Abstract
Feathers are the most prevalent agricultural waste generated by chicken farms, polluting the environment and wasting protein resources as a result of the accumulation of large amounts of feathers. Therefore, keratinase-producing microorganisms represent a promising potential technique for the degradation of feather waste. Streptomyces netropsis A-ICA and Bacillus subtilis ALICA, previously isolated from the rhizosphere of desert plants (Larrea tridentata and Prosopis juliflora) respectively, were assessed for their feather-degradation ability. Keratinase activity was optimized using various parameters, including incubation time, pH, temperature, and feather concentration. The maximum keratinase activity of S. netropsis A-ICA and B. subtilis ALICA (113.6 ± 5.1 and 135.6 ± 4.1 U/mL) was obtained at the 5th and 3rd day of incubation with initial pH of 7.0 and 7.5 at 25 and 30 °C, and 1% (w/v) of chicken feather, respectively. Under the optimized conditions, the concentration of soluble protein in the feather hydrolysate reached 423.3 ± 25 and 565.3 ± 7.7 µg/mL, with feathers weight loss of 84 ± 2 and 86± 1.5% by S. netropsis A-ICA and B. subtilis ALICA, respectively. The highest disulphide bond reductase activity reached 10.7 ± 0.4 and 10.96 ± 1.1 U/mL, after five and three days of inoculation with S. netropsis A-ICA and B. subtilis ALICA, respectively. Furthermore, the antioxidant activity of feather protein hydrolysate obtained by S. netropsis A-ICA and B. subtilis ALICA was evaluated using DPPH radical-scavenging activity, which exhibited a significant antioxidant potential with an IC50 value of 0.8 and 0.6 mg/mL. The 3D models of detected keratinases in both strains showed high similarity with subtilisin family. Further, the docking results clarified the importance of GSG and VVVFTP domains in B. subtilis and beta-keratin, respectively. The present study revealed the keratinolytic potential of S. netropsis A-ICA and B. subtilis ALICA in chicken feather degradation, which have potential application value and may be exploited as supplementary protein and antioxidant in animal feed formulations.
1. Introduction
Feathers are the principal waste by-product of the poultry industry, and annually millions of tons of feather waste are produced globally [1]. Chicken feathers contain approximately 90% crude protein, amino acids, high-value elements, growth factors, and vitamins [2]. A small proportion of these feathers is used in insulating materials, and down products, while the majority are burned or discarded as waste [2]. Due to the presence of keratin as the main constituent, chicken feathers exhibit extreme rigidity and resistance to chemical, physical, and biological agents as well as resistance to proteolysis by common proteases, such as pepsin, trypsin, and papain, thus slowing the process of feather degradation in the nature [3,4]. Chicken feathers mainly consist of β-keratin (over 90%), which is a highly stable and insoluble fibrous structural protein that represents a valuable source of amino acids, which are exploited in several applications such as animal feed, and fertilizers [5]. Keratin is an insoluble protein containing hydrogen and disulphide bonds that make it stable, rigid, and difficult to degrade [6]. Disulphide bonds can form crosslinks between protein peptide chains, resulting in a dense polymeric structure in combination with hydrogen bonding and hydrophobic forces, and this gives keratin quite good stability, rigidity, and strong mechanical resistance [2].
Traditional disposal of keratinous waste by burning has a harmful effect on environment, as well as is restricted or prohibited in several countries, thus, the developing of alternative techniques for this waste disposal is a quite important [7]. The conventional hydrothermal and chemical (acid and alkali) processes currently utilized in the industry of feather meal are energy-intensive and produce low soluble and a poorly digestible protein. The high pressure and temperatures used in these methods lead to product damage and release significant amounts of waste gases, such as ammonia and sulfur dioxide, making them polluting and unsustainable [2,8]. Furthermore, these techniques result in the loss of several thermo-sensitive amino acids, e.g., lysine, methionine, and tryptophan, as well as the addition of non-nutritive amino acids, such as lysinoalanine and lanthionine [9]. Therefore, the economic degradation of feathers is of great interest among researchers. Among the various techniques, the biodegradation of keratin by microbial keratinolytic proteases is viable as an effective alternative for physical and chemical approaches, due to their rapid growth, availability, high yield, cost, less space requirement, and sustainability [10]. The biodegradation of the keratinous substances by microorganisms is an eco-friendly and inexpensive approach for the effective processing of keratinous wastes that entails environmental safety, waste management, and resource generation, e.g., of peptides and amino acids [11]. Additionally, the enzymatic hydrolysis of feathers is able to protect the majority of the amino acids that are typically destroyed by physical and chemical treatments and improve their nutritional properties [5]. Several microorganisms, including fungi, Actinomycetes, and bacteria, can degrade the keratinous substrates [12]. Among bacteria, Bacillus spp., e.g., B. subtilis, B. cereus, and B. licheniformis, among others are effective for degrading feathers due to their fast growth rate and safety [13]. Streptomyces sp. is one of the most promising producers of extracellular keratinolytic enzymes [14].
Keratinases are proteolytic enzymes that can hydrolyze keratin and are exploited in various industries, such as detergent and leather industries, and in animal feed additives [5]. Keratinase activity involves disulphide reductase and polypeptide hydrolase. The main mechanisms of keratin degradation are denaturation, hydrolysis, and transamination. Firstly, the strongly bound structure is changed to its degenerative state by the reduction of the disulphide link in keratin from cystine (-S-S-) to cysteine (-SH). The subsequent step is keratin hydrolysis into polypeptides, oligopeptides, and free amino acids. Finally, the keratin is completely degraded into free amino acids and sulphides [6]. Thus, the biodegradation of chicken feathers using keratinolytic microorganisms presented a practical alternative for the efficient bioconversion of keratinous wastes into animal feed [15]. Natural antioxidants are among the many dietary components that are crucial for animal health and protect the body against free radicals. Feather wastes are an attractive source of proteins and antioxidant peptides [2,15].
