1. Introduction
The population’s constant growth has provoked noticeable food demand worldwide, which is expected to increase by up to 50% by 2050, according to the Food and Agriculture Organization of the United Nations (FAO) [
1]. However, no viable solution has been provided for the production of more and better food with the same or less use of resources [
2]. This has led to excessive resource consumption, which is accompanied by vast volumes of industrial waste [
3]. Annually, one-third of the produced food is wasted worldwide in the overall value chain, from farm to fork, corresponding to 1.3 billion tons yearly [
1]. Thus, the tendency of the European Commission to adapt to a circular economy is nowadays an undeniable fact. In light of the Green Deal’s recent reveal, that is, an aspirational plan to make Europe carbon-neutral by 2050, it is evident that companies will be forced to use closed resource loops in the future to limit waste and diminish the environmental effect of their processes [
4]. The Green Deal’s Farm to Fork strategy mainly emphasises the food system and intends to apply the circular economy approach to improve the sustainability of food production [
5]. Closing the loop in the framework of food production systems would entail improving the use of food surplus, waste and by-products.
As the world is trying to make a transition to a low-carbon economy, the European Potato Processors’ Association (EUPPA) members welcome green ambitions and desire to contribute to the shift to greater sustainability [
6]. Companies target reducing energy, freshwater intake, carbon emissions and zero waste to landfill in food processing plants. Thus, they are urged to demonstrate not just their decarbonization strategies, but also to reduce negative impacts on biodiversity. Potato is the third most important food crop on a global level [
7]. As a result, from 2001 to 2020, worldwide potato output climbed by around 18% [
1]. During the processing of these crops, potato companies produce enormous volumes of waste; for every ton of processed potatoes, 0.16 tons of solid waste are generated [
8]. The two primary sources of potato waste are potato agricultural operations (5–20%) and the potato chip industry (18%) [
9]. For instance, the production of potato chips generates waste from the following processes: peeling (potato peels), sorting (potato tubers), cutting (starch and potato slices) and frying (low-quality chips). These substrates are mainly composed of lignocellulosic materials, such as hemicellulose, cellulose and lignin, as well as carbohydrates, proteins, lipids and fatty acids. Given their properties, these waste streams may be characterised as promising feedstocks for the food-processing, pharmaceutical and biosynthetic industries. This has increased interest in valorising them for the production of bioproducts with added value [
10]. Turning them into biofuels might be another viable way for developing alternative energy sources and minimizing harmful gas emissions [
11].
Generated bioethanol through the microbial fermentation of sugar-based, starchy and lignocellulosic feedstock has been considered as a potential renewable fuel [
12]. The feedstock’s chemical and structural properties determine the difficulty of the production process. The total world ethanol production in 2021 is 27,310 million gallons, of which 60%, approximately, originates from starchy materials [
13,
14].
Few research studies for bioethanol production from potato processing industrial waste have been conducted, investigating the three steps of bioethanol synthesis by starchy and lignocellulosic matter: pretreatment, hydrolysis and fermentation. According to Khawla et. al. [
15], enzymatic hydrolysis has been proved to be preferable to acid hydrolysis, with significant concentrations of reducing sugars (69 g·L
−1), by the use of potato peel waste as feedstock. Another research study showed that 11.9 g·L
−1 of bioethanol was obtained from the enzymatic saccharification of potato peels using amylolytic and cellulolytic enzymes [
16]. Some of the steps mentioned above have been applied simultaneously, leading to even higher ethanol yields (simultaneous saccharification fermentation, SSF). Thus, SSF has replaced the separate hydrolysis and fermentation (SHF) process as a more economical and effective way to produce bioethanol. Specifically, a high ethanol yield was observed using
Saccharomyces cerevisiae after 48 h of incubation from potato peel waste at a temperature of 35 °C and pH of 6.0 [
17].
The objective of the present study is to produce bioethanol by degradation and valorisation of all the potato processing waste streams and not just one, as is the case in the literature. Four solid waste streams are utilised as substrates for bioethanol production. Firstly, the selection of the appropriate pretreatment was investigated in order to optimize the bioethanol production. Other operational conditions, such as solid loading, dosages of enzymes, etc., were also studied. Moreover, the kinetics of ethanol production under the optimal operational parameters were examined on a pilot scale of 200 L. Finally, based on the experimental results, a full-scale implementation plan is proposed as an innovative solution for the management of potato processing industrial waste, with remarkable perspectives from both the economic and environmental aspects.
3. Results and Discussion
3.1. Chemical Composition
A crucial first step to begin the investigation is the determination of the composition of the waste streams, which indicates their potential as bioethanol feedstocks. The composition of industrial waste varies, impacted by multiple factors, including the production line and the type of production, apart from the variety of the raw material. Hence, the four raw material waste streams were chemically characterised in their dry basis.
The potato peels and potato tubers and slices had a high moisture content, around 85% and 76%, respectively. From
Table 1, it is obvious that the four waste streams consist of carbohydrates, lipids and lignocellulosic substances, ingredients revealing an ideal substrate for biofuel production. More specifically, potato peels (PP) contain starch (17.3% ± 0.6%) and cellulose (18.9% ± 1.6%) in similar percentages, and 20.4% ± 0.6% acid insoluble residue (AIR). The high lignin content makes the saccharification of raw material difficult and less effective, thus a suitable pretreatment may be favourable. The potato tubers and slices (PT&S) contained 62.5% ± 5.6% starch. Starch waste (ST) is made of 83.4% ± 5.0% starch. Chips waste (CH) mainly consists of starch (51.0% ± 1.9%) and oils (36.6% ± 0.7%), implying that it is a rich medium for both bioethanol production and biodiesel.
In
Table 1, apart from the chemical composition of the four waste streams, a literature comparison is also presented. Regarding the potato peels, the experimental values fall within the literature range, apart from hemicellulose. This fact may be attributed to the different techniques applied and machinery used in the industrial plants for peeling, as well as the different potato varieties cultivated. The same also applies for the discrepancies noted for potato tubers and slices, along with the fact that there is not much literature available for this feedstock. Low-quality chips also fall well within the literature range.
