Abstract
Kitchen waste is an important component of domestic waste, and it is both harmful and rich in resources. Approximately 1.3 billion tons of kitchen waste are produced every year worldwide. Kitchen waste is high in moisture, is readily decayed, and has an unpleasant smell. Environmental pollution can be caused if this waste is treated improperly. Conventional treatments of kitchen waste (e.g., landfilling, incineration and pulverization discharge) cause environmental, economic, and social problems. Therefore, the development of a harmless and resource-based treatment technology is urgently needed. Profits can be generated from kitchen waste by converting it into biofuels. This review intends to highlight the latest technological progress in the preparation of gaseous fuels, such as biogas, biohythane and biohydrogen, and liquid fuels, such as biodiesel, bioethanol, biobutanol and bio-oil, from kitchen waste. Additionally, the pretreatment methods, preparation processes, influencing factors and improvement strategies of biofuel production from kitchen waste are summarized. Problems that are encountered in the preparation of biofuels from kitchen waste are discussed to provide a reference for its use in energy utilization. Optimizing the preparation process of biofuels, increasing the efficiency and service life of catalysts for reaction, reasonably treating and utilizing the by-products and reaction residues to eliminate secondary pollution, improving the yield of biofuels, and reducing the cost of biofuels, are the future directions in the biofuel conversion of kitchen waste.
1. Introduction
At present, the price of fossil fuels (such as coal, crude oil and natural gas), especially petroleum products (such as gasoline and diesel), is always rising. Moreover, fossil fuels result in various environmental and social implications, for example, the emission of greenhouse gases from combustion, the health threats derived from toxic and hazardous species, aquatic animal death due to the leakage of crude oil, currency inflation and even tensions among countries that contribute to fuel shortages [1,2,3]. Under the guidance of the “Double Carbon & Double Control” policy advocated by China, ensuring energy safety and environmental safety has become a critical topic worldwide for governments and researchers due to economic and social factors. In particular, producing higher-generation biofuels compared to first-generation biofuels that use advanced platforms, including those based on food wastes (FW), is also highly advantageous given the current challenges faced in the world; this is refers particularly to the Ukraine–Russia war and its adverse effects on the fuel and energy supply chains [4].
Therefore, biofuel development is an available pathway for solving or relieving the present greenhouse effect and energy crisis, and biofuels are alternatives to fossil fuels because of their major environmental advantages. Biofuels generally refer to solid, liquid or gaseous fuels made from biological organisms and their metabolic excretions [5]. Biofuels are pollution-free, locally available, sustainable and reliable. Biofuels differ from other petroleum feed-stocks in terms of their oxygen content. They have much oxygen, ranging from 10% to 45%, compared to petroleum products that have none. Biofuels also have poor sulfur and nitrogen levels when compared with petroleum sources [1]. Biodiesel, bioalcohols (ethanol and butanol), biomethane, and biohydrogen are the typical representatives of gaseous and liquid biofuels [6,7]. Additionally, biofuel production and application are considered effective methods for “carbon neutralization” due to the absorption of discharged carbon dioxide [8]. On a global scale, from 2007 to 2017, the average consumption of fuels increased by 11.4% annually [9].
For the production of biofuels, massive biomass, such as agricultural wastes, forest byproducts and municipal wastes, can be used as feedstocks. Recently, there have been numerous reviews and research works on biofuel production from biomass [10,11,12,13,14,15,16]. For example [17], the peel of petit grain bitter orange was used as feedstock to produce biofuel by steam distillation, and the result showed that the biofuel had minimal pollutant emissions; its performance and combustion characteristics were found to be advantageous over diesel.
In recent years, the conversion of kitchen waste into biofuels has been widely reported, which is one of the research hot spots in solid waste treatments [18,19,20,21,22]. Kitchen waste is a critical component of municipal domestic waste. Approximately 6.00 × 107~9.24 × 107 tons of kitchen waste is produced annually in China [23]; globally, almost 1.3 × 109 tons is produced [24]. Kitchen waste is a problematic food waste (FW), and is a municipal solid waste that produces an unpleasant odor and leachate during its collection, transportation, and temporary storage before disposal, and it is responsible for 6% of global greenhouse gas emissions [25]. The composition of kitchen waste varies with source, region, climate, time, cultural background, economic status, etc. [26]. In addition to the high contents of solids, organic matter, water, salt and fat, kitchen waste has the well-known characteristics of a wide source, a large quantity, a complex composition, an easy deterioration, and the coexistence of harmful components and resources [27]. If its disposal is delayed, kitchen waste can possibly contaminate surface water, underground water, soil and the atmosphere. It also can spread disease and impact municipal appearance [28]. Some governments, such as France, have adopted a policy of FW valorization after realizing the benefit of FW transformation into value-added materials and energy products, and have formed punitive laws so that the policy is strictly complied with by the public [29].
Reduction, harmlessness and recycling are general fundamental principles in kitchen waste treatment. Therefore, appropriate techniques should be employed based on local circumstances. The common disposal and utilization methods that are currently used for are shown in Figure 1. However, some methods of disposing kitchen waste (e.g., landfilling, incineration and pulverization discharge) cause environmental, economic, and social problems [18,25,30,31,32,33]. Waste edible oil from catering enterprises was used to produce solid alcohol biofuels, and high energy products were obtained [18,20]. Some researchers discussed the potential of kitchen waste as an effective and low-cost substrate for improved biohydrogen production technology, and proposed the application of nanomaterials to increase the yield of biohydrogen production and to make the entire process more economical and sustainable [18,20].
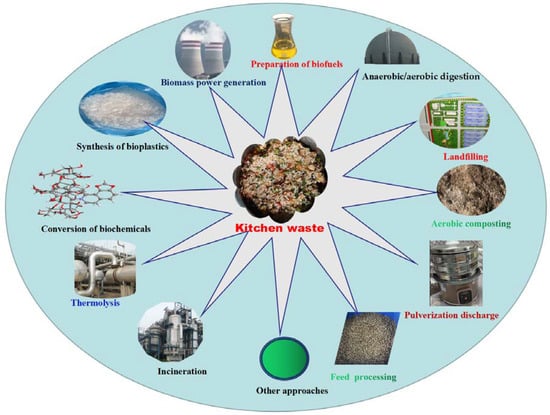
Figure 1.
Common disposal and utilization methods of kitchen waste.
At present, the major obstacle to biofuel commercialization is that the cost of oil feedstock is almost 70% of the production cost [34]; however, kitchen waste is rich in oil (about 15–35%), which can provide abundant and low-cost oil feedstock for the production of biofuels. For example, waste cooking oil (WCO) is used as a raw material, and thus the cost of biodiesel can be reduced since the cost of WCO accounts for approximately 70 to 95% of the total production cost of biodiesel. Research shows that biodiesel has no aromatics and only a small amount of sulfur, which helps to reduce carbon monoxide, hydrocarbon and particulate matter in the exhaust gas. Food wastes are cheap, abundant and renewable [19]. In the process of bioethanol production, after pretreatment and enzymatic hydrolysis, the glucose productivity is higher in comparison to other biomass, such as agricultural residues [35]. Thus, the production of biofuels from kitchen waste is considered to be an eco-friendly, economic, pollution-reducing and sustainable approach [29,36,37]
Compared with other biomass, the use of kitchen waste as feedstock for biofuel preparation has other advantages. First of all, kitchen waste is abundant in organic content, which can provide enough material and energy for its conversion into biofuel without the addition of other substances. On the basis of elemental analysis, kitchen waste contains 45–65% carbon, 6–7% hydrogen, 1–3% nitrogen, and 40–50% oxygen [26]. Secondly, the low content of refractory substances in kitchen waste can reduce the difficulties that are encountered in pretreatment [38].
Many technologies, such as catalysis, cracking, fermentation, pyrolysis and separation, are utilized in the preparation of biofuels from kitchen waste [18,32,39,40,41]. The general process of converting kitchen waste into biofuels is illustrated in Figure 2.
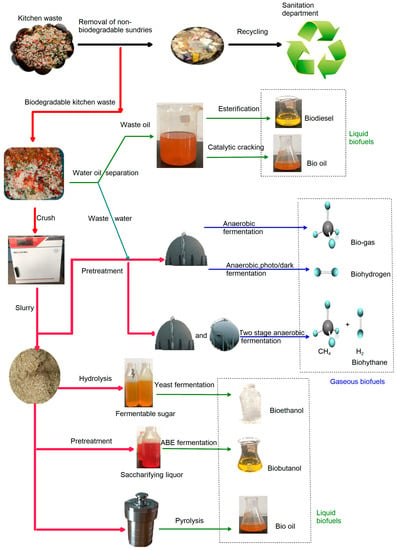
Figure 2.
General route for preparing biofuels from kitchen waste.
This review includes the pretreatment methods, preparation processes, influencing factors and improvement strategies that are involved in the production of biofuel from kitchen waste. However, the biological preparation process still faces various obstacles, for example, the complicated composition of the waste, the high impurity content, and variations among regions and durations of decay; these problems require additional attention. The technique discussed in this review could provide useful information for the application of biofuels produced from kitchen waste.
2. Sorting of Sundries and Solid–Liquid Separation in Kitchen Waste
2.1. Sorting
Kitchen waste usually contains 20% additional domestic waste. Sorting the waste is necessary for further treatment. The principle of sorting depends on the waste density, volume, elasticity, photoelectricity, friction, as well as surface-wetting behavior. Corresponding methods are adopted to remove non-degradable sundries, such as plastic bags, wood blocks, glass and metals, and other bulky substances [42].
2.2. Solid–Liquid Separation
Kitchen waste is heated to about 65 °C to obtain the solid residue and organic liquid (including oil and organic wastewater) phase by solid–liquid separation. The solid residue is pulverized into proper size particles for further processing into biodiesel. After separating the liquid phase into water and oil, oil is used to prepare liquid biofuels, such as biodiesel and bio-oil. The organic wastewater is transferred to a sewage plant or is used to prepare gaseous biofuel after anaerobic fermentation [43,44].
3. Preparation of Gaseous Biofuels from Kitchen Waste
3.1. Biogas
Biogas, which contains abundant methane (approximately 60%) and carbon dioxide (approximately 40%), as well as small amounts of hydrogen sulfide, ammonia, hydrogen, and oxygen, is a common fuel. Therefore, increasing the use of biogas will alleviate the rising global energy and environmental crises [45].
