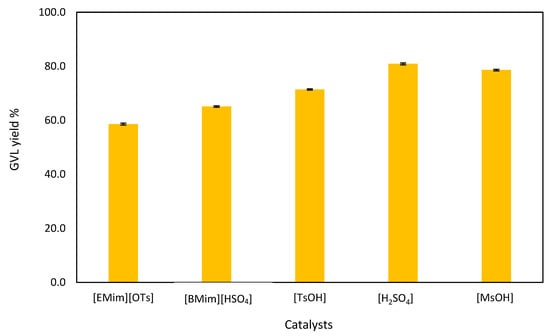
Figure 5.
Effect of catalysts on GVL production from LA.
Singh et al. [35] used Ni/NiO catalyst to produce GVL from commercial LA which showed high conversion of >90% and high selectivity of >90%; the LA conversion obtained in this study is higher by 7%, whereas the GVL selectivity is lower by 9% and the difference is due to the different catalysts used. Córdova-Pérez et al. [36] synthesized GVL from LA using a nickel (Ni)-supported nanoparticle, yielding 80% of GVL after 24 h at 170 °C. Because of the variable catalyst and reaction conditions, the GVL is greater than the GVL yield observed in this work, although the response time is extremely lengthy. Specific surface area, pore structure, functional groups, temperature, and time are all variables that can influence catalytic activity. As a result, depending on the above criteria for each catalyst, the product of the reaction might increase or decrease.
3.3. Effect of Catalysts on GVL Yield
Figure 5 illustrates the effect of the catalysts on the production of GVL. Various catalysts: tosylic acid [TsOH], 1-butyl-3-methylimidazolium hydrogen sulphate [BMim][HSO4], 1-ethyl-3-methylimidazolium tosylate [Emim][Ots], sulfuric acid [H2SO4], and methanesulfonic acid [MsOH] were used to study the catalysts’ effect on the production of GVL from commercial LA. The maximum GVL yield was for sulfuric acid (80.9%) followed by methanesulfonic acid (78.6%) and tosylic acid (71.4%). Although mineral acids usually produce high yields, their disadvantages include high cost of neutralization, separation, purification steps, and corrosiveness that needs special materials of construction. It is necessary to separate the mineral acid from the reaction products because it negatively affects downstream processes [20,37]. The two ionic liquids (1-butyl-3-methylimidazolium hydrogen sulphate and 1-ethyl-3-methylimidazolium tosylate) used in this work as catalysts yielded 65.1 and 58.6% of GVL, respectively, showing that ILs can catalyze LA to GVL in the presence of formic acid and triethylamine. The two ILs, namely, [BMim][HSO4] and [EMim][OTs], were chosen for this work because they are acidic ILs. Increases in IL acidity results in the enhancement in both LA (100%) conversion and GVL (99%) selectivity [21].
The advantages of ILs over minerals acids are their negligible vapor pressure, non-flammability, thermal and chemical stability, recoverability, and recyclability [38,39]. Therefore, the ILs are preferred as catalysts for GVL production from LA although [BMim][HSO4] yielded 15.8% less when compared to [H2SO4].
Sanchis et al. [40] used a synthesized nickel catalyst which presented a relatively high yield and selectivity to GVL (up to 40% and >98%, respectively) in the hydrogenation of LA. the maximum GVL yield obtained in this work is higher, however, as the nickel catalyst is highly selective compared to MsOH. López-Aguado et al. [41] reported on GVL production, where high LA conversion (>95%) and GVL yield (>90%) were achieved; however, the duration of the reaction was too long (20 days) at 170 °C using a Zr–Al-beta zeolite catalyst. Jori and Jadhav [42] produced GVL from biomass-derived levulinic acid using 150 wt % of catalyst (hafnium-based carbonaceous (Hf@CCSO3H)) at 200 °C for 24 h in isopropanol solvent as a hydrogen donor. In their study, 100% conversion of LA was achieved with a high yield of 96% with more than 99% selectivity of GVL, which is higher compared to the maximum yield obtained in this study which is due to the different catalysts and reaction conditions used. The catalyst used by López-Aguado et al. [41] and Jori and Jadhav [42] seem to be more effective under the harsh conditions, which is opposite of the catalyst used in this study. When the costs of the catalysts utilized in this investigation for 100 mL were evaluated, it was discovered that sulfuric acid was the least expensive and produced the highest GVL. [H2SO4] costs ZAR 75.7, [TsOH] costs ZAR 222.4, [MsOH] costs ZAR 718, [BMim][HSO4] costs ZAR 1 838, and [Emim][Ots] costs ZAR 9 360.
