Supplementing Proteolytic Enzymes Increased the In Vitro Nutrient Effective Degradability and Fermentation Characteristics of Pineapple Waste Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Chemical Composition Analysis
2.2. In Vitro Incubation to Measure Fermentation Characteristics
2.3. Estimation of Effective Degradability of Nutrients In Vitro
2.4. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of the PAS with or without PEs
3.2. Nutrient Disappearance and Effective Degradability of In Vitro-Incubated PAS with or without PE
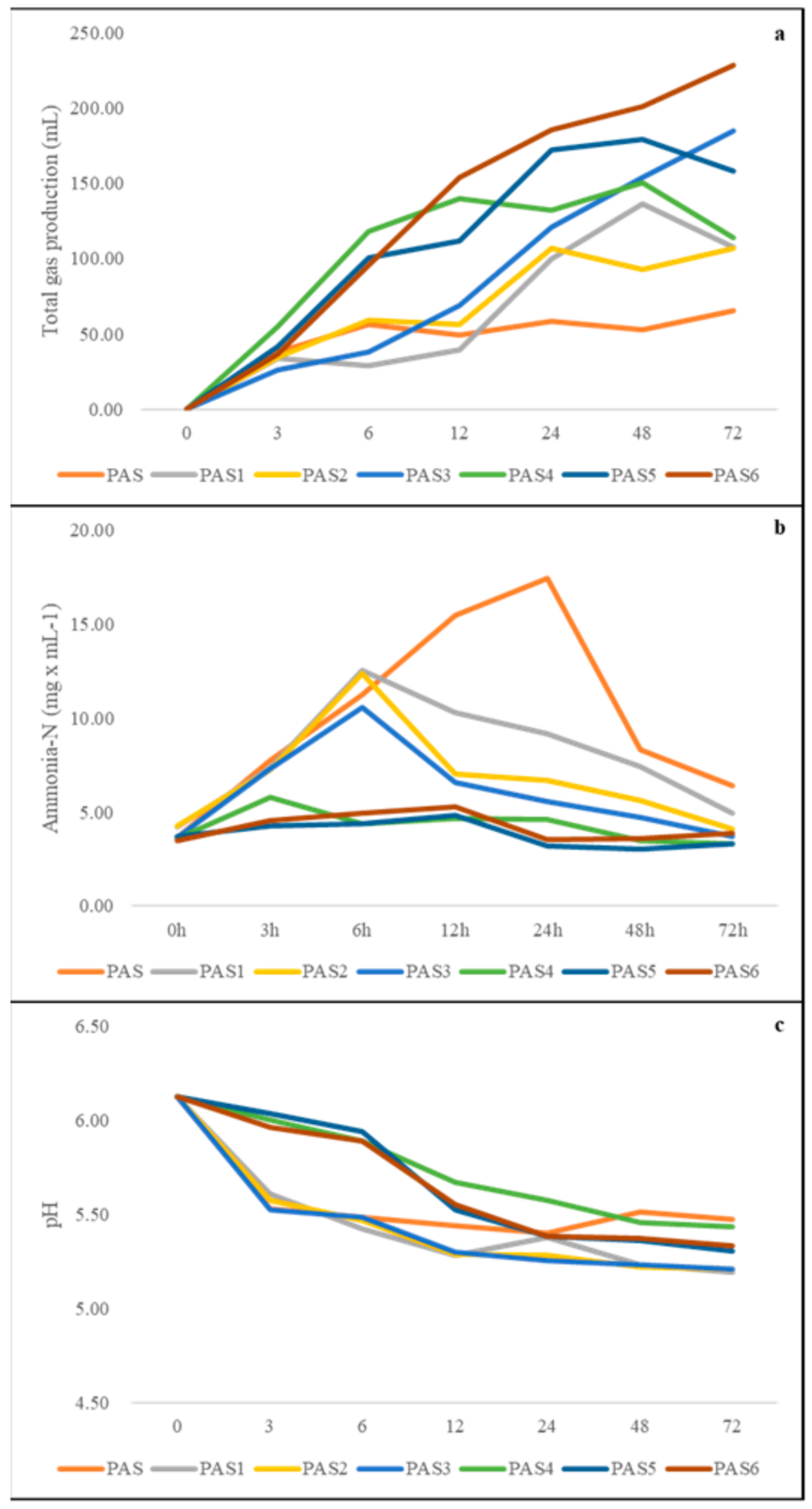
3.3. Fermentation Characteristics of In Vitro-Incubated PAS with or without PE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gowda, N.K.; Vallesha, N.C.; Awachat, V.B.; Anandan, S.; Pal, D.T.; Prasad, C.S. Study on evaluation of silage from pineapple (Ananas comosus) fruit residue as livestock feed. Trop Anim. Health Prod. 2015, 47, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Rawdkuen, S. Application of Bromelain Extract for Muscle Foods Tenderization. Food Nutr. Sci. 2011, 2, 393–401. [Google Scholar] [CrossRef]
- Azizan, A.; Xin, L.A.; Abdul Hamid, N.A.; Maulidiani, M.; Mediani, A.; Abdul Ghafar, S.Z.; Zulaikha Zolkeflee, N.K.; Abas, F. Potentially Bioactive Metabolites from Pineapple Waste Extracts and Their Antioxidant and alpha-Glucosidase Inhibitory Activities by (1)H NMR. Foods 2020, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Aruna, T. Production of value-added product from pineapple peels using solid state fermentation. Innov. Food Sci. Emerg. Technol. 2019, 57, 102193. [Google Scholar] [CrossRef]
- Müller, Z. Feeding potential of pineapple waste for [beef] cattle. World Anim. Rev. 1978, 25, 25–29. [Google Scholar]
- Choi, Y.; Park, K.; Lee, S.; Na, Y. Determination of in situ degradation parameters and feeding level of pineapple (Ananas comosus L.) cannery by-product to Hanwoo steers. Anim. Biosci. 2021, 34, 85–92. [Google Scholar] [CrossRef]
- Saenphoom, P.; Chimtong, S.; Chaokaur, A.; Kutdaeng, D.; Chanprecha, T.; Seesawhea, Y. Nutritive value, digestibility and gas production of fermented sugar palm peel with pineapple peel. Sci. Eng. Health Stud. 2016, 28, 32–37. [Google Scholar] [CrossRef]
- Colombatto, D.; Beauchemin, K.A. A protease additive increases fermentation of alfalfa diets by mixed ruminal microorganisms in vitro. J. Anim. Sci. 2009, 87, 1097–1105. [Google Scholar] [CrossRef]
