Effect of Vine Age, Dry Farming and Supplemental Irrigation on Color and Phenolic Extraction of cv. Zinfandel Wines from California
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viticulture Experiment
2.2. Winemaking
2.3. Wine Basic Analysis
2.4. Wine Spectrophotometric Analysis
2.5. Anthocyanin Analysis using an HPLC-Diode Array Detector (DAD)–Mass Spectrometry (MS)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Alcoholic Fermentation and Sugar Consumption
3.2. Basic Chemistry of the Wines
3.3. Phenolic Composition of the Wines
3.4. Chromatic Composition of the Wines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sullivan, C.L. Zinfandel: A History of a Grape and Its Wine; University of California Press: Berkeley, CA, USA, 2003. [Google Scholar]
- California Wine Institute—California Zinfandel. Available online: https://wineinstitute.org/our-industry/statistics/wine-fact-sheets/zinfandel/ (accessed on 20 July 2023).
- Maletic, E.; Pejic, I.; Karoglan Kontic, J.; Piljac, J.; Dangl, G.; Vokurka, A.; Lacombe, T.; Miroševic, N.; Meredith, C.P. The identification of Zinfandel in the Dalmatian coast of Croacia. Acta Hort. 2003, 603, 251–254. [Google Scholar] [CrossRef]
- Mafata, M.; Brand, J.; Panzeri, V.; Buica, A. Investigating the Concept of South African Old Vine Chenin Blanc. S. Afr. J. Enol. Vitic. 2020, 41, 168–182. [Google Scholar] [CrossRef]
- Priilaid, D.; Steyn, J. Evaluating the worth of nascent old vine cues for South African wines. Int. J. Wine Bus. Res. 2020, 32, 283–300. [Google Scholar] [CrossRef]
