Second Generation Bioethanol Production from Soybean Hulls Pretreated with Imidazole as a New Solvent
Abstract
1. Introduction
2. Materials and Methods
2.1. Lignocellulosic Biomass
2.2. Chemical Composition of Untreated and Imidazole-Pretreated Soybean Hull
2.3. Imidazole Pretreatment of Soybean Hulls
2.4. Enzymatic Digestibility
2.5. Bioethanol Production
2.6. Fourier Transforming Infrared Spectroscopy (FTIR), X-ray Diffraction (XRD) and SCANNING Electron Microscopy (SEM)
3. Results and Discussion
3.1. Effect of Imidazole Pretreatment on Soybean Hull’s Composition
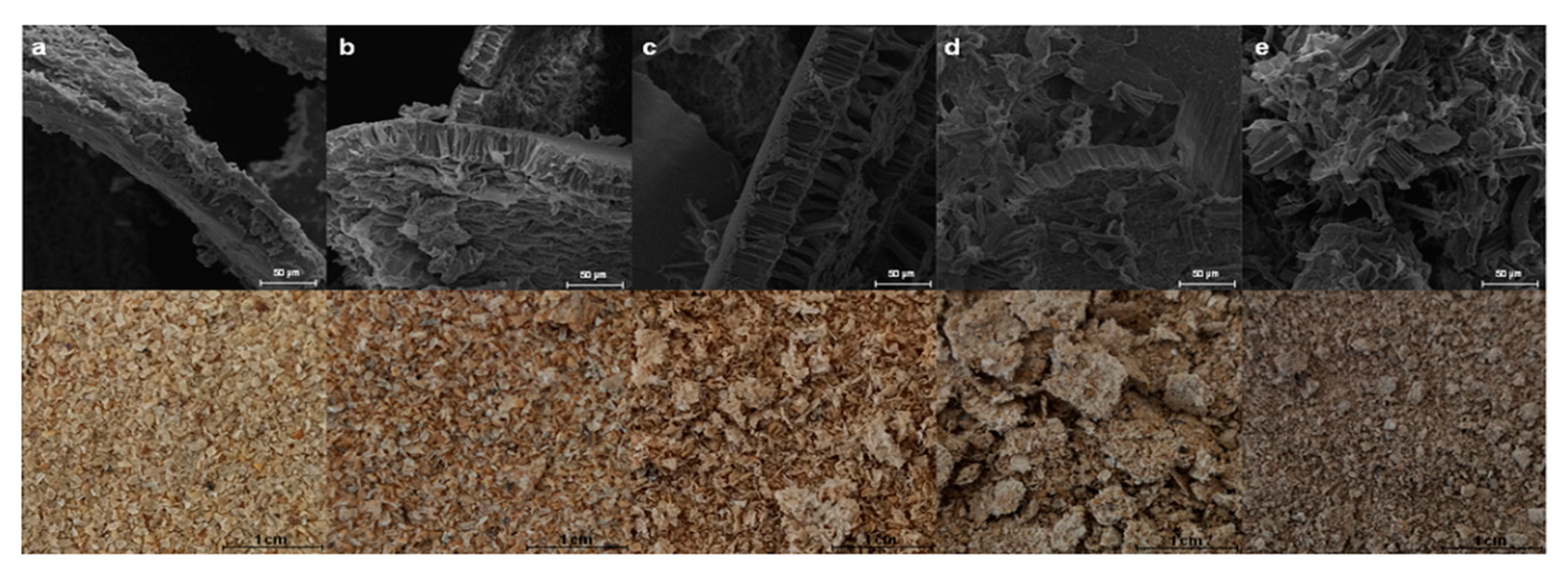
3.2. Effect of Imidazole Pretreatment on the Morphology of Soybean Hull
3.3. Effect of Imidazole Pretreatment on Crystallinity of Soybean Hull
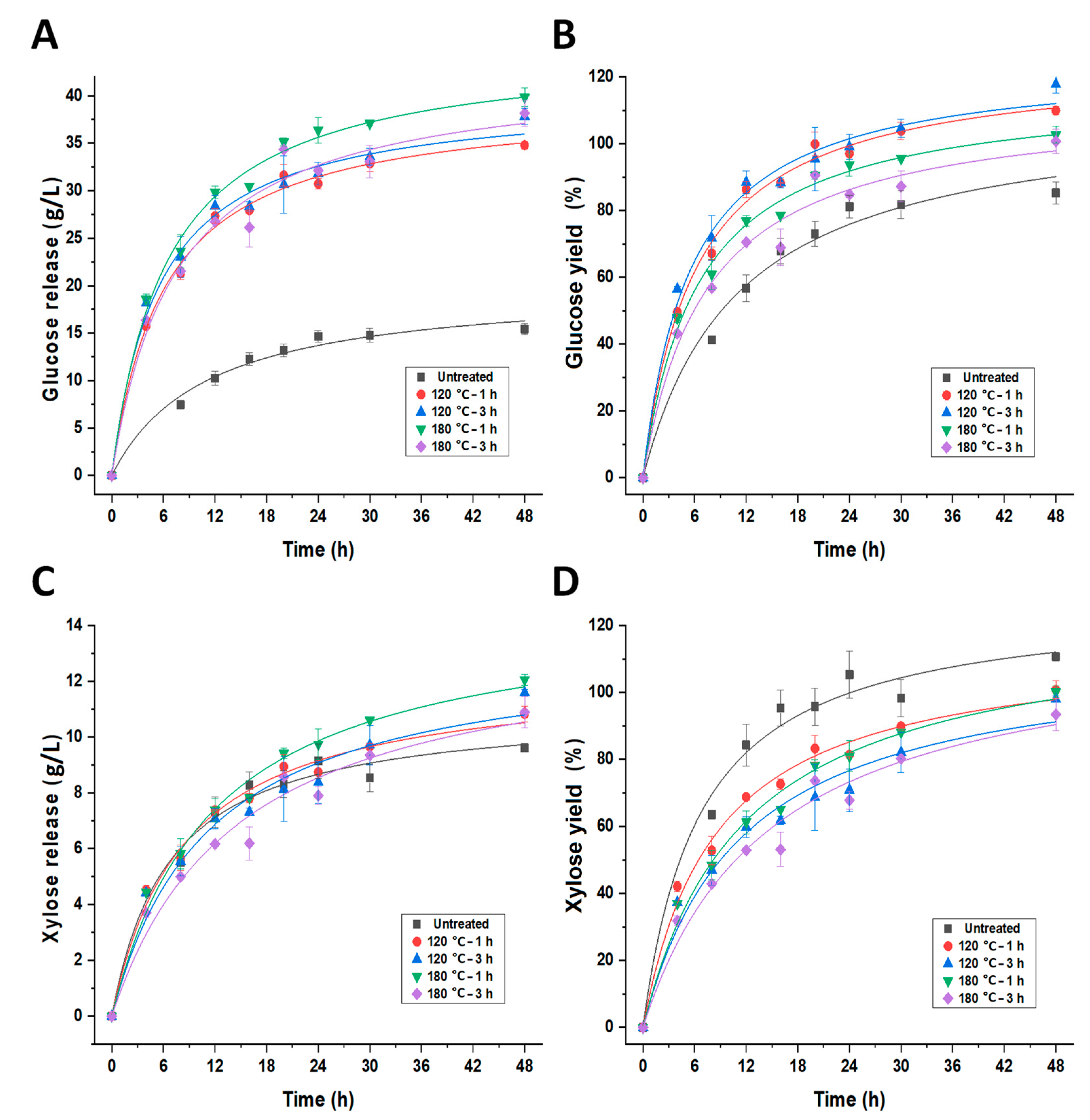
3.4. Enzymatic Hydrolysis of Untreated and Imidazole-Pretreated Soybean Hull
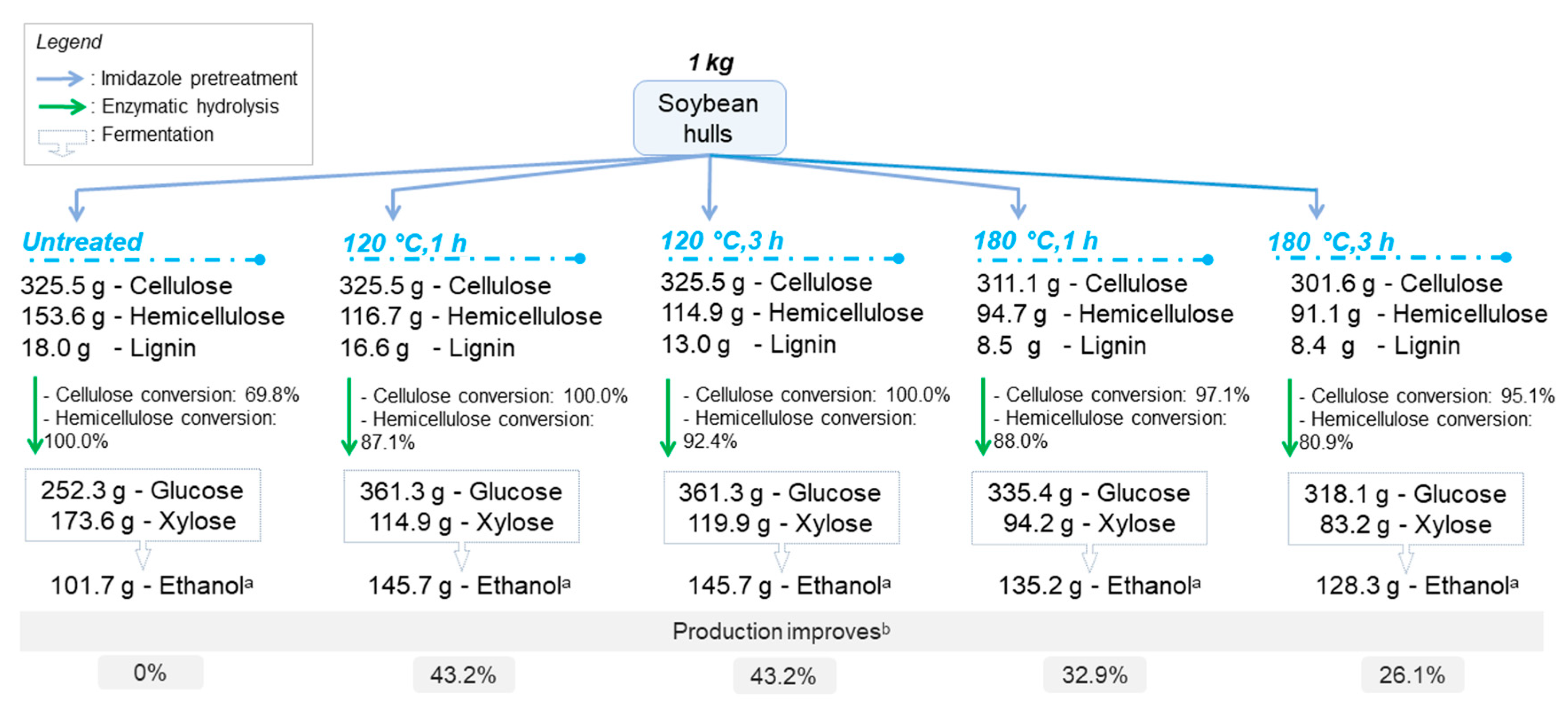
3.5. Bioethanol Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 2 February 2022).
- Luth, D.; Warnberg, K.; Wang, K. Soybean [Glycine Max (L.) Merr.]. Methods Mol. Biol. 2015, 1223, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, P.D. Soybean Production and Processing in Brazil. In Soybeans: Chemistry, Production, Processing, and Utilization; Elsevier: Amsterdam, The Netherlands, 2008; pp. 773–798. ISBN 9780128043523. [Google Scholar]
- Amaro Bittencourt, G.; Porto de Souza Vandenberghe, L.; Valladares-Diestra, K.; Wedderhoff Herrmann, L.; Fátima Murawski de Mello, A.; Sarmiento Vásquez, Z.; Grace Karp, S.; Ricardo Soccol, C. Soybean Hulls as Carbohydrate Feedstock for Medium to High-Value Biomolecule Production in Biorefineries: A Review. Bioresour. Technol. 2021, 339, 125594. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.A.; Gomez, L.D.; Steele-King, C.G.; Simister, R.; Bernardinelli, O.D.; Carvalho, M.A.; Rezende, C.A.; Labate, C.A.; Deazevedo, E.R.; McQueen-Mason, S.J.; et al. Evaluating the Composition and Processing Potential of Novel Sources of Brazilian Biomass for Sustainable Biorenewables Production. Biotechnol. Biofuels 2014, 7, 10. [Google Scholar] [CrossRef]