Kitchen waste is partially converted into biogas through anaerobic digestion (AD) by anaerobes in the digestion pool. The production of biogas through AD is one of the best feasible options due to its low energy requirement and eco-friendly nature, in comparison with other methods [46]. The mechanism of the process is still under discussion. To date, mainly two-stage, three-stage and four-stage theories [47] have been proposed. The four-stage theory of hydrolysis→acidogenesis→acetogenesis→methanogenesis is now usually applied (Figure 3). Various anaerobic stages are induced by the corresponding anaerobic bacterial communities [48]. Clostridium, Micrococci, Bacteroides, Butyrivibrio, Fusobacterium, and Selenomonas are involved in rate-controlling steps [49], namely, they participate in the hydrolysis stage to convert insoluble organics into soluble organics.
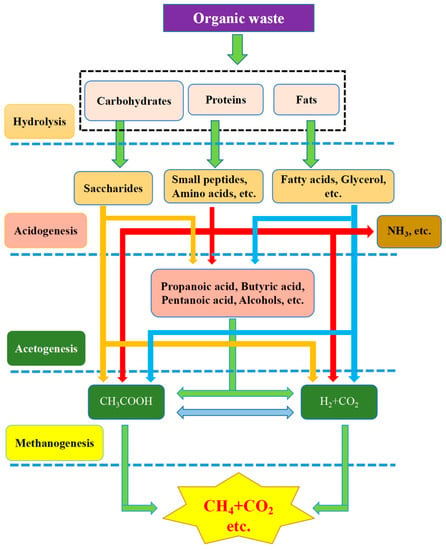
Figure 3.
Four-stage theory in the anaerobic digestion of organic waste [47].
Streptococcus, Lactobacillus, Bacillus, Escherichia, and Salmonella are involved in the conversion of hydrolytic products into low molecular weight products, such as propanoic acid, butanoic acid, ethanoic acid, aliphatic acid, alcohol, and carbon dioxide. All low molecular weight products in the previous step are transformed into acetic acid by acetic acidification. The products vary with the bacterial type and cultivation environment. Methane bacteria, such as Methanomicrobiales, Methanobacteriales, and Methanococcales, are used to generate methane via acetic acid, hydrogen and carbon dioxide, which are all formed in the second and third stages [50].
Although biogas production is a relatively mature technology, it has strict technical requirements because the composition of kitchen waste is complex and variable in time and space. The anaerobic or aerobic disposal of kitchen waste is challenging due to the presence of constituents that are difficult to decompose in kitchen waste [51]. However, only 40–60% of volatile solids are available in biomethane, which indicates that pretreatment is essential. The common pretreatment methods include chemical, thermal, pressure, biological, enzymatic, and ultrasonic methods, among others [52,53,54], as described in Table 1.

Table 1.
Effect of different pretreatment methods on methanogenesis of kitchen waste.
Anaerobic fermentation to yield biogas is affected by various factors, such as composition, salinity, alkalinity, residence time, solid–liquid ratio, temperature, pH, C/N ratio, organic loading, bacterial vaccination degree, toxicity type, hydraulic residence time, agitation/mixture, pretreatment process, and reactor [65,66,67]. The research showed that kitchen rice, vegetables, meat, and a mixture of kitchen waste produced 478.2, 433.3, 206.8 and 508.3 mL/g biogas, respectively, indicating that mixing waste is favorable for biogas production [65,66,67]. Salinity affects the normal growth of methane bacteria, resulting in an increase in salinity outside the bacterial cell and the loss of water from the cells, thereby reducing the activity of microorganisms and even resulting in the complete failure of the fermentation system [68]. Two methods can be performed as countermeasures to protect the bacterial cell from losing water in the high salinity system. The first method is the synthesis and accumulation of osmotic protectants. The second method is adding an influx of potassium and chloride into the cytoplasm [68,69]. Fermentation may produce free ammonia, volatile aliphatic acid and sulfide/sulfate, which are important nutrients for bacterial growth; however, their increased concentrations will inhibit biogas formation [70]. For example, in the fermentation process, ammonia nitrogen mainly exists in two forms, namely, free ammonia and ammonium ions, which directly or indirectly inhibit the anaerobic fermentation process, especially free ammonia, which has stronger toxicity. Free ammonia can penetrate the cell membranes of bacteria, disturb the proton balance, change the pH value in cells, inhibit the activity of specific enzymes, and eventually lead to the deterioration of the anaerobic digestion process [71,72]. A submersible microbial desalination cell was developed to lower the ammonia level by in situ ammonia recovery and electricity generation, with a significant increase in biogas production. By gradually increasing the ammonia nitrogen concentration, the bacteria could gradually adapt to the high ammonia concentration, which could also reduce the inhibition effect of ammonia on AD [71,72]. The research showed that mesophilic bacteria could be adapted to a total ammonia nitrogen (TAN) concentration up to 5 g/L by means of gradual TAN loading. The cumulative biogas production in reactors with gradual TAN loading (for maximum TAN of 10 g/L) was 1.9–3.1 times more than that with abrupt TAN addition [71,72].
In addition to the above factors, the addition of a third component, such as biocarbon or a trace element, can improve the biogas yield of kitchen waste. The gas production effect of the anaerobic digestion of food waste was the best, and the methane yield was 331.7 L/kg TS. When the inoculation amount of sludge was 20.98%, the initial pH was 7.05, and with the addition of 22.14 g/L biocarbon, the maximum kitchen waste anaerobic digestion was realized, namely, 331.7 L/kg TS [73]; however, the mechanisms of biocarbon synergism in anaerobic fermentation are still under discussion. For example, one mechanism is put forward that biocarbon can promote electron transfer between species, enhance cell colonization and increase enzyme activity to promote biogas production [64,65]; another proposed mechanism suggests that the addition of biocarbon can improve the availability of the trace elements and beneficial elements that are necessary for methane production, and can reduce the loss of nutrients during fermentation [74].
Proper supplementation with microelements can improve methane yield [75]. In semicontinuous acclimatization, the daily addition of Fe(0) species to kitchen waste in sludge for anaerobic fermentation resulted in a 17.74% higher average daily biogas production than the control group; i.e., Fe(0) was found to be more conducive to improving the stability and yield of biogas for the single-phase anaerobic digestion of kitchen waste [76]. The combination of FeCl2 and EDTA was shown to promote protein polysaccharide hydrolysis and improve the degradation of volatile acids in the 13 d reaction. The yield of gas was 4.1 times that of when only FeCl2 was added [77].
3.2. Biohythane
Biohythane is a hydrogen–methane blend with a hydrogen concentration of 10 to 30% v/v [78]. Biohythane is derived from organic degradable products in a secondary anaerobic digestion process. In two-stage anaerobic fermentation, hydrogen microbes play an important role in the short residence time in the first stage, even though they produce methane for a relatively long time. Compared with traditional fuels, biohythane is valuable on the market and is universally accepted as a commercial fuel for use in internal engines and automobiles [79]. Environmentally, biohythane is advantageous for decreasing greenhouse gases because the presence of hydrogen decreases the carbon content [80].
Biohythane is produced in a dark fermentation stage and an acetyl-hydrogen stage, and each stage is controlled by microbes. Each stage has its own optimal conditions, such as pH value, fermentation temperature, partial pressure of methane, hydraulic residence time, organic load and nutrient composition [78].
Compared with the one-stage system, the two-stage biohythane system achieved a higher COD removal rate and energy recovery rate. The first stage of hydrogen fermentation benefited subsequent biomethane production [80]. Furthermore, two-stage anaerobic methods are more commercially valuable than one-stage methods; this is because dark fermentation results in both the poor yield and productivity of hydrogen, and is economically unsustainable [81].
However, because of its high required investment and operation complexity, the applicability of the two-stage method is severely limited. Recent research on the anaerobic one-stage production of biohythane is confined to the use of one digestion pool in order to simultaneously produce hydrogen and methane [82,83]. The reactor design, reactor operation strategy, and collection mechanism of biohydrogen in the separation pattern are unknown [84].
Chen’s research on the two-phase fermentation of kitchen waste in order to produce biohythane reveals that a low ammonia nitrogen concentration has a positive effect on hydrogen formation. However, a high concentration has a negative effect or even causes complete interruption. A 9.4 g VS /L.d organic load, 4 d hydraulic residence time, 1.0 reflux ratio for the hydrogen-producing phase, and 20 d hydraulic residence time for the alkane-producing phase are favorable conditions for the capture of biohythane, with a 14.2% H2/CH4 ratio [85].
The accumulation yield of biohythane varies with the organic load (OL, Table 2). At various OL values, the methane fraction increased with time, contributing to the presence of hydrogen and volatile aliphatic (fatty) acids (VFAs) at the top of the reactor. VFAs are good precursor substrates for methane because the residue of acid-producing bacteria is the substrate of methane-producing bacteria. Several steps were used to induce the formation of methane, with a maximum methane content of 35% at an OL of 100 g/L COD. Increasing the organic load produced a considerable increase in VFA production. The improved yield in the fatty acid can be attributed to the resulting suppression of methanogenic activity during the feeding stage [86].

Table 2.
Cumulative biohythane and VFA production recorded at different OLs.
3.3. Biohydrogen
The combustion of hydrogen in air produces water with a high LHV (lower heating value, 120 MJ/kg) that is 2.5, 2.3, 2.6 and 2.4 times the LHV of the combustion with the same amount of CNG (compressed natural gas), LNG (liquefied natural gas) and LPG (propane and methane), respectively (Table 3). Hydrogen is considered to be the most extractable clean and renewable energy source [87]. The fermentation of anaerobic organic microbes is a key pathway for biological hydrogen production. There is a considerable amount of organic garbage in agricultural residue, kitchen waste and industrial wastewater.

Table 3.
Comparison of properties of different alternative gaseous fuels [88,89,90].
The microbial fermentation of organic matter under anaerobic conditions is an important method for biohydrogen production. Agricultural residues, food waste, industrial wastewater, vegetable wastes and other wastes containing high organic matter are economic raw materials for biohydrogen production. The combination of hydrogen production and pollutant removal is a promising method for energy recovery from wastes [46].
Many studies have investigated the use of kitchen waste as a substrate in order to produce biohydrogen, but this method is limited by various obstacles, such as the composition of the waste, the type of microbes, and the processing environment (pH, atmospheric pressure, temperature, hydraulic residence time, feeding rate, operating conditions, etc.). Reasonable pretreatment in hydrogen preparation is necessary in order for complex organics to improve the production performance. Biohydrogen is produced by different approaches, namely, biophotonolysis, electrolysis by microbes and fermentation (dark fermentation, dry fermentation, and photofermentation).
Dark fermentation has the suitable characteristics of low energy demand, high yield and productivity, in order to be considered for large-scale hydrogen production. In dark fermentation, in which a single bacterium or community is available, the microbe variety is critical to hydrogen production. Commonly used bacteria include Clostridium, Enterobacteriaceae, Bacteroides and Thermoanaerobium, among others [91].