3.2. Pretreatment Method Investigation in Lab-Scale
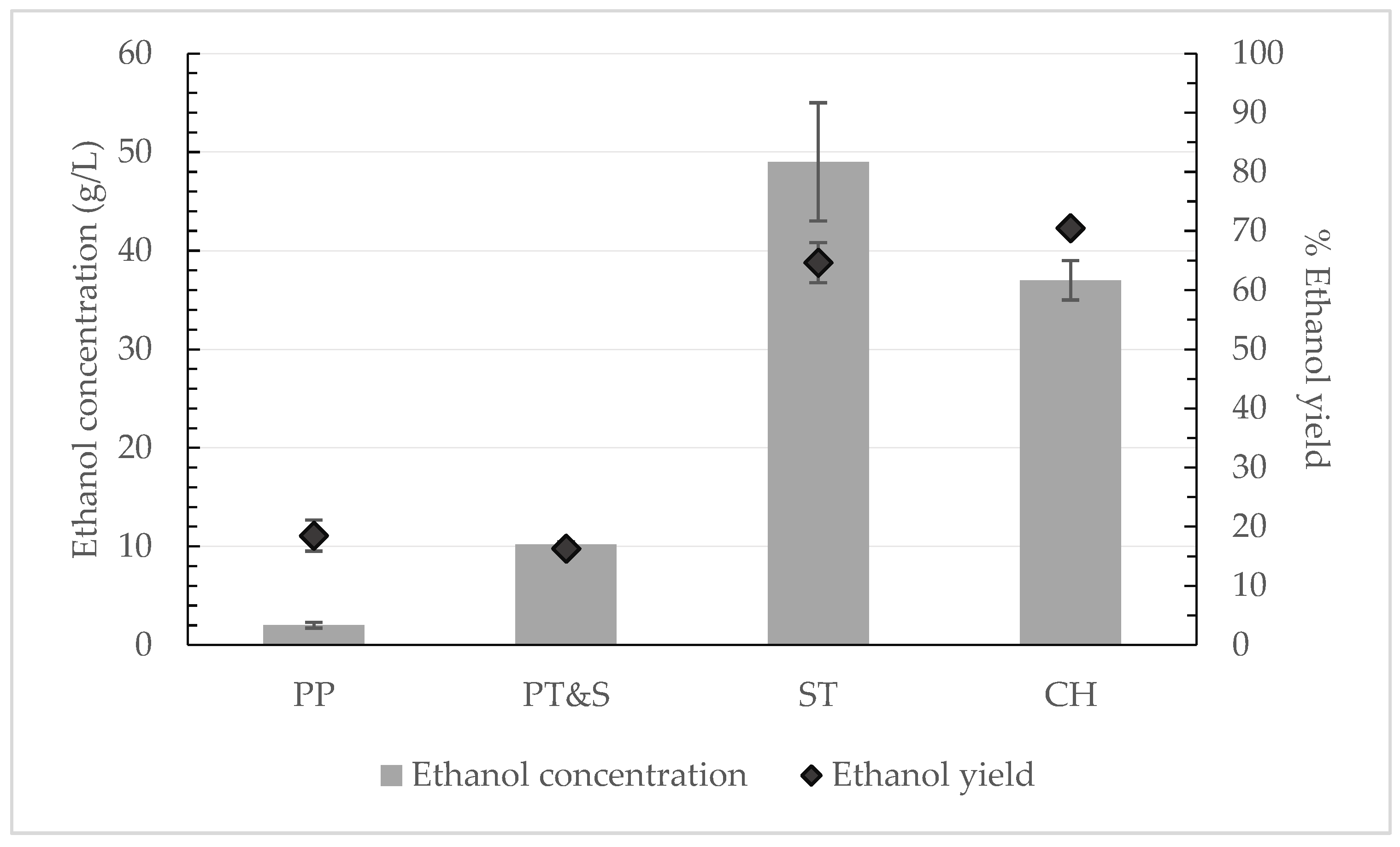
Each feedstock, although they all derive from potatoes, has a different chemical composition and structural characteristics. Thus, the need and the kind of pretreatment technique is dependent on the feedstock. Therefore, various pretreatment methods were examined. At first, no pretreatment was tested; instead, simultaneous saccharification and fermentation (SSF) was applied directly to each substrate. It was decided in advance that, for the substrates that would reach an ethanol yield over 60% without pretreatment, then no pretreatment would be applied, in order to keep the costs as low as possible.
According to
Figure 2, the most effective substrate for producing bioethanol, without the requirement for pretreatment, was found to be the potato chips, since 37.0 ± 2.0
of bioethanol was produced by the use of 40
and 2%
w/
w S. cerevisiae at 35 °C, for 24 h at 15% solid loading. The corresponding ethanol yield obtained was 72.4% ± 3.9%. The thermal processing pretreatment within the production line of the potato chips during frying may have affected positively the ethanol yield. Furthermore, the starch waste also achieved a high bioethanol concentration (49.0 ± 6.0
and rather high ethanol yield, 64.6% ± 3.4%.
Subsequently, three pretreatment methods were applied to the potato peels and the potato tubers and slices in order to select the appropriate one for further study. Firstly, an acid hydrolysis was tested, which can be considered as both pretreatment and hydrolysis methods for the raw material [
30]. Secondly, an alkali pretreatment was applied, which can break down the lignin, providing better access to enzymes for cellulose hydrolysis [
18]. Finally, a hydrothermal pretreatment was conducted, as it modifies the internal structure of the solid, enhancing the enzymatic degradation [
31].
