Influence of Fermentation Time on the Chemical and Functional Composition of Different Cocoa Clones from Southern Colombia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Selection of Cocoa Clones
2.2. Fermentation, Drying and Roasting of Cocoa Beans
2.3. Determination of Chemical Characteristics of Beans from Different Cocoa Clones during the Fermentation Process
2.3.1. Component Bromatological
2.3.2. Phenolic Compounds
2.3.3. Antioxidant Activity Compounds
2.4. Statistical Analysis
3. Results
3.1. Bromatological Characteristics of Beans from Different Cocoa Clones during the Fermentation Process
3.2. Phenolic Content and Antioxidant Activity of Beans from Different Cocoa Clones during the Fermentation Process
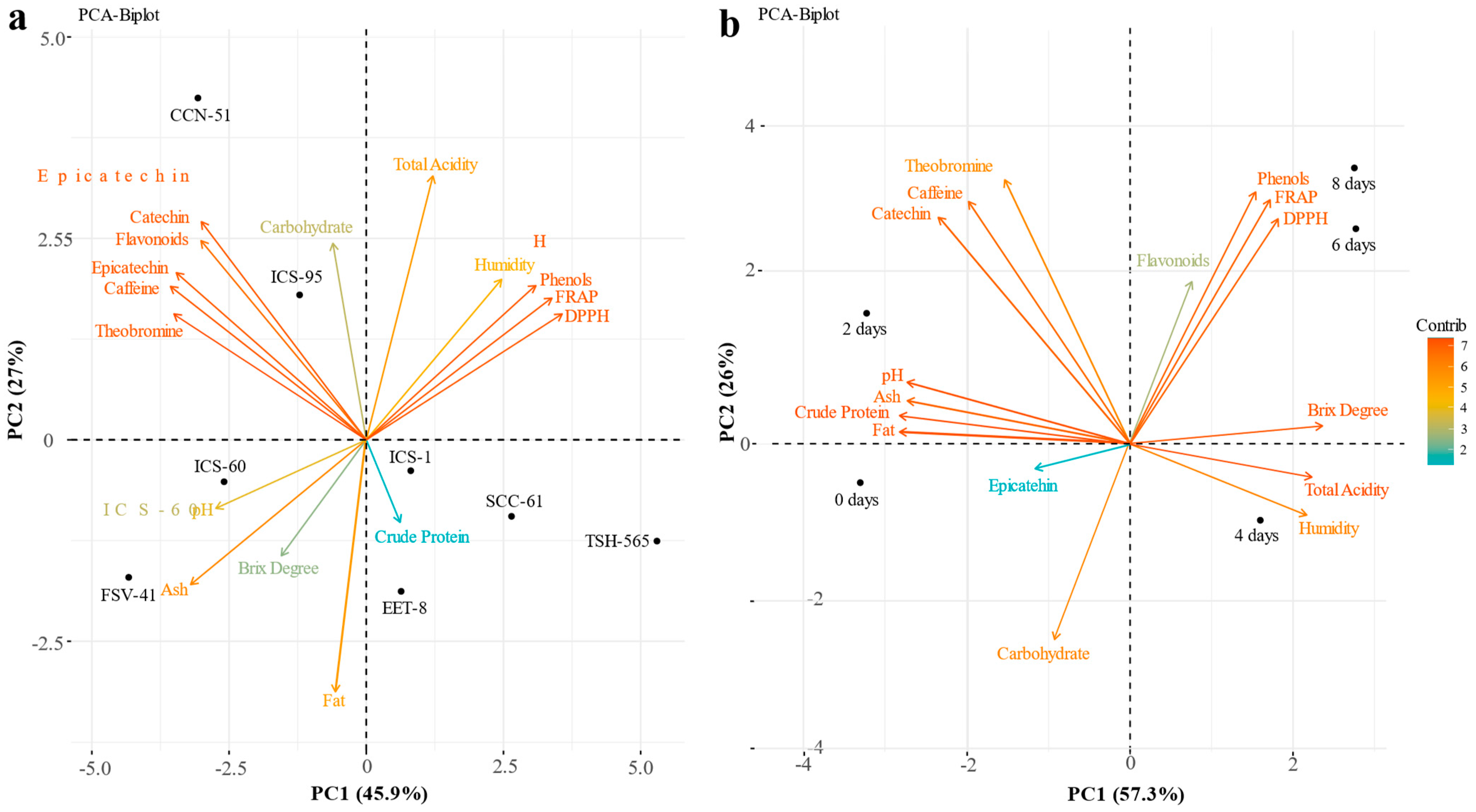
3.3. Correlations between the Different Bromatological Characteristics, Phenolic Compounds and Antioxidant Activity of Cocoa Beans
4. Discussion
4.1. Bromatological Characteristics of Beans from Different Cocoa Clones during the Fermentation Process
4.2. Phenolic Content and Antioxidant Activity of Beans from Different Cocoa Clones during the Fermentation Process
4.3. Correlation of Bromatological Characteristics, Phenolic Compounds, and Antioxidant Activity of Beans from Different Cocoa Clones during the Fermentation Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICCO. ICCO Quarterly Bulletin of Cocoa Statistics; The International Cocoa Organization (ICCO) Cocoa Producing and Cocoa Consuming Countries; Supply & Demand QBCS: London, UK, 2022; Volume XLVIII No. 1. [Google Scholar]
- ICCO. Quarterly Bulletin of Cocoa Statistics; ICCO: Côte d’Ivoire, France, 2017; pp. 20–29. [Google Scholar]
- Hernández-Núñez, H.E.; Gutiérrez-Montes, I.; Bernal-Núñez, A.P.; Gutiérrez-García, G.A.; Suárez, J.C.; Casanoves, F.; Flora, C.B. Cacao cultivation as a livelihood strategy: Contributions to the well-being of Colombian rural households. Agric. Hum. Values 2022, 39, 201–216. Available online: https://link.springer.com/article/10.1007/s10460-021-10240-y (accessed on 18 October 2023). [CrossRef]
- Barrera, V.; Alwang, J.; Casanova, T.; Domínguez, J.; Escudero, L.; Loor, G.; Tarqui, O.; Plaza, L.; Sotomayor, I.; Zambrano, F.; et al. La Cadena de Valor del Cacao en y el Bienestar de los Productores de la Provincia de Manabí-Ecuador. Iniap. 2019, pp. 1–204. Available online: http://repositorio.iniap.gob.ec/handle/41000/5382 (accessed on 19 February 2023).