- Silveira, M.H.L.; Morais, A.R.C.; da Costa Lopes, A.M.; Olekszyszen, D.N.; Bogel-Łukasik, R.; Andreaus, J.; Pereira Ramos, L. Current Pretreatment Technologies for the Development of Cellulosic Ethanol and Biorefineries. ChemSusChem 2015, 8, 3366–3390. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current Perspective on Pretreatment Technologies Using Lignocellulosic Biomass: An Emerging Biorefinery Concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Yoo, J.; Alavi, S.; Vadlani, P.; Amanor-Boadu, V. Thermo-Mechanical Extrusion Pretreatment for Conversion of Soybean Hulls to Fermentable Sugars. Bioresour. Technol. 2011, 102, 7583–7590. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Yu, J.H. The Production of Fermentable Sugar and Bioethanol from Acacia Wood by Optimizing Dilute Sulfuric Acid Pretreatment and Post Treatment. Fuel 2020, 275, 117943. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Chang, S.; Zhang, L.; Mao, Y.; Cui, T.; Yan, Z.; Luo, C.; Li, S. Bioethanol Production Using the Sodium Hydroxide Pretreated Sweet Sorghum Bagasse without Washing. Fuel 2016, 175, 20–25. [Google Scholar] [CrossRef]
- Sharma, M.; Kumar, A. Xylanases: An Overview. Br. Biotechnol. J. 2013, 3, 1–28. [Google Scholar] [CrossRef]
- Ju, X.; Engelhard, M.; Zhang, X. An Advanced Understanding of the Specific Effects of Xylan and Surface Lignin Contents on Enzymatic Hydrolysis of Lignocellulosic Biomass. Bioresour. Technol. 2013, 132, 137–145. [Google Scholar] [CrossRef]
