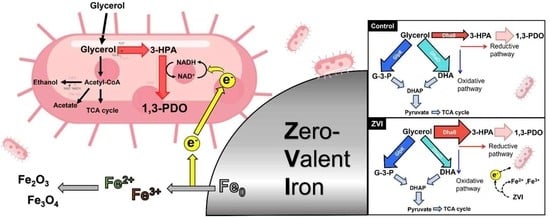
Bioconversion of Glycerol to 1,3-Propanediol Using Klebsiella pneumoniae L17 with the Microbially Influenced Corrosion of Zero-Valent Iron
Abstract
1. Introduction
2. Materials and Method
2.1. Strain and Cultivation
2.2. Analytical Methods
2.3. Real-Time qPCR
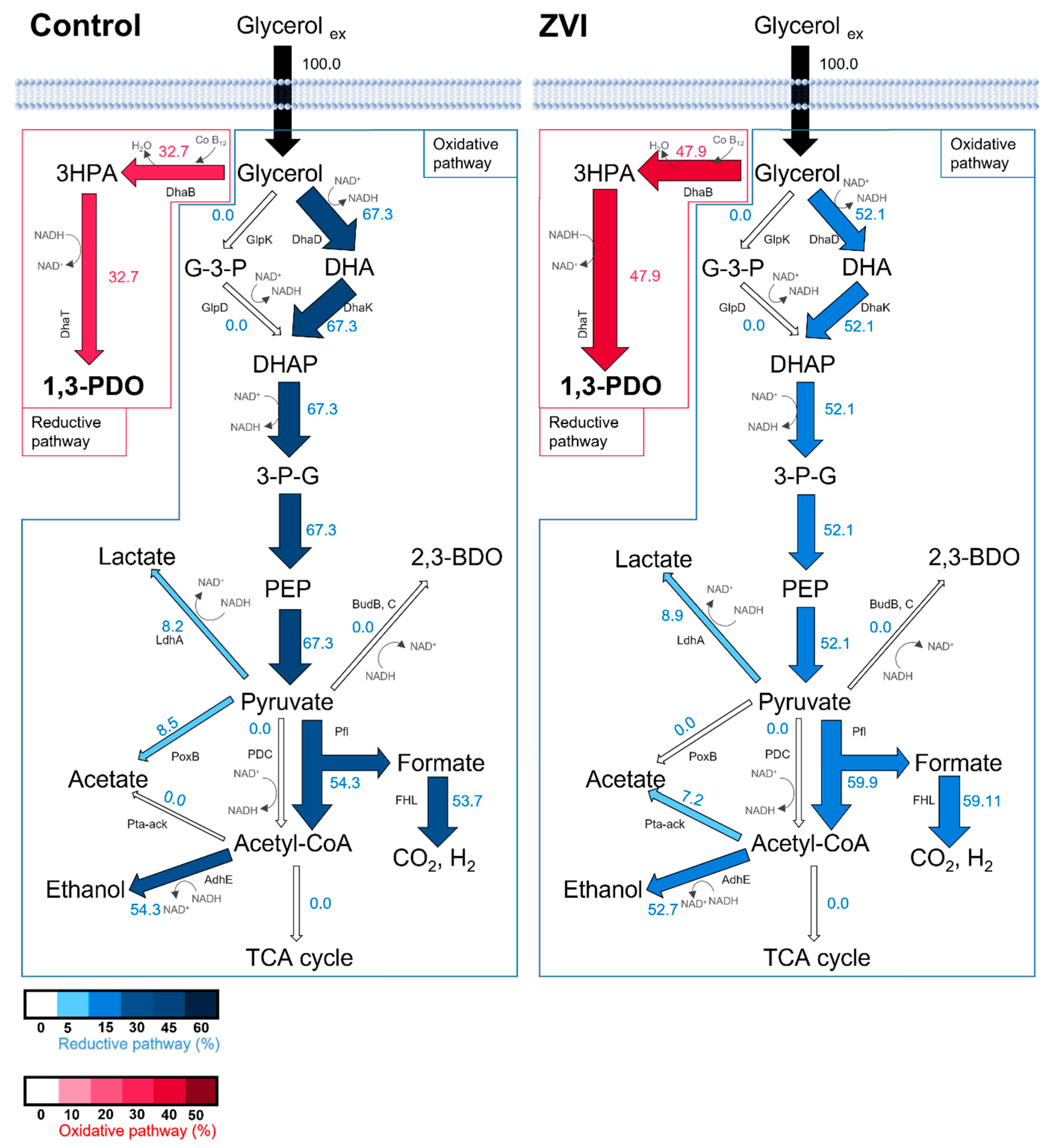
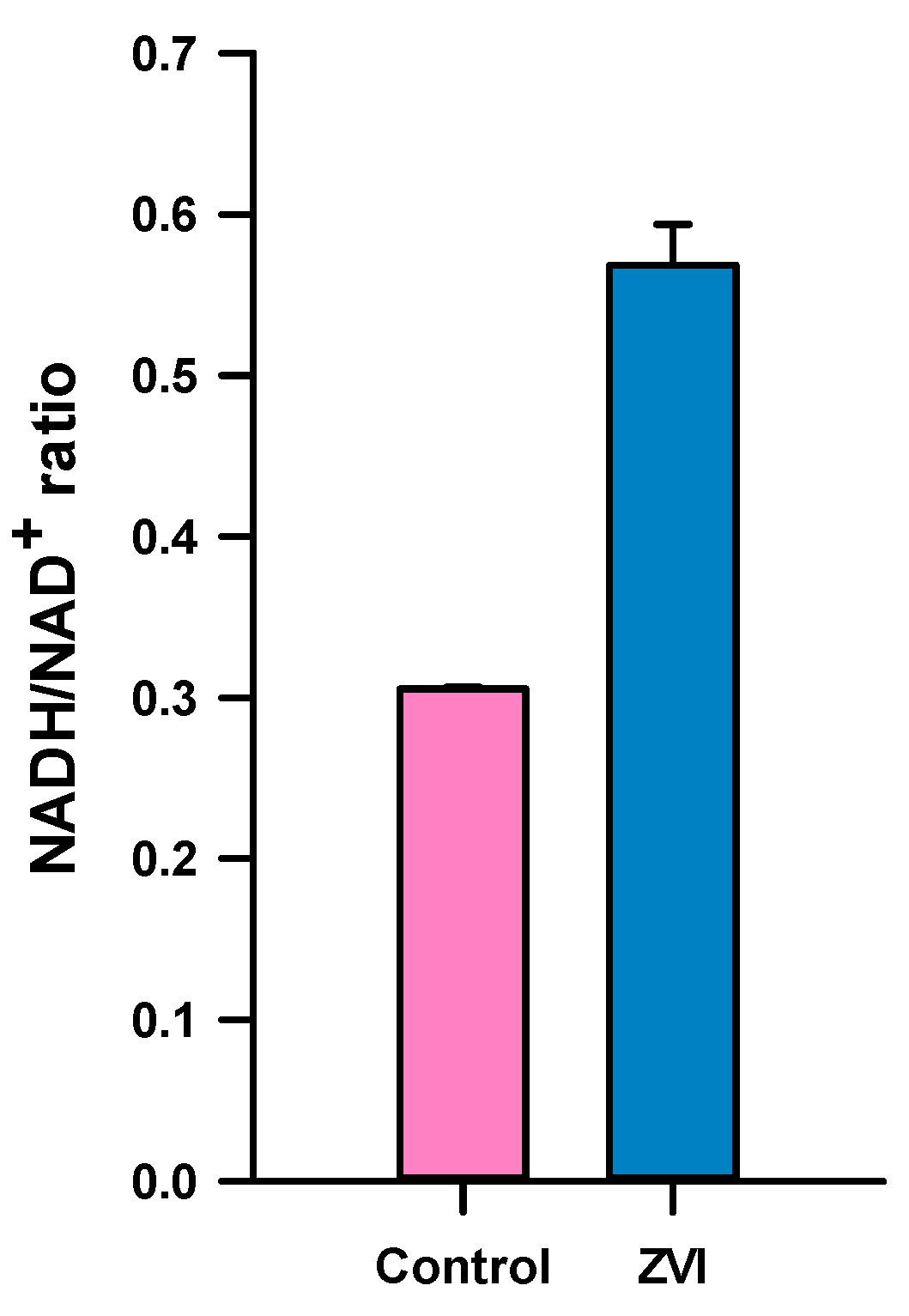
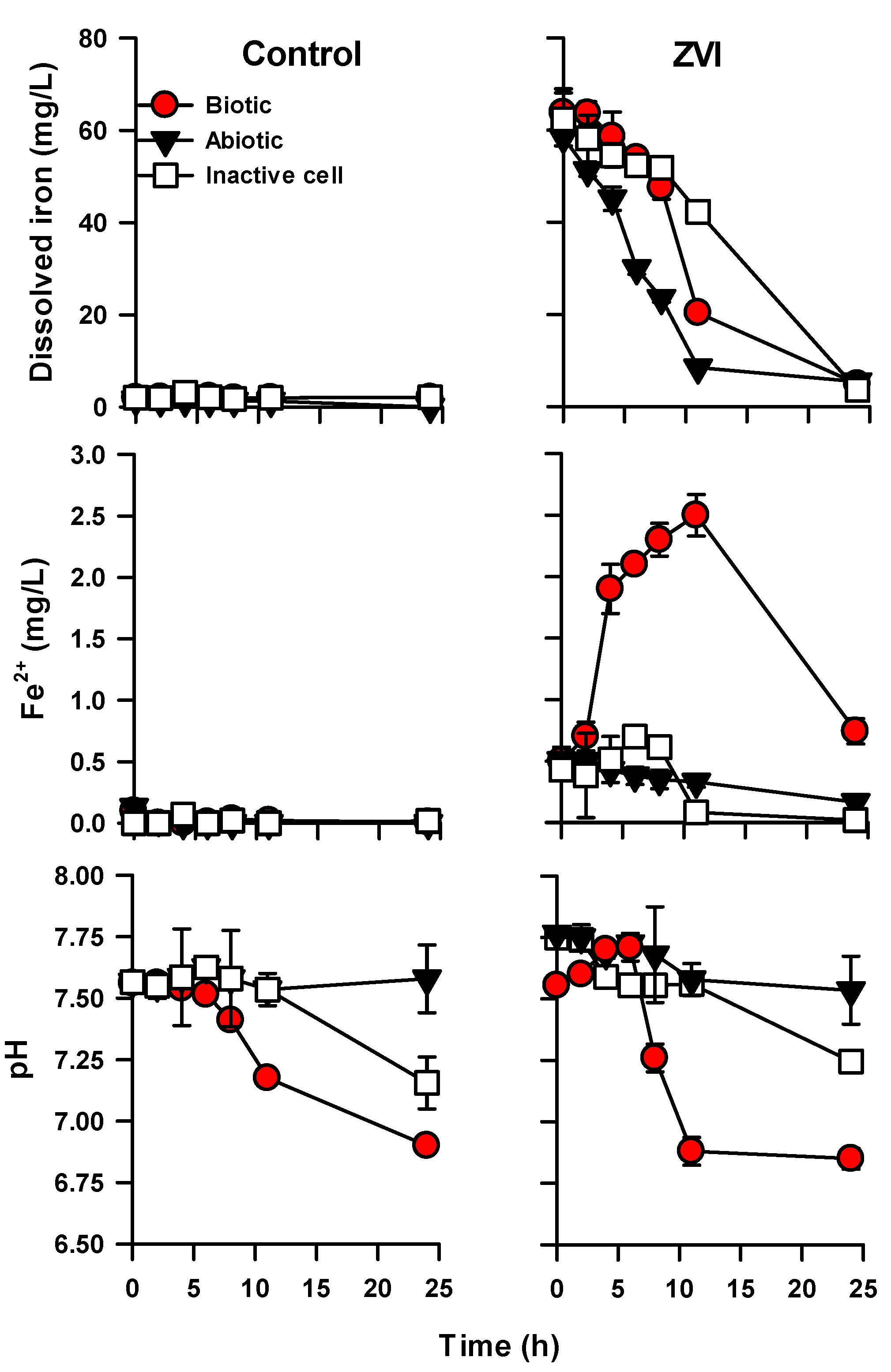
3. Results and Discussion
3.1. Metabolic Profile of K. pneumoniae L17 with ZVI
3.2. Change of Bacterial Redox State by ZVI
3.3. Oxidation of ZVI by Microbially Influenced Corrosion (MIC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koch, G. Cost of corrosion. In Trends in Oil and Gas Corrosion Research and Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–30. [Google Scholar]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. NPJ Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Emerson, D. The role of iron-oxidizing bacteria in biocorrosion: A review. Biofouling 2018, 34, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Little, B.; Blackwood, D.; Hinks, J.; Lauro, F.; Marsili, E.; Okamoto, A.; Rice, S.; Wade, S.; Flemming, H.-C. Microbially influenced corrosion—Any progress? Corros. Sci. 2020, 170, 108641. [Google Scholar] [CrossRef]
- Mand, J.; Park, H.S.; Jack, T.R.; Voordouw, G. The role of acetogens in microbially influenced corrosion of steel. Front. Microbiol. 2014, 5, 268. [Google Scholar] [CrossRef]
- Xu, D.; Li, Y.; Song, F.; Gu, T. Laboratory investigation of microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis. Corros. Sci. 2013, 77, 385–390. [Google Scholar] [CrossRef]
- Liu, B.; Li, Z.; Yang, X.; Du, C.; Li, X. Microbiologically influenced corrosion of X80 pipeline steel by nitrate reducing bacteria in artificial Beijing soil. Bioelectrochemistry 2020, 135, 107551. [Google Scholar] [CrossRef]
