New Insights into the Biosynthesis of Succinic Acid by Actinobacillus succinogenes with the Help of Its Engineered Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, and Culture Conditions
2.2. Plasmid Construction
2.3. Transformation and Gene-Silenced Mutant Screening of A. succinogenes
2.4. Determination of Extracellular and Intracellular Glucose and SA Concentrations
2.5. Fermentation and Analytical Methods
3. Results and Discussion
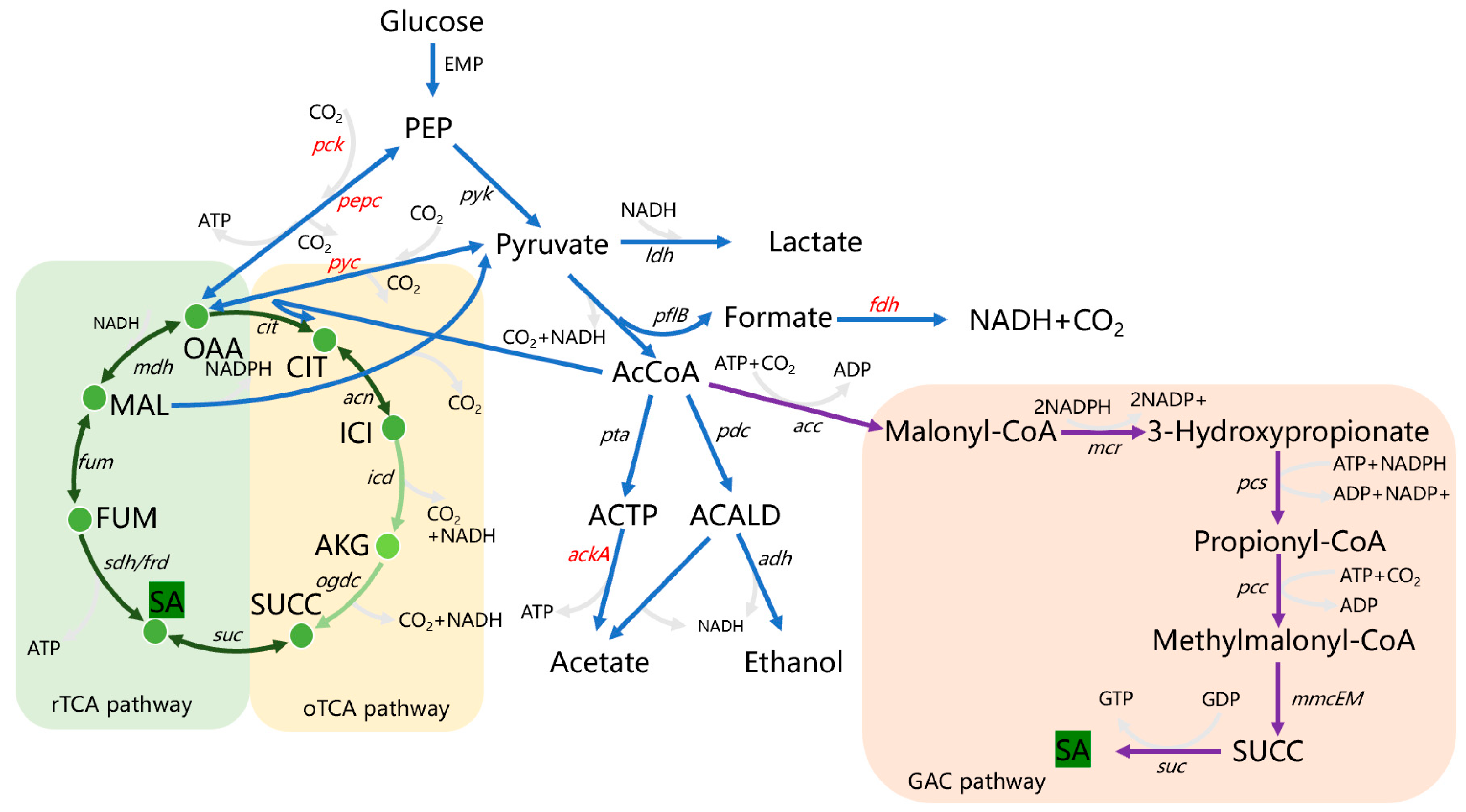
3.1. Succinic acid Biosynthesis Pathway
3.1.1. Effect of Strengthening CO2 Fixation Pathway
3.1.2. Effect of Preventing Oxaloacetic Acid Outflow by Inactivation of Oxaloacetic Acid Decarboxylase
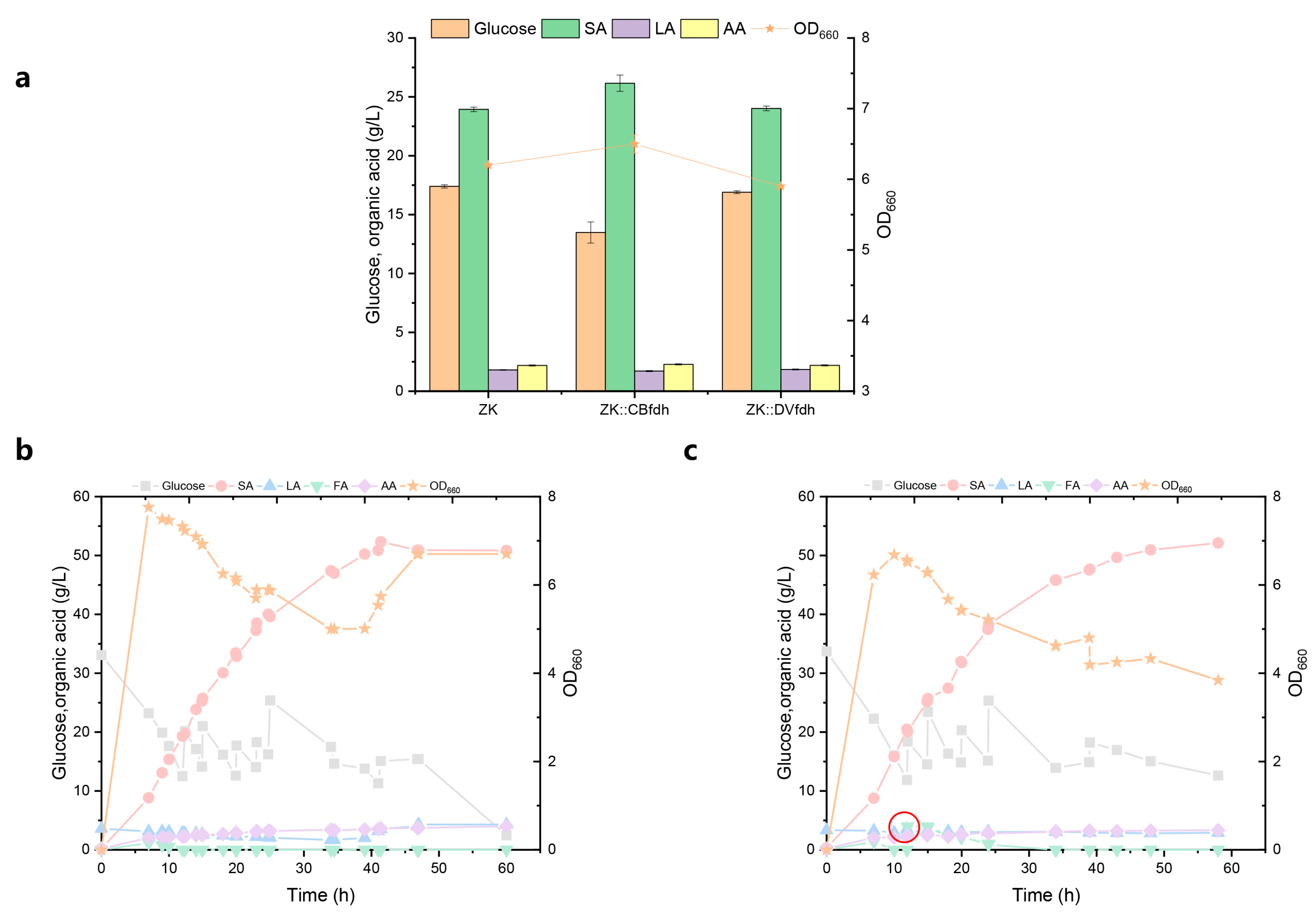
3.2. Effect of Formic Acid Pathway
3.3. Effect of Acetic Acid Pathway
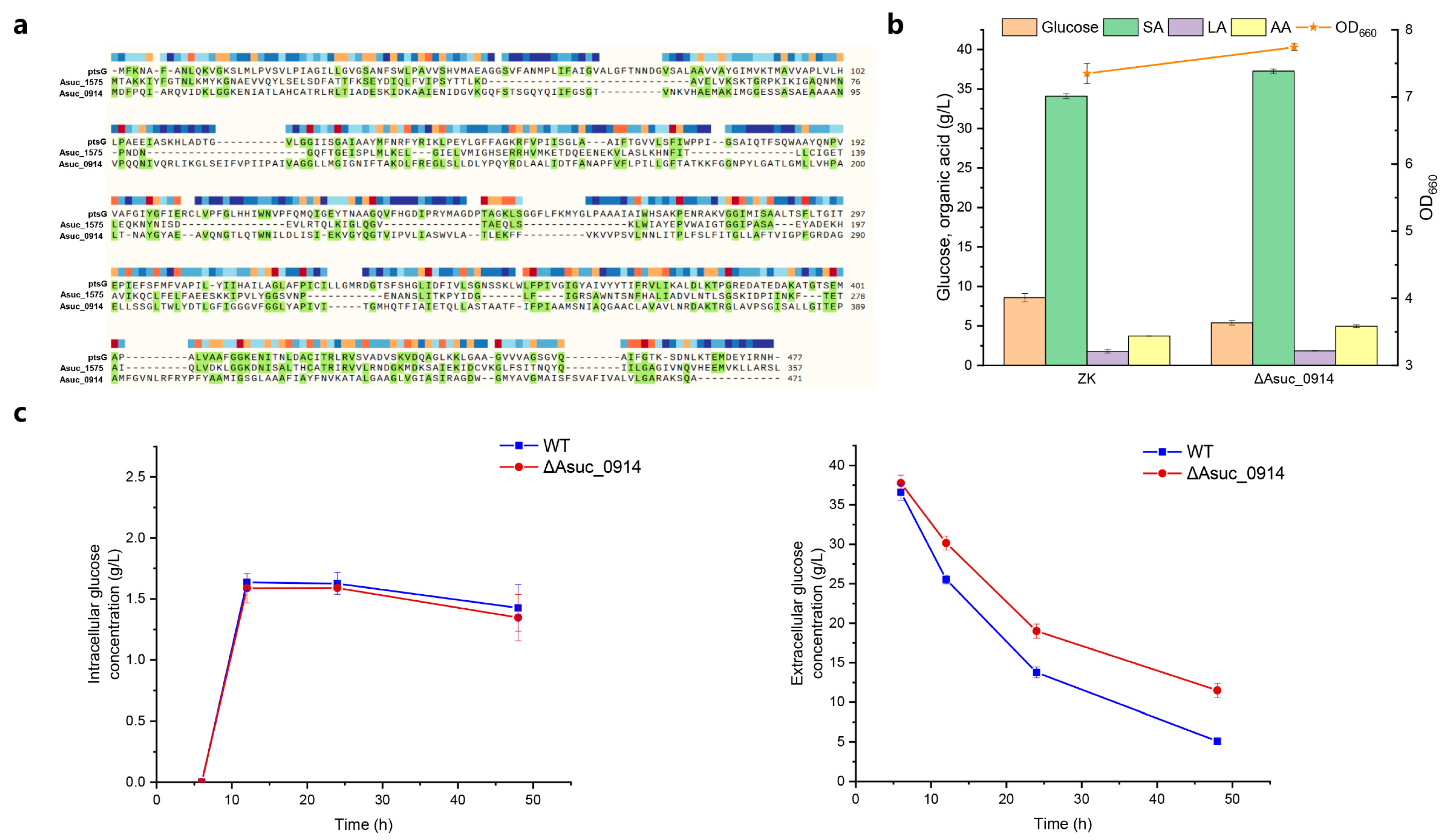
3.4. Mining and Identification of Glucose Transporter
3.5. Mining and Identification of SA Transporter
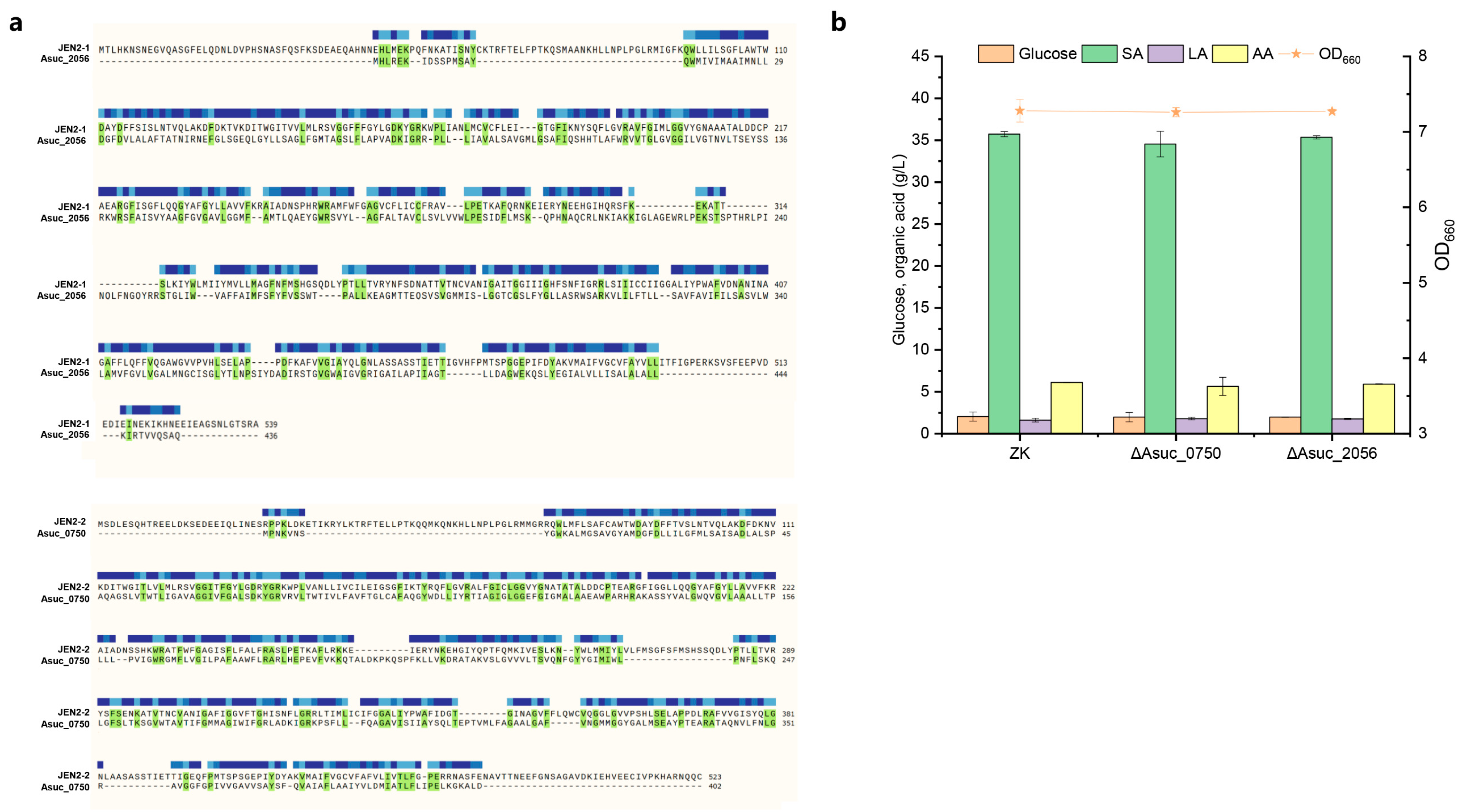
3.5.1. Mining and Identification of SA Importer
3.5.2. Mining and Identification of SA Exporter
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, S.F.; Zhang, Y.L.; Wei, Z.W.; Park, S. Recent advances in metabolic engineering of microorganisms for the production of monomeric C3 and C4 chemical compounds. Bioresour. Technol. 2023, 377, 128973. [Google Scholar] [CrossRef] [PubMed]
- Vivek, N.; Okibe, M.C.; Amulya, K.; Jokodola, E.O.; Coulon, F.; Kumar Tyagi, V.; Lens, P.N.L.; Binod, P.; Vinod, K. Technological advancements in valorization of second generation (2G) feedstocks for bio-based succinic acid production. Bioresour. Technol. 2022, 360, 127513. [Google Scholar] [CrossRef]