In photofermentation, which is dependent on rays, organic substrates are converted into hydrogen by photosynthetic bacteria [39]. Purple sulfur bacteria, purple non-sulfur bacteria and green sulfur bacteria are photosynthetic bacteria that use light and organic waste to produce hydrogen. The advantages of photofermentation include its good hydrogen yield and low COD level due to its utilization of dark fermentation products [92]. However, this technique has various shortcomings, such as poor photoefficiency, the requirement of transparent reactors and high energy consumption [63].
Dark fermentation produces hydrogen at a high rate, while photofermentation produces hydrogen at a good yield. Therefore, a suitable combination of the two could yield the maximum hydrogen output, which should be further investigated in the future [93]. For example, the researchers used the respective advantages of dark fermentation and light fermentation to implement the two-step coupling of dark–light fermentation, with kitchen waste as the substrate. By optimizing the H2 production conditions of dark fermentation and light fermentation, the following results were obtained: (1) at 37 °C, a 50% feed ratio, and an initial pH = 6, dark fermentation produced 25.18 mL/g of VS hydrogen, and (2) at 30 °C, 100 W illumination, an initial pH = 8, and 10% vaccination, photofermentation produced 34.62 mL/g of VS hydrogen. The total for the two fermentation methods produced up to 59.80 mL/g of VS hydrogen, significantly increasing the hydrogen yield of kitchen waste [94].
Anaerobic fermentation to generate hydrogen is classified into wet fermentation (less than 15% solid content) and dry fermentation (greater than 15% solid content). Compared with wet anaerobic fermentation, dry anaerobic fermentation has many advantages, such as lower water consumption, higher gas production, reduced biogas slurry, and the lower moisture content of biogas residues [84]. Synergistic dry fermentation with different substrates could improve hydrogen production efficiency. For example, when the ratio of kitchen waste to garden waste was 60:40, the maximum cumulative hydrogen production reached 85.28 NmL/g VS by dry fermentation at 55 °C, which was 1.35 times higher than that obtained using single kitchen waste and 1.93 times that obtained using a single garden waste [84].
Despite these results, industrial-scale anaerobic fermentation to produce biohydrogen has not been adopted due to technical obstacles, its high cost and poor efficiency. Improving the hydrogen yield is a continuous concern in academia. New ideas, such as substrate addition, the continuous removal of products, and removal inhibition caused by substrate and product accumulation, have been proposed [95]. Genetic engineering to modify bacterial strains to reduce side products [96] and the use of nanoparticle additives to enhance enzymatic activity [97] have also been proposed.
4. Preparation of Liquid Fuels with Kitchen Waste
4.1. Bioethanol
Bioethanol, with an oxygen content of ~34.7%, is a substitute for petroleum-based fuels. It completely burns to reduce particle pollutants [98].
The U.S. and Brazil are the primary countries that produce bioethanol globally. In 2020, over 13.8 billion gallons of bioethanol were manufactured in the U.S., and 7.9 billion gallons of bioethanol were produced in Brazil. The two countries were responsible for 87% of the global bioethanol production [99].
The feed materials for producing bioethanol by fermentation are classified into three categories: (1) Those used to directly transform into ethanol without complicated pretreatment, such as saccharide, sugar beet, and molasses. These materials have the advantages of simple processing and require less substrate, but they suffer from a high cost and are thus seldom used in practice. (2) Those that are hydrolyzed into saccharide with the help of enzymes, such as corn, wheat, potato, and rice. These materials have been adapted industrially, due to the risk of a “food crisis”. (3) Those that are transformed into saccharide by the pretreatment of industrial waste, such as cellulose. These materials have the advantages of a low cost and high yield, and pose no threat to people’s food safety, which is a concern among academics. Kitchen waste, which has abundant nutrients for producing methane, hydrogen, and bioethanol, is a primary component of municipal waste [100]. Saccharomyces cerevisiae has robust adaptability and is a common yeast strain, which grows in most fermentation substrates and has a high ethanol yield and ethanol tolerance [101]. The bioethanol production procedure is as follows: saccharification to transform starch into glucose and glucose fermentation into ethanol by yeast. Cellulose is difficult to hydrolyze, so kitchen waste with a low cellulose content is a good candidate for bioethanol production. Both starch and cellulose transform into ethanol via carbohydrate saccharification due to their indirect conversion into ethanol [102].
The enzymes that are usually used in saccharification are glucoamylase, α-amylase, β-amylase, branched amylase, and their mixtures. When wine yeast and enzymes were added together to conduct the simultaneous saccharification and fermentation of kitchen waste, it was found that glucoamylase and protease had a significant impact on the yield of bioethanol. After 120 h of fermentation, the yield of bioethanol reached 54.6 g/L with the addition of 150 U/g of protease, 100 U/g of glucoamylase or 100 U/g of cellulase [103].
Using 10 g/L of yeast extracts as a compensatory nitrogen source, 36 h/L of bioethanol was produced from 100 g/L of kitchen waste within 48 h. Moreover, 25 g/L of bioethanol was also obtained under identical conditions without the addition of a compensatory nitrogen source, which proved the high efficiency and economy of kitchen waste as a fermentation material [104].
To obtain ethanol from kitchen waste under non-sterilized fermentation conditions, the Zymomonas mobilis (a gram-negative ethanol-producing bacterium that can tolerate a high concentration of ethanol and a wide pH range) was used as a fermentation strain (control bacterium), and then the acid-resistant Zymomonas mobilis (GZNS1) was cultured under acidic conditions. A comparison between a contrasting bacterium and GZNS1 under different conditions revealed that the conditions, in decreasing order of ethanol yield, were sterilized kitchen waste (contrasting bacterium, 52 g/L, pH = 6), sterilized kitchen waste (GZNS1, 48 g/L, pH = 4), and non-sterilized kitchen waste (GZNS1, 46 g/L, pH = 4). Furthermore, via the recycled use of the waste in fermentation, 50 g/L of ethanol was obtained, which is a better result than that achieved with tap water. It is shown that CZNS1 is feasible for the production of ethanol with kitchen waste under non-sterilized fermentation conditions. Ethanol production under non-sterilized conditions is beneficial for reducing energy consumption for cooking and sterilization and, especially, simplifying the production process.
The hydrolysis of kitchen waste was a key step that directly affected the yield of bioethanol. The economic and efficient problems of starch and cellulose hydrolysis and saccharification could be solved by two-stage catalytic hydrolysis [105]. Additionally, the composite catalyst, acid concentration and hydrolytic temperature impacted on hydrolysis. It was experimentally reported that the use of a composite catalyst (0.5% H2SO4 + 0.01% aid-catalyst), 120 °C primary hydrolytic temperature and 180 °C secondary hydrolytic temperature produced better results [105].
Using three varieties of local yeast bacteria, namely, S. cerevisiae, Candida parasitosis and Lachancea fermentati as fermentation bacteria, and kitchen waste as the raw material, bioethanol was prepared via hydrolysis and fermentation. The fermentable saccharide quantities and saccharification ratio mainly depended on the pH, temperature, glucose amylase activity, feed quantity and hydrolysis time. The results showed that the optimal conditions were as follows: 60 °C temperature, pH = 5, 85 U/mL glucose amylase, 60 g/L of kitchen waste, and 22 h of hydrolysis time. The fermentable saccharide production exceeded 90%, which was equivalent to a bioethanol conversion rate of 82.06–98.19%.
Despite the many advantages discussed above, there are still no large-scale bioethanol production facilities that use kitchen waste as a substrate. Major research efforts on utilizing kitchen waste as a substrate for bioethanol production at scale and at a competitive level need to be carefully compared with the well-established bioethanol production methods that use other biomasses [32].
4.2. Biobutanol
Butanol is a better fuel than ethanol because it can be mixed with gasoline in any proportion, while ethanol can only be mixed at 85% [106]. Butanol is strongly hydrophobic, has a high boiling point, is poorly corrosive, is suitable for pump transportation and does not require alterations to the current pipeline facilities [107]. Butanol is classified into 1-butanol, 2-butanol, tert-butanol and isobutanol. Isobutanol is also considered to be a possible new biofuel and can be used as a substitute for gasoline. The octane number of isobutanol (RON, 113) is higher than that of n-butanol (RON, 96) (Table 4), and the price is slightly lower than that of gasoline [108].
In addition to the advantages described above, butanol offers other advantages over ethanol and methanol [109]: (a) it can be directly mixed with gasoline or diesel in different proportions in the refinery without any complex equipment; (b) due to its low volatility, it is convenient to transport, and it is less corrosive; (c) the air–fuel ratio of butanol is close to that of gasoline for existing vehicles. Although the replacement of gasoline by butanol requires a change in the air–fuel ratio, mixtures with over 20% butanol can be used in current vehicles without any modification; (d) the vaporization enthalpy of butanol is slightly higher than that of gasoline. Hence, the cold start problem that is encountered with ethanol and methanol fuels does not occur with butanol; and (e) the low solubility of butanol in water results in less water pollution.
Biobutanol is derived from the fermentation of saccharide and starch. Because of its low volatility and high potential energy, it is an ideal candidate for biofuel [110,111], as described in Table 4. Despite the advantages of butanol, its use is limited by its high cost and poor output. Traditional biobutanol production involves the extraction of saccharides from raw materials by pretreatment, followed by anaerobic fermentation with the help of Clostridiaceae microbes in order to form a mixture of acetone, butanol and ethanol [54]. The mixture is rectified to obtain the final product, and the fermentation of these products is defined as ABE (acetone, butanol and ethanol) fermentation [112,113]. In addition to acetone, butanol and ethanol, the ABE process generates lactic acid, isopropyl alcohol, acetic acid and other byproducts [110].

Table 4.
Comparison of properties of different alternative liquid fuels [109,114,115,116].
Table 4.