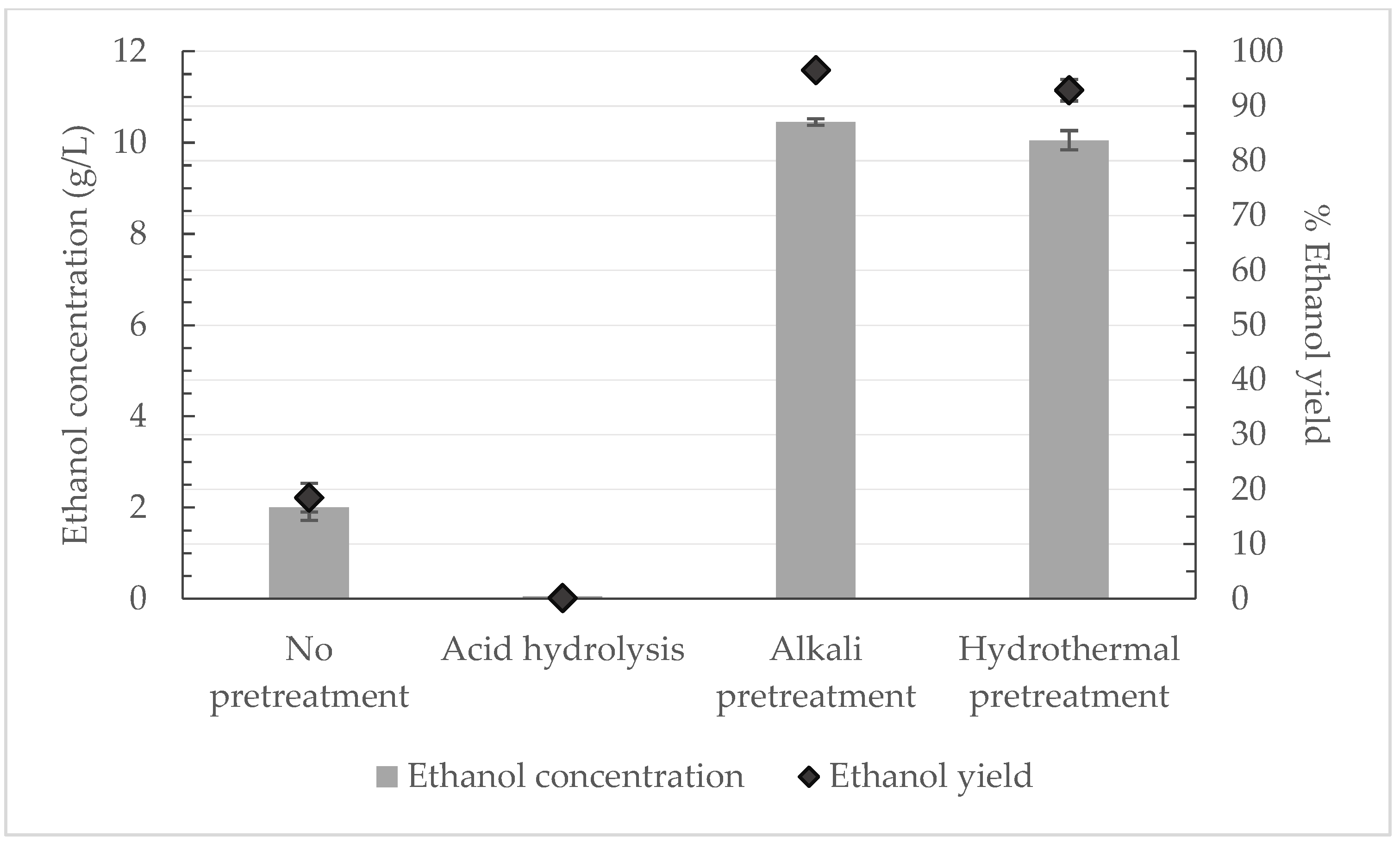
Figure 3 and
Figure 4 illustrate the ethanol concentration and yield that was obtained for each pretreatment method and for each waste stream.
For the potato peels, alkali pretreatment provided the best results, as the ethanol yield reached 96.6% ± 0.7%. Similar ethanol yields were observed from hydrothermal and alkali pretreatment of potato peels, according to Taher et al., which is also inferred from
Figure 3 [
28]. On the other hand, Taher et al. [
28] observed a 58% saccharification yield when potato peels were pretreated with 1%
w/
v NaOH and followed by incubation at 121 °C for 30 min. For the potato tubers and slices, hydrothermal pretreatment seemed favourable and was examined deeper, since the other pretreatment techniques examined did not adequately decompose the carbohydrates. Atitallah et al. [
32] stated that a 96% saccharification yield was obtained via hydrothermal pretreatment, demonstrating the effectiveness of the technique. Nevertheless, a direct comparison with the results of the present study cannot be performed, since a different fermentation mode was applied. Even though hydrothermal pretreatment seems beneficial for all the substrates, it is not always favourable because of its significant energy consumption.
It appears that acid hydrolysis did not benefit any substrate, contrary to the literature, in which the total hydrolysis of the starch and approximately 65–67% ethanol yield was achieved in some reports [
15,
32,
33]. Furthermore, Izmirlioglu et al. [
34] reached a 92% theoretical ethanol yield in biofilm reactors from potato waste hydrolysate at 34 °C. In addition, Hashem et al. [
35] reported that the maximum ethanol yield (97%) was achieved by fully hydrolysed starch (with 1% H
2SO
4 at 100 °C) at 35 °C by
S. cerevisiae.
3.3. Factorial Designs
Based on the optimum pretreatment, four factorial designs, one for each feedstock, were performed to evaluate the bioethanol production in terms of bioethanol concentration. For this purpose, the liquid phase of the residues after fermentation was analysed in terms of ethanol and glucose concentrations. These results are presented in
Table 2,
Table 3,
Table 4 and
Table 5.
The potato peels seem to offer the highest ethanol concentration (9.5 ± 0.4 ), when 1% w/v NaOH was added, with 40 and 175. The ethanol concentration from the potato tubers and slices reached 66.3 ± 1.8 , with 60 and 20% solid loading, after hydrothermal pretreatment and SSF. The starch waste produced 58.0 ± 2.8 of bioethanol, with 60 and 20% solid loading, when SSF took place for 48 h. As far as the chips are concerned, the optimum results were obtained under SSF, with a low dosage of enzyme (20 ) and 20% solid loading, which were the most favourable conditions within the range studied. It should be noted that, in all the cases and waste streams, a fairly high ethanol yield was acquired. Additionally, the fact that the glucose concentration is essentially zero at the end of fermentation implies that S. cerevisiae has completely metabolized it.
Taking into consideration the results of the factorial experiments presented
Table 2,
Table 3,
Table 4 and
Table 5, the following equations were constructed (in coded and physical values) in order to indicate the impact of the chosen operational conditions on the maximum ethanol concentration.