- Ewing, T.A.; Niels, N.; Lint, M.; Haveren, J.; Jeroen, H.; Es, D.S. Fermentation for the production of biobased chemicals in a circular economy: A perspective for the period 2022–2050. Green Chem. 2021, 24, 6373–6405. [Google Scholar] [CrossRef]
- Tong, U.; Jung, H.A.; Yoo-Sung, A.; Je, W.K.; Jong, A.L.; Eon, H.L. Metabolic engineering for the production of dicarboxylic acids and diamines. Metab. Eng. 2020, 58, 2–16. [Google Scholar] [CrossRef]
- Liu, X.T.; Zhao, G.; Sun, S.J.; Fan, C.L.; Feng, X.J.; Xiong, P. Biosynthetic pathway and metabolic engineering of succinic acid. Front. Bioeng. Biotechnol. 2022, 10, 843887. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Bennett, G.N.; San, K.Y. Fed-batch culture of a metabolically engineered Escherichia coli strain designed for high-level succinate production and yield under aerobic conditions. Biotechnol. Bioeng. 2005, 90, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Khai, L.O.; Cui, Z.Y.; Sang, Z.Y.; Li, X.T.; Patria, R.D.; Qi, Q.S.; Fickers, P.; Yan, J.B.; Lin, C.S.K. Promising advancement in fermentative succinic acid production by yeast hosts. J. Hazard. Mater. 2020, 401, 123414. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, J.; Wang, D.; Feng, Q.I. Advancement in genetic engineering for production of succinic acid by Escherichia coli. J. Chin. Biotechnol. 2009, 29, 108–117. [Google Scholar] [CrossRef]
- Ahn, J.H.; Seo, H.; Park, W.; Seok, J.; Lee, J.A.; Kim, W.J.; Kim, G.B.; Kim, K.J.; Lee, S.Y. Enhanced succinic acid production by Mannheimia employing optimal malate dehydrogenase. Nat. Commun. 2020, 11, 1970. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhou, Y.J.; Zhang, D. Engineering Corynebacterium crenatum for enhancing succinic acid production. J. Food Biochem. 2018, 42, 10. [Google Scholar] [CrossRef]