- Qian, H.; Zhang, D.; Lou, Y.; Li, Z.; Xu, D.; Du, C.; Li, X. Laboratory investigation of microbiologically influenced corrosion of Q235 carbon steel by halophilic archaea Natronorubrum tibetense . Corros. Sci. 2018, 145, 151–161. [Google Scholar] [CrossRef]
- Enning, D.; Garrelfs, J. Corrosion of iron by sulfate-reducing bacteria: New views of an old problem. Appl. Environ. Microbiol. 2014, 80, 1226–1236. [Google Scholar] [CrossRef]
- Kato, S. Microbial extracellular electron transfer and its relevance to iron corrosion. Microb. Biotechnol. 2016, 9, 141–148. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Zhang, R.; Guan, F.; Hou, B.; Duan, J. Microbiologically influenced corrosion of marine steels within the interaction between steel and biofilms: A brief view. Appl. Microbiol. Biotechnol. 2020, 104, 515–525. [Google Scholar] [CrossRef]
- Tremblay, P.-L.; Angenent, L.T.; Zhang, T. Extracellular electron uptake: Among autotrophs and mediated by surfaces. Trends Biotechnol. 2017, 35, 360–371. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Barco, R.A.; Emerson, D.; Roden, E.E. Comparative genomic analysis of neutrophilic iron (II) oxidizer genomes for candidate genes in extracellular electron transfer. Front. Microbiol. 2017, 8, 1584. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Khan, A.; Rao, T.S. Microbial fouling in water treatment plants. In Microbial and Natural Macromolecules; Elsevier: Amsterdam, The Netherlands, 2021; pp. 589–622. [Google Scholar]
- Schmidt, T.M. Encyclopedia of Microbiology; Academic Press: Oxford, UK, 2019. [Google Scholar]