Equations (4)–(7) refer to the coded values, while Equations (8)–(11) refer to the corresponding physical values. The ethanol generation from potato peels seems to be mostly impacted by the quantity of NaOH. For the ethanol production from potato tubers and slices, all the parameters are statistically important, along with their interaction. However, the statistically most important parameter is the solid loading. Contrarily, the ethanol production from starch waste seems to be positively influenced by solid loading, meaning that, as the solid loading increases, the ethanol concentration levels rise, which is beneficial. Of course, this was anticipated, but there are mass low restrictions regarding how high the solid loading may be. Finally, it seems that, in the case of chips, the amount of enzyme negatively affects the ethanol concentration, while the ethanol concentration is again mostly affected by the solid loading. Given the mild pretreatment that potato chips have undergone within the production line, the concentration of glucose may rise extremely quickly, inhibiting the functioning of the yeast.
3.4. Pilot Scale
The overall conversion efficiencies indicate the potential of potato processing industry waste as biomass for large-scale bioethanol production. Thus, experiments for each substrate were conducted on a pilot scale, applying the conditions that reached the maximum ethanol concentration. The potato peels were pretreated with 1% w/v NaOH for 6 h at 50 °C. After temperature and pH adjustment, the mixture of 5% solid loading was fermented at 35 °C by adding 40 , 175 and 2% w/w S. cerevisiae. The potato tubers and slices underwent hydrothermal pretreatment and then SSF of 20% solid loading with 60 and 2% w/w S. cerevisiae at 35 °C. Direct SSF was performed for the starch, with 60 and 2% w/w S. cerevisiae at 35 °C, and also for the chips, with 20 and 2% w/w S. cerevisiae at 35 °C.
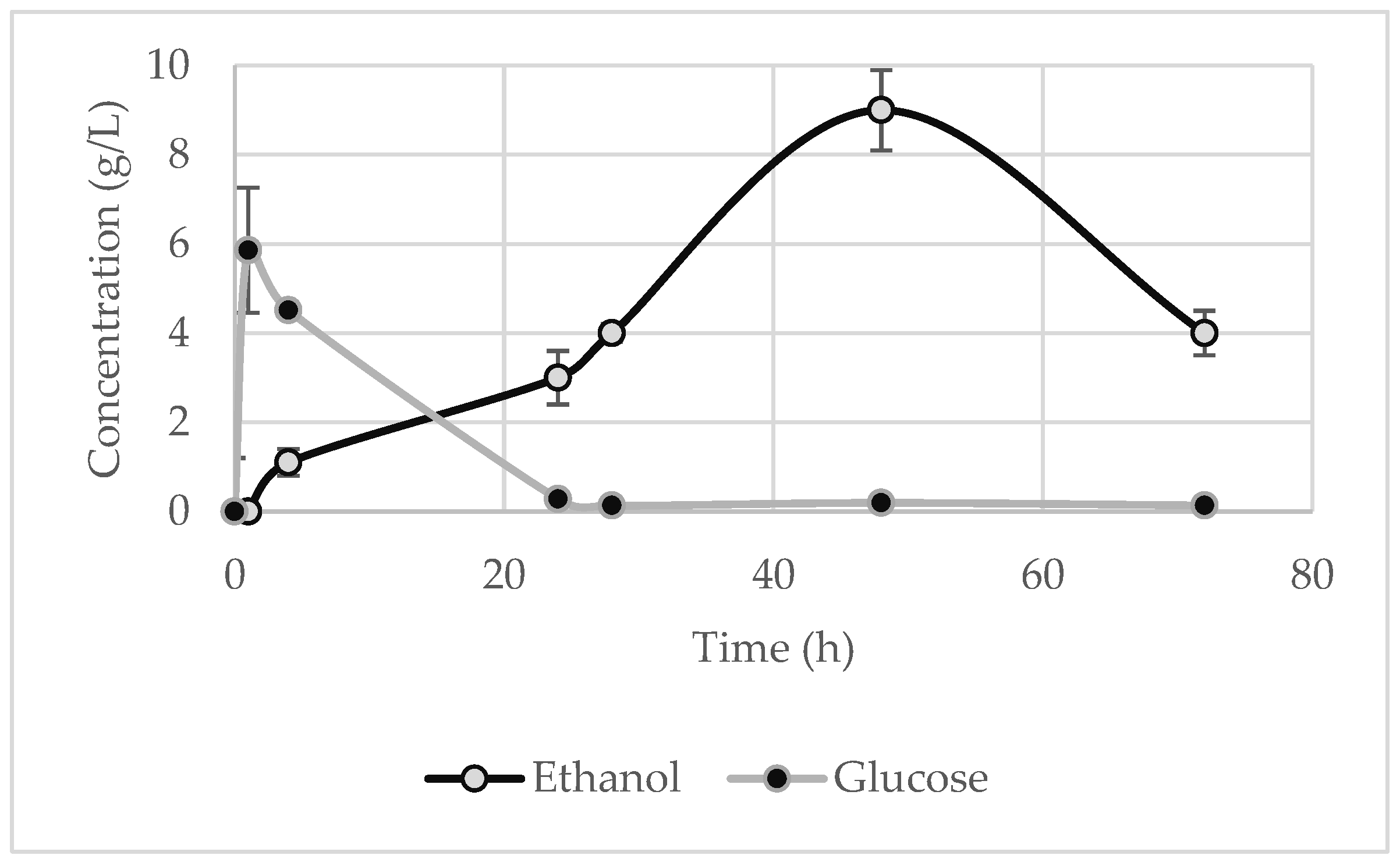
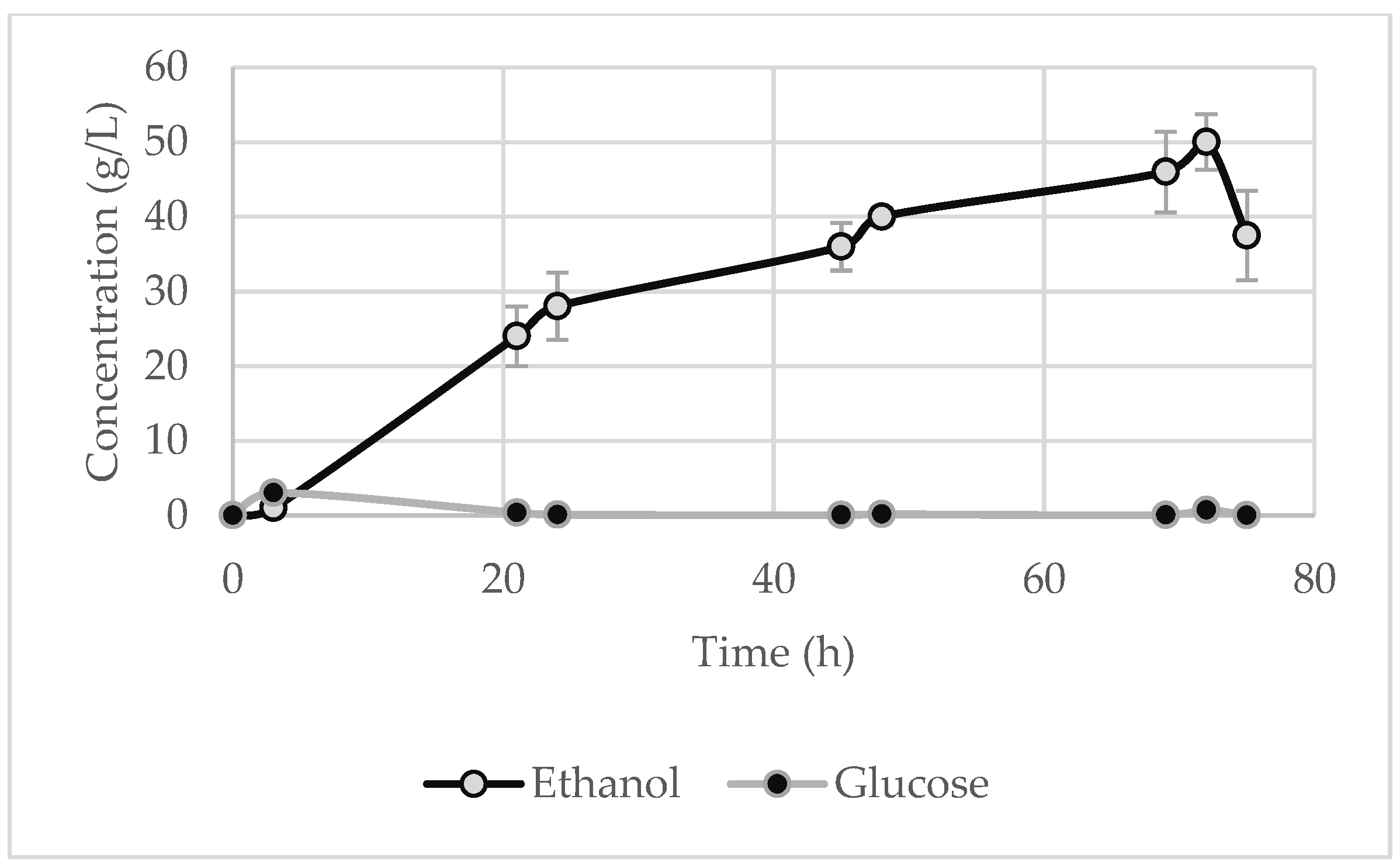
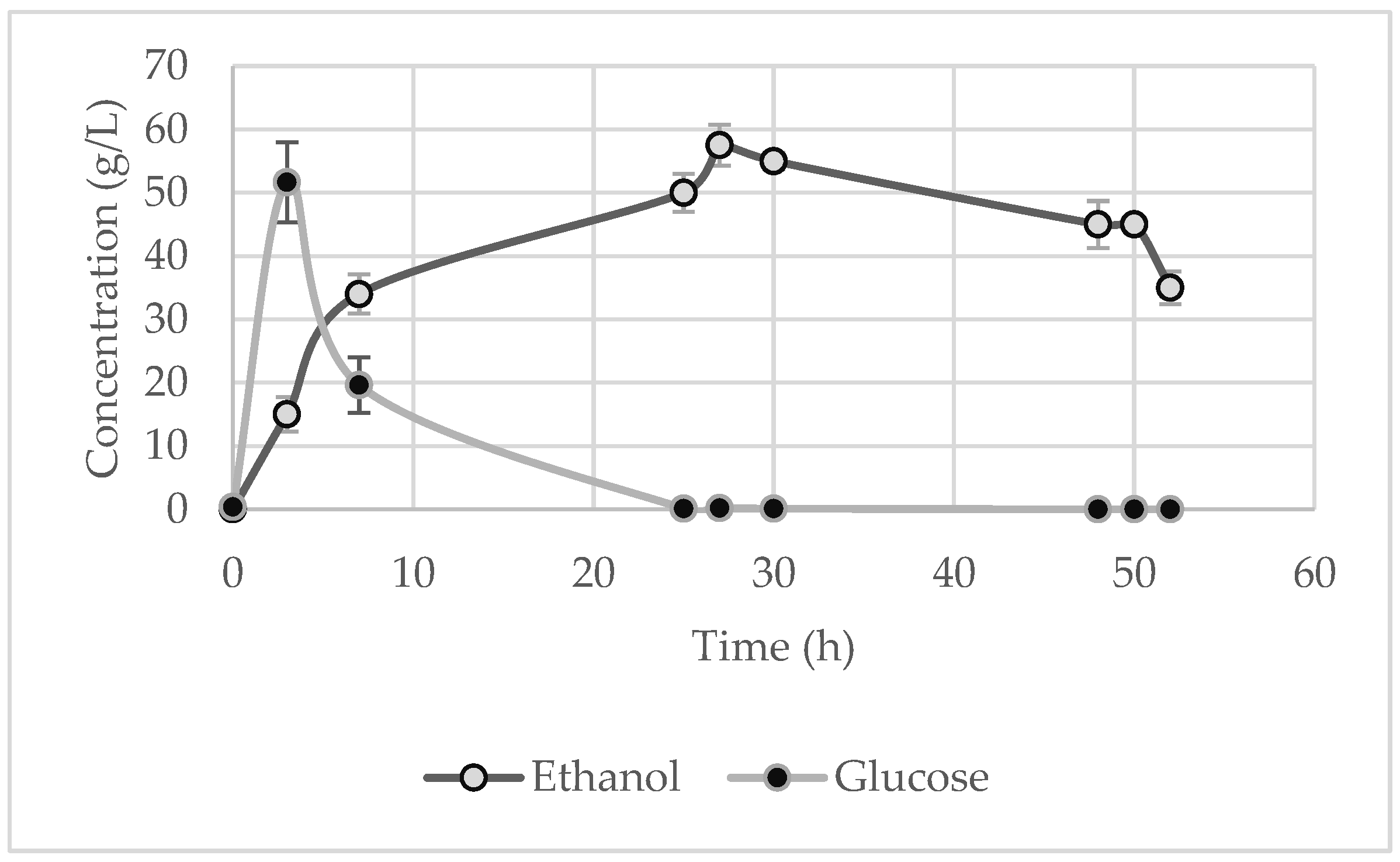
At regular time intervals, a sample was collected to measure the glucose and ethanol in order to study the kinetics of the reaction and to determine the maximum ethanol concentration. It is clear from the figures below that, during the first few hours, the glucose reached a peak before being entirely assimilated by the yeast. It should be noted, though, that for the starch waste (Figure 7), the glucose concentration did not rise as sharply, since probably the high starch content and the low temperature discouraged the enzymes’ ability to rapidly break down the starch. Thus, the microorganisms directly metabolise the produced glucose, as seen by the gradual generation of ethanol, and no sharp peaks are evident for glucose.