- Grigg, D.; Methven, D.; de Bei, R.; Rodríguez López, C.M.; Dry, P.; Collins, C. Effect of vine age on vine performance of Shiraz in the Barossa Valley, Australia. Aust. J. Grape Wine Res. 2018, 24, 75–87. [Google Scholar] [CrossRef]
- Riffle, V.; Alvarez Arredondo, J.; LoMonaco, I.; Appel, C.; Catania, A.A.; Dodson Peterson, J.C.; Casassa, L.F. Vine Age Affects Vine Performance, Grape and Wine Chemical and Sensory Composition of cv. Zinfandel from California. Am. J. Enol. Vitic. 2022, 73, 276–292. [Google Scholar] [CrossRef]
- Riffle, V.; Palmer, N.; Casassa, L.F.; Dodson Peterson, J.C. The Effect of Grapevine Age (Vitis vinifera L. cv. Zinfandel) on Phenology and Gas Exchange Parameters over Consecutive Growing Seasons. Plants 2021, 10, 311. [Google Scholar]
- Salón, J.L.; Chirivella, C.; Castel, J.R. Response of cv. Bobal to Timing of Deficit Irrigation in Requena, Spain: Water Relations, Yield, and Wine Quality. Am. J. Enol. Vitic. 2005, 56, 1–8. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Castel, J.R. Response of grapevine cv. Tempranillo to timing and amount of irrigation: Water relations, vine growth, yield and berry and wine composition. Irrig. Sci. 2010, 28, 113–125. [Google Scholar] [CrossRef]
- Casassa, L.F.; Harbertson, J.F. Extraction, evolution, and sensory impact of phenolic compounds during red wine maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef]
- Dooley, L.M.; Threlfall, R.T.; Meullenet, J.-F.; Howard, L.R. Compositional and Sensory Impacts from Blending Red Wine Varietals. Am. J. Enol. Vitic. 2012, 63, 241–250. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Hodgins, R.E.; Thurston, L.N.; Schaffer, L.J.; Reid, M.S.; Landon, J.L.; Ross, C.F.; Adams, D.O. Variability of Tannin Concentration in Red Wines. Am. J. Enol. Vitic. 2008, 59, 210–214. [Google Scholar] [CrossRef]