- Beese-Vasbender, P.F.; Nayak, S.; Erbe, A.; Stratmann, M.; Mayrhofer, K.J. Electrochemical characterization of direct electron uptake in electrical microbially influenced corrosion of iron by the lithoautotrophic SRB Desulfopila corrodens strain IS4. Electrochim. Acta 2015, 167, 321–329. [Google Scholar] [CrossRef]
- Chiu, B.K.; Kato, S.; McAllister, S.M.; Field, E.K.; Chan, C.S. Novel pelagic iron-oxidizing Zetaproteobacteria from the Chesapeake Bay oxic–anoxic transition zone. Front. Microbiol. 2017, 8, 1280. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Kim, C.; Song, Y.E.; Mutyala, S.; Seol, E.-H.; Kim, J.R. Bioelectrochemical metabolic regulation of a heterologously expressed glycerol reductive pathway in E. coli BL21 (DE3). Electrochim. Acta 2022, 434, 141260. [Google Scholar] [CrossRef]
- Mutyala, S.; Kim, C.; Song, Y.E.; Khandelwal, H.; Baek, J.; Seol, E.; Oh, Y.-K.; Kim, J.R. Enabling anoxic acetate assimilation by electrode-driven respiration in the obligate aerobe, Pseudomonas putida . Bioelectrochemistry 2021, 138, 107690. [Google Scholar] [CrossRef]
- Khandelwal, H.; Mutyala, S.; Kim, M.; Song, Y.E.; Li, S.; Jang, M.; Oh, S.-E.; Kim, J.R. Colorimetric isolation of a novel electrochemically active Pseudomonas strain using tungsten nanorods for bioelectrochemical applications. Bioelectrochemistry 2022, 146, 108136. [Google Scholar] [CrossRef]
- Kim, M.; Li, S.; Kong, D.S.; Song, Y.E.; Park, S.-Y.; Kim, H.-I.; Jae, J.; Chung, I.; Kim, J.-R. Polydopamine/Polypyrrole Modified Graphite Felt Enhances Biocompatibility for Electroactive Bacteria and Power Density of Microbial Fuel Cell. Chemosphere 2023, 313, 137388. [Google Scholar] [CrossRef]
- Huang, H.; Gong, C.S.; Tsao, G.T. Production of 1,3-propanediol by Klebsiella pneumoniae . In Biotechnology for Fuels and Chemicals; Springer: Totowa, NJ, USA, 2002; pp. 687–698. [Google Scholar]