3.4. Effect of Solvent on the Production of GVL
There are different types of solvents that have been used for GVL production, namely, water, ethanol, dichloromethane and water, 1,4-dioxane, and methanol [10]. Furthermore, alcohols have been used as an H-donor for the hydrogenation of GVL production [10]. Therefore, in this work, various solvents are studied to observe their effect on the GVL yield. The effect of the solvent in the hydrogenation of commercial LA to GVL using MsOH is shown in Figure 6. The following solvents were used: water (H2O), methanol (CH3OH), ethanol (C2H5OH), ethanol and water (C2H5OH and H2O), and butanol (C4H10O). Water is the best solvent for GVL production (78.6%). Water is also a preferred solvent because of its chemical and physical properties which makes water nontoxic to human and aquatic organisms, easily available, and environmentally friendly [43,44]. Ethanol also had a similar effect to water for the conversion process (76.5%). The ethanol and water mixture gave a lower yield of GVL (61%). This could be due to the intermolecular hydrogen bonding occurring between water and ethanol molecules preventing the conversion of LA to GVL. Polar mixed solvents do not increase the GVL conversion. To ensure a high degree of LA conversion, solvents such as alcohols (methanol, ethanol, butanol) or water are used [45,46]. Hengst et al. [47] reported LA conversion of 75–100% when various alcohols were used for GVL production with a Ni/Al2O3 catalyst. When water was used, the LA conversion was low with 2% compared to alcohols, even though similar reaction conditions (150 °C, 10 bar H2, 6 h) were used; however, the GVL selectivity of water was the highest (100%). The outcome of this study is the opposite of Hengst et al. [47], since when water was used the LA conversion was 97% and GVL selectivity was 81%, which were higher compared to alcohols (LA conversion, 85–95% and GVL selectivity, 60–80%). This is due to the different conditions and catalysts used. Carbon-laden wastewater might be utilized as a hydrogen source in the future instead of alcohols or formic acid with triethylamine [48]. Fu et al. [49] attained higher LA conversion (100%) and GVL selectivity (99.2%) by employing dioxane as the solvent under milder reaction conditions (180 °C, 2 h), even though the LA conversion and GVL selectivity is higher. However, the CHEM21 solvent selection guide, which ranks solvents according to the severity of safety (S), heath (H), and environmental (E) hazards, states dioxane as not recommended due to its toxicity [24].
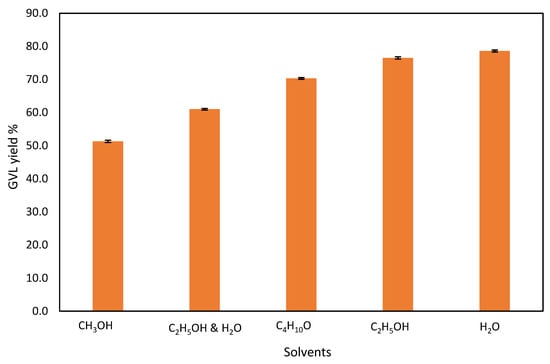
Figure 6.
Effect of solvents on the GVL production from commercial LA.