- Yuzbashev, T.V.; Yuzbasheva, E.Y.; Sobolevskaya, T.I.; Laptev, I.A.; Vybornaya, T.V.; Larina, A.S.; Matsui, K.; Fukui, K.; Sineoky, S.P. Production of succinic acid at low pH by a recombinant strain of the aerobic Yeast Yarrowia lipolytica. Biotechnol. Bioeng. 2010, 107, 673–682. [Google Scholar] [CrossRef]
- Wieschalka, S.; Michael, B.B.; Eikmanns, B.B.J. Bio-based production of organic acids with Corynebacterium glutamicum. Microb. Biotechnol. 2013, 6, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Franco-Duarte, R.; Bessa, D.; Goncalves, F.; Martins, R.; Silva-Ferreira, A.C.; Schuller, D.; Sampaio, P.; Pais, C. Genomic and transcriptomic analysis of Saccharomyces cerevisiae isolates with focus in succinic acid production. FEMS Yeast Res. 2017, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.G.; Mishra, S.; Bhagwat, S.S.; Shafaei, S.; Shen, Y.h.; Allen, J.L.; Crosly, B.A.; Tan, S.I.; Fatma, Z.; Rabinowitz, J.D.; et al. An end-to-end pipeline for succinic acid production at an industrially relevant scale using Issatchenkia orientalis. Nat. Commun. 2023, 14, 6152. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, X.F.; Gao, S.; Wang, H.M.; Lin, C.S.K. High efficiency succinic acid production from glycerol via in situ fibrous bed bioreactor with an engineered Yarrowia lipolytica. Bioresour. Technol. 2017, 225, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Guettler, M.V.; Rumler, D.; Jain, M.K. Actinobacillus succinogenes sp. nov., a novel succinic-acid-producing strain from the bovine rumen. Int. J. Syst. Bacteriol. 