- Zhu, F.H.; Liu, D.H.; Chen, Z. Recent advances in biological production of 1,3-propanediol: New routes and engineering strategies. Green Chem. 2022, 24, 1390–1403. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.H.; Baek, J.; Kong, D.S.; Na, J.G.; Lee, J.; Sundstrom, E.; Park, S.; Kim, J.R. Small Current but Highly Productive Synthesis of 1,3-Propanediol from Glycerol by an Electrode-Driven Metabolic Shift in Klebsiella pneumoniae L17. ChemSusChem 2020, 13, 564–573. [Google Scholar] [CrossRef]
- Jensen, T.O.; Kvist, T.; Mikkelsen, M.J.; Westermann, P. Production of 1,3-PDO and butanol by a mutant strain of Clostridium pasteurianum with increased tolerance towards crude glycerol. AMB Express 2012, 2, 44. [Google Scholar] [CrossRef]
- Mu, Y.; Teng, H.; Zhang, D.-J.; Wang, W.; Xiu, Z.-L. Microbial production of 1,3-propanediol by Klebsiella pneumoniae using crude glycerol from biodiesel preparations. Biotechnol. Lett. 2006, 28, 1755–1759. [Google Scholar] [CrossRef]
- Asopa, R.P.; Ikram, M.M.; Saharan, V.K.J. Valorization of glycerol into 1,3-propanediol and organic acids using biocatalyst Saccharomyces cerevisiae . Bioresour. Technol. Rep. 2022, 18, 101084. [Google Scholar] [CrossRef]
- Kong, D.S.; Kim, C.; Song, Y.E.; Baek, J.; Im, H.S.; Kim, J.R. Zero-valent iron driven bioconversion of glycerol to 1,3-propanediol using Klebsiella pneumoniae L17. Process Biochem. 2021, 106, 158–162. [Google Scholar] [CrossRef]
- Zhou, S.; Lama, S.; Sankaranarayanan, M.; Park, S. Metabolic engineering of Pseudomonas denitrificans for the 1,3-propanediol production from glycerol. Bioresour. Technol. 2019, 292, 121933. [Google Scholar] [CrossRef]