- Eun, J.S.; Beauchemin, K.A. Effects of a proteolytic feed enzyme on intake, digestion, ruminal fermentation, and milk production. J. Dairy Sci. 2005, 88, 2140–2153. [Google Scholar] [CrossRef]
- Zhu, W.Y.; Kingston-Smith, A.H.; Troncoso, D.; Merry, R.J.; Davies, D.R.; Pichard, G.; Theodorou, M.K. Evidence of a role for plant proteases in the degradation of herbage protein in the rumen of grazing cattle. J. Dairy Sci. 1999, 82, 2651–2658. [Google Scholar] [CrossRef]
- Tománková, O.; Kopečný, J. Prediction of feed protein degradation in the rumen with bromelain. Anim. Feed Sci. Technol. 1995, 53, 71–80. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Arlington, TX, USA, 1990. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and some Applications); US Agricultural Research Service: Washington, DC, USA, 1970. [Google Scholar]
- McDougall, E. Studies on ruminant saliva. 1. The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99. [Google Scholar] [CrossRef]
- Fawcett, J.; Scott, J. A rapid and precise method for the determination of urea. J. Clin. Pathol. 1960, 13, 156–159. [Google Scholar] [CrossRef]
- Ørskov, E.-R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Cody, R. An Introduction to SAS University Edition; SAS Institute: Cary, NC, USA, 2018. [Google Scholar]
- Kyawt, Y.Y.; Win, K.S.; Mu, K.S.; Aung, A.; Aung, M. Feeding pineapple waste silage as roughage source improved the nutrient intakes, energy status and growth performances of growing Myanmar local cattle. J. Adv. Vet. Anim. Res. 2020, 7, 436–441. [Google Scholar] [CrossRef]
- Ki, K.S.; Park, S.B.; Lim, D.H.; Seo, S. Evaluation of the nutritional value of locally produced forage in Korea using chemical analysis and in vitro ruminal fermentation. Asian-Australas J. Anim. Sci. 2017, 30, 355–362. [Google Scholar] [CrossRef]
- Braga, A.P.; Amâncio, A.V.d.A.F.; Gonçalves, J.D.S.; Assis, L.C.d.S.L.C.; Souza, C.M.S.; Maia, I.S.A.d.S.; Guerra, D.G.F. Ruminal degradability of agro-industrial fruit residues. Semin. Ciências Agrárias 2016, 37, 279–292. [Google Scholar] [CrossRef]
- Vera, J.M. Assessments of an Exogenous Proteolytic Enzyme in Beef Steer Diets to Improve Growth Performance and Ruminal Fermentation. Master’s Thesis, Utah State University, Logan, UT, USA, 2012. [Google Scholar]