- Bhatia, L.; Johri, S. Optimization of Simultaneous Saccharification and Fermentation Parameters for Sustainable Production of Ethanol from Wheat Straw by Pichia Stipitis NCIM 3498. Indian J. Exp. Biol. 2018, 56, 932–941. [Google Scholar]
- Kochepkaa, D.M.; Dill, L.P.; Fockink, D.H.; Łukasik, R.M. Contribution to the Production and Use of Biomass-Derived Solvents—A Review. Acta Innov. 2020, 9, 29–56. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.K.; Porto de Souza Vandenberghe, L.; Zevallos Torres, L.A.; Nishida, V.S.; Zandoná Filho, A.; Woiciechowski, A.L.; Soccol, C.R. Imidazole Green Solvent Pre-Treatment as a Strategy for Second-Generation Bioethanol Production from Sugarcane Bagasse. Chem. Eng. J. 2021, 420, 127708. [Google Scholar] [CrossRef]
- Toscan, A.; Fontana, R.C.; Andreaus, J.; Camassola, M.; Lukasik, R.M.; Dillon, A.J.P. New Two-Stage Pretreatment for the Fractionation of Lignocellulosic Components Using Hydrothermal Pretreatment Followed by Imidazole Delignification: Focus on the Polysaccharide Valorization. Bioresour. Technol. 2019, 285, 121346. [Google Scholar] [CrossRef]
- Morais, A.R.C.; Pinto, J.V.; Nunes, D.; Roseiro, L.B.; Oliveira, M.C.; Fortunato, E.; Bogel-Łukasik, R. Imidazole: Prospect Solvent for Lignocellulosic Biomass Fractionation and Delignification. ACS Sustain. Chem. Eng. 2016, 4, 1643–1652. [Google Scholar] [CrossRef]
- Jordan, T.; Schmidt, S.; Liebert, T.; Heinze, T. Molten Imidazole—A Starch Solvent. Green Chem. 2014, 16, 1967–1973. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; NREL/TP-510-42621; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Rojas, M.J.; Siqueira, P.F.; Miranda, L.C.; Tardioli, P.W.; Giordano, R.L.C. Sequential Proteolysis and Cellulolytic Hydrolysis of Soybean Hulls for Oligopeptides and Ethanol Production. Ind. Crop. Prod. 2014, 61, 202–210. [Google Scholar] [CrossRef]