3.5. DSB Conversion to GVL
3.5.1. Conversion of DSB to LA
The upscaled LA production from DSB (this work) gave an LA yield of 55% which is 0.4% higher compared to the LA yield produced for the laboratory scale [28]. This shows that there is no significant difference that occurred when the reaction was upscaled and that the optimized parameters can be used to reproduce the laboratory scale results. The only difference that was observed is the amount of water required—the mixture was too thick and therefore it required more water to be added for the mixture to be stirred. This shows that a process that requires combining raw biomass with liquid reagents does not scale linearly, i.e., the physical amount of biomass relative to the liquid reagents is far greater in the large-scale process. Ramli and Amin [50] produced 24.8% of LA yield from oil palm fronds (OPF) using 1-sulfonic acid-3-methyl imidazolium tetrachloroferrate [SMIM][FeCl4] which is lower than the yield obtained in this work, and this may be due to different catalysts used, or different reaction conditions.
3.5.2. Hydrogenation of LA Derived from DSB into GVL
The use of LA derived from DSB with the optimized conditions for the commercial LA conversion to GVL of 6 h, 112.5 °C, and 2.75 g of MsOH yielded a GVL of 77.6%, LA conversion of 95%, and 82% of GVL selectivity. The GVL yield obtained from DSB-derived LA is 1.0% less compared to the GVL yield produced from commercial LA. The difference may be due to impurities in the LA derived from DSB, but the difference is minor. Thus, LA derived from DSB is a promising reactant for GVL production. Barla et al. [51] used a cobalt-based catalyst for the hydrogenation of biomass-derived LA to GVL which resulted in a 99% conversion of LA and 80% selectivity of GVL. Although the catalysts used were different, the LA conversion difference was 4% and GVL selectivity was 2%, which is less, and this shows that MsOH can replace metal catalysts and that LA derived from DSB can be used as a starting material for GVL production. Figure 7 illustrates the GVL production from sugarcane bagasse including the reaction conditions for the two step reactions.
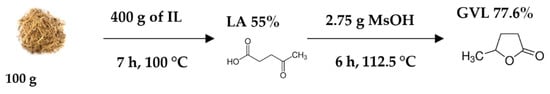
Figure 7.
The process of GVL production from sugarcane bagasse.
3.6. Techno-Economic Assessment of GVL Production from Sugarcane Bagasse
Comprehensive techno-economic feasibility studies on the technology development and industrial scale application of the conversion of biomass (sugarcane bagasse)-derived levulinic acid into γ-valerolactone using methanesulfonic acid determined the cost of using catalyst at a scaled-up level. The data in Table 4 extrapolated from the (TEA) report show that under optimum conditions it is more economical, financially sustainable, and cost effective to use the recyclable and recoverable catalysts. Detailed studies show the cost of catalyst measured against reactor capacity and capital costs.

Table 4.
Reactor size.
At scaled-up reactor capacity of 50 L and optimum conditions were attained in which the mass transfer was at its most efficient and more economically sustainable for the conversion of levulinic acid into γ-valerolactone using methanesulfonic acid. The cost of production in a 250 L reactor will need a catalyst quantity just above 10 percent of the overall volume. This implies that 90% of the reactor feedstock comprises active reagents that will contribute towards the yield. At the optimum conditions, the catalyst costs merely USD 34.72 for a recoverable mass of 4.167 kg of catalyst.
The amount of ionic liquid catalyst needed was varied while constantly increasing the reactor size, as shown in Table 5. The cost increased consistently as the volume increased. However, it remained more cost effective than using other heterogenous and homogenous catalyst materials.

Table 5.
Amount of catalyst needed.
The catalyst material is dispersed in the reagent solution of HCOOH and Et3N, and the catalyst forms an ionic solution that facilitates reduction of the levulinic acid to give the gamma-valerolactone. Table 6 presents the comprehensive capital expenditure determined from the feedstock and reactor capacity over the actual scaled-up reactor against the market penetration index (MPI), a unit of measurement used to show how reactor feedstock occupancy compares to a preselected set of competitors.

Table 6.
Comprehensive capital expenditure.