- Colombatto, D.; Hervas, G.; Yang, W.Z.; Beauchemin, K.A. Effects of enzyme supplementation of a total mixed ration on microbial fermentation in continuous culture, maintained at high and low pH. J. Anim. Sci. 2003, 81, 2617–2627. [Google Scholar] [CrossRef]
- Colombatto, D.; Morgavi, D.P.; Furtado, A.F.; Beauchemin, K.A. Screening of exogenous enzymes for ruminant diets: Relationship between biochemical characteristics and in vitro ruminal degradation. J. Anim. Sci. 2003, 81, 2628–2638. [Google Scholar] [CrossRef]
- Eun, J.-S.; Beauchemin, K. Exogenous proteolytic enzymes improve in vitro degradation of alfalfa hay but not alfalfa silage. In Journal of Dairy Science; AMER DAIRY SCIENCE ASSOC 1111 N DUNLAP AVE: Savoy, IL, USA, 2005; Volume 88, p. 316. [Google Scholar]
- Mertens, D.R. Creating a system for meeting the fiber requirements of dairy cows. J. Dairy Sci. 1997, 80, 1463–1481. [Google Scholar] [CrossRef]
- Erfle, J.D.; Boila, R.J.; Teather, R.M.; Mahadevan, S.; Sauer, F.D. Effect of pH on fermentation characteristics and protein degradation by rumen microorganisms in vitro. J. Dairy Sci. 1982, 65, 1457–1464. [Google Scholar] [CrossRef]
- Jin, G.L.; Shinekhuu, J.; Qin, W.-Z.; Kim, J.-K.; Ju, J.-K.; Suh, S.-W.; Song, M.-K. Effect of Protein Fractionation and Buffer Solubility of Forage Sources on In Vitro Fermentation Characteristics, Degradability and Gas Production. J. Korean Soc. Grassl. Forage Sci. 2012, 32, 59–74. [Google Scholar] [CrossRef]
- Satter, L.D.; Slyter, L.L. Effect of ammonia concentration of rumen microbial protein production in vitro. Br. J. Nutr. 1974, 32, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Grummer, R.R.; Clark, J.H.; Davis, C.L.; Murphy, M.R. Effect of ruminal ammonia-nitrogen concentration on protein degradation in situ. J. Dairy Sci. 1984, 67, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.J. Why don’t ruminal bacteria digest cellulose faster? J. Dairy Sci. 1996, 79, 1496–1502. [Google Scholar] [CrossRef]
| Chemical Composition | PAS | PAS1 | PAS2 | PAS3 | PAS4 | PAS5 | PAS6 |
|---|---|---|---|---|---|---|---|
| MC, % | 5.70 ± 0.36 a | 3.66 ± 0.16 b | 3.94 ± 0.29 b | 3.70 ± 0.19 b | 3.75 ± 0.28 b | 4.03 ± 0.18 c | 3.80 ± 0.34 b |
| NDF, % | 63.66 ± 0.37 | 62.32 ± 2.47 | 63.94 ± 0.35 | 62.34 ± 0.53 | 63.23 ± 0.09 | 63.41 ± 0.65 | 62.74 ± 0.93 |