- McCloskey, L.P.; Yengoyan, L.S. Analysis of Anthocyanins in Vitis Vinifera Wines and Red Color Versus Aging by HPLC and Spectrophotometry. Am. J. Enol. Vitic. 1981, 32, 257–261. [Google Scholar] [CrossRef]
- Casassa, L.F.; Huff, R.; Steele, N.B. Chemical consequences of extended maceration and post-fermentation additions of grape pomace in Pinot noir and Zinfandel wines from the Central Coast of California (USA). Food Chem. 2019, 300, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Generalić Mekinić, I.; Skračić, Ž.; Kokeza, A.; Soldo, B.; Ljubenkov, I.; Banović, M.; Skroza, D. Effect of winemaking on phenolic profile, colour components and antioxidants in Crljenak kaštelanski (sin. Zinfandel, Primitivo, Tribidrag) wine. J. Food Sci. Technol. 2019, 56, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Luković, J.; Chiang, J.C.H.; Blagojević, D.; Sekulić, A. A Later Onset of the Rainy Season in California. Geophys. Res. Lett. 2021, 48, e2020GL090350. [Google Scholar] [CrossRef]
- Alvarez Arredondo, J.; Muñoz, J.; Casassa, L.F.; Dodson Peterson, J.C. The Effect of Supplemental Irrigation on a Dry-Farmed Vitis vinifera L. cv. Zinfandel Vineyard as a Function of Vine Age. Agronomy 2023, 13, 1998. [Google Scholar]
- Harbertson, J.F.; Picciotto, E.A.; Adams, D.O. Measurement of polymeric pigments in grape berry extracts and wines using a protein precipitation assay combined with bisulfite bleaching. Am. J. Enol. Vitic. 2003, 54, 301–306. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Kennedy, J.A.; Adams, D.O. Tannin in skins and seeds of Cabernet Sauvignon, Syrah, and Pinot Noir berries during ripening. Am. J. Enol. Vitic. 2002, 53, 54–59. [Google Scholar] [CrossRef]