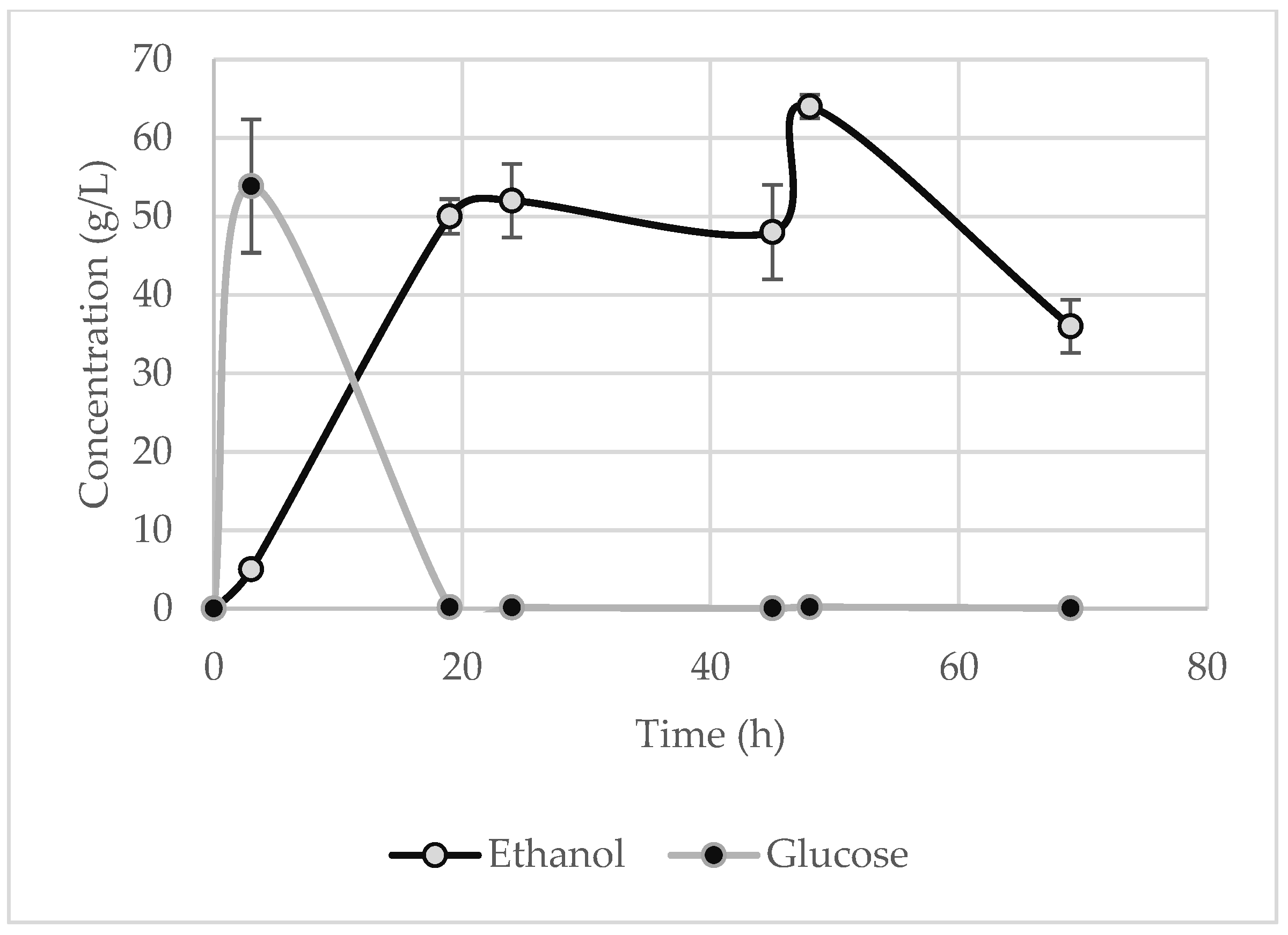
The maximum ethanol concentration was observed at 48 h for the potato peels and the potato tubers and slices, whose values are 9 ± 0.9 g·L
−1 (
Figure 5) and 64 ± 1.5 g·L
−1 (
Figure 6), respectively. These results are identical to those of the laboratory-scale trials. The highest ethanol concentration for the starch waste was recorded at 72 h (50 ± 3.7 g·L
−1) (
Figure 7), which corresponds to a 42.2% ± 2.2% ethanol yield. By comparing these values to the values that had derived from the experiments of the factorial design, lower ethanol concentrations were achieved for longer durations of fermentation. In contrast, the upscaling for the chips operated effectively, since the ethanol concentration reached 57.5 ± 3.2 g·L
−1 after 27 h (
Figure 8). Thus, it can be concluded that, in terms of ethanol production, scaling up by 1000 times generates comparable and, in some cases, greater results.