| ADF, % | 34.78 ± 1.51 a | 38.79 ± 0.28 b | 38.48 ± 0.83 b | 37.59 ± 0.95 b | 34.29 ± 0.48 a | 35.09 ± 0.26 a | 37.45 ± 0.10 b |
| CP, % | 14.18 ± 0.11 a | 14.92 ± 0.35 b | 14.90 ± 0.50 b | 14.10 ± 1.90 b | 13.12 ± 2.10 b | 13.97 ± 0.84 b | 13.79 ± 0.83 b |
| Ash, % | 7.71 ± 0.21 a | 7.85 ± 0.16 b | 7.76 ± 0.24 b | 7.95 ± 0.12 b | 8.10 ± 0.27 c | 8.12 ± 0.10 c | 7.95 ± 0.16 b |
| Parameters | PAS | PAS1 | PAS2 | PAS3 | PAS4 | PAS5 | PAS6 |
|---|---|---|---|---|---|---|---|
| 0 h | 30.40 | 30.69 | 32.85 | 32.28 | 31.17 | 29.13 | 29.11 |
| 3 h | 36.68 | 45.52 | 39.34 | 45.44 | 45.06 | 47.54 | 45.75 |
| 6 h | 37.64 | 47.07 | 40.81 | 39.55 | 46.14 | 42.60 | 39.11 |
| 12 h | 40.94 | 44.29 | 46.12 | 47.26 | 52.86 | 52.96 | 54.81 |
| 24 h | 39.05 | 59.38 | 58.69 | 61.80 | 64.91 | 60.98 | 63.17 |
| 48 h | 39.95 | 67.29 | 72.70 | 67.44 | 66.55 | 68.86 | 65.12 |
| 72 h | 39.23 | 71.85 | 71.87 | 71.97 | 70.83 | 66.29 | 70.37 |
| a, % | 31.78 ± 1.39 c,d | 33.55 ± 0.54 a,b | 31.53 ± 1.77 c,d | 33.97 ± 0.58 a | 33.44 ± 0.61 a,b | 32.58 ± 0.57 b,c | 31.35 ± 0.66 d |
| b, % | 26.85 ± 2.69 d | 37.68 ± 2.41 b | 41.48 ± 3.30 a | 37.37 ± 3.52 b | 34.88 ± 2.28 c | 32.57 ± 2.47 c | 35.28 ± 2.33 b,c |
| c, %/h | 0.45 ± 0.22 a | 0.07 ± 0.05 b | 0.10 ± 0.12 b | 0.06 ± 0.06 b | 0.29 ± 0.40 a,b | 0.32 ± 0.43 a,b | 0.29 ± 0.41 a,b |
| EDDM, % | 38.21 ± 1.32 b | 60.04 ± 3.83 a | 60.43 ± 3.55 a | 58.57 ± 6.94 a | 61.56 ± 2.82 a | 59.75 ± 2.24 a | 60.44 ± 2.94 a |
| Parameters | PAS | PAS1 | PAS2 | PAS3 | PAS4 | PAS5 | PAS6 |
|---|---|---|---|---|---|---|---|
| 0 h | 28.81 | 27.05 | 27.34 | 30.56 | 23.10 | 21.69 | 24.58 |
| 3 h | 21.53 | 39.61 | 37.08 | 35.49 | 49.13 | 41.92 | 40.09 |
| 6 h | 25.32 | 41.38 | 38.44 | 51.48 | 36.27 | 37.21 | 36.28 |
| 12 h | 21.87 | 44.62 | 43.89 | 44.78 | 48.41 | 51.55 | 53.2 |
| 24 h | 23.01 | 55.42 | 53.37 | 56.46 | 62.06 | 60.61 | 70.59 |
| 48 h | 23.31 | 64.52 | 65.36 | 63.82 | 62.67 | 64.99 | 74.28 |
| 72 h | 26.25 | 70.56 | 69.37 | 70.97 | 67.24 | 62.13 | 67.81 |
| a, % | 28.67 ± 0.82 a | 36.75 ± 0.30 d | 29.74 ± 2.10 b,c | 33.41 ± 0.4 d | 28.58 ± 1.07 b,c | 24.30 ± 0.52 b | 24.68 ± 0.72 b |
| b, % | 21.44 ± 0.37 a | 38.54 ± 91.12 b | 43.14 ± 3.35 c | 37.44 ± 1.30 b | 37.14 ± 2.48 b | 39.31 ± 4.12 b | 47.88 ± 4.04 c |
| c, %/h | 0.38 ± 0.14 d | 0.001 ± 0.00 a | 0.04 ± 0.15 b | 0.04 ± 0.02 b | 0.08 ± 0.45 b,c | 0.10 ± 0.77 c | 0.08 ± 0.81 b,c |