- FEDECACAO. Colombia Cacaotera. Federación Nacional de Cacaoteros. 2022. 24. Available online: https://drive.google.com/file/d/1w2tBLP8IG6faDgdYacgLn9mIdXI0qBNL/view (accessed on 19 February 2023).
- González, X. La Producción de Cacao Alcanzó Cifra Récord en 2020 y Llegó a las 63,416 Toneladas. La República. 2021. p. Agronegocios. Available online: https://www.google.com/search?q=La+producción+de+cacao+alcanzó+cifra+récord+en+2020+y+llegó+a+las+63.416+toneladas.+La+Nación&hl=es&sxsrf=AJOqlzWkylahtjJvPaEGAA5c847XMIBkWQ%3A1676821783080&source=hp&ei=F0XyY_XbAZeawbkPmsyU0AU&iflsig=AK50M_UAAAAAY_JTJzJknS (accessed on 19 February 2023).
- Gómez, J.; González, K.; Londoño, C.; López, V. Analysis of Variables for the Export of Colombian cocoa to European Countries. Bachelor’s Thesis, Fundación Universitaria Catolica, Cali, Colombia, 2017. Available online: https://repository.unicatolica.edu.co/handle/20.500.12237/862 (accessed on 18 October 2023).
- Kongor, J.E.; Hinneh, M.; de Walle, D.; Van Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—A review. In Food Research International; Elsevier: Amsterdam, The Netherlands, 2016; Volume 82, pp. 44–52. [Google Scholar]
- Hue, C.; Gunata, Z.; Breysse, A.; Davrieux, F.; Boulanger, R.; Sauvage, F.X. Impact of fermentation on nitrogenous compounds of cocoa beans (Theobroma cacao L.) from various origins. Food Chem. 2016, 192, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M.E. Dynamics of volatile and non-volatile compounds in cocoa (Theobroma cacao L.) during fermentation and drying processes using principal components analysis. Food Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- Zapata Bustamante, S.; Tamayo Tenorio, A.; Alberto Rojano, B. Efecto del Tostado Sobre los Metabolitos Secundarios y la Actividad Antioxidante de Clones de Cacao Colombiano. Rev. Fac. Nac. Agron. Medellín 2015, 68, 7497–7507. Available online: https://revistas.unal.edu.co/index.php/refame/article/view/47836 (accessed on 19 February 2023). [CrossRef]
- Martinez, N. Evaluación de Componentes Físicos, Químicos, Organolépticos y de Rendimiento de Clones Universales y regionales de Cacao (Theobroma cacao L.) en las Zonas Productoras de Santander, Arauca y Huila. Master’s Thesis, Universidad Nacional de Colombia, Facultad de Ciencias, Bogotá, Colombia, 2016. [Google Scholar]
- Ramírez González, M.B.; Cely Niño, V.H.; Ramírez, S.I. Actividad antioxidante de clones de cacao (Theobroma cacao L.) finos y aromáticos cultivados en el estado de Chiapas, México. Perspect. En Nutr. Humana 2013, 15, 27–47. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0124-41082013000100002&lng=en&nrm=iso&tlng=es (accessed on 19 February 2023). [CrossRef]
- Nazario, O.; Ordoñez, E.; Mandujano, Y.; Arévalo, J. Polifenoles totales, antocianinas, capacidad antioxidante de granos secos y análisis sensorial del licor de cacao (Theobroma cacao L.) criollo y siete clones. Investig. Amaz. 2013, 3, 51–59. [Google Scholar]
- Sukha, D.A.; Butler, D.R.; Umaharan, P.; Boult, E. The use of an optimised organoleptic assessment protocol to describe and quantify different flavour attributes of cocoa liquors made from Ghana and Trinitario beans. Eur. Food Res. Technol. 2008, 226, 405–413. Available online: https://link.springer.com/article/10.1007/s00217-006-0551-2 (accessed on 18 February 2023). [CrossRef]
- Quezada, L.; Quevedo, J.; García, R. Determinación del efecto del grado de madurez de las mazorcas en la producción y la calidad sensorial de (Theobroma cacao L.). Rev. Científica Agroecosistemas 2017, 5, 36–46. Available online: https://aes.ucf.edu.cu/index.php/aes/article/view/139 (accessed on 19 February 2023).