- Kellock, M.; Maaheimo, H.; Marjamaa, K.; Rahikainen, J.; Zhang, H.; Holopainen-Mantila, U.; Ralph, J.; Tamminen, T.; Felby, C.; Kruus, K. Effect of Hydrothermal Pretreatment Severity on Lignin Inhibition in Enzymatic Hydrolysis. Bioresour. Technol. 2019, 280, 303–312. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.K.; Porto de Souza Vandenberghe, L.; Nishida, V.S.; Zevallos Torres, L.A.; Zandoná Filho, A.; Soccol, C.R. Integrated Sugarcane Biorefinery for First- and Second-Generation Bioethanol Production Using Imidazole Pretreatment. J. Clean. Prod. 2022, 381, 135179. [Google Scholar] [CrossRef]
- Fockink, D.H.; Andreaus, J.; Ramos, L.P.; Łukasik, R.M. Pretreatment of Cotton Spinning Residues for Optimal Enzymatic Hydrolysis: A Case Study Using Green Solvents. Renew. Energy 2020, 145, 490–499. [Google Scholar] [CrossRef]
- da Cunha-Pereira, F.; Rech, R.; Záchia Ayub, M.A.; Pinheiro Dillon, A.; Dupont, J. Liberation of Fermentable Sugars from Soybean Hull Biomass Using Ionic Liquid 1-Butyl-3-Methylimidazolium Acetate and Their Bioconversion to Ethanol. Biotechnol. Prog. 2016, 32, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Camiscia, P.; Giordano, E.D.V.; Brassesco, M.E.; Fuciños, P.; Pastrana, L.; Cerqueira, M.F.; Picó, G.A.; Woitovich Valetti, N. Comparison of Soybean Hull Pre-Treatments to Obtain Cellulose and Chemical Derivatives: Physical Chemistry Characterization. Carbohydr. Polym. 2018, 198, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.K.C. The Plant Cell Wall; Blackwell: Oxford, UK, 2003; ISBN 1-84127-328-7. [Google Scholar]
- Cassales, A.; de Souza-Cruz, P.B.; Rech, R.; Záchia Ayub, M.A. Optimization of Soybean Hull Acid Hydrolysis and Its Characterization as a Potential Substrate for Bioprocessing. Biomass Bioenergy 2011, 35, 4675–4683. [Google Scholar] [CrossRef]
- Chaudhary, G.; Singh, L.K.; Ghosh, S. Alkaline Pretreatment Methods Followed by Acid Hydrolysis of Saccharum Spontaneum for Bioethanol Production. Bioresour. Technol. 2012, 124, 111–118. [Google Scholar] [CrossRef]