Comparison of properties of different alternative liquid fuels [109,114,115,116].
| Fuel | Gasoline | 1-Butanol | 2-Butanol | tert-Butanol | Isobutanol | Ethanol | Methanol | Diesel | Biodiesel |
|---|---|---|---|---|---|---|---|---|---|
| Composition | C4-C12 | C4H9OH | C4H9OH | C4H9OH | C4H9OH | C2H5OH | CH3OH | C12-C25 | C14-C24 |
| Energy density (MJ/L) | 32 | 29.2 | 29.07 | 37.16 | 28.87 | 19.6 | 16 | 39 | 31–33 |
| Vapor pressure (kPa) at 20 °C | 0.7–207 | 0.67 | 1.33 | 4.13 | 1.17 | 7.58 | 12.8 | <0.07 | <0.07 |
| Vapor pressure of mixture with gasoline (kPa) | 53.8–103.4 | 44.1 | — | — | 46.9 | 138 | 800 | — | |
| Air : fuel ratio | 14.6 | 11.2 | — | — | — | 9.0 | 6.5 | — | 12.5 |
| Heat of vaporization (MJ/kg) | 0.36 | 0.43 | — | — | — | 0.92 | 1.16 | — | — |
| Research octane number | 84–99 | 96 | 101 | 105 | 113 | 129 | 112–136 | — | — |
| Motor octane number | 81–89 | 78 | 32 | 89 | 94 | 102 | 97–104 | — | — |
| Cetane number * | 0–10 | 25 | — | — | — | 5–8 | 4 | 40–55 | 48–65 |
| Density (g/mL) at 20 °C | 0.72–0.78 | 0.809 | 0.806 | 0.789 | 0.802 | 0.789 | 0.792 | 0.82–0.86 | 0.82–0.86 |
| Flash point (°C) | −45 to −38 | 35 | 35 | 11 | 27.8 | 14 | 11.1 | 65–88 | 65–88 |
| Boiling point (°C) | 25–215 | 117.7 | 99.5 | 82.4 | 108 | 78.5 | 64.5 | 180–370 | 180–370 |
| Flammability (% vol) | 0.6–8 | 1.4–11.2 | 1.7–9.8 | 2.4–8.0 | 1.2–10.9 | 3.3–19 | 6–36.5 | 1.5–7.6 | 1.5–7.6 |
| Self-ignition temperature (°C) | 427 | 343 | 406.3 | 477.8 | 415.6 | 363 | 473 | 220 | 220 |
| Viscosity (mpa.s, 25 °C) | 0.4–0.8 | 2.544 | 3.096 | 3.411 | 4.312 | 1.096 | 0.545 | 1.9–4.1 | 1.9–4.1 |
| Freezing temperature (°C) | <−60 | −89.5 | −115 | 23–26 | −108 | −114.5 | −97.6 | −30 to −9.9 | 7.5 to −16 |
| Hygroscopicity | Low | Low | — | — | Low | High | High | Very low | Very low |
| Compatibility with existing infrastructure | Yes | Yes | — | — | Yes | No | No | Yes | No |
* Cetane number is a measurement of the combustion quality or ignition delay of diesel fuel during compression ignition.
The ABE anaerobic process to form biobutanol is carried out in two stages, namely, the acid production stage and the solvent production stage. In the acid production stage, the bacteria convert the substrate into acetic acid and butanoic acid. When the acid accumulation reaches a threshold, cell metabolism enters the solvent-produced stage, where acetone, butanol and ethanol formation is accomplished by acid conversion. The resultant products are recovered as side products, suspension species and biomass [114,117].
At present, the primary feed used to produce biobutanol is starch compounds, such as corn, rice, and dry potato. The use of these foodstuffs results in high production costs. Improving biobutanol conversion and finding cheap non-foodstuff sources are the focus of research. A promising way to produce biobutanol is the fermentation of lignocellulose waste and kitchen waste. The production of biobutanol from wastes not only provides an ideal, eco-friendly clean/green energy source, but also has the potential to address the global issues of pollution, global warming, and the greenhouse effect, etc., to a greater extent [118].
It is feasible to use lignocelluloses (wheat and barley straw, corn straw, cassava residue, and sugar cane residue) to prepare biobutanol; however, by-products, such as carboxylic acids, aldehydes, and lignin, are generated in the process. Simultaneously, sulfate ions and chloride ions enter the reaction system, which inhibit normal fermentation from producing butanol via the following mechanisms: destruction of the H+ gradient and the stability of the cell membrane, inhibition of enzymatic activity, and alteration of the cell permeability [38,119]. Several strategies can be adopted in view of the above problems: (1) development of modified cellulose hydrolyzing organisms as well as enzymes; (2) development of improved biomass pretreatment process; (3) development of metabolically engineered biomass that can be more easily hydrolyzed with less inhibition compounds; and (4) development of metabolically engineered microorganisms that are resistant to the inhibitory compounds present in lignocellulosic hydrolysates [120].
Due to its abundant carbohydrates, high C/N ratio and high water content, kitchen waste is considered a good substitute for the production of biobutanol [110]. The carbon source in kitchen waste is derived from starch, so the production of biobutanol is feasible [119]. FW (dry basis: 63.5% starch, 4.3% glucose, 13.9% protein, 4.1% oil, and 5.2% neutral washing cellulose) was used as a raw material to obtain biobutanol by ABE fermentation with the Clostridium beijerinckii P260 bacterium. In contrast to fermentation with expensive glucose (control group), ABE fermentation using FW has a lower cost, higher yield and less residue [118,119]. The final ABE concentration of the fermentation product was 18.9 g/L, while that of the control group was only 14.2 g/L. The ABE generation rate was 0.46 g/(L.h), which was greater than that of the control group [118]. Furthermore, FW fermentation to produce biobutanol did not require the addition of hydrolase; instead, it was generated from culture secretions. ABE fermentation coupled with vacuum stripping technology completely controlled the butanol concentration to below 6 g/L. When the FW concentration reached 129 g/L, the saccharide in the fermented liquid was almost completely utilized. Vacuum stripping technology potentially decreases the energy cost, water consumption and facility space, thereby reducing the production cost of biobutanol [118,119].
The pretreatment of raw material achieves physiochemical or enzymatic hydrolysis, such as steam explosion, organic solution treatment and enzymatic hydrolysis. Enzymatic hydrolysis is environmentally friendly, whereas physiochemical pretreatment has poor selectivity. Hydrolysis promotes saccharide release from carbohydrates. FW can contain a mixture of glucose, xylose, lactose, arabinose, etc. However, Clostridium cannot consume all these sugars, which limits the production of butanol, resulting in the inhibition of carbon catabolism [102,110]. Researchers have attempted to avoid such inhibition via genetic engineering. By modifying Clostridium’s genes, glucose and xylose could be used simultaneously to inhibit carbohydrate loss in fermentation [121].
When the saccharification liquid of kitchen waste was used as a substrate, and Clostridium beijerinckii NCIMB 8052 was used as a fermentative bacterium, ABE fermentation was carried out under non-control conditions (without the addition of any nutrients or pH adjustment) to produce biobutanol. The biobutanol yield was only 6 g/L under the non-control conditions, and “acid corruption” occurred; meanwhile, a 0.3% addition (w/v) of CaCO3 enhanced the saccharification liquid’s pH buffer. Thereby, the “acid corruption” was solved, the biobutanol yield could increase to 57.8%, the total solvent production could increase to 53.1% and the butanol production rate could increase to 90.6% [38].
Using Clostridium saccharoperbutylacetonicum as a butanol strain, the effects of different starches from different sources and configurations, and various substrate concentrations on fermentation were studied [112]. The results verified that the direct fermentation of kitchen waste exhibited a higher efficiency than saccharification fermentation and avoided substrate inhibition. At a 1:1 solid/liquid ratio, biobutanol production reached 12.1 g/L, and the maximum biobutanol production rate was 0.705 g/(L.h), while at a 1:2 solid/liquid ratio, the maximum production rate was 2.05 times that of saccharification fermentation, and amylose was easier to use. Thus, direct kitchen waste fermentation is feasible. It outperforms saccharification fermentation in terms of the butanol production rate and carbon conversion [112].
Although kitchen waste is a suitable substrate for biobutanol production, there are still some challenges, such as effectively pretreating kitchen waste from different sources and selecting appropriate microbial strains to fully utilize different sugars. Thus, subsequent studies must be carried out.
4.3. Biodiesel
Biodiesel, a clean renewable energy source, is a low-carbon ester species that is derived from methyl aliphate or ethyl aliphate by esterification between animal and plant oil, food oil, nonfeed oil or microbic lipids, and methanol or ethanol [122].
A biodiesel with a high hexadecane value, high combustion performance, high flash point, safe storage, low environmental hazard, self-degradability, and renewability is widely desired [123].
Traditional biodiesel manufacturing can be traced back to rapeseed, palm oil and corn. Growing these crops requires large amounts of arable land and labor, as well as suitable weather conditions. The cost limits biodiesel development. To overcome this obstacle, oil-producing microorganisms, such as yeast, fungi and microalgae, are widely studied because of their production of biodiesel. Some yeast and fungi may carry out lipid synthesis by using a variety of wastes, such as lignocellulose, fruit, vegetable, and food wastes. Yeast (for example, Lipomyces sp. and Cryptococcus sp.) and fungi (for example, Mortierella alliacea and Aspergillus sp.) may collectively reach a lipid yield of 70% [124,125].
Waste food oil has become a hot spot in biodiesel research due to its low cost (2.5–3.5 times lower than that of plant oil) [126]. Waste oil can be used to prepare biodiesel by transesterification. Acid catalysis, alkaline catalysis, enzymatic catalysis, supercritical extraction and ionic liquid methods have been developed, of which acid–base catalysis is the most mature. The main procedure is as follows: the extraction of lipids from kitchen waste oil, followed by esterification or ester exchange to convert kitchen waste lipids into biodiesel, or esterification exchange and acid–base catalysis to convert them into biodiesel by fungal hydrolytic pretreatment [123]. In addition to ester exchange, direct co-mixing, catalytic cracking and microemulsion agents are also used for biodiesel production [41]. Traditional lipid extraction transforms a mixture of food waste and water into a pulp-like species, followed by solvent extraction. Fungus hydrolysis is an additional approach for producing lipids from carbohydrate-rich, phosphate-rich, amino-acid-rich, and aliphatic-acid-rich food waste. After lipid extraction, acid- (H2SO4 or HNO3) or alkali-catalyzed transesterification (KOH or NaOH) is carried out on the basis of the water content, acid content and aliphatic acid content in the lipid [127].
The transesterification reaction with waste food oil as a raw material and sodium hydroxide as a catalyst was carried out, and a biodiesel with a yield of 94.6% was obtained [128]. The fatty acid composition affects the biodiesel’s properties. For example, free fatty acids in waste food oil can induce saponification under alkaline aqueous conditions, consume catalysts, and cause the formation of emulsions; therefore, researchers have proposed using acid catalysts for esterification, followed by transesterification to reduce the influence of free fatty acids [129]. The presence of exceedingly saturated fatty acids leads to the poor cold flow properties of biodiesel, limiting its use in cold climates, whereas highly unsaturated fatty acids in waste oil decrease the stability of biodiesel due to oxidation [126]. Blending with a cold flow modifier, such as petroleum diesel, ethanol or other short-chain alcohols, can improve the cold flow properties of biodiesel at a low temperature [130,131]. Although oxidation cannot be prevented entirely, however, antioxidants can improve the biodiesel’s oxidative stability to slow down the biodiesel’s oxidative rate [132,133]. The use of synthetic phenolic antioxidants, such as butylated hydroxytoluene, ethoxyquin, pyrogallol and dodecyl gallate, in order to prevent oxidation in biodiesel, has been widely reported [132,133].
