Abstract
Microalgae are considered to have great potential as a source of biodiesel. Currently, algae culture has three different trophic modes, i.e., autotrophic, heterotrophic, and mixotrophic, but not all kinds of algae are suitable for heterotrophic and mixotrophic cultivation. In this study, Parachlorella kessleri TY, screened from the soil of Shanxi Province, was heterotrophically and mixotrophically treated with glucose as an organic carbon source, and the physiological and biochemical levels of its growth and lipid accumulation were measured. The results showed that the highest biomass and biomass productivity (1.53 g·L−1 and 218.57 mg·L−1d−1) were attained by P. kessleri TY under mixotrophic cultivation. In comparison, the lowest (0.55 g·L−1 and 78.57 mg·L−1d−1) were attained under heterotrophic culture. Furthermore, heterotrophic and mixotrophic conditions could accumulate more lipids (total lipid contents: 39.85% and 42.92%, respectively), especially the neutral lipids. Additionally, the contents of fatty acids suitable for use as biodiesel raw materials in both heterotrophic and mixotrophic cultures increased, especially the content of C18:1. Moreover, due to the lower biomass of heterotrophic cultivation compared with that from mixotrophic cultivation, the total lipid productivity of heterotrophic conditions decreased. In summary, the conditions of mixotrophic cultivation are more conducive to the accumulation of lipids in P. kessleri TY.
1. Introduction
As worldwide energy demand continues to increase, biodiesel, a source of clean energy, is presently receiving extensive research attention. For this reason, microalgae are considered to be extremely valuable due to their numerous advantages, such as their small size, the possibility of cultivation without the need for arable land, and their high photosynthetic efficiency and biomass productivity, especially in terms of their storage of rich lipids [,]. Therefore, they are currently considered to be a potential source of biodiesel. To date, some oil-rich microalgae have already been studied, such as Chlorella vulgaris, Nannochloropsis oceanica, Scenedesmus obliquus, Selenastrum capricornutum, and so on [,,,]. A native oil-rich algal strain from Shanxi Province, Parachlorella kessleri TY, was previously screened by the authors of the current study []. P. kessleri is a single-celled immobile green microalgae belonging to the class Trebouxiophyceae; it has spherical cells of less than 10 μm in diameter and contains many chloroplasts []. However, of the few reports about P. kessleri in the literature, most have focused on chloroplasts, its use in wastewater treatment, or the effect on it of metal ions [,,,,,,]. Nonetheless, P. kessleri is considered to be a promising strain that can grow in a variety of media []. It has also been shown to promote CO2 capture and inorganic carbon assimilation in gaseous fermentation waste []. In addition, it has been reported that P. kessleri has a positive effect on the compound conversion and natural detoxification of cadmium Cd2+ and chromium Cr2O72− ions []. However, there are currently few studies on the lipid accumulation qualities of P. kessleri.
Microalgae can be cultured in a variety of ways, either autotrophically or using organic carbon sources. Carbon sources are of particular importance because they are essential for the development and formation of any organism. Previous studies have shown that the carbon source is crucial to the biomass and lipid yields of microalgae []. Heterotrophic cultivation offers a way of adding carbon sources such as glucose, sucrose, acetic acid, and other organic matter to the medium used for microalgae in an autotrophic culture system under low-light conditions. Microalga culture under heterotrophic conditions is considered to be preferable to culture under autotrophic conditions because it effectively avoids the issues related to light simulation that occur with autotrophic cultivation []. Many studies have compared the growth of microalgae that have been cultured using different carbon sources. In the case of green algae, not all the algae strains that were cultured under heterotrophic conditions were able to absorb organic carbon sources other than glucose; however, the use of glucose resulted in lower levels of bacterial contamination than other carbon sources. In addition, the use of glucose in a heterotrophic culture system was found to improve the growth parameters of green algae because the supplementation of glucose in the medium induced the movement of passive transport cells from the medium to the cells, therefore increasing the growth rate [,,]. Another reason for choosing glucose is that it is a byproduct of other industries [,,]. Furthermore, glucose is easily metabolized by heterotrophic microalgae. Therefore, glucose was selected as the heterotrophic carbon source in this study.