- Toscan, A.; Morais, A.R.C.; Paixão, S.M.; Alves, L.; Andreaus, J.; Camassola, M.; Dillon, A.J.P.; Lukasik, R.M. Effective Extraction of Lignin from Elephant Grass Using Imidazole and Its Effect on Enzymatic Saccharification to Produce Fermentable Sugars. Ind. Eng. Chem. Res. 2017, 56, 5138–5145. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Lin, F.; Zhang, H.; Xiao, R. Structural Elucidation of Industrial Bioethanol Residual Lignin from Corn Stalk: A Potential Source of Vinyl Phenolics. Fuel Process. Technol. 2018, 169, 50–57. [Google Scholar] [CrossRef]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. Nanofibrillated Cellulose Obtained from Soybean Hull Using Simple and Eco-Friendly Processes Based on Reactive Extrusion. Cellulose 2020, 27, 1975–1988. [Google Scholar] [CrossRef]
- Merci, A.; Urbano, A.; Grossmann, M.V.E.; Tischer, C.A.; Mali, S. Properties of Microcrystalline Cellulose Extracted from Soybean Hulls by Reactive Extrusion. Food Res. Int. 2015, 73, 38–43. [Google Scholar] [CrossRef]
- Fung, W.Y.; Yuen, K.H.; Liong, M.T. Characterization of Fibrous Residues from Agrowastes and the Production of Nanofibers. J. Agric. Food Chem. 2010, 58, 8077–8084. [Google Scholar] [CrossRef]
- French, A.D. Idealized Powder Diffraction Patterns for Cellulose Polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Duchemin, B. Size, Shape, Orientation and Crystallinity of Cellulose Iβ by X-ray Powder Diffraction Using a Free Spreadsheet Program. Cellulose 2017, 24, 2727–2741. [Google Scholar] [CrossRef]
- Rosso, D.F.; Negrão, D.R.; Driemeier, C. Unveiling the Variability and Multiscale Structure of Soybean Hulls for Biotechnological Valorization. Waste Biomass Valorization 2022, 13, 2095–2108. [Google Scholar] [CrossRef]