Enzyme catalysis is another alternative method for biodiesel production. Lipase is used to convert kitchen waste oil into biodiesel. The reaction can catalyze the conversion of free fatty acids and triacylglycerol into fatty acid methyl ester biodiesel under mild conditions and in the presence of water. However, the application of lipase in production is an important obstacle to its stability, reuse and cost. One way to solve the problem is to use fixed lipase, which favors a multi-recycling process of diesel conversion to remove lipase or to recycle lipase. The common method for immobilizing lipase involves the expression and purification of the lipase enzyme, the synthesis of a carrier and the anchoring of lipase on the carrier [134]. Under mild conditions, the fungal hydrolysis (through intracellular enzymes) and lipase-mediated catalysis was used to produce biodiesel [127]. Another potential method is to modify the genetic code to directly produce immobilized enzymes in bacterial cells. Lipase catalysts can also be improved by orientation evolution in order to produce mutants with a higher thermal and/or organic solvent stability and to enhance the methanol resistance of biodiesel production. The methods discussed above may lead to differences in function between screened enzymes and immobilized enzymes [134,135].
4.4. Bio-Oil
Bio-oil is an eco-friendly energy source with the potential to substitute for fossil-derived fuels [136]. Bio-oil can be derived from waste biomass, such as agricultural wastes, aquatic plants, and other organic wastes [137]. At present, there are two methods for converting biomass into fuel oil: pyrolysis into bio-oil and the esterification of raw oil and alcohol to form biodiesel. Bio-oil is readily available via the pyrolytic liquefaction of cellulose-rich, hemicellulose, and lignin components, and is significantly advantageous over traditional fossil fuels [138]. Pyrolytic products, which are quite different from biodiesel in composition, contain alkanes, alkenes, aromatics, carbonyl compounds and aliphatic acids. At low temperatures, bio-oil has good fluidity, and its combustion heat is similar to that of ordinary diesel [30]. Bio-oil derived from the catalytic cracking of kitchen waste is of interest. Using waste oil separated from kitchen waste as a raw material and ZSM-5 with different silicon:aluminium ratios (Si:Al) as catalysts, bio-oil was prepared by catalytic cracking [30]. The optimum catalytic cracking process conditions were a silicon:aluminium ratio of 25:1 and a temperature of 470 °C. The yield of bio-oil was 68%. The major components of the bio-oil were C5–C9 species, 38.13% of which were toluene, as well as hydrocarbons, alcohols, acetone, and naphthalene [30].
Hydrothermal liquefaction refers to the thermochemical conversion of organic biomass into bio-oil at the subcritical point of water. This technique avoids extra pretreatment drying to distinctly reduce the energy cost [139,140]. In subcritical water, biomass molecules are degraded and condensed repeatedly to form four phases: a bio-oil phase (35–40 MJ/kg thermal value), an aqueous phase with partially polar organics, a scorched solid phase, and a gaseous phase containing CO2. Compared to pyrolytic oil, hydrothermal oil contains less water and has a higher thermal value [141].
The hydrothermal treatment of kitchen waste can perform in a batch reactor. The effects of the key parameters and the characteristic of the products were studied. The optimal parameters of the hydrothermal treatment of kitchen waste and the characteristics of bio-oil are described in Table 5 [142]. Suitable acidic additives were able to reduce the nitrogen content and viscosity of bio-oil and were able to improve the fluidity of bio-oil. Under the conditions of a high temperature and high pressure, hydrothermal treatment does not require the pre-dehydration treatment of kitchen waste, which is rapid, efficient, and adaptable to various raw materials. Thus, this technique has high commercial prospects.

Table 5.
Optimal parameters of bio-oil production from kitchen waste by hydrothermal reaction and characteristics of bio-oil [142].
Studies have proved for most biomass that the optimal hydrothermal reaction temperature for preparing bio-oil is approximately 300 °C [139,140,143]. Below 250 °C, the yield of bio-oil is poor due to insufficient hydrolysis, whereas above the optimal temperature, the oil phase decreases, and the gaseous phase and solid phase increase.
Despite considerable progress in the preparation of bio-oil by hydrothermal liquefaction in the laboratory, the hydrothermal–liquefaction mechanism is still uncertain due to its complexity, dependence on various parameters and the many reactions involved. Furthermore, the commercial cost, commercial feasibility, and processing still need to be deeply investigated.
5. Conclusions and Future Directions
Kitchen waste produces an unpleasant odor and leachate during its collection, transportation, and temporary storage before disposal. Over 1.3 billion tons/year of kitchen waste are generated globally. Reduction, harmlessness and recycling are general fundamental principles in kitchen waste treatment. Compared with other treatment methods, recycling and converting kitchen waste into valuable biofuel products is a promising solution. The production of biofuels from kitchen waste is considered to be an eco-friendly, economic, pollution-reducing and sustainable approach. Many technologies, such as catalysis, cracking, fermentation, pyrolysis and separation, are utilized in the preparation of biofuels from kitchen waste. This review intends to highlight the latest technological progress in the preparation of gaseous fuels, such as biogas, biohythane and biohydrogen, and liquid fuels, such as biodiesel, bioethanol, biobutanol and bio-oil, from kitchen waste. Additionally, the preparation processes and influencing factors are summarized. According to the above discussion, the transformation of kitchen waste into biofuels reduces pollution and carbon emissions, and changes it into resources, while increasing the economy. This approach conforms to the “decrement, harmlessness and resource recovery” principle for the reasonable utilization of kitchen waste.
Breakthroughs have been made in the conversion of kitchen waste into biofuels. However, it is still difficult to produce biofuels on a large scale because of technical and economic obstacles, and biofuels cannot yet compete with fossil fuels. For example, a cheap, rapid, efficient, common pretreatment method is required to ensure a continuous stable reaction due to the extensive variations in kitchen waste with region and time. Moreover, the complex components in biofuels require complex separation processes in order to meet industrial and automobile needs. The collection and transportation cost of kitchen waste is also a problem that must be faced.
Optimizing the preparation process of biofuels, increasing the efficiency and service life of catalysts for reaction, reasonably treating and utilizing the by-products and reaction residues to eliminate secondary pollution, improving the yield of biofuels, and reducing the cost of biofuels, are the future development directions in the biofuel conversion of kitchen waste. In addition to the preparation of biofuels, the conversion of kitchen waste into bio-fertilizers, bio-feeds, bio-chemicals and bioelectric energy can also be considered. Proper policies are also needed to minimize the generation of kitchen waste.
Funding
This research was funded by [the Chongqing Special Project for Technological Innovation and Application Development] grant number [cstc2020jscx-msxmX0076], [the Chongqing Talent Plan for Technological Innovation and Application Development] grant number [cstc2021ycjh-bgzxm0242], [the Chongqing Talent Innovation and Entrepreneurship Demonstration Team] grant number [CQYC201903189], and [the Science and Technology Project of Chongqing Technology and Business University] grant number [1952024].
Acknowledgments
The authors are thankful to Sheguang Ding—Chongqing Technology and Business University for supplying part translation of the draft.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ABE | acetone:butanol:ethanol |
| AD | anaerobic digestion |
| C/N | carbon-to-nitrogen ratio |
| CNG | compressed natural gas |
| COD | chemical oxygen demand |
| EDTA | ethylene diamine tetraacetic acid |
| FW | food waste |
| LHV | lower heating value |
| LNG | liquefied natural gas |
| LPG | liquefied petroleum gas |
| MON | motor octane number |
| OL | organic load |
| RON | research octane number |
| TAN | total ammonia nitrogen |
| TS | total solid |
| VFA | volatile fatty (aliphatic) acid |
| VS | volatile solid |
| WCO | waste cooking oil |
References
- Mahapatra, S.; Kumar, D.; Singh, B.; Sachan, P.K. Biofuels and their sources of production: A review on cleaner sustainable alternative against conventional fuel, in the framework of the food and energy nexus. Energy Nexus 2021, 4, 100036. [Google Scholar] [CrossRef]
- Akinwumi, A.R.; Nwinyi, O.C.; Ayeni, A.O.; Ahuekwe, E.F.; Chukwu, M.N. An overview of the production and prospect of polyhydroxyalkanote (PHA)-based biofuels: Opportunities and limitations. Sci. Afr. 2022, 16, e01233. [Google Scholar] [CrossRef]
- Mohan, R.; Ray, P. Indian Monetary Policy in the Time of Inflation Targeting and Demonetization. Asian Econ. Policy Rev. 2019, 14, 67–92. [Google Scholar] [CrossRef]