- Pérez-Caballero, V.; Ayala, F.; Echávarri, J.F.; Negueruela, A.I. Proposal for a New Standard OIV Method for Determination of Chromatic Characteristics of Wine. Am. J. Enol. Vitic. 2003, 54, 59–62. [Google Scholar] [CrossRef]
- Downey, M.O.; Rochfort, S. Simultaneous separation by reversed-phase high-performance liquid chromatography and mass spectral identification of anthocyanins and flavonols in Shiraz grape skin. J. Chromatogr. A 2008, 1201, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. 7—Fermentation. In Wine Science, 4th ed.; Jackson, R.S., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 427–534. [Google Scholar] [CrossRef]
- Postiglione, D.; Casassa, L.F.; Dodson Peterson, J.C. Effects of variations in berry size and manipulations of fermentation solids in Zinfandel grapes and wines. In Proceedings of the 69th National Conference of the American Society for Enology and Viticulture, Monterey, California, USA, 18–21 June 2018. [Google Scholar]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef] [PubMed]
- Vivar-Quintana, A.M.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Anthocyanin-derived pigments and colour of red wines. Anal. Chim. Acta 2002, 458, 147–155. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Spayd, S. Measuring Phenolics in the Winery. Am. J. Enol. Vitic. 2006, 57, 280–288. [Google Scholar] [CrossRef]