- Roos, W. Índice Mip de Algunos Cultivos Tropicales. EDICIONES. 2015. 119p. Available online: https://www.cabi.org/wp-content/uploads/Rogg-2000b-IPM-in-tropical-crops.pdf (accessed on 19 February 2023).
- Boza, E.J.; Motamayor, J.C.; Amores, F.M.; Cedeño-Amador, S.; Tondo, C.L.; Livingstone, D.S.; Schnell, R.J.; Gutiérrez, O.A. Genetic characterization of the cacao cultivar CCN 51: Its impact and significance on global cacao improvement and production. J. Am. Soc. Hortic. Sci. 2014, 139, 219–229. Available online: https://journals.ashs.org/jashs/view/journals/jashs/139/2/article-p219.xml (accessed on 19 February 2023). [CrossRef]
- Bekele, F.L.; Bekele, I.; Butler, D.R.; Bidaisee, G.G. Patterns of morphological variation in a sample of cacao (Theobroma cacao L.) germplasm from the International Cocoa Genebank, Trinidad. Genet. Resour. Crop Evol. 2006, 53, 933–948. [Google Scholar] [CrossRef]
- Johnson, E.S.; Bekele, F.L.; Brown, S.J.; Song, Q.; Zhang, D.; Meinhardt, L.W.; Schnell, R.J. Population Structure and Genetic Diversity of the Trinitario Cacao (Theobroma cacao L.) from Trinidad and Tobago. Crop Sci. 2009, 49, 564–572. Available online: https://onlinelibrary.wiley.com/doi/full/10.2135/cropsci2008.03.0128 (accessed on 20 February 2023). [CrossRef]
- Hernández-Núñez, H.E.; Gutiérrez-Montes, I.; Sánchez-Acosta, J.R.; Rodríguez-Suárez, L.; Gutiérrez-García, G.A.; Suárez-Salazar, J.C.; Casanoves, F. Agronomic conditions of cacao cultivation: Its relationship with the capitals endowment of Colombian rural households. Agrofor. Syst. 2020, 94, 2367–2380. [Google Scholar] [CrossRef]
- Loureiro, G.A.H.A.; Araujo, Q.R.; Sodré, G.A.; Valle, R.R.; Souza, J.O.; Ramos, E.M.L.S.; Comerford, N.B.; Grierson, P.F. Cacao quality: Highlighting selected attributes. In Food Reviews International; Taylor & Francis: Abingdon, UK, 2017; Volume 33, pp. 382–405. Available online: https://www.tandfonline.com/doi/abs/10.1080/87559129.2016.1175011 (accessed on 19 February 2023).
- Ramón, V.; Hernández, H.E.; Polania, P.; Suárez, J.C. Spatial Distribution of Cocoa Quality: Relationship between Physicochemical, Functional and Sensory Attributes of Clones from Southern Colombia. Agronomy 2023, 13, 15. Available online: https://www.mdpi.com/2073-4395/13/1/15/htm (accessed on 20 February 2023). [CrossRef]
- Samaniego, I.; Espín, S.; Quiroz, J.; Ortiz, B.; Carrillo, W.; García-Viguera, C.; Mena, P. Effect of the growing area on the methylxanthines and flavan-3-ols content in cocoa beans from Ecuador. J. Food Compos. Anal. 2020, 88, 103448. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; (970.22); Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- García, E.; Fernández, I. Determinación de Proteínas de un Alimento por el Método Kjeldahl. Valoración con un Ácido Fuerte. ETSIAMN. Universidad Politécnica de Valencia. Universitat Politècnica de València. June 2020. Available online: https://riunet.upv.es/handle/10251/29832 (accessed on 19 February 2023).
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Galeano, P.G.; Paladines, M.B. Actividad antioxidante de extractos de granos de copoazú (Theobroma grandiflorum). Vitae 2012, 19, 436–438. Available online: https://www.redalyc.org/pdf/1698/169823914137.pdf (accessed on 19 February 2023).
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Hatzidimitriou, E.; Nenadis, N.; Tsimidou, M.Z. Changes in the catechin and epicatechin content of grape seeds on storage under different water activity (aw) conditions. Food Chem. 2007, 105, 1504–1511. [Google Scholar] [CrossRef]
- Brunetto, M.d.R.; Gutiérrez, L.; Delgado, Y.; Gallignani, M.; Zambrano, A.; Gómez, Á.; Ramos, G.; Romero, C. Determination of theobromine, theophylline and caffeine in cocoa samples by a high-performance liquid chromatographic method with on-line sample cleanup in a switching-column system. Food Chem. 2007, 100, 459–467. [Google Scholar] [CrossRef]
- Velásquez, H.; Galeano, P. Evaluación fitoquímica y de actividad antioxidante de los rizomas de tres especies del género Cyperus. Momentos Cienc. 2012, 9, 15–21. [Google Scholar]
- Balzarini, M.; Di Rienzo, J.; Tablada, M.; Gonzalez, L.; Bruno, C.; Córdoba, M.; Casanoves, F. Estadística y Biometrías. Ilustraciones del uso de InfoStat en Problemas de Agronomía, 2nd ed.; Editorial Brujas: Córdoba, Argentina, 2012; 380p. [Google Scholar]
- Rienzo, D.; Alejandro, J.; Alicia, L.; Margot, E.; Pilar, M. Estadistica Para Las Ciencias Agropecuarias; Potencia: Córdoba, Argentina, 2005; pp. 1–329. [Google Scholar]
- R Development Core Team. R: The R Project for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2023; ISBN 3-900051-07-0. Available online: https://www.r-project.org/ (accessed on 18 October 2023).