- Shams Esfandabadi, Z.; Ranjbari, M.; Scagnelli, S. The imbalance of food and biofuel markets amid Ukraine-Russia crisis: A systems thinking perspective. Biofuel Res. J. 2022, 9, 1640–1647. [Google Scholar] [CrossRef]
- Lan, Z. Study on Biofuels Development and its Impacts; Wuhan University of Technology: Wuhan, China, 2009. [Google Scholar]
- Ko, J.K.; Lee, J.H.; Jung, J.H.; Lee, S.-M. Recent advances and future directions in plant and yeast engineering to improve lignocellulosic biofuel production. Renew. Sustain. Energy Rev. 2020, 134, 110390. [Google Scholar] [CrossRef]
- Soltanian, S.; Aghbashlo, M.; Almasi, F.; Hosseinzadeh-Bandbafha, H.; Nizami, A.-S.; Ok, Y.S.; Lam, S.S.; Tabatabaei, M. A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Convers. Manag. 2020, 212, 112792. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Bonilla-Petriciolet, A.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Emerging technologies for biofuel production: A critical review on recent progress, challenges and perspectives. J. Environ. Manag. 2021, 290, 112627. [Google Scholar] [CrossRef] [PubMed]
- Ebadian, M.; van Dyk, S.; McMillan, J.D.; Saddler, J. Biofuels policies that have encouraged their production and use: An international perspective. Energy Policy 2020, 147, 111906. [Google Scholar] [CrossRef]
- Ashani, P.N.; Shafiei, M.; Karimi, K. Biobutanol production from municipal solid waste: Technical and economic analysis. Bioresour. Technol. 2020, 308, 123267. [Google Scholar] [CrossRef]
- Beig, B.; Riaz, M.; Raza Naqvi, S.; Hassan, M.; Zheng, Z.; Karimi, K.; Pugazhendhi, A.; Atabani, A.E.; Thuy Lan Chi, N. Current challenges and innovative developments in pretreatment of lignocellulosic residues for biofuel production: A review. Fuel 2021, 287, 119670. [Google Scholar] [CrossRef]
- Ab Rasid, N.S.; Shamjuddin, A.; Abdul Rahman, A.Z.; Amin, N.A.S. Recent advances in green pre-treatment methods of lignocellulosic biomass for enhanced biofuel production. J. Clean. Prod. 2021, 321, 129038. [Google Scholar] [CrossRef]
- Boro, M.; Verma, A.K.; Chettri, D.; Yata, V.K.; Verma, A.K. Strategies involved in biofuel production from agro-based lignocellulose biomass. Environ. Technol. Innov. 2022, 28, 102679. [Google Scholar] [CrossRef]
- Sawasdee, V.; Pisutpaisal, N. Potential of Napier grass Pak Chong 1 as feedstock for biofuel production. Energy Rep. 2021, 7, 519–526. [Google Scholar] [CrossRef]
- Echaroj, S.; Pannucharoenwong, N.; Duanguppama, K.; Santikunaporn, M.; Rattanadecho, P. Supercritical ethanol liquefaction of rice husk to bio-fuel over modified graphene oxide. Energy Rep. 2022, 8, 173–183. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Sivakumar, N.; Lukk, T.; Pecoraro, L.; Thakur, V.K.; Roberts, D.; Newbold, J.; Gupta, V.K. Bioprocessing of waste biomass for sustainable product development and minimizing environmental impact. Bioresour. Technol. 2021, 322, 124548. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, K.; Ikhsan Taipabu, M.; Wu, W. Novel Petit grain bitter orange waste peel oil biofuel investigation in diesel engine with modified fuel injection pressure and bowl geometry. Fuel 2022, 319, 123660. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Abd_Allah, E.F.; Singh, R.; Hashem, A.; Gupta, V.K. Biohydrogen production using kitchen waste as the potential substrate: A sustainable approach. Chemosphere 2021, 271, 129537. [Google Scholar] [CrossRef] [PubMed]
- Suzihaque, M.U.H.; Syazwina, N.; Alwi, H.; Ibrahim, U.K.; Abdullah, S.; Haron, N. A sustainability study of the processing of kitchen waste as a potential source of biofuel: Biodiesel production from waste cooking oil (WCO). Mater. Today: Proc. 2022, 63, S484–S489. [Google Scholar] [CrossRef]
- Norouzian Baghani, A.; Sadjadi, S.; Yaghmaeian, K.; Hossein Mahvi, A.; Yunesian, M.; Nabizadeh, R. Solid alcohol biofuel based on waste cooking oil: Preparation, properties, micromorphology, heating value optimization and its application as candle wax. Renew. Energy 2022, 192, 617–630. [Google Scholar] [CrossRef]
- Ajay, C.M.; Mohan, S.; Dinesha, P. Decentralized energy from portable biogas digesters using domestic kitchen waste: A review. Waste Manag. 2021, 125, 10–26. [Google Scholar] [CrossRef]
- Kanchanatip, E.; Chansiriwat, W.; Palalerd, S.; Khunphonoi, R.; Kumsaen, T.; Wantala, K. Light biofuel production from waste cooking oil via pyrolytic catalysis cracking over modified Thai dolomite catalysts. Carbon Resour. Convers. 2022, 5, 177–184. [Google Scholar] [CrossRef]
- He, M.; Xu, C.; Li, B.; Hu, T.; Shi, Z. The influence of ventilation rate on nitrogen conversion and N2O releasing in composting of kitchen waste. J. Ningbo Univ. 2021, 34, 114–120. [Google Scholar]
- Zhao, C.; Xin, L.; Xu, X.; Qin, Y.; Wu, W. Dynamics of antibiotics and antibiotic resistance genes in four types of kitchen waste composting processes. J. Hazard. Mater. 2022, 424, 127526. [Google Scholar] [CrossRef]
- Kim, S.; Mostafa, A.; Im, S.; Lee, M.-K.; Kang, S.; Na, J.-G.; Kim, D.-H. Production of high-calorific biogas from food waste by integrating two approaches: Autogenerative high-pressure and hydrogen injection. Water Res. 2021, 194, 116920. [Google Scholar] [CrossRef] [PubMed]
- Negri, C.; Ricci, M.; Zilio, M.; D’Imporzano, G.; Qiao, W.; Dong, R.; Adani, F. Anaerobic digestion of food waste for bio-energy production in China and Southeast Asia: A review. Renew. Sustain. Energy Rev. 2020, 133, 110138. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Merrylin, J.; Poornima Devi, T.; Kavitha, S.; Sivashanmugam, P.; Kumar, G.; Rajesh Banu, J. Food waste valorization: Biofuels and value added product recovery. Bioresour. Technol. Rep. 2020, 11, 100524. [Google Scholar] [CrossRef]
- De Medina-Salas, L.; Castillo-González, E.; Giraldi-Díaz, M.R.; Jamed-Boza, L.O. Valorisation of the organic fraction of municipal solid waste. Waste Manag. Res. 2018, 37, 59–73. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Varjani, S.; Hyoun Kim, S.; Wong, J.W.C. Sustainable processing of food waste for production of bio-based products for circular bioeconomy. Bioresour. Technol. 2021, 325, 124684. [Google Scholar] [CrossRef]
- Miao, Z. Study on Co-Production of Biogas and Fuel Oil for Kitchen Waste; Kunming University of Science and Technology: Kunming, China, 2019. [Google Scholar]
- Bing, J.; Luo, E.; Jing, Y.; Li, Y.; Liu, D. Research on Construction Status of Kitchen Waste Resource Utilization System in China. Environ. Sci. Manag 2018, 43, 39–43. [Google Scholar]
- Hafid, H.S.; Rahman, N.A.A.; Shah, U.K.M.; Baharuddin, A.S.; Ariff, A.B. Feasibility of using kitchen waste as future substrate for bioethanol production: A review. Renew. Sustain. Energy Rev. 2017, 74, 671–686. [Google Scholar] [CrossRef]
- Li, M.; Li, F.; Zhou, J.; Yuan, Q.; Hu, N. Fallen leaves are superior to tree pruning as bulking agents in aerobic composting disposing kitchen waste. Bioresour. Technol. 2022, 346, 126374. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Long, F.; Cao, X.; Zhao, J.; Liu, P.; Xu, J. Catalytic cracking of waste cooking oil followed with hydro-isomerization for high-quality biofuel production. J. Clean. Prod. 2022, 345, 131027. [Google Scholar] [CrossRef]
- Singh, A.; Singhania, R.R.; Soam, S.; Chen, C.-W.; Haldar, D.; Varjani, S.; Chang, J.-S.; Dong, C.-D.; Patel, A.K. Production of bioethanol from food waste: Status and perspectives. Bioresour. Technol. 2022, 360, 127651. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, E.; Lorentz, K.O.; Stein, G.J.; Mitchell, P.D. Prehistoric schistosomiasis parasite found in the Middle East. Lancet Infect. Dis. 2014, 14, 553–554. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Kim, S.H.; Pandey, A. Microbial strategies for bio-transforming food waste into resources. Bioresour. Technol. 2020, 299, 122580. [Google Scholar] [CrossRef]
- Shi, S.; Gao, M.; Wang, J.; Zhen, J.; Yuan, Y.; Tian, D. Study on bio-butanol production from food waste by abe fermentation. Environ. Eng 2017, 35, 117–121. [Google Scholar] [CrossRef]
- Hoàng, T.Y.; Khoo, K.S.; Ngọc, H.L.T.; Thu, Q.T.T.; Thị, T.Đ.; Thu, H.Đ.T.; Hoàng, H.C.; Chinthalapati, S.; Lay, C.-H.; Show, P.L. Sustainable cultivation via waste soybean extract for higher vaccenic acid production by purple non-sulfur bacteria. Clean Technol. Environ. Policy 2021, 23, 103–112. [Google Scholar] [CrossRef]
- Yadav, A. Biofuel from waste cooking oil of hospitality laboratory. Mater. Today Proc. 2021, 57, 2121–2123. [Google Scholar] [CrossRef]
- Ginni, G.; Adish Kumar, S.; Mohamed Usman, T.M.; Pakonyi, P.; Rajesh Banu, J. Chapter 13—Integrated biorefineries of food waste. In Food Waste to Valuable Resources; Banu, J.R., Kumar, G., Gunasekaran, M., Kavitha, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 275–298. [Google Scholar]
- Chen, B. Food Waste pretreatment technologies. Environ. Sanit. Eng. 2015, 23, 10–12. [Google Scholar]
- Ban, F.; Sun, X.; Liu, X.; Li, M. Introduction to a food waste treatment project with automatic sorting, solid-liquid separation, and oil-water separation process. Environ. Eng. 2016, 34, 145. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, Z.; Li, H.; Cui, J.; He, Q.; Zhang, H.; Zhang, H.; Yang, L. Effect of two-stage separation on the removal of sundries in kitchen waste. Guanndong Chem. Ind. 2022, 49, 109–110, 122. [Google Scholar]