Due to the promising applications of keratinase in various industries, it became crucial to investigate various conditions for bacterial diversity with the potential to develop novel keratinases [16]. In the present study, we have used two strains previously identified as Streptomyces netropsis A-ICA and Bacillus subtilis ALICA, which have been isolated from the rhizosphere of Larrea tridentata and Prosopis juliflora respectively [17,18]. Therefore, this study was conducted to evaluate the keratinolytic potentials of these strains using chicken feathers as a sole carbon and nitrogen source. The fermentation conditions were optimized for maximum keratinase production. In addition, the potential of keratinase-producing strains to degrade chicken feathers and produce antioxidant hydrolysate was assessed under optimized conditions. Finally, bioinformatic analysis, including proteases detection, 3d modelling of potential keratinases, as well as protein–protein interaction simulation, have been performed to define the hot spots either in beta-keratin or detected keratinases.
2. Materials and Methods
2.1. Microorganisms
S. netropsis strain A-ICA and B. subtilis strain ALICA were previously isolated as plant growth promoting and antagonistic strains from rhizosphere of L. tridentata and P. juliflora respectively, from the desert region of Baja California, Mexico. These strains were identified based on 16S rDNA sequences and submitted to the GenBank with accession number MN535765 and KX137176 respectively [17,18].
2.2. Poultry Feathers and Chemical Materials Used in This Study
Chicken feathers were collected from the chicken slaughtering market and thoroughly cleaned by washing with tap water, then with distilled water. The clean feathers were dried at 55 °C for 2 days, and then feathers were cut into pieces of 2–3 cm and stored until used.
The chemical materials used in this study include casein, potato dextrose media, CaCl2, MgSO4·7 H2O, K2HPO4, KH2PO4, NaH2PO4, Na2HPO4, Trichloroacetic acid, Na2CO3, Folin–Ciocalteu’s phenol reagent, tyrosine, NaOH, 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and methanol.
2.3. Primary Detecting of Keratinase Activity
The keratinolytic activity of both S. netropsis strain A-ICA and B. subtilis strain ALICA was qualitatively tested on casein agar plates containing 2% (w/v) casein. S. netropsis A-ICA was grown in 50 mL potato dextrose broth at 30 °C for one week at 120 rpm. Hence, 5 µL of spore suspension was spot inoculated in the center of casein plates, while B. subtilis strain ALICA was directly streaked on casein agar plates. The plates were incubated at 33 ± 2 °C for 72 h, and the keratinolytic activity was observed by the appearance of a clear hydrolysis zone around the colony after the incubation time [13].
2.4. Optimization of Culture Conditions and Quantitative Assay of Keratinase Activity
Culture conditions: 2 mL of S. netropsis A-ICA (106 CFU/mL) and B. subtilis ALICA (108 CFU/mL) were inoculated separately in 500 mL Erlenmeyer flask containing 200 mL of keratinase production media (Components g/L): CaCl2 0.22, MgSO4·7 H2O 0.2, K2HPO4 0.3, KH2PO4 0.4, poultry feather 5) [19]. Several culture conditions were tuned to get the most keratinase possible from S. netropsis A-ICA and B. subtilis ALICA as follows: (a) Incubation period: To assess the effect of incubation period on keratinase production, the inoculated media (pH = 7) was incubated at 30 °C and 120 rpm for 10 days. Every 24 h, 2-mL samples were taken and the microbial growth removed by centrifugation of the culture at 12,000 rpm and 4 °C for 8 min, and the supernatant was used as a crude enzyme solution for keratinase assay. (b) pH: To check the impact of initial pH on keratinase activity, S. netropsis A-ICA and B. subtilis ALICA were inoculated in basal salt medium containing 1% feathers with different pH 6.0, 6.5, 7, 7.5, 8, 8.5, and 9.0 for 6 days at 30 °C and 120 rpm. Keratinase activity was estimated using cell free supernatant. (c) Temperature: For determining the optimum temperature for keratinase production and feather degradation by S. netropsis A-ICA and B. subtilis ALICA, these strains were grown in basal medium adjusted with 1% feathers, pH = 7, for 6 days at diverse temperatures ranging from 20 to 40 °C with 5 °C interval. After incubation, the supernatant was used for keratinase activity estimation; (d) feather concentration: The effect of feather concentration on keratinase production was studied by the inoculation of microorganisms in the basal medium prepared with different feather concentrations (0.5, 1, 1.5, 2, and 2.5%). (e) Keratinase assay: Casein solution 2% (w/v) was prepared in 50 mM phosphate buffer (pH 7) and used as substrate. Equal volumes (1 mL) of both crude enzyme and substrate solution were mixed and incubated for 20 min at 40 °C in a water bath. Then, 2 mL of 0.4 M Trichloroacetic acid was added to the mixture for terminating the reaction, and kept at room temperature for 30 min. The mixture was centrifuged for 10 min at 12,000 rpm and 4 °C to remove the precipitated proteins, and finally 0.5 mL of supernatant, 2.5 mL of 0.4 M Na2CO3 and 0.75 mL of Folin–Ciocalteu’s phenol reagent: water (1:3 v/v) were mixed and incubated under dark condition for 30 min at room temperature. The absorbance of the developed color was measured using UV–vis spectroscopy (Thermo Scientific BioMate 3 Spectrophotometer, Waltham, MA, USA) at 660 nm, and the optical density of the samples was compared against tyrosine standard curve. The required amount of the enzyme to liberate 1 µg of tyrosine per minute under the standard assay conditions was defined as one unit of keratinase activity [13].
2.5. Feathers Degradation under Optimized Fermenting Conditions
The feather degradation experiments were verified under the optimized conditions in 2-L Erlenmeyer flasks containing 1 L of the basal media supplemented by 1% (w/v) of chicken feather waste. The pH media was adjusted to 7 and 7.5 for S. netropsis A-ICA and B. subtilis ALICA, which were inoculated separately and incubated at 25 and 30 °C for 10 days, respectively.