- Del, M.; Amaíz, C.L.; Gutiérrez, R.; Pérez, E.; Álvarez, C. Efecto del Tostado Sobre las Propiedades Físicas, Fisicoquímicas, Composición Proximal y Perfil de Ácidos Grasos de la Manteca de Granos de Cacao del Estado Miranda, Venezuela Effect of Roasting Process on Physical and Physicochemical Properties, Proximat. Volume 12, Revista Científica UDO Agrícola. Universidad de Oriente. 2012. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=4688626&info=resumen&idioma=ENG (accessed on 19 February 2023).
- Álvarez, C.; Pérez, E.; Lares, M.C. Caracterización física y química de almendras de cacao fermentadas, secas y tostadas cultivadas en la región de Cuyagua, estado Aragua. Agron. Trop. 2007, 57, 249–256. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0002-192X2007000400001&lng=es&nrm=iso&tlng=es (accessed on 18 February 2023).
- Perea, J.A.; Ramirez, O.L.; Villamizar, A.R. Caracterización fisicoquimica de materiales regionales de cacao colombiano. Biotecnol. Sect. Agropecu Agroindustrial 2011, 9, 35–42. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S1692-35612011000100005&lng=en&nrm=iso&tlng=es (accessed on 18 February 2023).
- Vera Chang, J.F.; Vallejo Torres, C.; Párraga Morán, D.E.; Macías Véliz, J.; Ramos Remache, R.; Morales Rodríguez, W. Atributos físicos-químicos y sensoriales de las almendras de quince clones de cacao nacional (Theobroma cacao L.) en el Ecuador. Cienc. Tecnol. 2015, 7, 21–34. Available online: https://revistas.uteq.edu.ec/index.php/cyt/article/view/139 (accessed on 18 February 2023). [CrossRef]
- AOAC. Ash (Acid-Insoluble) of Cacao Products. AOAC 975.12. 2000. Available online: http://www.aoacofficialmethod.org/index.php?main_page=product_info&products_id=407 (accessed on 20 February 2023).
- Álvarez, R.; Portillo, E.; Portillo, A.; Villasmil, R. Evaluación de las propiedades sensoriales del licor de cacao (Theobroma cacao L.) obtenido en forma artesanal e industrial. Rev. Agrollanía 2018, 15, 1690–8066. Available online: http://localhost:8080/xmlui/handle/654321/2290 (accessed on 18 February 2023).
- Mustiga, G.M.; Morrissey, J.; Stack, J.C.; DuVal, A.; Royaert, S.; Jansen, J.; Bizzotto, C.; Villela-Dias, C.; Mei, L.; Cahoon, E.B.; et al. Identification of Climate and Genetic Factors That Control Fat Content and Fatty Acid Composition of Theobroma cacao L. Beans. Front. Plant Sci. 2019, 10, 1159. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario Castro, M.; Hernández, J.A.; Marcilla, S.; Córdova, J.S.; Solari, F.A.; Chire, G.C. Efecto del contenido de grasa en la concentración de polifenoles y capacidad antioxidante de Theobroma cacao L. “Cacao”. Cienc. Investig. 2017, 19, 19–23. Available online: https://www.researchgate.net/profile/Armando-Solari-Godino/publication/308140856_EFECTO_DEL_CONTENIDO_DE_GRASA_EN_LA_CONCENTRACION_DE_POLIFENOLES_Y_CAPACIDAD_ANTIOXIDANTE_DE_Theobroma_cacao_L_CACAO/links/5df011af4585159aa473ecc8/EFECTO-DEL-CONTENIDO-DE-GR (accessed on 19 February 2023). [CrossRef]
- Servant, A.; Boulanger, R.; Davrieux, F.; Pinot, M.N.; Tardan, E.; Forestier-Chiron, N.; Hue, C. Assessment of cocoa (Theobroma cacao L.) butter content and composition throughout fermentations. Food Res. Int. 2018, 107, 675–682. [Google Scholar] [CrossRef]
- Andrade, J.A.; Rivera-García, J.; Chire-Fajardo, G.C.; Ureña-Peralta, M.O. Propiedades físicas y químicas de cultivares de cacao (Theobroma cacao L.) de Ecuador y Perú. Enfoque UTE 2019, 10, 1–12. Available online: http://scielo.senescyt.gob.ec/scielo.php?pid=S1390-65422019000400001&script=sci_arttext (accessed on 19 February 2023). [CrossRef]
- Barrientos, L.D.P.; Oquendo, J.D.T.; Garzón, M.A.G.; Álvarez, O.L.M. Effect of the solar drying process on the sensory and chemical quality of cocoa (Theobroma cacao L.) cultivated in Antioquia, Colombia. Food Res. Int. 2019, 115, 259–267. [Google Scholar] [CrossRef]
- Imru, N.O.; Wogderess, M.D.; Gidada, T.V. A study of the effects of shade on growth, production and quality of coffee (Coffea arabica) in Ethiopia. Int. J. Agric. Sci. 2015, 5, 748–752. Available online: http://www.academicjournals.org/JHF (accessed on 19 February 2023).