Table 6 presents the degradation of the total solids and starch of the feedstocks after SSF. As expected, the starch was almost fully converted to glucose, which, in turn, was fully consumed, thereby achieving a high percentage of starch degradation in all the substrates. The solid degradations achieved were also quite high (apart from the potato peels), which is very important given that the solid waste is converted to a bioproduct and to a liquid waste that is more easily handled. It is also worth noting that, for the potato peels, the degradation of cellulose was 69.1% of the initial solid, a quite high degradation efficiency for lignocellulosic feedstocks.
3.5. Full Scale Implementation
3.5.1. Mass Flows
A potato chip company, in 2021, processed 7800 tons of potatoes, producing 2200 tons of chips as the final product. Four distinct waste streams are produced from the potato chip production line: waste from peeling (potato peels), from sorting (potato tubers), from slicing (potato slices and starch) and from frying (bad-quality chips). According to the record data from the company for 2021, 330 tons of potato tubers and slices, 76 tons of potato peels, 201 tons of starch and 76 tons of rejected chips were derived as waste.
Considering 260d annual operation, the potato chips company is processing 30 tons/d potatoes to produce 8.5 tons/d of chips, and the daily mass flows of waste are presented in
Table 7. The quantities of waste are expressed on a dry basis.
3.5.2. Current Waste Management
In regard to the current waste management of the potato chip company, the potato peels along, with the sewage sludge from the existing aerobic wastewater treatment plant, end up in a landfill, resulting in an additional cost for the company. The potato slices and tubers are distributed to farmers free of charge, while the bad-quality potato chips are sold for 80–90 €/ton as animal feed for pigs. Finally, the starch from the slicing step is sold for 120 €/ton to a company for starch glue production. As a result, the company’s profit from waste is approximately 2300 € per month. However, it must be considered that the use of raw waste as animal feed is restricted by European legislation, which outlines the regulations that control the trade of raw ingredients used in animal feed and compound feed [
36]. Apart from that, the landfilling of waste results in an extra environmental cost that burdens the carbon footprint of the industry. Hence, there is a pressing need to strive toward producing food with a low carbon footprint, reducing food waste, and finding new uses for the waste produced during food production.
3.5.3. Valorisation of Waste
According to the results presented in this study, the conditions shown in
Figure 9 were considered optimal for bioethanol production. As mentioned, each feedstock requires different pretreatment, due to the different chemical and structural properties. More specifically, potato peels and potato tubers and slices have high moisture content (85 and 75%), therefore, minimising storage is necessary. Potato peels require alkali pretreatment, while potato tubers and slices need to undergo hydrothermal pretreatment. For the starch and the chips flows, no pretreatment is needed.
A potato chip production line operates five days per week (Monday–Friday), which corresponds to the days of waste generation. However, the bioethanol production unit may operate seven days per week. The assumptions made are as follows: (a) five days waste generation, (b) seven days waste processing, (c) 6 h idle time (loading—temperature and pH adjustment—unloading) for each batch, (d) 6 h alkali pretreatment or 15 min hydrothermal pretreatment at 121 °C, (e) 48 h fermentation, (f) 6 h distillation. Taking into consideration these assumptions, a reactor was designed to process the potato peels in two fermentation batches per week and a second reactor for the potato tubers and slices in three fermentation batches per week. For the processing of the starch and chips, a third reactor is proposed, which will perform three fermentation batches per week.
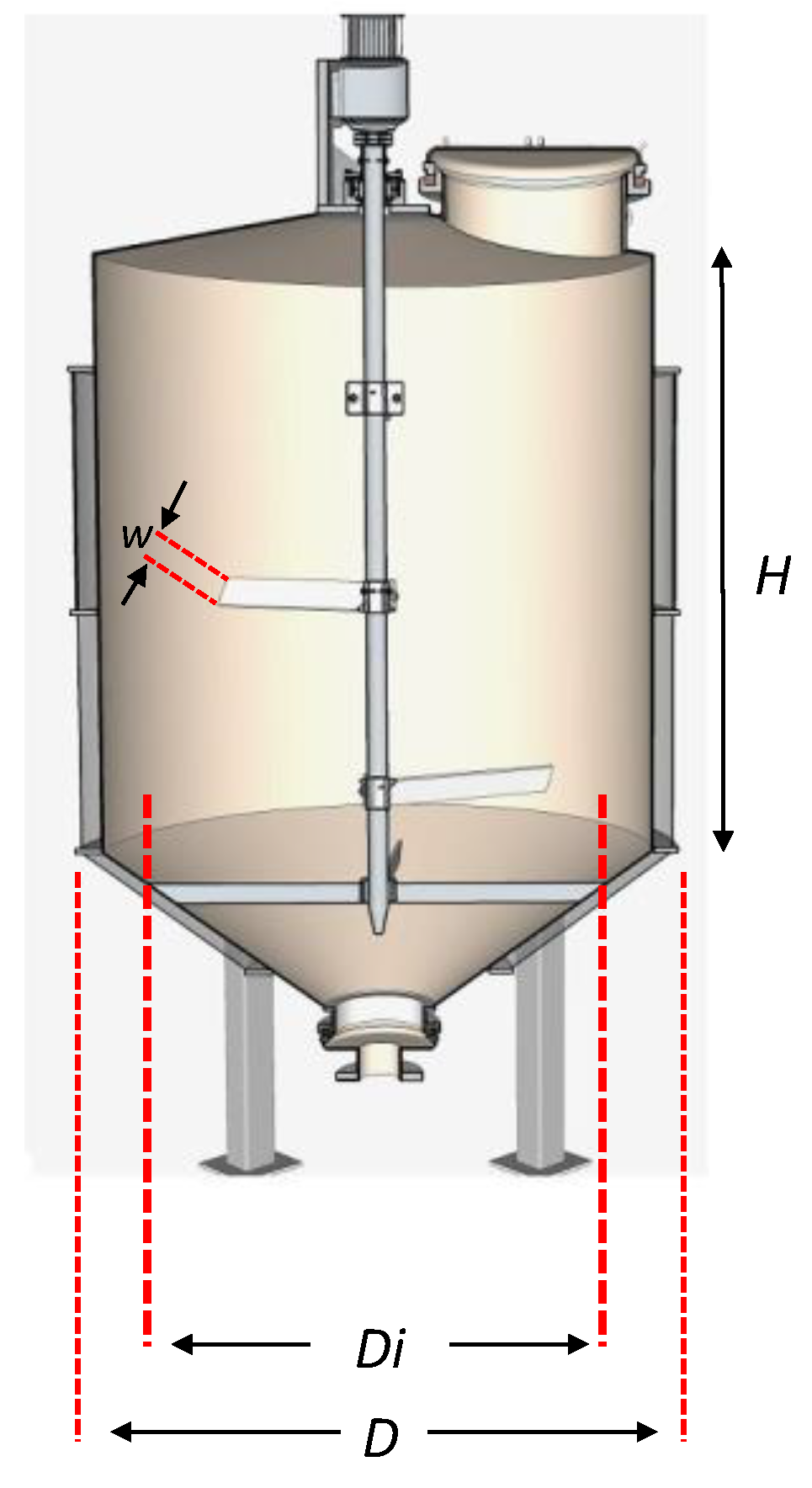
Figure 10 is a graphical representation of the reactor, while
Table 8 presents the dimensions of the three bioreactors.