At the end of incubation period, the feathers degradation rate (% of substrate weight loss) was calculated using the difference between the dry weight of pre- and post-decomposition [2]. Unhydrolyzed feathers were removed from the hydrolysate by passing it through Whatman No. 1 filter paper. The feathers were then thoroughly washed with deionized water to remove any soluble materials and microorganisms, and then dried for 24 h at 60 °C. The formula used to determine the feather degradation rate (DR) is as follows:
where A represents the dry weight of the feathers prior to decomposition and B represents the dry weight of the feathers following decomposition. Total protein of the supernatant has been estimated after 1, 3, 5, 7, and 9 days of incubation according to Bradford method [20] and using bovine serum albumin standard curve.
(DR) = ((A − B)/A) × 100
2.6. Disulphide Bond Reductase Activity and Keratinase Activity under Optimized Conditions
The reductase activity of disulphide bond was measured spectrophotometrically at 412 nm in the supernatant by evaluating the yellow-colored sulphide generated upon reduction of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) to confirm the occurrence of thiolysis [21]. Briefly, 1 mL of DTNB was mixed with 0.5 mL of supernatant, and the mixture was left at room temperature for 5 min to develop the color. The absorbance of yellow 2-nitro-5-thiobenzoic acid (TNB) generated from the reduction of DTNB was measured by a spectrophotometer at 412 nm. Under the given conditions, 0.01 increase in absorbance was expressed as one unit of disulphide bond reductase activity [13]. Keratinase assay was measured as described above.
2.7. Antioxidant Activity of Chicken Feather Hydrolysate
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging activity of the feather hydrolysate was estimated. The mixture of 1.9 mL of DPPH in methanol and 0.1 mL of hydrolysate sample at several concentrations (0.0, 0.1, 0.5, 1.5, 2.0, and 2.5 mg/mL) was incubated in the darkness at room temperature for 60 min. The scavenging activity of DPPH (absorbance decrease) was measured by UV–visible spectrophotometer at 517 nm [15]. The % inhibition of the DPPH radical (scavenging activity) was determined using the following formula:
% of scavenging activity = ((Control OD − Sample OD)/(Control OD)) × 100
The graph displaying the percentage of inhibition against the concentration of the feather hydrolysate was used to calculate the sample concentration that provides 50% inhibition (IC50).
2.8. Bioinformatics Analysis for Keratinases in Studied Strains
The complete genomes of S. netropsis and B. subtilis (strain 168) were downloaded from Uniprot (Release 2022_04) [22]. Further, 14 different protease families were retrieved from MEROPS database (Release 12.1) [23]. The Peptidase_S8 (PF00082.24), the characteristic profile hidden Markov model (pHMM) of keratinases, and the 3D structure of β-keratin (PF02422) were downloaded from InterPro [24].
To detect all the existent proteases of studied strains, a BLAST search was performed for the complete genomes of S. netropsis and B. subtilis (strain 168) against the 14 families of MEROPS database. An E-value of 0.0001 was set as the cutoff value. Moreover, we assigned the existed keratinases in both strains using HMMER package [25] by running hmmsearch of the PF00082.24 against the two studied genomes. Finally, the redundancy between the detected keratinases was reduced to 30% using MMseqs2 [26].
SWISS-MODEL server [27] was used to predict the 3D structure of the keratinase hits in both strains. For every hit, a protein–protein interaction was executed with the 3D model of β-keratin (PF02422) via ClusPro server [28].
2.9. Statistical Analysis
One-way analysis of variance (ANOVA) was used to conduct statistical analyses of all data using PASW statistics 21.0. (IBM Inc., Chicago, IL, USA). Tukey’s honestly significant difference (HSD) was used to compare the different means of traits for different treatments, and all results were considered significant at p ≤ 0.05. All data were expressed as mean of triplicates ± standard deviation.
3. Results and Discussion
3.1. Screening of Keratinase Activity
In this study, S. netropsis A-ICA and B. subtilis ALICA previously isolated from the rhizosphere of L. tridentata and P. juliflora respectively were subjected for preliminary screening on casein agar plates. Keratinase activity was indicated in both strains by observing the clear zone formation around their colonies (Figure 1).
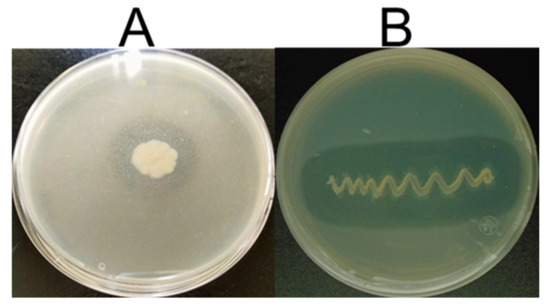
Figure 1.
Preliminary screening of keratinase production by two strains (A) S. netropsis A-ICA; (B) B. subtilis ALICA exhibiting zone of hydrolysis around the colonies on the casein plate.
Both strains showed a hydrolysis clear zone of 14 and 17 mm, respectively. Feathers are a keratinous waste with high protein content, which can be utilized in a variety of applications using developing biotechnological strategies. However, these strategies face significant difficulties as keratin is difficult to digest and demonstrates considerable resistance to physical and chemical treatments and typical proteases enzymes, although keratin can be degrading by the proteolytic enzymes known as keratinases [5]. Keratinase producing microorganisms can be used as alternative and cheap method to get rid of feather wastes by converting into peptides and amino acids which can use as low-cost supplements for foodstuff and animal feed [29,30]. The microbial keratinases are of special interest in the context of biotechnological processes due to their role to degrade feather wastes. Keratinase production from soil bacteria has been documented for industrial uses. Most Gram-positive bacteria, particularly soil microorganisms Bacilli and Actinomycetes, produce keratinase [10].