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the Structural and Chemical Changes of Plant Biomass Following Steam Explosion Pretreatment. Biotechnol. Biofuels 2017, 10, 36. [Google Scholar] [CrossRef]
- Gaur, R.; Agrawal, R.; Kumar, R.; Ramu, E.; Bansal, V.R.; Gupta, R.P.; Kumar, R.; Tuli, D.K.; Das, B. Evaluation of Recalcitrant Features Impacting Enzymatic Saccharification of Diverse Agricultural Residues Treated by Steam Explosion and Dilute Acid. RSC Adv. 2015, 5, 60754–60762. [Google Scholar] [CrossRef]
- Sun, X.F.; Xu, F.; Sun, R.C.; Fowler, P.; Baird, M.S. Characteristics of Degraded Cellulose Obtained from Steam-Exploded Wheat Straw. Carbohydr. Res. 2005, 340, 97–106. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A Critical Review of Analytical Methods in Pretreatment of Lignocelluloses: Composition, Imaging, and Crystallinity. Bioresour. Technol. 2016, 200, 1008–1018. [Google Scholar] [CrossRef]
- Cui, T.; Li, J.; Yan, Z.; Yu, M.; Li, S. The Correlation between the Enzymatic Saccharification and the Multidimensional Structure of Cellulose Changed by Different Pretreatments. Biotechnol. Biofuels 2014, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, J.; Yu, H.; Li, Y.; Wang, Y.; Gao, H.; Peng, H.; Hu, Z.; Wang, H.; Zhang, G.; et al. Optimizing Two Green-like Biomass Pretreatments for Maximum Bioethanol Production Using Banana Pseudostem by Effectively Enhancing Cellulose Depolymerization and Accessibility. Sustain. Energy Fuels 2021, 5, 3467–3478. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Ra, C.H.; Sunwoo, I.Y.; Sukwong, P.; Jeong, G.-T.; Kim, S.-K. Bioethanol Production from Soybean Residue via Separate Hydrolysis and Fermentation. Appl. Biochem. Biotechnol. 2018, 184, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-J.; Wen, J.-L.; Mei, Q.-Q.; Chen, X.; Sun, D.; Yuan, T.-Q.; Sun, R.-C. Facile Fractionation of Lignocelluloses by Biomass-Derived Deep Eutectic Solvent (DES) Pretreatment for Cellulose Enzymatic Hydrolysis and Lignin Valorization. Green Chem. 2019, 21, 275–283. [Google Scholar] [CrossRef]