- Zhao, Q.; Du, G.; Zhao, P.; Guo, A.; Cao, X.; Cheng, C.; Liu, H.; Wang, F.; Zhao, Y.; Liu, Y.; et al. Investigating wine astringency profiles by characterizing tannin fractions in Cabernet Sauvignon wines and model wines. Food. Chem. 2023, 414, 135673. [Google Scholar] [CrossRef]
- Adams, D.O.; Harbertson, J.F.; Picciotto, E.A. Fractionation of red wine polymeric pigments by protein precipitation and bisulfite bleaching. In Red Wine Color; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2004; Volume 886, pp. 275–288. [Google Scholar]
- Landon, J.L.; Weller, K.; Harbertson, J.F.; Ross, C.F. Chemical and Sensory Evaluation of Astringency in Washington State Red Wines. Am. J. Enol. Vitic. 2008, 59, 153–158. [Google Scholar] [CrossRef]
- Casassa, L.F.; Fanzone, M.L.; Sari, S.E. Comparative phenolic, chromatic, and sensory composition of five monovarietal wines processed with microwave technology. Heliyon 2022, 8, e12332. [Google Scholar] [CrossRef]
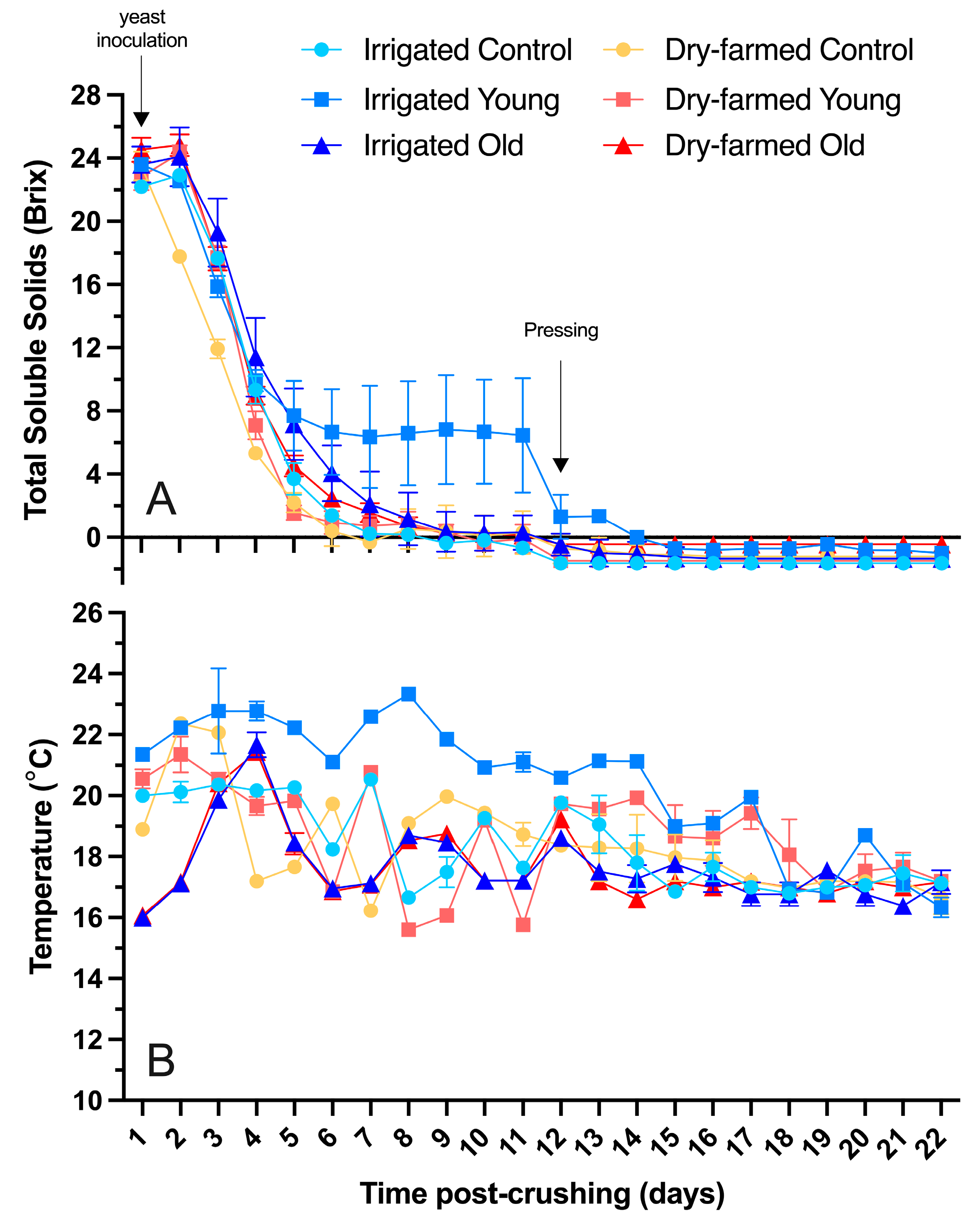

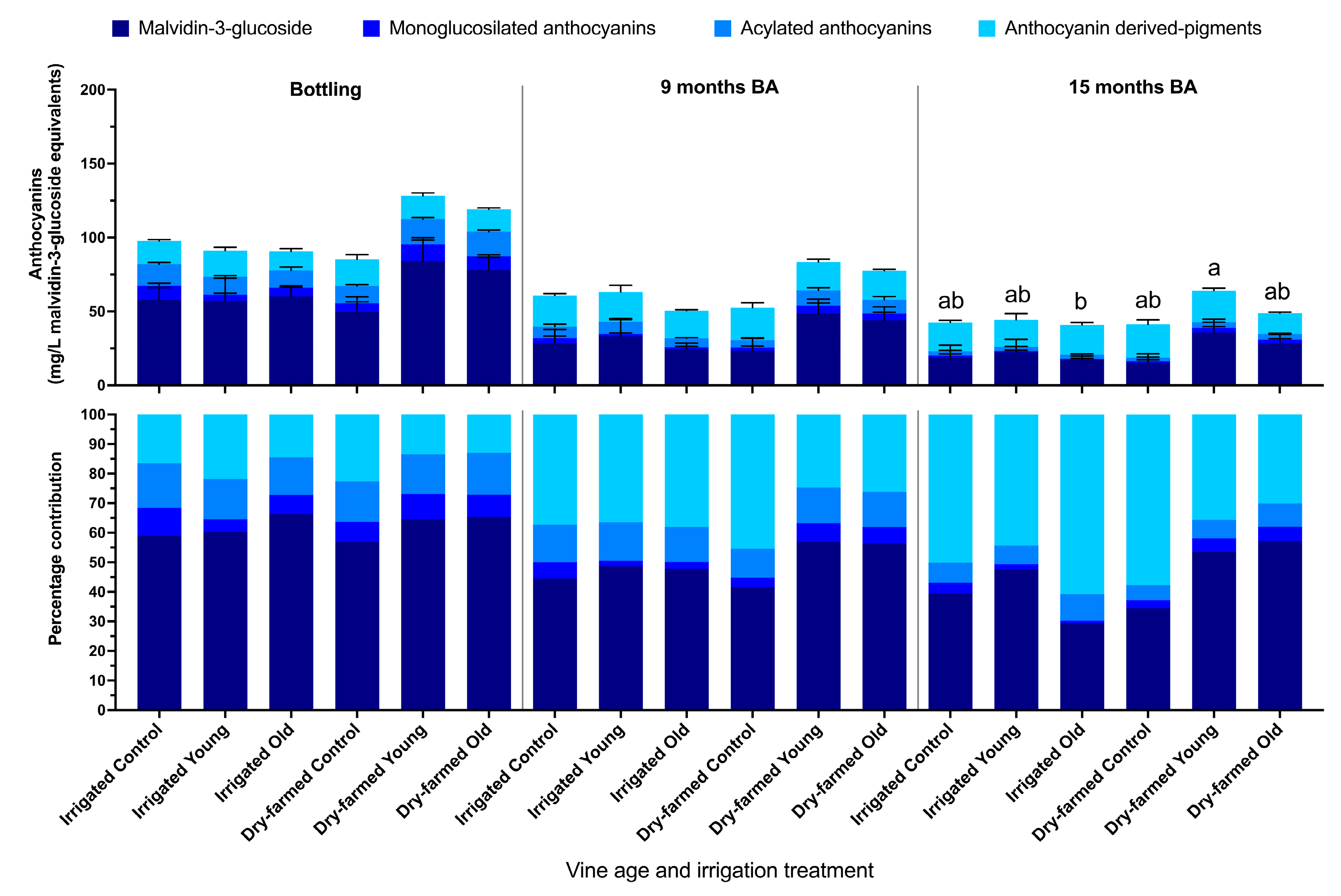
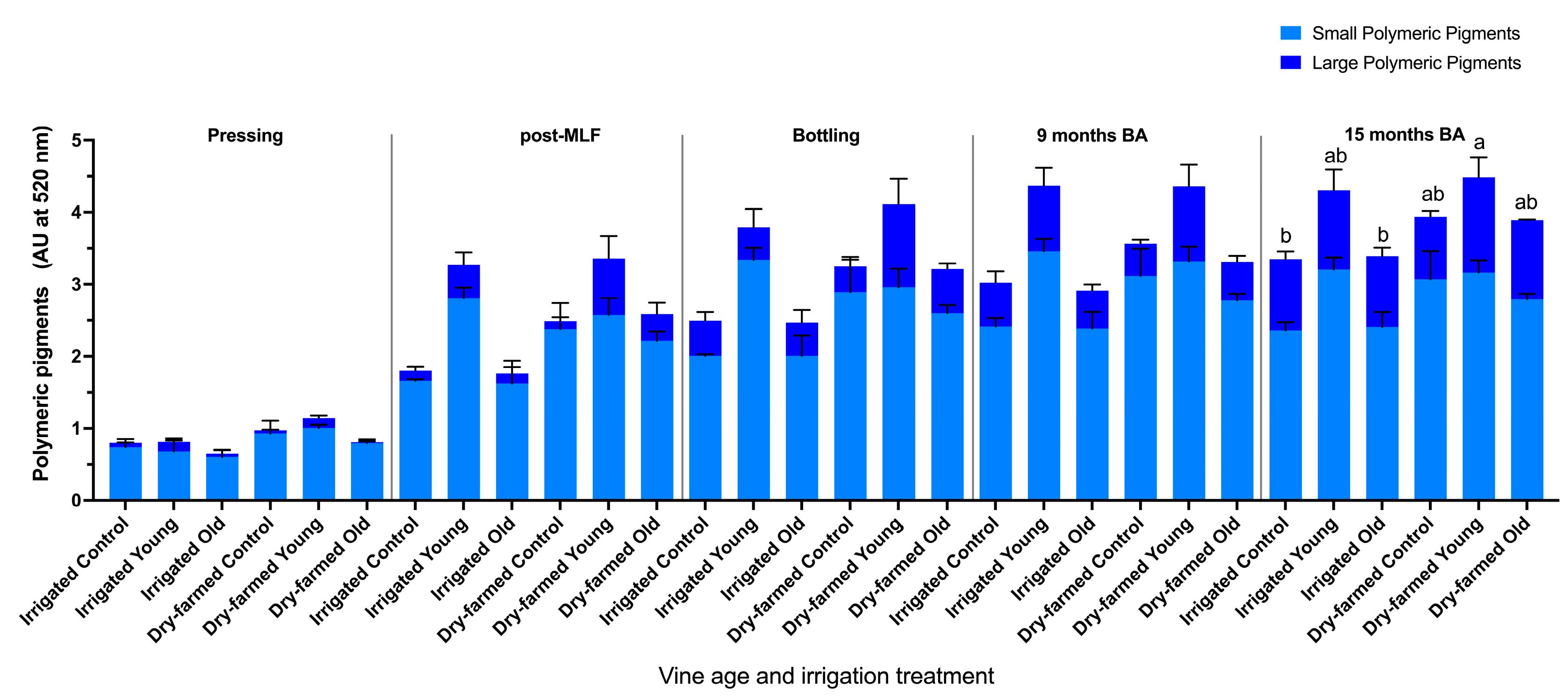

| Irrigation | Vine Age | Ethanol (% v/v) | pH | Titratable Acidity (g/L Tartaric Acid) | Glucose + Fructose (g/L) | Malic Acid h(g/L) | Lactic Acid (g/L) | Acetic Acid (g/L) |
|---|---|---|---|---|---|---|---|---|
| Irrigated | Control | 16.1 ± 0.44 ab | 3.36 ± 0.09 b | 8.05 ± 0.53 a | 0.50 ± 0.09 a | 0.09 ± 0.01 b | 1.21 ± 0.12 bc | 0.24 ± 0.03 c |
| Young | 14.2 ± 1.14 b | 3.79 ± 0.12 a | 6.78 ± 0.23 c | 0.81 ± 0.31 a | 0.09 ± 0.03 b | 1.81 ± 0.17 a | 0.75 ± 0.08 a | |
| Old | 16.7 ± 0.86 a | 3.36 ± 0.11 b | 7.42 ± 0.01 abc | 2.96 ± 1.49 a | 0.12 ± 0.01 b | 1.07 ± 0.11 c | 0.44 ± 0.09 bc | |