Detailed analysis of the comprehensive economic impact of the technology against a set of competing technologies shows that the pre-eminent feature of the conversion of biomass-derived levulinic acid into γ-valerolactone using methanesulfonic acid as the catalyst is in the mechanism of the mass transfer that translates to a cost effective and sustainable process at optimized conditions as indicated in Table 6. At a fixed volume of 50 L reactor size and optimum temperature and pressure, the catalyst cost will be ZAR 9 604 for a 7 h residence of the reacting materials. The ease with which the catalyst material is recovered makes the choice of the ionic liquid catalyst more desirable and sustainable even at scaled-up operations as shown in Table 6.
4. Conclusions
The optimization study of LA conversion to GVL showed that time, temperature, and catalyst loading have a significant effect on the GVL yield.
The maximum GVL yield of 78.6% was obtained at 6 h, 112.5 °C, and 2.75 g of MsOH.
IL: 1-butyl-3-methylimidazolium hydrogen sulphate produced relatively high GVL yield (65.1%) and could be considered for environmentally green technologies. Sulfuric acid yielded the highest GVL, but it is toxic and corrosive.
Water produced the highest GVL yield, and the mixed solvent of water and ethanol yielded the second-lowest GVL yield.
The GVL yield produced from DSB-derived LA is 1.0% less compared to the GVL produced from the commercial sample of LA. LA derived from DSB is a promising replacement of non-biomass-derived LA.
Author Contributions
Methodology, L.D.M.; Software, L.D.M.; Formal analysis, L.D.M.; Investigation, L.D.M. and F.D.; Writing—original draft, L.D.M.; Writing—review and editing, R.G., D.L., F.D. and N.D.; Supervision, D.L. and N.D.; Project administration, L.D.M. and N.D.; Funding acquisition, L.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Research Foundation (NRF), UID: 138554, Durban University of Technology, and L’Oréal-UNESCO for Women in Science Sub-Saharan Africa Regional fellowship.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Council for Scientific and Industrial Research (CSIR) Forestry and Forest Products Research Centre, Durban for the facility to upscale levulinic acid production from depithed sugarcane bagasse.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gustavsson, L.; Svenningsson, P. Substituting fossil fuels with biomass. Energy Convers. Manag. 1996, 37, 1211–1216. [Google Scholar] [CrossRef]
- Li, W.; Xie, J.-H.; Lin, H.; Zhou, Q.-L. Highly efficient hydrogenation of biomass-derived levulinic acid to γ-valerolactone catalyzed by iridium pincer complexes. Green Chem. 2012, 14, 2388–2390. [Google Scholar] [CrossRef]
- Delhomme, C.; Schaper, L.-A.; Zhang-Preße, M.; Raudaschl-Sieber, G.; Weuster-Botz, D.; Kühn, F.E. Catalytic hydrogenation of levulinic acid in aqueous phase. J. Organomet. Chem. 2013, 724, 297–299. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Mutrakulcharoen, P.; Chuetor, S.; Cheenkachorn, K.; Tantayotai, P.; Panakkal, E.J.; Sriariyanun, M. Recent situation and progress in biorefining process of lignocellulosic biomass: Toward green economy. Appl. Sci. Eng. Prog. 2020, 13, 299–311. [Google Scholar] [CrossRef]
- Sriariyanun, M.; Heitz, J.H.; Yasurin, P.; Asavasanti, S.; Tantayotai, P. Itaconic acid: A promising and sustainable platform chemical? Appl. Sci. Eng. Prog. 2019, 12, 75–82. [Google Scholar]