In view of examining the mass balances and the consumables needed,
Table 9 presents the inputs required per week in mass terms for bioethanol production; waste, water, NaOH, H
2SO
4, enzymes and yeast. There is no need for fresh water, since the treated effluent from the already existing activated sludge system could be used. Alternatively, the water from the starch filtration could be used as input water, with a positive effect on the bioethanol production.
In this way, the weekly outputs produced are bioethanol, water and solid residue. The respective quantities are presented in
Table 9. The solid residue, in the form of a slurry, could be treated along with the wastewater in the activated sludge system. The produced bioethanol, after further dehydration, could be used either within the plant or be promoted to the market, either as a biofuel or fuel additive or as an industrial biosolvent. The bioethanol market is very dynamic in Europe, while there is no production of bioethanol in Greece [
37]. The required quantities are imported. From the experimentation, it was estimated that the total solids of the waste are degraded by 84%. Thus, the solid waste that needs management from the industry on a weekly basis is reduced from 7.0 tons to 1.1 tons.
3.5.4. Economic Considerations
Capital Expenditure
The capital expenditure for the full-scale implementation of the proposed process includes the construction and installation costs of the required number of prototype units. In order to calculate the cost of the necessary equipment, the following equation, which expresses the rule of six-tenths, was used for the capital expenditure:
where
is the cost of equipment of size
(m
2);
is the known cost of equipment of size
(m
2); and
is the dimensionless size factor [
38]. Thus, the total construction cost for a capacity of 17.1m
3 was estimated at 189,655 €.
Moreover, another important aspect of the capital expenditure is the installation cost. In the economic analysis of the pilot plant, it was calculated that the installation cost reached a percentage of 10% of the total capital cost. Therefore, the same percentage can be used to calculate the installation of the full-scale equipment, which reaches the amount of 18,965.5 €. The total capital expenditure for a plant to valorise the waste of the potato chips company is calculated to be 208,620.5 €.
Operating Expenditure
Concerning the operating expenditure of the plant, the annual staff costs are considered low, since the plant will be fully automated and the existing staff of the industry could operate it. Regarding the cost of consumables,
Table 10 presents their cost on a weekly basis, based on the optimum conditions and the applied pretreatment method.
Another aspect of the operational cost is the cost of energy consumption from
Table 11. According to the operation of the pilot plant of a 200L capacity, the energy consumption of the proposed plant was calculated, taking into consideration the upscaling factor.
Conclusively, the total operational cost is calculated at 1647 €/week.
Economic Benefits
As described above, the potential uses of the bioethanol produced will be as biofuel or as biosolvent. It has been estimated that 1928 L/week bioethanol could be produced by the valorisation of potato chips industry waste. The mean price of ethanol is 1.10 USD/L globally. Nevertheless, there is a great price range among countries, from 0.771 in France to 1.433 in Sweden and 1.898 in Spain. Product prices are determined based on four critical factors: (a) product quality, (b) market demand for bioethanol and competing product prices, (c) production and distribution costs, and (d) results from market research, based on how much the consuming public would be willing to spend to obtain the product. The high volatility of the bioethanol price has led to selecting the average value as the price of the bioethanol for the examined business case at 1.2 €/L.
Regarding the capacity of the unit, the plant is expected to produce 1928L per week. Based on the aforementioned, the annual economic benefit from the bioethanol sales is expected to reach 103,649 €.
4. Conclusions
In conclusion, because of the high carbohydrate content in waste derived from the potato chips industry, a wide range of bioprocesses can be utilized to generate value-added bioproducts. An investment in waste treatment could be profitable for a company from an economic and environmental point of view. The volatility of the biofuels market renders it much more interesting.
Simultaneous saccharification and fermentation (SSF) was applied after the appropriate pretreatment, which rendered the desired carbohydrates available to the enzymes, producing glucose, which, in turn, was converted to bioethanol. Under the optimal conditions a typical potato processing company can obtain 1928 L/week of bioethanol. As a result, the valorisation of waste via ethanolic fermentation provides an innovative solution for a potato chips company, since an 84% reduction of solid waste that needs treatment could be achieved, and, at the same time, the monthly economic benefit from the ethanol sales could reach 8639 €, which is 3.7 times greater than the profit from the waste sales to farmers and the glue industry.
It is noteworthy that residues from ethanol distillation may be used as fuel for anaerobic digestion, which produces biogas and biofertilizer, and that the oil content of potato chips can be recovered and used to produce biodiesel. This constitutes a biorefinery approach, based on the circular economy, aiming to enhance the sustainability of the potato industry.
Conclusively, the proposed treatment scheme could improve significantly the industry’s carbon footprint, which may be easily calculated by the application of life cycle assessment techniques.