| Dry-farmed | Control | 16.7 ± 0.73 a | 3.57 ± 0.11 ab | 7.77 ± 0.18 ab | 2.64 ± 2.12 a | 1.75 ± 0.15 a | 0.07 ± 0.02 d | 0.41 ± 0.13 bc |
| Young | 17.1 ± 0.43 a | 3.64 ± 0.06 ab | 7.05 ± 0.05 bc | 3.46 ± 2.82 a | 0.11 ± 0.01 b | 1.52 ± 0.11 ab | 0.37 ± 0.09 bc | |
| Old | 17.8 ± 0.38 a | 3.43 ± 0.05 b | 7.51 ± 0.12 abc | 3.03 ± 0.83 a | 0.13 ± 0.02 b | 0.94 ± 0.07 c | 0.59 ± 0.06 ab | |
| p-value | 0.0663 | 0.0340 | 0.0393 | 0.6939 | <0.0001 | <0.0001 | 0.0235 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casassa, L.F.; Alvarez Arredondo, J.; Dodson Peterson, J.C. Effect of Vine Age, Dry Farming and Supplemental Irrigation on Color and Phenolic Extraction of cv. Zinfandel Wines from California. Fermentation 2023, 9, 974. https://doi.org/10.3390/fermentation9110974
Casassa LF, Alvarez Arredondo J, Dodson Peterson JC. Effect of Vine Age, Dry Farming and Supplemental Irrigation on Color and Phenolic Extraction of cv. Zinfandel Wines from California. Fermentation. 2023; 9(11):974. https://doi.org/10.3390/fermentation9110974
Chicago/Turabian StyleCasassa, L. Federico, Jocelyn Alvarez Arredondo, and Jean Catherine Dodson Peterson. 2023. "Effect of Vine Age, Dry Farming and Supplemental Irrigation on Color and Phenolic Extraction of cv. Zinfandel Wines from California" Fermentation 9, no. 11: 974. https://doi.org/10.3390/fermentation9110974
APA StyleCasassa, L. F., Alvarez Arredondo, J., & Dodson Peterson, J. C. (2023). Effect of Vine Age, Dry Farming and Supplemental Irrigation on Color and Phenolic Extraction of cv. Zinfandel Wines from California. Fermentation, 9(11), 974. https://doi.org/10.3390/fermentation9110974