- Afoakwa, E.O.; Ofosu-Ansah, E.; Takrama, J.F.; Budu, A.S.; Mensah-Brown, H. Changes in chemical quality of cocoa butter during roasting of pulp pre-conditioned and fermented cocoa (Theobroma cacao) beans. Int. Food Res. J. 2014, 21, 2221–2227. Available online: https://www.researchgate.net/profile/Emmanuel-Ohene-Afoakwa/publication/276371202_Changes_in_Biochemical_and_Physico-chemical_Qualities_during_Drying_of_Pulp_Preconditioned_and_Fermented_Cocoa_Theobroma_cacao_Beans/links/56a795e008ae860e025577d3/Changes-i (accessed on 19 February 2023).
- Rohan, T.A.; Stewart, T. The Precursors of Chocolate Aroma: Changes in the Sugars During the Roasting of Cocoa Beans. J. Food Sci. 1966, 31, 206–209. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2621.1966.tb00480.x (accessed on 19 February 2023). [CrossRef]
- Zapata Bustamante, S.; Tamayo Tenorio, A.; Alberto Rojano, B. Efecto de la fermentación sobre la actividad antioxidante de diferentes clones de cacao Colombiano. Rev. Cuba Plantas Med. 2013, 18, 391–404. Available online: http://scielo.sld.cu/scielo.php?pid=S1028-47962013000300007&script=sci_arttext&tlng=pt (accessed on 20 February 2023).
- Zzaman, W.; Bhat, R.; Abedin, M.Z.; Yang, T.A. Comparison between superheated steam and convectional roasting on changes in the phenolic compound and antioxidant activity of cocoa beans. Food Sci. Technol. Res. 2013, 19, 949–956. [Google Scholar] [CrossRef][Green Version]
- Lujano, E.; Manganiello, L.; Contento, A.; Rios, A. Identification and quantification of (+)-Catechins and Procyanidins in Cocoa from Ocumare de la Costa, Venezuela. Rev. Ing. UC 2019, 26, 192–201. [Google Scholar]
- Álvarez, L.C.; Álvarez, N.C.; García, P.G.; Salazar, J.C.S. Effect of fermentation time on phenolic content and antioxidant potential in Cupuassu (Theobroma grandiflorum (Willd. ex Spreng.) K. Schum.) beans. Acta Agron. 2017, 66, 473–479. Available online: http://www.scielo.org.co/scielo.php?pid=S0120-28122017000400473&script=sci_arttext&tlng=en (accessed on 19 February 2023). [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Ciência Tecnol. Aliment. 2005, 25, 726–732. Available online: http://www.scielo.br/j/cta/a/B58T9S5zLLxjBL5PVzZXHCF/abstract/?lang=es (accessed on 19 February 2023). [CrossRef]
- Roginsky, V.; Lissi, E.A. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005, 92, 235–254. [Google Scholar] [CrossRef]
- Perea, J.; Cadena, T.; Herrera, J. El cacao y sus productos como fuente de antioxidantes: Efecto del procesamiento. Salud UIS 2009, 41, 128–134. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0121-08072009000200003 (accessed on 19 February 2023).
- Apriyanto, M. Changes in Chemical Properties of Dreid Cocoa (Theobroma cacao) Beans during Fermentation. Int. J. Fermented Foods 2016, 5, 11. Available online: https://www.indianjournals.com/ijor.aspx?target=ijor:ijff&volume=5&issue=1&article=002 (accessed on 19 February 2023). [CrossRef]
- Acevedo, L.; Mejía, D.; Acosta, E.; Valencia, W.; Penagos, L. Efecto de la temperatura del conchado sobre los polifenoles en un chocolate semi-amargo. Aliment. Hoy 2017, 25, 31–50. Available online: https://alimentoshoy.acta.org.co/index.php/hoy/article/view/447 (accessed on 19 February 2023).