Other research reports have shown that some microalgae can be cultivated under autotrophic and heterotrophic conditions, as well as by a combination of autotrophic and heterotrophic conditions, i.e., mixotrophic conditions, which can promote an increase in resource utilization. In mixotrophic culture methods, microalgae not only use organic matter as a carbon source but also perform light-intensity irradiation on it. In this way, the carbon dioxide that is released by the microalgae through respiration can be further absorbed and utilized under conditions of autotrophic culture. This behavior can overcome the issue of biomass loss caused by the failure of the autotrophic growth of microalgae in heterotrophic cultivation under dark conditions []. In addition, some studies have also reported the influence of mixotrophic cultivation on the lipid accumulation of microalgae; the results indicated that employing mixotrophic culture methods can improve the lipid productivity of microalgae [] but that not all algae species are suitable for heterotrophic and mixotrophic cultivation.
In this study, Parachlorella kessleri TY was grown in heterotrophic and mixotrophic cultivation conditions with added glucose. Subsequently, the adaptability of P. kessleri TY to heterotrophic and mixotrophic culture systems was explored and analyzed by measuring physiological indexes such as growth, pigment content, chlorophyll fluorescence activity, lipid accumulation, carbohydrate content, and protein content. To the best of our knowledge, no prior report exists on the heterotrophic and mixotrophic culture of Parachlorella algae using an efficient culture method, i.e., with the addition of organic carbon sources to accelerate the cell growth rate and shorten the cell growth time. Improving these parameters would be conducive to achieving high biomass and a good accumulation of bioactive substances in the future. Therefore, this study also serves as theoretical support for the expansion of heterotrophic and mixotrophic alga cultivation.
2. Materials and Methods
2.1. Algal Strains and Cultivation
The experimental algal species used in this study was the high-lipid soil-based green algae Parachlorella kessleri TY, which was screened from soil samples sourced from Shanxi Province in China. The algal strains were first cultured in an improved tris-acetate-phosphate (TAP) [] medium (without glacial acetic acid) with a pH = 7.0 ± 0.1. The culture conditions were as follows: temperature—25 ± 1 °C; light/dark ratio—12 h:12 h; light intensity—3000 lux. Samples were placed on a shaking table (HY-5A, Guohua, Jiangsu, China), which was set to shake continuously at a rate of 2.5 g. After the algal strains had been cultured to the logarithmic stage, they were centrifuged (2795× g for 10 min) to obtain an algal pellet, which was then placed in a 1 L conical flask for culturing (with no more than 700 mL of culture medium). The initial dry weight used for inoculation was about 0.07 g·L−1. The experiment was divided into a heterotrophic group (TAP full medium, i.e., a mixture containing glacial acetic acid, to which a certain concentration of glucose was added; light training was avoided), a mixotrophic group (TAP full medium, to which a certain concentration of glucose was added; the sample was in normal light conditions), and an autotrophic group (the control). Each group comprised three experimental replicates. Culturing occurred over 7 days, with sampling analyses performed at the same time every day.
2.2. Determination of Biomass Concentration and Biomass Productivity
Since the heterotrophic and mixotrophic experiment was conducted by adding an appropriate volume of glucose to the medium as an organic carbon source, it was, thus, necessary to begin by identifying the appropriate concentration of glucose for use under heterotrophic and mixotrophic conditions to carry out the follow-up experiments. First, different concentrations of glucose were added to the new total TAP medium, and then the logarithmic algae were transferred into the mixture for heterotrophic and mixotrophic cultivation. The glucose concentrations selected for the experiment were 5 g·L−1, 10 g·L−1, 15 g·L−1, 20 g·L−1, and 40 g·L−1, and the control group presented an autotrophically cultured alga (glucose concentration: 0 g·L−1). Three replicates were set for each group of experiments, and the culture conditions were the same as above for a period of 7 d. Sampling and analysis were conducted at the same time every day.