- Mthembu, L.D.; Gupta, R.; Deenadayalu, N. Conversion of cellulose into value-added products. In Cellulose Science and Derivatives; BoD–Books on Demand: Germany; Intech Open: London, UK, 2021; Volume 125. [Google Scholar]
- Bozell, J.J.; Moens, L.; Elliott, D.; Wang, Y.; Neuenscwander, G.; Fitzpatrick, S.; Bilski, R.; Jarnefeld, J. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels. Energy Environ. Sci. 2011, 4, 83–99. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Tang, X.; Zeng, X.; Li, Z.; Hu, L.; Sun, Y.; Liu, S.; Lei, T.; Lin, L. Production of γ-valerolactone from lignocellulosic biomass for sustainable fuels and chemicals supply. Renew. Sustain. Energy Rev. 2014, 40, 608–620. [Google Scholar] [CrossRef]
- de Souza, R.O.; Miranda, L.S.; Luque, R. Bio (chemo) technological strategies for biomass conversion into bioethanol and key carboxylic acids. Green Chem. 2014, 16, 2386–2405. [Google Scholar] [CrossRef]
- Chauvier, C.; Tlili, A.; Gomes, C.D.N.; Thuéry, P.; Cantat, T. Metal-free dehydrogenation of formic acid to H 2 and CO 2 using boron-based catalysts. Chem. Sci. 2015, 6, 2938–2942. [Google Scholar] [CrossRef] [PubMed]
- Kopetzki, D.; Antonietti, M. Transfer hydrogenation of levulinic acid under hydrothermal conditions catalyzed by sulfate as a temperature-switchable base. Green Chem. 2010, 12, 656–660. [Google Scholar] [CrossRef]
- Deng, L.; Li, J.; Lai, D.M.; Fu, Y.; Guo, Q.X. Catalytic conversion of biomass-derived carbohydrates into γ-valerolactone without using an external H2 supply. Angew. Chem. 2009, 121, 6651–6654. [Google Scholar] [CrossRef]
- Ortiz-Cervantes, C.; García, J.J. Hydrogenation of levulinic acid to γ-valerolactone using ruthenium nanoparticles. Inorg. Chim. Acta 2013, 397, 124–128. [Google Scholar] [CrossRef]
- Qing, Q.; Gao, X.; Wang, P.; Guo, Q.; Xu, Z.; Wang, L. Dilute acid catalyzed fractionation and sugar production from bamboo shoot shell in γ-valerolactone/water medium. RSC Adv. 2018, 8, 17527–17534. [Google Scholar] [CrossRef]
- Rye, L.; Blakey, S.; Wilson, C.W. Sustainability of supply or the planet: A review of potential drop-in alternative aviation fuels. Energy Environ. Sci. 2010, 3, 17–27. [Google Scholar] [CrossRef]
- Alonso, D.M.; Gallo, J.M.R.; Mellmer, M.A.; Wettstein, S.G.; Dumesic, J.A. Direct conversion of cellulose to levulinic acid and gamma-valerolactone using solid acid catalysts. Catal. Sci. Technol. 2013, 3, 927–931. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, J.-M.; Hwang, D.W.; Halligudi, S.B.; Hwang, Y.K.; Chang, J.-S. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts. J. Ind. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Rodiansono, R.; Astuti, M.D.; Ghofur, A.; Sembiring, K.C. Catalytic hydrogenation of levulinic acid in water into g-valerolactone over bulk structure of inexpensive intermetallic Ni-Sn alloy catalysts. Bull. Chem. React. Eng. Catal. 2015, 10, 192. [Google Scholar] [CrossRef]
- Kondawar, S.; Rode, C. Ionic liquids for the sustainable transformation of levulinic acid to gamma-valerolactone (GVL). Curr. Opin. Green Sustain. Chem. 2022, 35, 100607. [Google Scholar] [CrossRef]
- Gamma Valerolactone (CAS 108-29-2) Market Research Report. Available online: https://dataintelo.com/report/global-gamma-valerolactone-%28cas-108-29-2%29-market/#:~:text=The%20Global%20Gamma%20Valerolactone%20(CAS,5.02%25%20from%202022%20to%202030 (accessed on 1 November 2021).