- Wong, N.; Jantama, K. Engineering Escherichia coli for a high yield of 1,3-propanediol near the theoretical maximum through chromosomal integration and gene deletion. Appl. Microbiol. Biotechnol. 2022, 106, 2937–2951. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Ainala, S.K.; Oh, Y.K.; Jeon, B.H.; Park, S.; Kim, J.R. Metabolic flux change in Klebsiella pneumoniae L17 by anaerobic respiration in microbial fuel cell. Biotechnol. Bioproc. Eng. 2016, 21, 250–260. [Google Scholar] [CrossRef]
- Sakuntala, M.; Kim, J.R. Recent advances and challenges in the bioconversion of acetate to value-added chemicals. Bioresour. Technol. 2022, 364, 128064. [Google Scholar]
- Kim, M.; Li, S.; Song, Y.E.; Lee, D.-Y.; Kim, J.R. Electrode-attached cell-driven biogas upgrading of anaerobic digestion effluent CO2 to CH4 using a microbial electrosynthesis cell. Chem. Eng. J. 2022, 446, 137079. [Google Scholar] [CrossRef]
- Im, H.S.; Kim, C.; Song, Y.E.; Baek, J.; Im, C.H.; Kim, J.R. Isolation of novel CO converting microorganism using zero valent iron for a bioelectrochemical system (BES). Biotechnol. Bioproc. Eng. 2019, 24, 232–239. [Google Scholar] [CrossRef]
- Kong, D.S.; Park, E.J.; Mutyala, S.; Kim, M.; Cho, Y.; Oh, S.E.; Kim, C.; Kim, J.R. Bioconversion of Crude Glycerol into 1,3-Propanediol (1,3-PDO) with Bioelectrochemical System and Zero-Valent Iron Using Klebsiella pneumoniae L17. Energies 2021, 14, 6806. [Google Scholar] [CrossRef]
- Hidaka, T.; Tsuno, H.; Yagi, H.; Kosaka, Y. Fundamental analysis of real-time PCR quantification and modeling for thermophilic L-lactate fermentation by Bacillus coagulans from glucose. Biotechnol. Bioproc. Eng. 2012, 17, 290–297. [Google Scholar] [CrossRef]
- Liu, J.L.; Ren, X.M.; Li, C.Z.; Wang, M.D.; Li, H.; Yang, Q.H. Assembly of COFs layer and electron mediator on silica for visible light driven photocatalytic NADH regeneration. Appl. Catal. B-Environ. 2022, 310, 121314. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yun, H.; Park, S.; Lee, S.Y. MetaFluxNet: The management of metabolic reaction information and quantitative metabolic flux analysis. Bioinformatics 2003, 19, 2144–2146. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Park, S.J. Potential and limitations of Klebsiella pneumoniae as a microbial cell factory utilizing glycerol as the carbon source. Biotechnol. Adv. 2018, 36, 150–167. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Forage, R.; Lin, E. Glycerol kinase as a substitute for dihydroxyacetone kinase in a mutant of Klebsiella pneumoniae. J. Bacteriol. 1982, 152, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, T.; Pujilaksono, B.; Hallstrom, S.; Agren, J.; Svensson, J.E.; Johansson, L.G.; Halvarsson, M. An ESEM in situ investigation of the influence of H2O on iron oxidation at 500 degrees C. Corros. Sci. 2009, 51, 1914–1924. [Google Scholar] [CrossRef]
- Bertrand, N.; Desgranges, C.; Poquillon, D.; Lafont, M.-C.; Monceau, D.J. Iron oxidation at low temperature (260–500 °C) in air and the effect of water vapor. Oxid. Met. 2010, 73, 139–162. [Google Scholar] [CrossRef]
- Jonsson, T.; Pujilaksono, B.; Fuchs, A.; Svensson, J.E.; Johansson, L.G.; Halvarsson, M. The influence of H2O on iron oxidation at 600 C: A microstructural study. Mater. Sci. Forum 2008, 595, 1005–1012. [Google Scholar] [CrossRef]
- Ravi, S.; Karthikeyan, A.J. Magnetically tunable superparamagnetic cobalt doped iron oxide colloidal nanocluster. Adv. Mater. Lett. 2013, 4, 562–566. [Google Scholar] [CrossRef]
- Deutzmann, J.S.; Sahin, M.; Spormann, A.M. Extracellular enzymes facilitate electron uptake in biocorrosion and bioelectrosynthesis. mBio 2015, 6, e00496-15. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Xu, D.K.; Chen, C.F.; Li, X.G.; Jia, R.; Zhang, D.W.; Sand, W.; Wang, F.H.; Gu, T.Y. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: A review. J. Mater. Sci. Technol. 2018, 34, 1713–1718. [Google Scholar] [CrossRef]