Working according to the method used by Lv et al. [], some changes were made, and the biomass of the algal culture was measured. The biomass of the algal culture was expressed in terms of its dry weight. First, 10 mL of algal cultivation broth was filtered by pre-drying, weighing, and passing through a membrane with an aperture of 0.45 μm. Then, the sample was dried to a constant weight and weighed again. The difference in quality between the two values was recorded as the algal dry weight. This was calculated using Equation (1), as follows:
where DW is the microalgal dry weight (g·L−1), m1 (g) is the constant dry weight of the filter membrane, m2 (g) is the total weight of the filter membrane and algal culture after extraction and filtration, and V (L) is the volume of the algal cultivation broth. Equation (2) incorporates the dry weight value calculated in Equation (1), as follows:
where BP (mg·L−1d−1) is the biomass productivity of the algae, DW (g) is the dry weight of the algal powder, and T (d) is the time of the cultivation.
2.3. Determination of Pigment Contents
Working according to the method developed by Mera et al. [], the chlorophyll and carotenoid contents were determined. First, 3 mL of alga culture was extracted every day and then centrifuged (2795× g for 10 min), after which the supernatant was discarded. Then, 3 mL of 95% ethanol solution was added, and the resulting solution was mixed. Afterward, the cell wall of the algal strains was broken (at 20% for 10 min) with an ultrasonic crushing instrument (SCIIENTZ-ⅡD, Scientz, Ningbo, China). The resulting mixture was wrapped in aluminum foil and placed in a refrigerator set at 4 °C (in the dark and at low temperature) for 24 h. After 24 h, the mixture was taken out and centrifuged (2795× g, 10 min) to obtain the supernatant for use in subsequent experiments. A solution of 95% alcohol was taken solution as the blank control, and the absorbances at 649 nm, 665 nm, and 470 nm were determined with a UV-visible spectrophotometer (TU-1810, Persee, Beijing, China). The pigment content was calculated according to the following formulae:
where Chl a (mg·L−1) is the content of chlorophyll a (mg·L−1) in the algal cells for use in Equation (3), Chl b (mg·L−1) is the content of chlorophyll b (mg·L−1) in the algal cells for use in Equation (4), Chl (a + b) is the content of total chlorophyll for use in Equation (5), and Car (mg·L−1) is the content of carotenoids for use in Equation (6).
2.4. Determination of Chlorophyll Fluorescence
To study further the effect of trophic modes on the photosynthetic activity of P. kessleri TY, three indicators of chlorophyll fluorescence PSII activity were measured using a portable PAM fluorometer (AquaPen-C AP-C 100, EcoTech, Beijing, China) to calculate the photosynthetic capacity of algae. The chlorophyll fluorescence was determined according to the procedure developed by Markou et al. [], whereby the fluorescence was measured after 3 mL of algal culture underwent dark reaction conditions for 20 min. The measured indexes include the maximum quantum yield of photosystem II photochemistry (Fv/Fm), the potential activity of photosystem II (Fv/Fo), and the total light energy flux (PIABS values).
2.5. Determination of Total Carbohydrate Content
Similarly, 0.1 g of algal powder was accurately weighed after production under different trophic modes, and 6 M HCl was put into a water bath at 100 °C for 30 min. After cooling, the sample was neutralized with 6 M NaOH, and the supernatant was taken after centrifugation had been performed []. Total carbohydrate was extracted using the phenol sulfuric acid method, after which a certain amount of the extract was taken. First, 1.5 mL of distilled water was added, then 1 mL of 9% phenol solution and 5 mL of concentrated sulfuric acid solution were added, and the mixture was left to stand for 30 min. A UV spectrophotometer was used to determine the spectrum at 485 nm, and a sucrose solution was used as the standard. Afterward, a standard curve was created, and the content was calculated against the standard curve [,]. It was also necessary for the carbohydrate content to be converted, the formula for which is as follows:
where CSc represents the content of carbohydrates in the sample (% DW), C2 represents the content of carbohydrates according to the standard curve (μg), VT2 represents the total volume of the extract (mL), dr represents the dilution factor, VS2 represents the amount added in the determination process (mL), and WF2 represents the dry weight of the sample [].