- Quiñones, M.; Miguel, M.; Aleixandre, A. Los polifenoles, compuestos de origen natural con efectos saludables sobre el sistema cardiovascular. Nutr. Hosp. 2012, 27, 76–89. [Google Scholar]
- Jinap, S.; Dimick, P.S. Acidic Characteristics of Fermented and Dried Cocoa Beans from Different Countries of Origin. J. Food Sci. 1990, 55, 547–550. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2621.1990.tb06806.x (accessed on 19 February 2023). [CrossRef]
- Afoakwa, E.O.; Quao, J.; Takrama, J.; Budu, A.S.; Saalia, F.K. Chemical composition and physical quality characteristics of Ghanaian cocoa beans as affected by pulp pre-conditioning and fermentation. J. Food Sci. Technol. 2013, 50, 1097–1105. Available online: https://link.springer.com/article/10.1007/s13197-011-0446-5 (accessed on 19 February 2023). [CrossRef] [PubMed]
- Locatelli, M.; Travaglia, F.; Giovannelli, L.; Coïsson, J.D.; Bordiga, M.; Pattarino, F.; Arlorio, M. Clovamide and phenolics from cocoa beans (Theobroma cacao L.) inhibit lipid peroxidation in liposomal systems. Food Res. Int. 2013, 50, 129–134. [Google Scholar] [CrossRef]
- Ordoñez, E.S.; Leon-Arevalo, A.; Rivera-Rojas, H.; Vargas, E. Cuantificación de polifenoles totales y capacidad antioxidante en cáscara y semilla de cacao (Theobroma cacao L.), tuna (Opuntia ficus indica Mill), uva (Vitis vinífera) y uvilla (Pourouma cecropiifolia). Sci. Agropecu. 2019, 10, 175–183. Available online: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S2077-99172019000200003&lng=es&nrm=iso&tlng=pt (accessed on 19 February 2023). [CrossRef]
- Ramos-Escudero, F.; Casimiro-Gonzales, S.; Fernández-Prior, Á.; Cancino Chávez, K.; Gómez-Mendoza, J.; de la Fuente-Carmelino, L.; Muñoz, A.M. Colour, fatty acids, bioactive compounds, and total antioxidant capacity in commercial cocoa beans (Theobroma cacao L.). LWT 2021, 147, 111629. [Google Scholar] [CrossRef]
- Othman, A.; Ismail, A.; Abdul Ghani, N.; Adenan, I. Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 2007, 100, 1523–1530. [Google Scholar] [CrossRef]
- Szeto, Y.T.; Tomlinson, B.; Benzie, I.F.F. Total antioxidant and ascorbic acid content of fresh fruits and vegetables: Implications for dietary planning and food preservation. Br. J. Nutr. 2002, 87, 55–59. Available online: https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/total-antioxidant-and-ascorbic-acid-content-of-fresh-fruits-and-vegetables-implications-for-dietary-planning-and-food-preservation/F457978920144B378888C03DEEA2885A (accessed on 19 February 2023). [CrossRef]
- Sotelo, C.L.; Alvis, B.A.; Arrázola, P.G. Evaluación de epicatequina, teobromina y cafeína en cáscaras de cacao (Theobroma cacao L.), determinación de su capacidad antioxidante. Rev. Colomb. Cienc. Hortícolas 2015, 9, 124. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S2011-21732015000100011&lng=en&nrm=iso&tlng=es (accessed on 19 February 2023). [CrossRef]
- Belščak, A.; Komes, D.; Horžić, D.; Ganić, K.K.; Karlović, D. Comparative study of commercially available cocoa products in terms of their bioactive composition. Food Res. Int. 2009, 42, 707–716. [Google Scholar] [CrossRef]
- Quizhpe, P.A.C. Efecto Inhibitorio de la Pulpa que Recubre las Semillas del Cacao (Theobroma cacao) a Diferentes Concentraciones sobre la cepa de Streptococcus mutans: Estudio in estudio in Vitro. Quito: UCE. 2018. Available online: http://www.dspace.uce.edu.ec/handle/25000/15186 (accessed on 19 February 2023).
- Peláez, P.P.; Bardón, I.; Camasca, P. Methylxanthine and catechin content of fresh and fermented cocoa beans, dried cocoa beans, and cocoa liquor. Sci. Agropecu. 2016, 7, 355–365. Available online: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S2077-99172016000500001&lng=es&nrm=iso&tlng=en (accessed on 19 February 2023). [CrossRef]
- Carrillo, L.C.; Londoño-Londoño, J.; Gil, A. Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia. Food Res. Int. 2014, 60, 273–280. [Google Scholar] [CrossRef]