3.2. Optimization of Culture Condition for Keratinase Activity
The strains S. netropsis A-ICA and B. subtilis ALICA exhibited remarkable feather degradation in the broth medium amended with feathers as a sole nitrogen and carbon source. The extracellular keratinase production by these strains was evaluated daily during 10 days of incubation. The results of both strains revealed that keratinase activity was low at the first of incubation period. Then, the enzyme activity significantly increased until reaching the peak of keratinolytic activity. After that, the enzyme activity was gradually decreased with more incubation times. The data presented in Figure 2A illustrate that the highest significant (p < 0.05) keratinase activity of 36.8 ± 3.1 and 50.56 ± 0.7 U/mL was observed at the 5th and 3rd day of incubation by S. netropsis A-ICA and B. subtilis ALICA respectively. The highest keratinase activity was occurred at the exponentially microbial growth phase, indicating that the tested microorganisms secreted keratinase enzyme as principal metabolite [29]. Similar results were reported by several researchers [2,13,16]. The metabolites released by the microorganisms and the byproducts of enzyme activities would be a logical explanation for the observed pattern. The organic acid may be one of the early-stage metabolites released that increased the acidity of the media. In later stages, alkaline-based metabolites, including those connected to nitrogen, would have prevailed. Deamination of amino acids resulting from keratin degradation is the primary cause of alkalization [16].
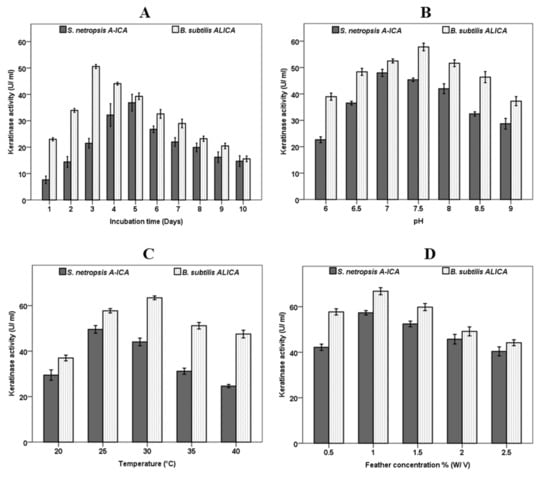
Figure 2.
Effect of incubation time (A), pH (B), Temperature (C) and Feathers concentration (D) on Keratinase activity produced by S. netropsis A-ICA and B. subtilis ALICA.
3.3. Effect of Culture pH and Fermentation Temperature on Keratinase Activity
The effect of initial pH of culture media on keratinase production was observed at different pH levels ranging from 6 to 9. Generally, the keratinase production was significantly impacted by the initial pH in both microorganisms. The results presented in Figure 2B revealed that the highest keratinase production of 47.9 ± 1.3 and 57.8 ± 1.4 U/mL was observed at pH 7 and 7.5 by S. netropsis A-ICA and B. subtilis ALICA, respectively. Similarly, it was reported that the optimum pH for keratinase production by B. tropicus Gxun-17 was neutral to weakly alkaline [13]. Moreover, S. coelicoflavus showed maximum keratinase production at pH 8.0 [31]. Generally, the most suitable pH for keratinase production from Actinomycetes, fungi, and bacteria was found to neutral to alkaline range [29]. On the other hand, it has been observed that temperature has a remarkable impact on keratinase activity in both examined strains (Figure 2C). The variation of culture temperature exhibited that the keratinase activity was at its peak (49.5 ± 1.7 and 63.4 ± 0.8 U/mL) at 25 and 30 °C in S. netropsis A-ICA and B. subtilis ALICA, respectively. Keratinase activity continuously reduced as fermentation temperature increased which suggests the mesophilic nature of S. netropsis A-ICA and B. subtilis ALICA.
In this context, keratinase enzyme was optimally released by Bacillus sp. FPF-1 at 25 °C, then the enzyme activity dropped continuously with increasing temperature [16]. Moreover, S. coelicoflavus exhibited maximum keratinase activity at 40 °C [31]. Numerous cultural conditions have an impact on feather deterioration. The efficiency of feather decomposition depends on the medium pH and fermentation temperature, which differ from strain to other. The optimal pH and temperature for degradation of chicken feathers by S. netropsis A-ICA and B. subtilis ALICA were neutral pH (pH 7.0 and 7.5) and moderate temperature (25 and 30 °C), respectively. At higher pH and temperature, feather degradation was slowed down. The capacity of these strains to degrade the feather at neutral pH and moderate temperature is considered a desirable and attractive outcome due to the possibility of several essential amino acids being destroyed during feather digestion at higher alkaline (pH > 9) levels [15].
3.4. Effect of Feather Concentration on Keratinase Activity
Among different feather concentrations, 1% (w/v) significantly increased keratinase production (Figure 2D). The maximum keratinase activity of 57.3 ± 0.9 and 66.8 ± 1.5 U/mL by S. netropsis A-ICA and B. subtilis ALICA, respectively was recorded at 1% (w/v) of chicken feather in the fermentation media. Then, keratinase activity was constantly decreased with further increase of feather concentration more than 1%. Similarly, B. tropicus Gxun-17 reached its peak of keratinase production at 15 g/L of feathers, then decreased at higher concentrations of feather [13]. This may be due to the fermentation broth’s comparatively high viscosity, caused by high concentrations of feather, which affect the bacterial growth and the release of keratinase by reducing the dissolved oxygen supply to the fermentation system [15,16,32]. The higher feather concentration leads to an increase in viscosity and decrease in aeration in the fermentation medium, which impedes the microbial growth as well as retards feather degradation [15]. It has been reported that the majority of bacteria achieved their peak of keratinase activity at feather concentrations range of 5–20 g/L [13]. S. coelicoflavus produced the most keratinase when feather concentrations were 1% (w/v), and this production marginally decreased as feather concentrations increased [31].