1999, 49, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, M.; Dai, Z.X.; Xin, F.X.; Zhou, J.; Dong, W.L.; Ma, J.F.; Jiang, M.; Zhang, W.M. Comprehensive investigation of succinic acid production by Actinobacillus succinogenes: A promising native succinic acid producer. Biofuels Bioprod. Biorefin. 2020, 14, 950–964. [Google Scholar] [CrossRef]
- Hu, S.M.; You, Y.; Xia, F.F.; Liu, J.M.; Dai, W.C.; Liu, J.S.; Wang, Y.H. Genome shuffling improved acid-tolerance and succinic acid production of Actinobacillus succinogenes. Food Sci. Biotechnol. 2018, 28, 817–822. [Google Scholar] [CrossRef]
- Zhang, W.M.; Tao, W.X.; Wu, M.; Xin, F.X.; Dong, W.L.; Zhou, J.; Gu, J.C.; Ma, J.F.; Jiang, M. Adaptive evolution improves acid tolerance and succinic acid production in Actinobacillus succinogenes. Process Biochem. 2020, 98, 76–82. [Google Scholar] [CrossRef]
- McKinlay, J.B.; Laivenieks, M.; Schindler, B.D.; McKinlay, A.A.; Siddaramappa, S.; Challacombe, J.F.; Lowry, S.R.; Clum, A.; Lapidus, A.L.; Burkhart, K.B.; et al. A genomic perspective on the potential of Actinobacillus succinogenes for industrial succinate production. BMC Genom. 2010, 11, 680. [Google Scholar] [CrossRef]
- Dessie, W.; Xin, F.; Zhang, W.; Jiang, Y.; Wu, H.; Ma, J.; Jiang, M. Opportunities, challenges, and future perspectives of succinic acid production by Actinobacillus succinogenes. Appl. Microbiol. Biotechnol. 2018, 102, 9893–9910. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.T.; Zhou, Z.H.; Wang, C.; Chen, Z.J.; Cai, H. Enhanced succinic acid production in Corynebacterium glutamicum with increasing the available NADH supply and glucose consumption rate by decreasing H+-ATPase activity. Biotechnol. Lett. 2016, 38, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Wang, C.; Chen, Y.L.; Zhang, K.; Xu, H.T.; Cai, H.; Chen, Z.J. Increasing available NADH supply during succinic acid production by Corynebacterium glutamicum. Biotechnol. Prog. 2015, 31, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.T.; Feng, X.J.; Ding, Y.M.; Gao, W.J.; Xian, M.; Wang, J.C.; Zhao, J. Characterization and directed evolution of propionyl-CoA carboxylase and its application in succinate biosynthetic pathway with two CO2 fixation reactions. Metab. Eng. 2020, 62, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Song, H.; Lee, S.Y. Genome-based metabolic engineering of Mannheimia succiniciproducens for succinic acid production. Appl. Environ. Microbiol. 2006, 72, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Kildegaard, K.R.; Niaei, A.; Hosseini, M.; Ebrahimi, S.; Sudarsan, S.; Angelidaki, I.; Borodina, I. Engineering oleaginous yeast as the host for fermentative succinic acid production from glucose. Front. Bioeng. Biotechnol. 2019, 7, 361. [Google Scholar] [CrossRef]
- Guarnieri, M.T.; Chou, Y.C.; Salvachúa, D.; Mohagheghi, A.; Beckham, G.T. Metabolic engineering of Actinobacillus succinogenes provides insights into succinic acid biosynthesis. Appl. Environ. Microb. 2017, 83, e00996-17. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Q.; Wu, M.; Liu, H.J.; Zhou, J.; Dong, W.L.; Ma, J.F.; Jiang, M.; Xin, F.X. Metabolic regulation of organic acid biosynthesis in Actinobacillus succinogenes. Front. Bioeng. Biotechnol. 2019, 7, 216. [Google Scholar] [CrossRef]