- Sindhu, R.; Gnansounou, E.; Rebello, S.; Binod, P.; Varjani, S.; Thakur, I.S.; Nair, R.B.; Pandey, A. Conversion of food and kitchen waste to value-added products. J. Environ. Manag. 2019, 241, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.K.; Ramadoss, G.; Jain, A.K.; Dhiman, R.K.; Bhatia, S.K.; Bhatt, A.K. Conversion of Waste Biomass into Gaseous Fuel: Present Status and Challenges in India. BioEnergy Res. 2020, 13, 1046–1068. [Google Scholar] [CrossRef]
- Gao, Y. Study on the Domestication of Propionic Acid Methanogens and the Enhancement of Anaerobic Fermentation of Food Waste; Northeast Agricultural University: Harbin, China, 2018. [Google Scholar]
- Pasalari, H.; Gholami, M.; Rezaee, A.; Esrafili, A.; Farzadkia, M. Perspectives on microbial community in anaerobic digestion with emphasis on environmental parameters: A systematic review. Chemosphere 2021, 270, 128618. [Google Scholar] [CrossRef]
- Caruso, M.C.; Braghieri, A.; Capece, A.; Napolitano, F.; Romano, P.; Galgano, F.; Altieri, G.; Genovese, F. Recent Updates on the Use of Agro-Food Waste for Biogas Production. Appl. Sci. 2019, 9, 1217. [Google Scholar] [CrossRef]
- Kothari, R.; Pandey, A.K.; Kumar, S.; Tyagi, V.V.; Tyagi, S.K. Different aspects of dry anaerobic digestion for bio-energy: An overview. Renew. Sustain. Energy Rev. 2014, 39, 174–195. [Google Scholar] [CrossRef]
- Ma, Y.; Gu, J.; Liu, Y. Evaluation of anaerobic digestion of food waste and waste activated sludge: Soluble COD versus its chemical composition. Sci. Total Environ. 2018, 643, 21–27. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y. Turning food waste to energy and resources towards a great environmental and economic sustainability: An innovative integrated biological approach. Biotechnol. Adv. 2019, 37, 107414. [Google Scholar] [CrossRef]
- Panepinto, D.; Genon, G. Analysis of the extrusion as a pretreatment for the anaerobic digestion process. Ind. Crops Prod. 2016, 83, 206–212. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Frunzo, L.; Esposito, G.; Lens, P.N.L.; Pirozzi, F. Enhanced anaerobic digestion of food waste by thermal and ozonation pretreatment methods. J. Environ. Manag. 2014, 146, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Ariunbaatar, J.; Panico, A.; Yeh, D.H.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Enhanced mesophilic anaerobic digestion of food waste by thermal pretreatment: Substrate versus digestate heating. Waste Manag. 2015, 46, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, Y. Effects of thermal pretreatment on acidification phase during two-phase batch anaerobic digestion of kitchen waste. Renew. Energy 2015, 77, 550–557. [Google Scholar] [CrossRef]
- Ma, J.; Duong, T.H.; Smits, M.; Verstraete, W.; Carballa, M. Enhanced biomethanation of kitchen waste by different pre-treatments. Bioresour. Technol. 2011, 102, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H. Study on Anaerobic Digestion of Food Waste: Effect of Pretreatment; Zhejiang University: Hangzhou, China, 2018. [Google Scholar]
- Feng, L.; LI, R. Efficiency of anaerobic digestion of kitchen waste by low intensity ultrasound pretreatment. Chin. J. Environ. Eng. 2012, 6, 3280–3286. [Google Scholar]
- Sun, Y. Effect of Different Pretretments on Fermentative Hydrogen and Methane Production from Food Waste and Mechanism Investigation; Beijing University of Chemical Technology: Beijing, China, 2013. [Google Scholar]
- Linyi, C.; Yujie, Q.; Buqing, C.; Chenglong, W.; Shaohong, Z.; Renglu, C.; Shaohua, Y.; Lan, Y.; Zhiju, L. Enhancing degradation and biogas production during anaerobic digestion of food waste using alkali pretreatment. Environ. Res. 2020, 188, 109743. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.; Chen, X.; Ren, L. Effects of bentonite on antibiotic resistance genes in biogas slurry and residue from thermophilic and mesophilic anaerobic digestion of food waste. Bioresour. Technol. 2021, 336, 125322. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, W.; Zhang, H.; Wang, Z.; Fan, C.; Zang, L. Recent achievements in enhancing anaerobic digestion with carbon-based functional materials. Bioresour. Technol. 2018, 266, 555–567. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, N.; Tian, H.; Lin, C.; Zhang, Y.; Liu, Z. Comparing two enhancing methods for improving kitchen waste anaerobic digestion: Bentonite addition and autoclaved de-oiling pretreatment. Process Saf. Environ. Prot. 2018, 115, 116–124. [Google Scholar] [CrossRef]
- Yin, X. Optimization of Anaerobic Fermentation Process with Mixed Materials of Kitchen Waste and Vetiveria Zizanioides; Nanyang Normal University: Nanyang, China, 2018. [Google Scholar]
- Yi, L.; Rao, L.; Wang, X.; Wang, H. Effect of ventilation on nitrogen conversion and nitrogen loss in kitchen waste compost. J. Cent. South Univ. 2012, 43, 1584–1588. [Google Scholar]
- Enzmann, F.; Mayer, F.; Rother, M.; Holtmann, D. Methanogens: Biochemical background and biotechnological applications. AMB Express 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Microbial life at high salt concentrations: Phylogenetic and metabolic diversity. Saline Syst. 2008, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhu, N. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion. Renew. Sustain. Energy Rev. 2016, 58, 429–438. [Google Scholar] [CrossRef]
- Akindele, A.A.; Sartaj, M. The toxicity effects of ammonia on anaerobic digestion of organic fraction of municipal solid waste. Waste Manag. 2018, 71, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Angelidaki, I. Submersible microbial desalination cell for simultaneous ammonia recovery and electricity production from anaerobic reactors containing high levels of ammonia. Bioresour. Technol. 2015, 177, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Ma, S. Study on Biochar Promoting Anaerobic Digestion of Gas Production from Kitchen Waste; Huazhong University of Science and Technology: Wuhan, China, 2018. [Google Scholar]
- Qi, Q.; Sun, C.; Zhang, J.; He, Y.; Wah Tong, Y. Internal enhancement mechanism of biochar with graphene structure in anaerobic digestion: The bioavailability of trace elements and potential direct interspecies electron transfer. Chem. Eng. J. 2021, 406, 126833. [Google Scholar] [CrossRef]
- Choong, Y.Y.; Norli, I.; Abdullah, A.Z.; Yhaya, M.F. Impacts of trace element supplementation on the performance of anaerobic digestion process: A critical review. Bioresour. Technol. 2016, 209, 369–379. [Google Scholar] [CrossRef]
- Wei, T.; Wen, H.; Chen, J. The effect of zero-valent iron acclimated sludge on methane production from kitchen wastes by anaerobic digestion. Hubei Agr. Sci. 2016, 55, 3618–3621. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Z.; Meng, X.; Ding, q.; Zhang, H. Effects of Fe2+ dosing methods on anaerobic digestion of kitchen waste. Appl. Chem. Ind. 2019, 48, 838–840. [Google Scholar] [CrossRef]
- Bolzonella, D.; Battista, F.; Cavinato, C.; Gottardo, M.; Micolucci, F.; Lyberatos, G.; Pavan, P. Recent developments in biohythane production from household food wastes: A review. Bioresour. Technol. 2018, 257, 311–319. [Google Scholar] [CrossRef]
- David, B.; Federico, B.; Cristina, C.; Marco, G.; Federico, M.; Paolo, P. Chapter 13—Biohythane Production from Food Wastes. In Biohydrogen, 2nd ed.; Pandey, A., Mohan, S.V., Chang, J.-S., Hallenbeck, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 347–368. [Google Scholar]
- Si, B.; Liu, Z.; Zhang, Y.; Li, J.; Shen, R.; Zhu, Z.; Xing, X. Towards biohythane production from biomass: Influence of operational stage on anaerobic fermentation and microbial community. Int. J. Hydrogrn Energy 2016, 41, 4429–4438. [Google Scholar] [CrossRef]
- Abreu, A.A.; Tavares, F.; Alves, M.M.; Pereira, M.A. Boosting dark fermentation with co-cultures of extreme thermophiles for biohythane production from garden waste. Bioresour. Technol. 2016, 219, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, S.B.; Venkata Mohan, S. Single-stage fermentation process for high-value biohythane production with the treatment of distillery spent-wash. Bioresour. Technol. 2015, 189, 177–185. [Google Scholar] [CrossRef]
- Vo, T.-P.; Lay, C.-H.; Lin, C.-Y. Effects of hydraulic retention time on biohythane production via single-stage anaerobic fermentation in a two-compartment bioreactor. Bioresour. Technol. 2019, 292, 121869. [Google Scholar] [CrossRef] [PubMed]
- Ta, D.T.; Lin, C.-Y.; Ta, T.M.N.; Chu, C.-Y. Biohythane production via single-stage anaerobic fermentation using entrapped hydrogenic and methanogenic bacteria. Bioresour. Technol. 2020, 300, 122702. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Ammonia Nitrogen Characteristics and Control Strategies for Two-Phase Anaerobic Digestion of Food Waste; Zhejiang University: Hangzhou, China, 2014. [Google Scholar]
- Sarkar, O.; Venkata Mohan, S. Pre-aeration of food waste to augment acidogenic process at higher organic load: Valorizing biohydrogen, volatile fatty acids and biohythane. Bioresour. Technol. 2017, 242, 68–76. [Google Scholar] [CrossRef]
- Dong, L.; Cao, G.; Zhao, L.; Liu, B.; Ren, N. Alkali/urea pretreatment of rice straw at low temperature for enhanced biological hydrogen production. Bioresour. Technol. 2018, 267, 71–76. [Google Scholar] [CrossRef]
- Stępień, Z. A Comprehensive Overview of Hydrogen-Fueled Internal Combustion Engines: Achievements and Future Challenges. Energies 2021, 14, 6504. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- The Engineering ToolBox. Fuels-Higher and Lower Calorific Values. Available online: https://www.engineeringtoolbox.com (accessed on 1 February 2023).