| Introduced Commercial | ||
|---|---|---|
| Nomenclature | Identification and Origin | Characteristics of Interest |
| CCN-51 | Castro Naranjal (Ecuador) | Commercially grown. High yield. Resistance to Monilia [18]. |
| EET-8 | United Fruit Company (Costa Rica) | Commercially grown. Good grain index [19]. |
| TSH-565 | Trinidad Selection Hybrid (Trinidad) | Resistance to Monilliphthora perniciosa, high productivity [20]. |
| ICS-1 | Imperial College Selection (Trinidad, Nicaragua y Venezuela) | Present in commercial crops in several countries. Good grain and cob index [19]. |
| ICS-60 | Imperial College Selection (Trinidad, Nicaragua y Venezuela) | Present in commercial crops in several countries. Good grain and cob index [19]. |
| ICS-95 | Imperial College Selection (Trinidad, Nicaragua y Venezuela) | Present in commercial crops in several countries. Good grain and cob index [19]. |
| Regional | ||
| SCC-61 | Selección Colombia Corpoica (Santander), Hibrido trinitario | High grain index [12]. |
| FSV-41 | Fedecacao San Vicente (Santander), Híbrido trinitario | High grain rate, yield and quality [12]. |
| Component | Variable | Unit | Method |
|---|---|---|---|
| Bromatological | pH | Potentiometric [24] | |
| Acidity | % | Titling [24] | |
| Moisture | % | Gravimetric [24] | |
| Ash | % | Incineration [25] | |
| Fat | % | Soxhlet [24] | |
| Crude protein | % | Kjeldahl [26] | |
| Sucrose | °Brix | Refractometry [22] | |
| Total carbohydrates | mg g−1 | Phenol-Sulfuric [27] | |
| Phenolic compounds | Total phenols | mg g−1 | Folin-Ciocalteu colorimetry [28] |
| Total flavonoids | mg g−1 | Aluminum chloride [29] | |
| Catechin | mg g−1 | HPLC [30] | |
| Epicatechin | mg g−1 | HPLC [30] | |
| Theobromine | mg g−1 | HPLC [31] | |
| Caffeine | mg g−1 | HPLC [31] | |
| Antioxidant activity | DPPH | (µmol g−1) | Colorimetric [32] |
| FRAP | (µmol g−1) | Colorimetric [32] |
| Factor | Level | Moisture (%) | Ash (%) | pH | Acidity (%) | Fat (%) | Crude Protein (%) | Sucrose °Brix | Total Carbohydrates (mg g−1) |
|---|---|---|---|---|---|---|---|---|---|
| Clone | CCN-51 | 2.98 ± 0.16 a | 3.03 ± 0.09 c | 5.14 ± 0.09 | 0.56 ± 0.03 ab | 53.25 ± 2.56 | 12.07 ± 0.18 c | 2.78 ± 0.54 b | 1.63 ± 0.12 |
| EET-8 | 2.54 ± 0.18 abc | 3.17 ± 0.10 bc | 5.30 ± 0.10 | 0.46 ± 0.03 c | 63.61 ± 3.10 | 12.40 ± 0.21 bc | 3.42 ± 0.61 ab | 1.35 ± 0.13 | |
| FSV-41 | 1.98 ± 0.26 c | 3.53 ± 0.14 a | 5.32 ± 0.13 | 0.49 ± 0.05 bc | 60.66 ± 4.99 | 12.60 ± 0.28 abc | 4.43 ± 0.81 ab | 1.55 ± 0.18 | |
| ICS-1 | 2.38 ± 0.15 bc | 3.35 ± 0.09 ab | 5.16 ± 0.09 | 0.54 ± 0.03 abc | 58.26 ± 2.56 | 12.85 ± 0.18 a | 3.51 ± 0.52 ab | 1.62 ± 0.11 | |
| ICS-60 | 2.65 ± 0.18 ab | 3.21 ± 0.10 bc | 5.17 ± 0.10 | 0.49 ± 0.03 bc | 64.45 ± 2.98 | 12.19 ± 0.20 c | 3.23 ± 0.59 b | 1.55 ± 0.13 | |
| ICS-95 | 2.90 ± 0.13 a | 3.37 ± 0.07 ab | 5.10 ± 0.08 | 0.59 ± 0.02 a | 55.66 ± 2.07 | 12.50 ± 0.16 bc | 4.57 ± 0.46 a | 1.55 ± 0.10 | |
| SCC-61 | 3.01 ± 0.23 a | 3.31 ± 0.13 abc | 5.35 ± 0.12 | 0.52 ± 0.04 abc | 59.83 ± 3.72 | 12.82 ± 0.26 ab | 3.97 ± 0.76 ab | 1.22 ± 0.17 | |
| TSH-565 | 2.61 ± 0.15 ab | 3.15 ± 0.08 c | 5.11 ± 0.09 | 0.55 ± 0.03 ab | 60.80 ± 2.50 | 12.12 ± 0.18c | 3.91 ± 0.52 ab | 1.53 ± 0.11 | |
| p value | 0.0031 | 0.0017 | 0.1937 | 0.0420 | 0.0624 | 0.0009 | 0.0324 | 0.2789 | |
| Fermentation time (days) | 0 | 2.52 ± 0.15 ab | 3.47 ± 0.08 a | 5.49 ± 0.08 a | 0.42 ± 0.03 b | 65.44 ± 2.38 a | 12.90 ± 0.17 a | 3.29 ± 0.51 | 1.49 ± 0.11 |
| 2 | 2.21 ± 0.14 b | 3.58 ± 0.08 a | 5.51 ± 0.08 a | 0.43 ± 0.03 b | 62.37 ± 2.10 ab | 12.87 ± 0.16 a | 3.28 ± 0.48 | 1.52 ± 0.11 | |
| 4 | 2.75 ± 0.14 a | 3.15 ± 0.08 b | 4.99 ± 0.08 b | 0.61 ± 0.03 a | 56.69 ± 2.14 bc | 12.21 ± 0.16 b | 3.97 ± 0.49 | 1.47 ± 0.11 | |
| 6 | 2.86 ± 0.14 a | 3.07 ± 0.08 b | 4.98 ± 0.08 b | 0.60 ± 0.03 a | 55.70 ± 2.10 c | 12.14 ± 0.16 b | 3.98 ± 0.48 | 1.56 ± 0.11 | |