- Chen, C.M.; Zhang, Q.; Qian, J.J.; Wu, D.; Chen, P.C.; Zheng, P. Effect of the Gad system on Actinobacillus succinogenes during acid stress. Syst. Microbiol. Biomanuf. 2022, 2, 9. [Google Scholar] [CrossRef]
- Chen, C.M.; Zheng, P. Effects of down-regulation of ackA expression by CRISPR-dCpf1 on succinic acid production in Actinobacillus succinogenes. AMB Express 2023, 13, 12. [Google Scholar] [CrossRef]
- Litsanov, B.; Kabus, A.; Brocker, M.; Bott, M. Efficient aerobic succinate production from glucose in minimal medium with Corynebacterium glutamicum. Microb. Biotechnol. 2012, 5, 116–128. [Google Scholar] [CrossRef]
- Lin, H.; San, K.Y.; Bennett, G.N. Effect of Sorghum vulgare phosphoenolpyruvate carboxylase and Lactococcus lactis pyruvate carboxylase coexpression on succinate production in mutant strains of Escherichia coli. Appl. Microbiol. Biotechnol. 2005, 67, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Lexow, W.G.; Mokwatlo, S.C.; Brink, H.G.; Nicol, W. Identifying energy extraction optimisation strategies of Actinobacillus succinogenes. Catalysts 2021, 11, 1016. [Google Scholar] [CrossRef]
- Chatterjee, R.; Millard, C.S.; Champion, K.; Clark, D.P.; Donnelly, M.I. Mutation of the ptsG gene results in increased production of succinate in fermentation of glucose by Escherichia coli. Appl. Environ. Microb. 2001, 67, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.Y.; Zhan, T.; Xu, H.T.; Chen, J.; Bi, C.H.; Fan, F.Y.; Zhang, X.L. Characterization of JEN family carboxylate transporters from the acid-tolerant yeast Pichia kudriavzevii and their applications in succinic acid production. Microb. Biotechnol. 2021, 14, 1130–1147. [Google Scholar] [CrossRef]