- Liu, X.; Bao, Z.; Pen, J.; Yang, L.; Shi, Y.; Liu, H. Review of biohydrogen production by anaerobic fermentation of food waste. J. Tianjin Agric. Univ. 2017, 24, 95–99. [Google Scholar]
- Putatunda, C.; Behl, M.; Solanki, P.; Sharma, S.; Bhatia, S.K.; Walia, A.; Bhatia, R.K. Current challenges and future technology in photofermentation-driven biohydrogen production by utilizing algae and bacteria. Int. J. Hydrogrn Energy, 2022; in press. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Khoo, K.S.; Show, P.-L.; Femina Carolin, C.; Fetcia Jackulin, C.; Jeevanantham, S.; Karishma, S.; Show, K.-Y.; Lee, D.-J.; et al. Biohydrogen from organic wastes as a clean and environment-friendly energy source: Production pathways, feedstock types, and future prospects. Bioresour. Technol. 2021, 342, 126021. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X. tudies on hydrogen production from kitchen waste by sequential dark-and photo-fermentation. Environ. Sci. Manag. 2013, 38, 152–155. [Google Scholar]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Mishra, P.; Wahid, Z.A.; Karim, A.; Pant, K.K.; Ghosh, P.; Kumar, D.; Singh, L. Chronological perspective on fermentative-hydrogen from hypothesis in early nineteenth century to recent developments: A review. Biomass Convers. Biorefin. 2022, 12, 3711–3723. [Google Scholar] [CrossRef]
- Kumar, G.; Mathimani, T.; Rene, E.R.; Pugazhendhi, A. Application of nanotechnology in dark fermentation for enhanced biohydrogen production using inorganic nanoparticles. Int. J. Hydrogrn Energy 2019, 44, 13106–13113. [Google Scholar] [CrossRef]
- Maxa, D.; Rychtera, M.; Linhova, M.; Fribert, P.; Muzikova, Z.; Lipovský, J.; Paulová, L.; Pospisil, M.; Sebor, G.; Melzoch, K. Perspectives of Biobutanol Production and Use; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Bajpai, P. (Ed.) Global Production of Bioethanol. In Developments in Bioethanol; Springer: Singapore, 2021; pp. 177–196. [Google Scholar]
- Ma, H.; Wang, Q.; Qian, D.; Gong, L.; Zhang, W. The utilization of acid-tolerant bacteria on ethanol production from kitchen garbage. Renew. Energy 2009, 34, 1466–1470. [Google Scholar] [CrossRef]
- Chatterjee, S.; Venkata Mohan, S. Fungal biorefinery for sustainable resource recovery from waste. Bioresour. Technol. 2022, 345, 126443. [Google Scholar] [CrossRef]
- Uçkun Kiran, E.; Trzcinski, A.P.; Ng, W.J.; Liu, Y. Bioconversion of food waste to energy: A review. Fuel 2014, 134, 389–399. [Google Scholar] [CrossRef]
- Zhang, Q. Fuel ethanol production from kitchen garbage by simultaneous Saccharification and fermentation (SSF). Chem. Ind. Eng. Prog. 2015, 34, 91–94. [Google Scholar] [CrossRef]
- Hong, Y.S.; Yoon, H.H. Ethanol production from food residues. Biomass Bioenergy 2011, 35, 3271–3275. [Google Scholar] [CrossRef]
- Jiang, L.; Guo, D.; Yuan, K.; Du, A.; Chen, G. Secondary catalytic hydrolysis reaction of fuel ethanol production from kitchen garbag. Renew. Energ. Source 2012, 30, 66–69. [Google Scholar] [CrossRef]
- Amaro, H.M.; Guedes, A.C.; Malcata, F.X. Advances and perspectives in using microalgae to produce biodiesel. Appl. Energy 2011, 88, 3402–3410. [Google Scholar] [CrossRef]
- Sakuragi, H.; Kuroda, K.; Ueda, M. Molecular breeding of advanced microorganisms for biofuel production. J. Biomed. Biotechnol. 2011, 2011, 416931. [Google Scholar] [CrossRef]
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R.; Chu, D.-T.; Show, P.-L. Waste to bioenergy: A review on the recent conversion technologies. BMC Energy 2019, 1, 4. [Google Scholar] [CrossRef]
- Ranjan, A.; Moholkar, V.S. Biobutanol: Science, engineering, and economics. Int. J. Energy Res. 2012, 36, 277–323. [Google Scholar] [CrossRef]
- Abo, B.O.; Gao, M.; Wu, C.; Zhu, W.; Wang, Q. A review on characteristics of food waste and their use in butanol production. Rev. Environ. Health 2019, 34, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Ndaba, B.; Chiyanzu, I.; Marx, S. n-Butanol derived from biochemical and chemical routes: A review. Biotechnol. Rep. 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, M.; Abo, B.O.; Wang, F.; Wang, Q. Bio-butanol production from direct fermentation of kitchen wastes. Environ. Eng. 2019, 37, 137–142. [Google Scholar] [CrossRef]
- Inui, M.; Suda, M.; Kimura, S.; Yasuda, K.; Suzuki, H.; Toda, H.; Yamamoto, S.; Okino, S.; Suzuki, N.; Yukawa, H. Expression of Clostridium acetobutylicum butanol synthetic genes in Escherichia coli. Appl. Microbiol. Biotechnol. 2008, 77, 1305–1316. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Mathimani, T.; Varjani, S.; Rene, E.R.; Kumar, G.; Kim, S.-H.; Ponnusamy, V.K.; Yoon, J.-J. Biobutanol as a promising liquid fuel for the future-recent updates and perspectives. Fuel 2019, 253, 637–646. [Google Scholar] [CrossRef]
- Bankar, S.B.; Survase, S.A.; Ojamo, H.; Granström, T. Biobutanol: The outlook of an academic and industrialist. RSC Adv. 2013, 3, 24734–24757. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, J.H.; Jang, S.H.; Nielsen, L.K.; Kim, J.; Jung, K.S. Fermentative butanol production by clostridia. Biotechnol. Bioeng. 2008, 101, 209–228. [Google Scholar] [CrossRef]
- Veza, I.; Muhamad Said, M.F.; Latiff, Z.A. Recent advances in butanol production by acetone-butanol-ethanol (ABE) fermentation. Biomass Bioenergy 2021, 144, 105919. [Google Scholar] [CrossRef]
- Rathour, R.K.; Ahuja, V.; Bhatia, R.K.; Bhatt, A.K. Biobutanol: New era of biofuels. Int. J. Energy Res. 2018, 42, 4532–4545. [Google Scholar] [CrossRef]
- Huang, H.; Singh, V.; Qureshi, N. Butanol production from food waste: A novel process for producing sustainable energy and reducing environmental pollution. Biotechnol. Biofuels 2015, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-S.; Malaviya, A.; Cho, C.; Lee, J.; Lee, S.Y. Butanol production from renewable biomass by clostridia. Bioresour. Technol. 2012, 123, 653–663. [Google Scholar] [CrossRef]
- Bruder, M.; Moo-Young, M.; Chung, D.A.; Chou, C.P. Elimination of carbon catabolite repression in Clostridium acetobutylicum—A journey toward simultaneous use of xylose and glucose. Appl. Microbiol. Biotechnol. 2015, 99, 7579–7588. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Akhtar, M.N.; Nisar, N.; Rashid, N. Optimized production and advanced assessment of biodiesel: A review. Int. J. Energy Res. 2018, 42, 2070–2083. [Google Scholar] [CrossRef]
- Zhong, C.; Liang, K.; Li, A. Preparation of biodiesel from kitchen waste assisted by microwave. Mod. Chem. Ind. 2019, 39, 144–148. [Google Scholar] [CrossRef]
- Han, S.; Kim, G.-Y.; Han, J.-I. Biodiesel production from oleaginous yeast, Cryptococcus sp. by using banana peel as carbon source. Energy Rep. 2019, 5, 1077–1081. [Google Scholar] [CrossRef]
- Fei, Q.; O’Brien, M.; Nelson, R.; Chen, X.; Lowell, A.; Dowe, N. Enhanced lipid production by Rhodosporidium toruloides using different fed-batch feeding strategies with lignocellulosic hydrolysate as the sole carbon source. Biotechnol. Biofuels 2016, 9, 130. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Tsang, D.C.W.; Bolan, N.S.; Sik Ok, Y.; Igalavithana, A.D.; Kirkham, M.B.; Kim, K.-H.; Vikrant, K. Value-added chemicals from food supply chain wastes: State-of-the-art review and future prospects. Chem. Eng. J. 2019, 375, 121983. [Google Scholar] [CrossRef]
- Karmee, S.K. Liquid biofuels from food waste: Current trends, prospect and limitation. Renew. Sustain. Energy Rev. 2016, 53, 945–953. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Ueki, Y.; Saiki, S.; Hoshina, H.; Seko, N. Biodiesel fuel production from waste cooking oil using radiation-grafted fibrous catalysts. Radiat. Phys. Chem. 2018, 143, 41–46. [Google Scholar] [CrossRef]
- Edith, O.; Janius, R.; Yunus, R. Factors Affecting the Cold Flow Behaviour of Biodiesel and Methods for Improvement—A Review. Pertanika J. Sci. Technol. 2012, 20, 1–14. [Google Scholar]
- Verma, P.; Sharma, M.P.; Dwivedi, G. Evaluation and enhancement of cold flow properties of palm oil and its biodiesel. Energy Rep. 2016, 2, 8–13. [Google Scholar] [CrossRef]
- Focke, W.W.; Westhuizen, I.v.d.; Grobler, A.B.L.; Nshoane, K.T.; Reddy, J.K.; Luyt, A.S. The effect of synthetic antioxidants on the oxidative stability of biodiesel. Fuel 2012, 94, 227–233. [Google Scholar] [CrossRef]
- Amran, N.A.; Bello, U.; Hazwan Ruslan, M.S. The role of antioxidants in improving biodiesel’s oxidative stability, poor cold flow properties, and the effects of the duo on engine performance: A review. Heliyon 2022, 8, e09846. [Google Scholar] [CrossRef]
- Heater, B.S.; Chan, W.S.; Lee, M.M.; Chan, M.A.-O. Directed evolution of a genetically encoded immobilized lipase for the efficient production of biodiesel from waste cooking oil. Biotechnol. Biofuels 2019, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Tai, K.; Chua, B.J.W.; Li, Z. Directed evolution of Thermomyces lanuginosus lipase to enhance methanol tolerance for efficient production of biodiesel from waste grease. Bioresour. Technol. 2017, 245, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Praserttaweeporn, K.; Vitidsant, T.; Charusiri, W. Ni-modified dolomite for the catalytic deoxygenation of pyrolyzed softwood and non-wood to produce bio-oil. Results Eng. 2022, 14, 100461. [Google Scholar] [CrossRef]
- Wakatuntu, J.; Olupot, P.W.; Jjagwe, J.; Menya, E.; Okure, M. Optimization of pyrolysis conditions for production of rice husk-based bio-oil as an energy carrier. Results Eng. 2023, 17, 100947. [Google Scholar] [CrossRef]
- Makarfi Isa, Y.; Ganda, E.T. Bio-oil as a potential source of petroleum range fuels. Renew. Sustain. Energy Rev. 2018, 81, 69–75. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, P.; Reddy, S.N. Catalytic (copper) hydrothermal liquefaction for lignin to produce high quality bio-oil and nano Cu carbon hybrids material. Chem. Eng. Sci. 2023, 270, 118548. [Google Scholar] [CrossRef]
- Zhao, K.; Li, W.; Yu, Y.; Chen, G.; Yan, B.; Cheng, Z.; Zhao, H.; Fang, Y. Speciation and transformation of nitrogen in the hydrothermal liquefaction of wastewater-treated duckweed for the bio-oil production. Renew. Energy 2023, 204, 661–670. [Google Scholar] [CrossRef]
- Déniel, M.; Haarlemmer, G.; Roubaud, A.; Weiss-Hortala, E.; Fages, J. Energy valorisation of food processing residues and model compounds by hydrothermal liquefaction. Renew. Sustain. Energy Rev. 2016, 54, 1632–1652. [Google Scholar] [CrossRef]
- Shu, D. Study on Bio-Oil/Char Production from Kitchen Food Waste by Hydrothermal Treatment; Zhejiang University: Hangzhou, China, 2019. [Google Scholar]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).