| 8 | 2.82 ± 0.14 a | 3.05 ± 0.08 b | 5.05 ± 0.08 b | 0.58 ± 0.03 a | 54.96 ± 2.10 c | 12.12 ± 0.16 b | 4.12 ± 0.48 | 1.46 ± 0.11 | |
| p value | 0.0004 | <0.0001 | <0.0001 | <0.0001 | 0.0028 | <0.0001 | 0.2568 | 0.8975 |
| Factor | Level | Total Phenols (mg g−1) | Total Flavonoids (mg g−1) | Catechin (mg g−1) | Epicatechin (mg g−1) | Theobromine (mg g−1) | Caffeine (mg g−1) | DPPH (µmol g−1) | FRAP (µmol g−1) |
|---|---|---|---|---|---|---|---|---|---|
| Clone | CCN-51 | 64.56 ± 11.74 a | 3.30 ± 0.57 a | 1.95 ± 0.06 a | 0.49 ± 0.01 a | 0.30 ± 0.01 a | 0.37 ± 0.01 a | 325.55 ± 33.30 | 331.00 ± 34.29 |
| EET-8 | 42.71 ± 7.63 abc | 2.73 ± 0.54 a | 1.90 ± 0.07 a | 0.48 ± 0.01 ab | 0.29 ± 0.01 a | 0.37 ± 0.01 a | 251.47 ± 40.26 | 244.72 ± 41.45 | |
| FSV-41 | 46.46 ± 19.37 abc | 2.25 ± 0.78 abc | 1.82 ± 0.09 abc | 0.47 ± 0.02 abc | 0.29 ± 0.01 ab | 0.37 ± 0.01 ab | 168.71 ± 64.91 | 169.51 ± 66,84 | |
| ICS-1 | 47.55 ± 7.30 abc | 1.72 ± 0.44 bc | 1.78 ± 0.05 bc | 0.46 ± 0.01 bc | 0.29 ± 0.01 ab | 0.36 ± 0.01 ab | 245.23 ± 33.30 | 248.29 ± 34.29 | |
| ICS-60 | 39.93 ± 7.54 bc | 2.84 ± 0.53 a | 1.93 ± 0.07 a | 0.48 ± 0.01 a | 0.30 ± 0.01 a | 0.38 ± 0.01 a | 224.42 ± 38.79 | 226.87 ± 39.94 | |
| ICS-95 | 52.23 ± 7.73 ab | 2.55 ± 0.43 ab | 1.88 ± 0.05 ab | 0.48 ± 0.01 ab | 0.30 ± 0.01 a | 0.37 ± 0.01 a | 256.22 ± 26.95 | 259.61 ± 27.75 | |
| SCC-61 | 35.36 ± 7.66 c | 2.41 ± 0.69 abc | 1.87 ± 0.08 abc | 0.48 ± 0.02 ab | 0.29 ± 0.01 ab | 0.37 ± 0.01 ab | 275.00 ± 48.38 | 278.95 ± 49.82 | |
| TSH-565 | 60.97 ± 8.27 a | 1.29 ± 0.42 c | 1.71 ± 0.05 c | 0.44 ± 0.01c | 0.28 ± 0.01 b | 0.35 ± 0.01 b | 350.01 ± 32.56 | 356.18 ± 33.42 | |
| p value | 0.0238 | 0.0063 | 0.0022 | 0.0213 | 0.0195 | 0.0117 | 0.0678 | 0.0678 | |
| Fermentation time (days) | 0 | 39.32 ± 8.77 | 2.64 ± 0.46 | 1.90 ± 0.06 | 0.48 ± 0.01 | 0.30 ± 0.01 | 0.37 ± 0.01 | 256.11 ± 32.88 | 259.50 ± 30.43 |
| 2 | 48.91 ± 8.62 | 2.44 ± 0.44 | 1.88 ± 0.05 | 0.47 ± 0.01 | 0.29 ± 0.01 | 0.37 ± 0.01 | 272.95 ± 29.00 | 276.84 ± 29.86 | |
| 4 | 52.91 ± 8.64 | 2.36 ± 0.46 | 1.85 ± 0.05 | 0.47 ± 0.01 | 0.29 ± 0.01 | 0.37 ± 0.01 | 291.95 ± 29.55 | 296.40 ± 30.43 | |
| 6 | 50.50 ± 8.62 | 2.18 ± 0.41 | 1.82 ± 0.05 | 0.47 ± 0.01 | 0.29 ± 0.01 | 0.36 ± 0.01 | 260.08 ± 29.00 | 263.59 ± 29.86 | |
| 8 | 51.97 ± 8.62 | 2.31 ± 0.43 | 1.83 ± 0.05 | 0.47 ± 0.01 | 0.29 ± 0.01 | 0.37 ± 0.01 | 283.11 ± 29.00 | 287.30 ± 29.86 | |
| p value | 0.5806 | 0.8314 | 0.3330 | 0.8945 | 0.2962 | 0.2282 | 0.9101 | 0.9101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polanía-Hincapié, P.A.; Suárez, J.C.; Hernández, H.E.; Ramón-Triana, V.Y.; Cuéllar-Álvarez, L.N.; Casanoves, F. Influence of Fermentation Time on the Chemical and Functional Composition of Different Cocoa Clones from Southern Colombia. Fermentation 2023, 9, 982. https://doi.org/10.3390/fermentation9110982
Polanía-Hincapié PA, Suárez JC, Hernández HE, Ramón-Triana VY, Cuéllar-Álvarez LN, Casanoves F. Influence of Fermentation Time on the Chemical and Functional Composition of Different Cocoa Clones from Southern Colombia. Fermentation. 2023; 9(11):982. https://doi.org/10.3390/fermentation9110982
Chicago/Turabian StylePolanía-Hincapié, Paola Andrea, Juan Carlos Suárez, Héctor Eduardo Hernández, Vivian Yorlady Ramón-Triana, Liceth N. Cuéllar-Álvarez, and Fernando Casanoves. 2023. "Influence of Fermentation Time on the Chemical and Functional Composition of Different Cocoa Clones from Southern Colombia" Fermentation 9, no. 11: 982. https://doi.org/10.3390/fermentation9110982
APA StylePolanía-Hincapié, P. A., Suárez, J. C., Hernández, H. E., Ramón-Triana, V. Y., Cuéllar-Álvarez, L. N., & Casanoves, F. (2023). Influence of Fermentation Time on the Chemical and Functional Composition of Different Cocoa Clones from Southern Colombia. Fermentation, 9(11), 982. https://doi.org/10.3390/fermentation9110982