| EDOM, % | 23.60 ± 0.16 a | 57.49 ± 0.95 c,d | 55.80 ± 4.66 b,c | 57.64 ± 2.64 c,d | 57.25 ± 3.01 c,d | 56.34 ± 1.59 b | 61.95 ± 2.29 d |
| Parameters | PAS | PAS1 | PAS2 | PAS3 | PAS4 | PAS5 | PAS6 |
|---|---|---|---|---|---|---|---|
| 0 h | 24.89 | 31.58 | 24.96 | 25.83 | 27.81 | 26.71 | 29.17 |
| 3 h | 43.07 | 49.15 | 47.33 | 44.76 | 43.92 | 42.85 | 55.19 |
| 6 h | 46.46 | 56.43 | 51.94 | 49.64 | 41.14 | 43.80 | 60.88 |
| 12 h | 46.92 | 55.93 | 56.3 | 56.05 | 57.5 | 56.57 | 62.89 |
| 24 h | 46.83 | 63.14 | 60.38 | 61.66 | 57.19 | 66.30 | 79.61 |
| 48 h | 45.83 | 59.44 | 62.97 | 62.12 | 64.29 | 67.91 | 70.65 |
| 72 h | 44.80 | 69.59 | 69.32 | 70.85 | 68.68 | 69.68 | 68.94 |
| a, % | 29.81 ± 4.40 a | 34.13 ± 2.86 b | 28.67 ± 3.23 b | 29.13 ± 2.16 b | 30.56 ± 0.75 b | 28.92 ± 0.74 b | 33.33 ± 4.63 a |
| b, % | 13.11 ± 7.44 c | 28.13 ± 4.09 b | 33.78 ± 4.69 a | 35.01 ± 3.48 a | 34.09 ± 2.19 a | 36.84 ± 4.23 a | 28.44 ± 9.61 b |
| c, %/h | 2.41 ± 0.24 b | 0.35 ± 0.34 c | 0.42 ± 0.48 c | 0.27 ± 0.40 c | 0.24 ± 0.35 c | 0.49 ± 0.68 c | 3.35 ± 0.16 a |
| EDNDF, % | 42.80 ± 3.83 c | 59.93 ± 0.55 a,b | 59.53 ± 0.60 a,b | 59.82 ± 0.81 a,b | 58.87 ± 2.26 b | 60.76 ± 1.50 a,b | 61.58 ± 5.86 a |
| Parameters | PAS | PAS1 | PAS2 | PAS3 | PAS4 | PAS5 | PAS6 |
|---|---|---|---|---|---|---|---|
| 0 h | 31.39 | 30.46 | 33.06 | 30.01 | 33.9 | 34.65 | 33.54 |
| 3 h | 32.13 | 35.25 | 33.73 | 35.11 | 43.23 | 43.55 | 45.65 |
| 6 h | 30.36 | 42.92 | 45.88 | 49.83 | 45.33 | 47.91 | 47.89 |
| 12 h | 33.06 | 48.87 | 51.23 | 56.32 | 55.72 | 51.46 | 59.88 |
| 24 h | 36.1 | 52.06 | 61.38 | 73.82 | 67.09 | 69.35 | 66.22 |
| 48 h | 35.06 | 58.36 | 62.54 | 73.89 | 71.78 | 77.43 | 70.85 |
| 72 h | 35.71 | 68.4 | 66.64 | 71.87 | 79.02 | 77.87 | 78.56 |
| a, % | 30.77 ± 0.05 d | 32.34 ± 0.17 c | 31.15 ± 0.49 d | 28.21 ± 0.39 e | 34.30 ± 1.11 b | 34.70 ± 1.24 b | 35.29 ± 0.71 a |
| b, % | 31.54 ± 10.02 c | 34.54 ± 0.38 b,c | 32.43 ± 2.32 c | 41.03 ± 5.24 a | 41.97 ± 1.88 a | 43.61 ± 2.16 a | 39.07 ± 1.76 a,b |
| c, %/h | 0.06 ± 0.03 b | 0.04 ± 0.01 b | 0.29 ± 0.41 a,b | 0.51 ± 0.76 a | 0.13 ± 0.19 b | 0.13 ± 0.19 b | 0.20 ± 0.29 a,b |
| EDADF, % | 53.29 ± 7.26 c | 54.85 ± 0.86 b,c | 57.56 ± 3.42 b | 63.33 ± 2.47 a | 65.94 ± 4.19 a | 66.98 ± 4.76 a | 66.29 ± 3.56 a |
| Parameters | PAS | PAS1 | PAS2 | PAS3 | PAS4 | PAS5 | PAS6 |
|---|---|---|---|---|---|---|---|