- Mori, K.; Tsurumaru, H.; Harayama, S. Iron corrosion activity of anaerobic hydrogen-consuming microorganisms isolated from oil facilities. J. Biosci. Bioeng. 2010, 110, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.T.; Chang, W.W.; Cui, T.Y.; Qian, H.C.; Huang, L.Y.; Ma, L.W.; Hao, X.P.; Zhang, D.W. Microbiologically influenced corrosion inhibition of carbon steel via biomineralization induced by Shewanella putrefaciens . NPJ Mater. Degrad. 2021, 5, 59. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.S.; Kim, M.; Li, S.; Mutyala, S.; Jang, M.; Kim, C.; Kim, J.R. Bioconversion of Glycerol to 1,3-Propanediol Using Klebsiella pneumoniae L17 with the Microbially Influenced Corrosion of Zero-Valent Iron. Fermentation 2023, 9, 233. https://doi.org/10.3390/fermentation9030233
Kong DS, Kim M, Li S, Mutyala S, Jang M, Kim C, Kim JR. Bioconversion of Glycerol to 1,3-Propanediol Using Klebsiella pneumoniae L17 with the Microbially Influenced Corrosion of Zero-Valent Iron. Fermentation. 2023; 9(3):233. https://doi.org/10.3390/fermentation9030233
Chicago/Turabian StyleKong, Da Seul, Minsoo Kim, Shuewi Li, Sakuntala Mutyala, Min Jang, Changman Kim, and Jung Rae Kim. 2023. "Bioconversion of Glycerol to 1,3-Propanediol Using Klebsiella pneumoniae L17 with the Microbially Influenced Corrosion of Zero-Valent Iron" Fermentation 9, no. 3: 233. https://doi.org/10.3390/fermentation9030233
APA StyleKong, D. S., Kim, M., Li, S., Mutyala, S., Jang, M., Kim, C., & Kim, J. R. (2023). Bioconversion of Glycerol to 1,3-Propanediol Using Klebsiella pneumoniae L17 with the Microbially Influenced Corrosion of Zero-Valent Iron. Fermentation, 9(3), 233. https://doi.org/10.3390/fermentation9030233