- Xu, C.; Asraful Alam, M.; Wang, Z.; Chen, H.; Zhang, J.; Huang, S.; Zhuang, W.; Xu, J. Mechanisms of Bio-Additives on Boosting Enzymatic Hydrolysis of Lignocellulosic Biomass. Bioresour. Technol. 2021, 337, 125341. [Google Scholar] [CrossRef] [PubMed]
- Usmani, Z.; Sharma, M.; Gupta, P.; Karpichev, Y.; Gathergood, N.; Bhat, R.; Gupta, V.K. Ionic Liquid Based Pretreatment of Lignocellulosic Biomass for Enhanced Bioconversion. Bioresour. Technol. 2020, 304, 123003. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Luo, F.; Huang, W.; Huang, W.; Wu, Y. Comparison of Three Fermentation Strategies for Alleviating the Negative Effect of the Ionic Liquid 1-Ethyl-3-Methylimidazolium Acetate on Lignocellulosic Ethanol Production. Appl. Energy 2017, 197, 124–131. [Google Scholar] [CrossRef]
- Kennes, D.; Abubackar, H.N.; Diaz, M.; Veiga, M.C.; Kennes, C. Bioethanol Production from Biomass: Carbohydrate vs. Syngas Fermentation. J. Chem. Technol. Biotechnol. 2016, 91, 304–317. [Google Scholar] [CrossRef]
- Choi, I.S.; Kim, Y.G.; Jung, J.K.; Bae, H.-J. Soybean Waste (Okara) as a Valorization Biomass for the Bioethanol Production. Energy 2015, 93, 1742–1747. [Google Scholar] [CrossRef]
- Cortivo, P.R.D.; Hickert, L.R.; Hector, R.; Ayub, M.A.Z. Fermentation of Oat and Soybean Hull Hydrolysates into Ethanol and Xylitol by Recombinant Industrial Strains of Saccharomyces Cerevisiae under Diverse Oxygen Environments. Ind. Crop. Prod. 2018, 113, 10–18. [Google Scholar] [CrossRef]
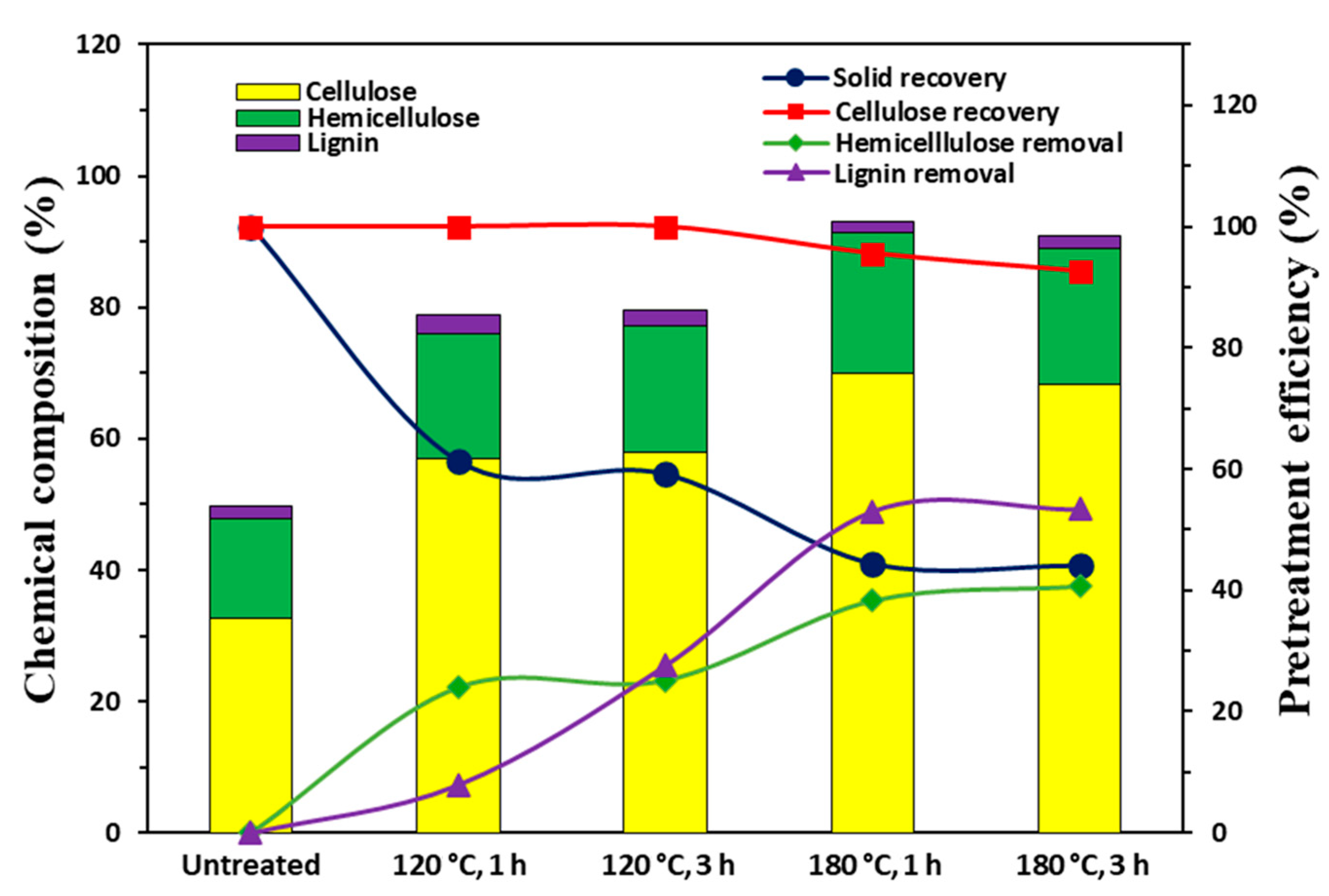


| Composition (%) | Untreated a | 120–1 | 120–3 | 180–1 | 180–3 |
|---|---|---|---|---|---|
| Glucan | 32.5 | 57.1 | 57.9 | 69.9 | 68.3 |