3.5. Feathers Degradation under Optimized Condition
The concentration of soluble protein in the hydrolysate gradually increased throughout the course of the incubation period, reaching 423.3 ± 25 and 565.3 ± 7.7 µg/mL by S. netropsis A-ICA and B. subtilis ALICA respectively, after nine days of hydrolysis, (Figure 3A). Generally, with the prolongation of the fermentation time, the enzyme activities decreased, whilst the soluble protein concentration was continuing to increase. Total protein in the cell free broth was increased as time progressed, indicating keratin degradation. The high potential of microbial keratinases degrades keratinous biomass quicker than the producing bacteria can utilize it, resulting in an increase the total protein concentration in the fermentation broth [16]. Our results are in accordance with previous reports [7,33]. On the other hand, the weight loss of feathers was measured after one, three, five, seven, and nine days of fermentation. The rate of feather weight loss significantly increased over time, reaching its peak of 84 ± 2 and 86± 1.5% for S. netropsis A-ICA and B. subtilis ALICA after seven and five days respectively, showing the complete degradation of feathers by both strains (Figure 3B). It was observed that the medium’s total protein level remarkably increased in response to the degradation of the feather substrate, providing a measure of the degradation process’s effectiveness. These outcomes are consistent with those attained by Kshetri et al. [15], who got 84% feather degradation in addition to an increase in soluble protein concentration. Similarly, the degradation rate of feathers by Bacillus velezensis NCIM 5802 was found to about 90% under optimized conditions [11]. The weight loss of the keratin substrate is the strongest evidence of the keratinolytic capacity of microorganisms [6].
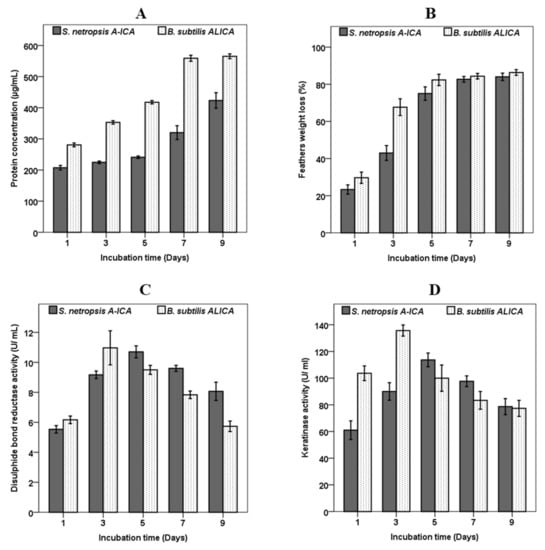
Figure 3.
Total protein concentration (A), feather weight loss (B), and disulphide bond reductase activity (C), and keratinase activity (D) by S. netropsis A-ICA and B. subtilis ALICA separately, in the basal feather medium during 9 days of fermentation.
Disulphide Bond Reductase Activity and Keratinase Activity under Optimized Conditions
As illustrated in Figure 3C, disulphide bond reductase activity gradually increased and peaked at five and three days after inoculation with S. netropsis A-ICA and B. subtilis ALICA, with the highest disulphide bond reductase activity reaching 10.7 ± 0.4 and 1.96 ± 1.1 U/mL, respectively. Disulphide bond reductase activity declined after five and three days of fermentation by S. netropsis A-ICA and B. subtilis ALICA, respectively. Under the optimized conditions, the keratinase activity of S. netropsis A-ICA and B. subtilis ALICA reached 113.6 ± 5.1 and 135.6 ± 4.1 U/mL, which was 2.4- and 2.36-fold that obtained before optimizing conditions (47 and 57 U/mL) respectively (Figure 3D). There are four theories that explain the mechanisms of feather degradation, including mechanical pressure theory, biological membrane potential theory, thiolysis theory, and enzymolysis theory. However, the essence of each theory depends on the fracture of disulphide bonds [13]. In the present study, both S. netropsis A-ICA and B. subtilis ALICA produced a significant amount of keratinase and disulphide bond reductase, which simultaneously contribute to the process of feather degradation. It was found that disulphide bond reduction significantly facilitates the complete degradation of rigid keratin through structural modification, and this structural alteration makes keratin more susceptible to proteolytic hydrolysis [16]. The extraordinarily complicated structure of keratinous substrates, which contains a significant number of disulfide bonds, prevents traditional proteases from acting upon them. In contrast, the keratinase enzymes are able to easily degrade the keratin’s complicated structure by reducing the number of disulphide bonds and breaking complex keratin structures [34]. The keratin degradation mechanism by microorganisms depends on two protein enzymes’ cooperation. Here, disulfide reductase will decrease disulfide bonds and form denatured keratin (opened keratin structure), which will be degraded by protease enzymes into peptides and amino acids [6,34]. Although the activity of two enzymes produced by B. tropicus Gxun-17 decreased with the prolongation of fermentation time, the feather degradation rate was continuously increased, and this indicated that the two enzymes’ impacts may not be the only action of the feather degradation mechanism [13]. There are a large variety of keratinolytic microbes that exist in nature and are capable of decreasing the disulfide bonds in complex and rigid keratin structures, rendering them susceptible to proteolytic destruction, thereby resolving the issue of excessive waste produced by keratinous wastes [34,35].