- Manzer, L.E. Catalytic synthesis of α-methylene-γ-valerolactone: A biomass-derived acrylic monomer. Appl. Catal. A Gen. 2004, 272, 249–256. [Google Scholar] [CrossRef]
- Dutta, S.; Iris, K.; Tsang, D.C.; Ng, Y.H.; Ok, Y.S.; Sherwood, J.; Clark, J.H. Green synthesis of gamma-valerolactone (GVL) through hydrogenation of biomass-derived levulinic acid using non-noble metal catalysts: A critical review. Chem. Eng. J. 2019, 372, 992–1006. [Google Scholar] [CrossRef]
- Mthembu, L.D.; Lokhat, D.; Deenadayalu, N. Valorization of sugarcane bagasse to a platform chemical (levulinic acid) catalysed by 1-butyl-2, 3-dimethylimidazolium tetrafluoroborate ([BMMim][BF4]). Waste Biomass Valoriz. 2021, 12, 199–209. [Google Scholar] [CrossRef]
- Guo, H.; Tomoka, S.; Smith Jr, R.L. Catalytic hydrogenation of levulinic acid in ionic liquid mixtures using hydrogen gas in high-pressure CO2. J. Supercrit. Fluids 2020, 164, 104891. [Google Scholar] [CrossRef]
- Selva, M.; Gottardo, M.; Perosa, A. Upgrade of biomass-derived levulinic acid via Ru/C-catalyzed hydrogenation to γ-valerolactone in aqueous–organic–ionic liquids multiphase systems. ACS Sustain. Chem. Eng. 2013, 1, 180–189. [Google Scholar] [CrossRef]
- Mthembu, L.D.; Lokhat, D.; Gupta, R.; Deenadayalu, N. Optimization of Levulinic Acid Production from Depithed Sugarcane Bagasse in 1-Ethyl-3-methylimidazolium hydrogen sulfate [EMim][HSO4]. Waste Biomass Valoriz. 2021, 12, 3179–3191. [Google Scholar] [CrossRef]
- Johnson, T.C.; Morris, D.J.; Wills, M. Hydrogen generation from formic acid and alcohols using homogeneous catalysts. Chem. Soc. Rev. 2010, 39, 81–88. [Google Scholar] [CrossRef]
- Grasemann, M.; Laurenczy, G. Formic acid as a hydrogen source–recent developments and future trends. Energy Environ. Sci. 2012, 5, 8171–8181. [Google Scholar] [CrossRef]
- Rackemann, D.W.; Bartley, J.P.; Doherty, W.O. Methanesulfonic acid-catalyzed conversion of glucose and xylose mixtures to levulinic acid and furfural. Ind. Crops Prod. 2014, 52, 46–57. [Google Scholar] [CrossRef]
- Mkhize, T.; Mthembu, L.D.; Gupta, R.; Kaur, A.; Kuhad, R.C.; Reddy, P.; Deenadayalu, N. Enzymatic saccharification of acid/alkali pre-treated, millrun, and depithed sugarcane bagasse. BioResources 2016, 182, 136–143. [Google Scholar]
- Silva-Fernandes, T.; Marques, S.; Rodrigues, R.C.; Loureiro-Dias, M.C.; Fonseca, C.; Gírio, F. Enzymatic hydrolyses of pretreated eucalyptus residues, wheat straw or olive tree pruning, and their mixtures towards flexible sugar-based biorefineries. Biomass Convers. Biorefinery 2016, 6, 385–396. [Google Scholar] [CrossRef]
- De Haan, J. Hydrogenation of Levulinic Acid to γ-in a Continuous Packed Bed ReactorValerolactone. Master’s Thesis, Faculty of Science and Engineering, University of Groningen, Groningen, The Netherlands, 2013. [Google Scholar]
- Singh, H.; Iyengar, N.; Yadav, R.; Rai, A.; Sinha, A.K. Facile conversion of levulinic acid to γ-valerolactone using a high surface area magnetically separable Ni/NiO catalyst. Sustain. Energy Fuels 2018, 2, 1699–1706. [Google Scholar] [CrossRef]
- Córdova-Pérez, G.E.; Cortez-Elizalde, J.; Silahua-Pavón, A.A.; Cervantes-Uribe, A.; Arévalo-Pérez, J.C.; Cordero-Garcia, A.; de Los Monteros, A.E.E.; Espinosa-González, C.G.; Godavarthi, S.; Ortiz-Chi, F. γ-Valerolactone Production from Levulinic Acid Hydrogenation Using Ni Supported Nanoparticles: Influence of Tungsten Loading and pH of Synthesis. Nanomaterials 2022, 12, 2017. [Google Scholar] [CrossRef]