| 0 h | 30.58 | 32.66 | 33.82 | 35.24 | 30.32 | 31.14 | 31.75 |
| 3 h | 33.66 | 32.43 | 35.08 | 35.61 | 35.88 | 35.17 | 39.29 |
| 6 h | 47.86 | 52.37 | 49.75 | 41.16 | 42.66 | 45.07 | 44.55 |
| 12 h | 49.12 | 56.04 | 51.42 | 53.06 | 55.27 | 44.18 | 53.74 |
| 24 h | 55.33 | 63.32 | 63.18 | 66.96 | 62.56 | 59.55 | 64.93 |
| 48 h | 49.41 | 64.75 | 78.02 | 83.37 | 65.71 | 70.22 | 68.79 |
| 72 h | 52.26 | 84.71 | 82.47 | 82.96 | 82.25 | 75.01 | 77.58 |
| a, % | 30.55 ± 1.69 c,d | 33.36 ± 0.53 a | 32.08 ± 2.31 b | 31.87 ± 0.71 b,c | 31.27 ± 0.54 b,c | 29.86 ± 2.25 d | 31.84 ± 1.07 b,c |
| b, % | 22.02 ± 1.47 f | 45.58 ± 1.66 c,d | 50.44 ± 3.18 b | 55.62 ± 3.10 a | 46.83 ± 2.27 c | 44.04 ± 3.73 d,e | 42.31 ± 1.74 e |
| c, %/h | 0.38 ± 0.46 a | 0.05 ± 0.04 b | 0.12 ± 0.21 b | 0.04 ± 0.01 b | 0.06 ± 0.05 b | 0.12 ± 0.17 b | 0.13 ± 0.19 b |
| EDCP, % | 50.46 ± 1.04 d | 64.10 ± 3.98 a,b,c | 66.26 ± 6.29 a,b | 67.21 ± 3.81 a | 62.44 ± 5.19 b,c | 60.73 ± 5.82 c | 63.86 ± 4.08 a,b,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogoy, K.M.C.; Lee, J.I.; Yu, J.; Sang, J.I.; Hong, H.K.; Ji, Y.G.; Li, X.Z.; Choi, S.H. Supplementing Proteolytic Enzymes Increased the In Vitro Nutrient Effective Degradability and Fermentation Characteristics of Pineapple Waste Silage. Fermentation 2023, 9, 218. https://doi.org/10.3390/fermentation9030218
Nogoy KMC, Lee JI, Yu J, Sang JI, Hong HK, Ji YG, Li XZ, Choi SH. Supplementing Proteolytic Enzymes Increased the In Vitro Nutrient Effective Degradability and Fermentation Characteristics of Pineapple Waste Silage. Fermentation. 2023; 9(3):218. https://doi.org/10.3390/fermentation9030218
Chicago/Turabian StyleNogoy, Kim Margarette Corpuz, Jae Ik Lee, Jia Yu, Jung In Sang, Hyoung Ki Hong, Yoon Gwang Ji, Xiang Zi Li, and Seong Ho Choi. 2023. "Supplementing Proteolytic Enzymes Increased the In Vitro Nutrient Effective Degradability and Fermentation Characteristics of Pineapple Waste Silage" Fermentation 9, no. 3: 218. https://doi.org/10.3390/fermentation9030218
APA StyleNogoy, K. M. C., Lee, J. I., Yu, J., Sang, J. I., Hong, H. K., Ji, Y. G., Li, X. Z., & Choi, S. H. (2023). Supplementing Proteolytic Enzymes Increased the In Vitro Nutrient Effective Degradability and Fermentation Characteristics of Pineapple Waste Silage. Fermentation, 9(3), 218. https://doi.org/10.3390/fermentation9030218