| Xylan | 12.3 | 16.3 | 17.4 | 21.3 | 20.6 |
| Anhydroarabinose | 3.2 | 2.7 | 2.0 | 0.0 | 0.0 |
| Acetyl groups | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Acid-soluble lignin | 1.1 | 1.4 | 1.5 | 1.4 | 1.6 |
| Acid-insoluble lignin | 0.7 | 1.3 | 0.7 | 0.5 | 0.3 |
| Ash | 4.3 | - | - | - | - |
| Extractives-Water | 13.5 | - | - | - | - |
| Extractives-EtOH | 4.0 | - | - | - | - |
| Solid Yield (%) | - | 61.38 | 59.23 | 44.48 | 44.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishida, V.S.; Woiciechowski, A.L.; Valladares-Diestra, K.K.; Zevallos Torres, L.A.; Vandenberghe, L.P.d.S.; Zandoná Filho, A.; Soccol, C.R. Second Generation Bioethanol Production from Soybean Hulls Pretreated with Imidazole as a New Solvent. Fermentation 2023, 9, 93. https://doi.org/10.3390/fermentation9020093
Nishida VS, Woiciechowski AL, Valladares-Diestra KK, Zevallos Torres LA, Vandenberghe LPdS, Zandoná Filho A, Soccol CR. Second Generation Bioethanol Production from Soybean Hulls Pretreated with Imidazole as a New Solvent. Fermentation. 2023; 9(2):93. https://doi.org/10.3390/fermentation9020093
Chicago/Turabian StyleNishida, Verônica Sayuri, Adenise Lorenci Woiciechowski, Kim Kley Valladares-Diestra, Luis Alberto Zevallos Torres, Luciana Porto de Souza Vandenberghe, Arion Zandoná Filho, and Carlos Ricardo Soccol. 2023. "Second Generation Bioethanol Production from Soybean Hulls Pretreated with Imidazole as a New Solvent" Fermentation 9, no. 2: 93. https://doi.org/10.3390/fermentation9020093
APA StyleNishida, V. S., Woiciechowski, A. L., Valladares-Diestra, K. K., Zevallos Torres, L. A., Vandenberghe, L. P. d. S., Zandoná Filho, A., & Soccol, C. R. (2023). Second Generation Bioethanol Production from Soybean Hulls Pretreated with Imidazole as a New Solvent. Fermentation, 9(2), 93. https://doi.org/10.3390/fermentation9020093