3.6. Antioxidant Activity of Chicken Feather Hydrolysate
DPPH radical scavenging activity was used to assess the antioxidant capacity of feather protein hydrolysate at different concentrations (0.1, 0.5, 1.0, 1.5, 2.0, and 2.5 mg/mL), and the results revealed that the inhibitory activity increases as hydrolysate concentration increased. As shown in Figure 4, feather hydrolysate exhibited remarkable radical scavenging activity with an IC50 value of 0.8 and 0.6 mg/mL using S. netropsis A-ICA and B. subtilis ALICA, respectively. The feather hydrolysate with S. netropsis A-ICA and B. subtilis ALICA exhibited lower IC50 values (indicating higher antioxidant abilities) than chemically prepared keratin hydrolysate [36]. It was observed that the antioxidant activity of feather hydrolysate obtained using S. netropsis A-ICA (IC50 = 0.8 mg/mL) and B. subtilis ALICA (IC50 = 0.6 mg/mL) is higher than feather hydrolysate prepared using Streptomyces sp. MAB18 (IC50 = 78 mg/mL) [37]. Moreover, antioxidants not only increase the nutritional value of animal feed, but also protect it against deterioration [15].
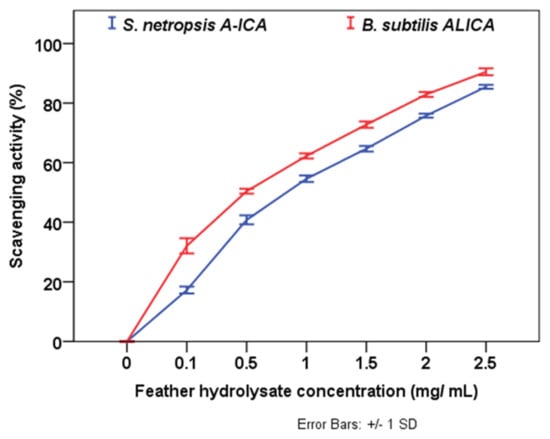
Figure 4.
Inhibition percentage of DPPH antioxidant activity of feather hydrolysates.
3.7. Bioinformatics Analysis for Keratinases in Studied Strains
Keratinases are formerly ordered with a full four-digit EC number, and multiple keratinase sequences are accessible in the NCBI database. The common sequenced keratinases are under one of 14 different protease families, namely M3, M4, M14, M16, M28, M32, M36, M38, M55, S1, S8, S9, S10, and S16. The families of M4, M16, M36, S1, S8, and S16 act as endo-attack proteases, while M14, M28, M38, M55, S9, and S10 families work as exo-attack proteases, while M3 and M32 behave as oligopeptides [4]. Figure 5 and Figure 6 depict the detected hits of proteases in B. subtilis (strain 168) and S. netropsis, respectively. While the former has 12,679 hits in 67 unique proteins, the latter contains 19,660 different hits in 120 in distinctive proteins. Both strains contain seven different keratinase hits that belong to S8 family. Members of this family are endopeptidases with a catalytic triad in the order Asp, His, and Ser in their sequences. A standard S8 protein structure encompasses three layers with a seven-stranded β sheet squeezed by two layers of helices [23]. Molecular docking enabled us to predict the interactions between beta-keratin and keratinases from both strains and cause them to be in a conjugated system. A molecular docking technique was employed in this work to support the results of the analysis. Figure 7 reveals the possible interaction between beta-keratin and the predicted 3D models of eight different keratinases (two of B. subtilis (strain 168) and six of S. netropsis). Among these interactions, some interesting findings have been found. For example, the GSG interacted with the domain in both keratinases of B. subtilis where the serine acts as the nucleophilic amino acid at the keratinase active sites [38]. Moreover, the studied keratinases bind to their substrate via active sites which are distributed commonly along the first and second loops. In addition, beta-keratin interacts with the aforementioned keratinases with a common binding domain (VVVFTP) located at its second loop.
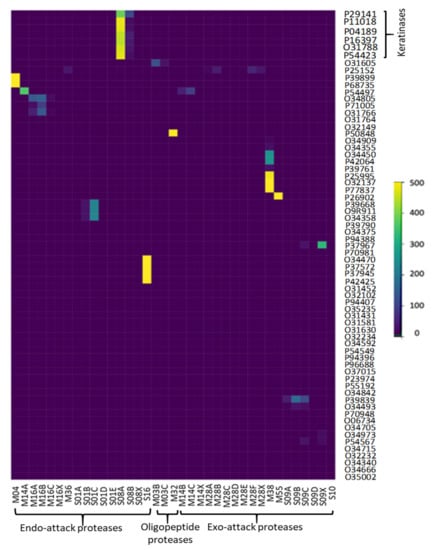
Figure 5.
Detected proteases of B. subtilis (strain 168).
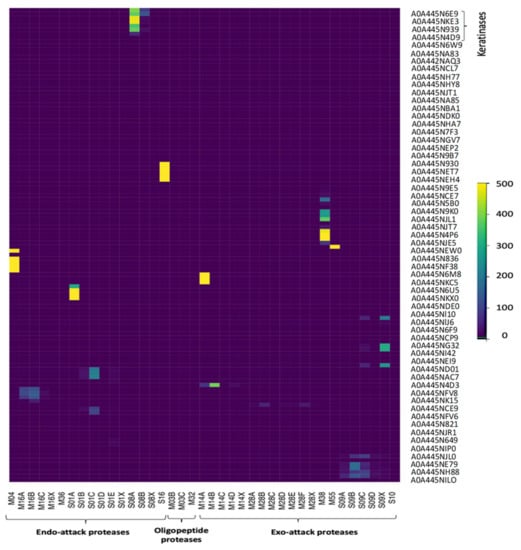
Figure 6.
Detected proteases of S. netropsis.
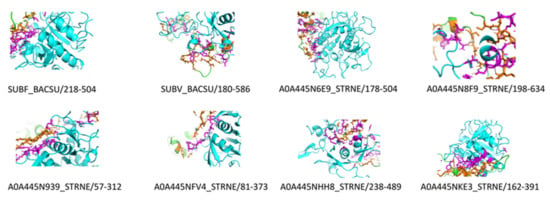
Figure 7.