- Fukui, K.; Nanatani, K.; Hara, Y.; Yamakami, S.; Yahagi, D.; Chinen, A.; Tokura, M.; Abe, K. Escherichia coli yjjPB genes encode a succinate transporter important for succinate production. Biosci. Biotechnol. Biochem. 2017, 81, 1837–1844. [Google Scholar] [CrossRef][Green Version]
- Fukui, K.; Koseki, C.; Yamamoto, Y.; Nakamura, J.; Sasahara, A.; Yuji, R.; Hashiguchi, K.; Usuda, Y.; Matsui, K.; Kojima, H.; et al. Identification of succinate exporter in Corynebacterium glutamicum and its physiological roles under anaerobic conditions. J. Biotechnol. 2011, 154, 25–34. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Zheng, P. New Insights into the Biosynthesis of Succinic Acid by Actinobacillus succinogenes with the Help of Its Engineered Strains. Fermentation 2023, 9, 1026. https://doi.org/10.3390/fermentation9121026
Chen C, Zheng P. New Insights into the Biosynthesis of Succinic Acid by Actinobacillus succinogenes with the Help of Its Engineered Strains. Fermentation. 2023; 9(12):1026. https://doi.org/10.3390/fermentation9121026
Chicago/Turabian StyleChen, Chunmei, and Pu Zheng. 2023. "New Insights into the Biosynthesis of Succinic Acid by Actinobacillus succinogenes with the Help of Its Engineered Strains" Fermentation 9, no. 12: 1026. https://doi.org/10.3390/fermentation9121026
APA StyleChen, C., & Zheng, P. (2023). New Insights into the Biosynthesis of Succinic Acid by Actinobacillus succinogenes with the Help of Its Engineered Strains. Fermentation, 9(12), 1026. https://doi.org/10.3390/fermentation9121026