- Braden, D.J.; Henao, C.A.; Heltzel, J.; Maravelias, C.C.; Dumesic, J.A. Production of liquid hydrocarbon fuels by catalytic conversion of biomass-derived levulinic acid. Green Chem. 2011, 13, 1755–1765. [Google Scholar] [CrossRef]
- Jimenez de la Parra, C.; Navarrete, A.; Dolores Bermejo, M.; Jose Cocero, M. Patents review on lignocellulosic biomass processing using ionic liquids. Recent Pat. Eng. 2012, 6, 159–181. [Google Scholar] [CrossRef]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Sanchis, R.; García, T.; Dejoz, A.M.; Vázquez, I.; Llopis, F.J.; Solsona, B. Easy method for the transformation of levulinic acid into gamma-valerolactone using a nickel catalyst derived from nanocasted nickel oxide. Materials 2019, 12, 2918. [Google Scholar] [CrossRef]
- López-Aguado, C.; Paniagua, M.; Melero, J.A.; Iglesias, J.; Juárez, P.; López Granados, M.; Morales, G. Stable continuous production of γ-valerolactone from biomass-derived levulinic acid over zr–al-beta zeolite catalyst. Catalysts 2020, 10, 678. [Google Scholar] [CrossRef]
- Jori, P.K.; Jadhav, V.H. Efficient Synthesis of γ-Valerolactone-A Potential Fuel from Biomass Derived Levulinic Acid Using Catalytic Transfer Hydrogenation Over Hf@ CCSO3H Catalyst. Catal. Lett. 2020, 150, 2038–2044. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dumont, M.-J.; Raghavan, V. Sustainable production of hydroxymethylfurfural and levulinic acid: Challenges and opportunities. Biomass Bioenergy 2015, 72, 143–183. [Google Scholar] [CrossRef]
- Mthembu, L.D. Production of Levulinic Acid from Sugarcane Bagasse. Master’s Thesis, Department of Chemistry, Faculty of Applied Sciences, Durban University of Technology, Durban, South Africa, 2016. [Google Scholar]
- Al-Shaal, M.G.; Wright, W.R.; Palkovits, R. Exploring the ruthenium catalysed synthesis of γ-valerolactone in alcohols and utilisation of mild solvent-free reaction conditions. Green Chem. 2012, 14, 1260–1263. [Google Scholar] [CrossRef]
- Protsenko, I.I.; Nikoshvili, L.Z.; Matveeva, V.G.; Sulman, E.M.; Rebrov, E. Selective hydrogenation of levulinic acid to gamma-valerolactone using polymer-based Ru-containing catalysts. Chem. Eng. Trans. 2016, 52, 679–684. [Google Scholar]
- Hengst, K.; Schubert, M.; Carvalho, H.W.; Lu, C.; Kleist, W.; Grunwaldt, J.-D. Synthesis of γ-valerolactone by hydrogenation of levulinic acid over supported nickel catalysts. Appl. Catal. A Gen. 2015, 502, 18–26. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous phase reforming process for the valorization of wastewater streams: Application to different industrial scenarios. Catal. Today 2022, 387, 224–236. [Google Scholar] [CrossRef]
- Fu, J.; Sheng, D.; Lu, X. Hydrogenation of levulinic acid over nickel catalysts supported on aluminum oxide to prepare γ-valerolactone. Catalysts 2015, 6, 6. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Optimization of biomass conversion to levulinic acid in acidic ionic liquid and upgrading of levulinic acid to ethyl levulinate. BioEnergy Res. 2017, 10, 50–63. [Google Scholar] [CrossRef]
- Barla, M.K.; Velagala, R.R.; Minpoor, S.; Madduluri, V.R.; Srinivasu, P. Biomass derived efficient conversion of levulinic acid for sustainable production of γ-valerolactone over cobalt based catalyst. J. Hazard. Mater. 2021, 405, 123335. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).