Molecular docking complexes of detected keratinases in both strains (B. subtilis strain 168, and S. netropsis) and beta-keratin (Keratinases in cartoon Cyan, beta-keratin in cartoon Green, Interacted residues of keratinases in stick Magenta, Interacted residues of beta-keratin in stick orange, probable interaction in dashed black).
4. Conclusions
The bioprocessing of waste into useful products is an important strategy for sustainable development and the exploitation of renewable resources. Thus, the potential demonstrated by S. netropsis A-ICA and B. subtilis ALICA are suggestive of the great production of keratinases that bio-convert chicken feathers into soluble proteins and other related products. A neutral to weakly alkaline initial pH medium, 1% (w/v) of the substrate (chicken feather), and the mesophilic temperature (25 and 30 °C) were the process conditions that exhibited the optimal keratinase production. The ability of S. netropsis A-ICA and B. subtilis ALICA to effectively use chicken feathers as a sole carbon and nitrogen source indicates that a variety of enzymes are synthesized to efficiently decompose the complex pertinacious polymer. The high number of total proteins and the high rate of featherweight loss in the fermentation broth are strong indicators of feather degradation by both studied strains. Furthermore, this study exhibited that feather protein hydrolysate possessed an antioxidant potential. Further studies should be conducted to assess the application of these feather hydrolysates as an animal feed additive in vivo. Moreover, bioinformatics analysis provides a comprehensive survey for proteases of B. subtilis (strain 168) and S. netropsis, the active sites of their keratinases, and possible interaction with beta-keratin. As a result, these hot spots at beta-keratin and keratinases could be a fertile field for molecular biologists to maximize the keratinolytic process, hence accelerating the feather industry wheel.
Author Contributions
Conceptualization, A.A., V.M.-T., D.G.-M. and A.F.R.; methodology, A.A., C.C.-D., O.T.-C. and V.M.-T.; supervision, A.F.R., C.M.-C., A.A. and O.G.-J.; writing—original draft preparation, A.A., D.G.-M. and A.F.R.; writing—review and editing, D.G.-M., A.A. and A.F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The 16S rRNA gene sequences of identified bacteria were deposited in GenBank with accession number MN535765 and KX137176, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mazotto, A.M.; Cedrola, S.M.L.; de Souza, E.P.; Couri, S.; Vermelho, A.B. Enhanced Keratinase Production by Bacillus subtilis Amr Using Experimental Optimization Tools to Obtain Feather Protein Lysate for Industrial Applications. 3 Biotech 2022, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Mao, X.; Zhang, J.; Du, G.; Chen, J. Effective Biodegradation of Chicken Feather Waste by Co-Cultivation of Keratinase Producing Strains. Microb. Cell Factories 2019, 18, 84. [Google Scholar] [CrossRef]
- Jeevana Lakshmi, P.; Kumari Chitturi, C.M.; Lakshmi, V.V. Efficient Degradation of Feather by Keratinase Producing Bacillus sp. Int. J. Microbiol. 2013, 2013, e608321. [Google Scholar] [CrossRef]
- Qiu, J.; Barrett, K.; Wilkens, C.; Meyer, A.S. Bioinformatics Based Discovery of New Keratinases in Protease Family M36. New Biotechnol. 2022, 68, 19–27. [Google Scholar] [CrossRef]
- Emran, M.A.; Ismail, S.A.; Abdel-Fattah, A.M. Valorization of Feather via the Microbial Production of Multi-Applicable Keratinolytic Enzyme. Biocatal. Agric. Biotechnol. 2020, 27, 101674. [Google Scholar] [CrossRef]
- Fitriyanto, N.A.; Ramadhanti, Y.; Rismiyati; Rusyadi, I.; Pertiwiningrum, A.; Prasetyo, R.A.; Erwanto, Y. Enzymatic Activity and Amino Acid Production by Indigenous Keratinolytic Strains on the Various Poultry Feather Substrate. IOP Conf. Ser. Earth Environ. Sci. 2022, 1059, 012026. [Google Scholar] [CrossRef]
- Bezus, B.; Ruscasso, F.; Garmendia, G.; Vero, S.; Cavello, I.; Cavalitto, S. Revalorization of Chicken Feather Waste into a High Antioxidant Activity Feather Protein Hydrolysate Using a Novel Psychrotolerant Bacterium. Biocatal. Agric. Biotechnol. 2021, 32, 101925. [Google Scholar] [CrossRef]
- Li, Z.-W.; Liang, S.; Ke, Y.; Deng, J.-J.; Zhang, M.-S.; Lu, D.-L.; Li, J.-Z.; Luo, X.-C. The Feather Degradation Mechanisms of a New Streptomyces sp. Isolate SCUT-3. Commun. Biol. 2020, 3, 191. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Timorshina, S.; Osmolovskiy, A.; Misri, J.; Singh, R. Chicken Feather Waste Valorization Into Nutritive Protein Hydrolysate: Role of Novel Thermostable Keratinase From Bacillus pacificus RSA27. Front. Microbiol. 2022, 13, 882902. [Google Scholar] [CrossRef]
- Moonnee, Y.A.; Foysal, M.J.; Hashem, A.; Miah, M.F. Keratinolytic Protease from Pseudomonas aeruginosa for Leather Skin Processing. J. Genet. Eng. Biotechnol. 2021, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Pranaw, K.; Soni, H.; Rawat, H.K.; Kango, N. Parametrically Optimized Feather Degradation by Bacillus velezensis NCIM 5802 and Delineation of Keratin Hydrolysis by Multi-Scale Analysis for Poultry Waste Management. Sci. Rep. 2022, 12, 17118. [Google Scholar] [CrossRef]
- He, Z.; Sun, R.; Tang, Z.; Bu, T.; Wu, Q.; Li, C.; Chen, H. Biodegradation of Feather Waste Keratin by the Keratin-Degrading Strain Bacillus subtilis 8. J. Microbiol. Biotechnol. 2018, 28, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Yang, M.; Xie, C.; Pan, J.; Pang, K.; Zhang, H.; Wang, Y.; Jiang, M. Isolation and Identification of a Feather Degrading Bacillus tropicus Strain Gxun-17 from Marine Environment and Its Enzyme Characteristics. BMC Biotechnol. 2022, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Demir, T.; Hameş, E.E.; Öncel, S.S.; Vardar-Sukan, F. An Optimization Approach to Scale up Keratinase Production by Streptomyces sp. 2M21 by Utilizing Chicken Feather. Int. Biodeterior. Biodegrad. 2015, 103, 134–140. [Google Scholar] [CrossRef]
- Kshetri, P.; Roy, S.S.; Sharma, S.K.; Singh, T.S.; Ansari, M.A.; Prakash, N.; Ngachan, S.V. Transforming Chicken Feather Waste into Feather Protein Hydrolysate Using a Newly Isolated Multifaceted Keratinolytic Bacterium Chryseobacterium sediminis RCM-SSR-7. Waste Biomass Valorization 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Nnolim, N.E.; Okoh, A.I.; Nwodo, U.U. Bacillus sp. FPF-1 Produced Keratinase with High Potential for Chicken Feather Degradation. Molecules 2020, 25, 1505. [Google Scholar] [CrossRef]
- Abdelmoteleb, A.; Troncoso-Rojas, R.; Camacho, O.; Gonzalez-Mendoza, D.; Duran, C.; Grimaldo, O.; Aviles-Marin, M.; Durán-Hernández, D. Biocontrol of Fusarium spp., Causal Agents of Damping-off in Cotton Plants by Native Bacillus subtilis Isolated from Prosopis juliflora. Int. J. Agric. Biol. 2017, 19, 713–718. [Google Scholar] [CrossRef]
- Abdelmoteleb, A.; González-Mendoza, D. A Novel Streptomyces Rhizobacteria from Desert Soil with Diverse Anti-Fungal Properties. Rhizosphere 2020, 16, 100243. [Google Scholar] [CrossRef]
- Selvam, K.; Vishnupriya, B.; Yamuna, M. Isolation and Description of Keratinase Producing Marine Actinobacteria from South Indian Coastal Region. Afr. J. Biotechnol. 2013, 12, 19–26. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS Database of Proteolytic Enzymes, Their Substrates and Inhibitors in 2017 and a Comparison with Peptidases in the PANTHER Database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef] [PubMed]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Profile Hidden Markov Models. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef]
- Hauser, M.; Steinegger, M.; Söding, J. MMseqs Software Suite for Fast and Deep Clustering and Searching of Large Protein Sequence Sets. Bioinformatics 2016, 32, 1323–1330. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling Protein Tertiary and Quaternary Structure Using Evolutionary Information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Desta, I.T.; Porter, K.A.; Xia, B.; Kozakov, D.; Vajda, S. Performance and Its Limits in Rigid Body Protein-Protein Docking. Structure 2020, 28, 1071–1081.e3. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Das, A.; Thatoi, H.; Mondal, K.C.; Mohapatra, P.K.D. Keratinase Production and Biodegradation of Whole Chicken Feather Keratin by a Newly Isolated Bacterium Under Submerged Fermentation. Appl. Biochem. Biotechnol. 2012, 167, 1040–1051. [Google Scholar] [CrossRef]
- Huang, H.-J.; Weng, B.-C.; Lee, Y.-S.; Lin, C.-Y.; Hsuuw, Y.-D.; Chen, K.-L. The Effects of Two-Stage Fermented Feather Meal-Soybean Meal Product on Growth Performance, Blood Biochemistry, and Immunity of Nursery Pigs. Fermentation 2022, 8, 634. [Google Scholar] [CrossRef]
- Jadhav, R.S.; Oberoi, J.K.; Rokade, T.; Shingade, R.; Yadav, M.; Momin, T. Optimizing the Fermentation Conditions and Enhances the Keratinase Production from Streptomyces coelicoflavus. Acta Sci. Microbiol. 2021, 3, 14–24. [Google Scholar]
- Reddy, M.R.; Reddy, K.S.; Chouhan, Y.R.; Bee, H.; Reddy, G. Effective Feather Degradation and Keratinase Production by Bacillus pumilus GRK for Its Application as Bio-Detergent Additive. Bioresour. Technol. 2017, 243, 254–263. [Google Scholar] [CrossRef]
- Alahyaribeik, S.; Sharifi, S.D.; Tabandeh, F.; Honarbakhsh, S.; Ghazanfari, S. Bioconversion of Chicken Feather Wastes by Keratinolytic Bacteria. Process Saf. Environ. Prot. 2020, 135, 171–178. [Google Scholar] [CrossRef]
- Verma, A.; Singh, H.; Anwar, S.; Chattopadhyay, A.; Tiwari, K.K.; Kaur, S.; Dhilon, G.S. Microbial Keratinases: Industrial Enzymes with Waste Management Potential. Crit. Rev. Biotechnol. 2017, 37, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Możejko, M.; Bohacz, J. Optimization of Conditions for Feather Waste Biodegradation by Geophilic Trichophyton ajelloi Fungal Strains towards Further Agricultural Use. Int. J. Environ. Res. Public Health 2022, 19, 10858. [Google Scholar] [CrossRef] [PubMed]
- Alahyaribeik, S.; Ullah, A. Methods of Keratin Extraction from Poultry Feathers and Their Effects on Antioxidant Activity of Extracted Keratin. Int. J. Biol. Macromol. 2020, 148, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.-K. Production, Characterization and Antioxidant Potential of Protease from Streptomyces sp. MAB18 Using Poultry Wastes. BioMed Res. Int. 2013, 2013, e496586. [Google Scholar] [CrossRef]
- Hedstrom, L. Serine Protease Mechanism and Specificity. Chem. Rev. 2002, 102, 4501–4524. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).