2.6. Determination of Total Protein Content
The algal cultures produced under different nutrient methods were centrifuged and freeze-dried, after which 0.1 g algal powder was weighed. PBS buffer was added to the powder, and then the cells were crushed with a cell breaker for 10 min to ensure that the cell walls of the algal cells were fully broken to better extract the protein content. The determination of protein content was conducted according to the method employed by He et al. [] and Guo [], after which the content was calculated against the standard curve. To achieve this, the protein content in the sample needed to be converted, and the formula for which is as follows:
where CSP represents the protein content of the sample (% DW), C1 represents the protein content according to the standard curve (μg), VT1 represents the total volume of the extract (mL), VS1 represents the amount of sample added during the determination process (mL), and WF1 represents the dry weight to the sample [].
2.7. Determination of Neutral Lipid Content
Nile Red (NR) is a fluorescent dye that is soluble in lipids and can be used to detect the neutral lipid content of biological cells. The neutral lipids react with the NR dye to produce bright yellow fluorescence; therefore, the detected fluorescence intensity can be used to show changes in neutral lipid content [].
2.8. Determination of Total Lipid Content and Fatty Acid Composition
2.8.1. Determination of Total Lipid Content
First, the algal culture was centrifuged (2795× g, 10 min), after which the algal pellet was put into a freeze dryer (18ND, Scientz, Ningbo, China) for freeze-drying after centrifugation. The dried algal powder was accurately weighed at 0.1 g (w1, g) after being frozen and was then put into an empty 5 mL glass bottle. The empty 5 mL glass bottles were weighed in advance (w2, g), after which 3 mL of methanol and 1.5 mL of chloroform were dissolved into them. The contents were mixed well using the vortex oscillator, crushed with an ultrasonic crushing machine (at 25% for 10 min), and, finally, centrifuged (2795× g, 10 min). The upper liquid was drawn off, and 1.5 mL of 0.75% KCl solution and 1.5 mL of chloroform solution were added. The contents were shaken and mixed evenly, then they were centrifuged at 2795× g for 10 min, and the lower liquid was removed. The above steps were repeated twice, then all liquids were combined in a 5 mL glass bottle, blown dry with nitrogen, and weighed (w3, g) []; three repetitions were performed for each group. The calculation formula is as follows:
where LC (% DW) is the weight percentage of the total lipid content in the algal powder, w1 (g) is the dry weight of the algal powder, w2 (g) is the weight of the empty bottle, and w3 (g) is the total weight of the algal lipids and the glass bottle after the algal lipids were extracted, calculated using Equation (7). LP (mg·L−1d−1) is the lipid productivity of the alga, calculated using Equation (8).
2.8.2. Determination of Fatty Acid Composition
First, the extracted algal oil was methylated, then the dried algal oil was dissolved in chloroform and then transferred to a 1.5 mL Agilent bottle. Then 1 mL 1 mol/L of sulfuric acid methanol solution was added, after which the bottle was filled with nitrogen and sealed. Afterward, the bottle was heated in a water bath at 100 °C for 1 h, cooled at room temperature, and 200 μL ultra pure water was added, followed by shaking the bottle to mix the liquid thoroughly. The organic phase was added to another 1.5 mL Agilent bottle and dried using nitrogen for testing [].
Afterward, the above sample was analyzed using a gas chromatography-mass spectrometry instrument (GC-MS) (7890A-5975C, Agilent, Los Angeles, CA, USA). For the GC-MS analysis, an RTW-WAX column (30 m × 0.25 mm and 0.5 μm) with a temperature program was used to bring the sample from 50 °C to 150 °C. This temperature was maintained for 2 min, then it was raised to 200 °C at a speed of 10 °C/min for 6 min, raised to 230 °C at a speed of 10 °C/min for 30 min, and finally raised to 240 °C at a speed of 10 °C/min for 10 min. Acting as a carrier gas, nitrogen was used at a flow velocity of 0.35 mL/min with an electron ionization (EI) source set at an electron energy level of 70 eV. The mass spectrum scanning range was m/z 20–450, and the sample size was 0.2 μL. The mass spectral database NIST 05 was employed to determine the molecular structure of each component, the relative content of which was calculated using the peak area normalization method [].
2.9. Statistical Analysis
The experiment was replicated three times, and all data were expressed as the mean ± standard error. A completely randomized design was used for the study, and a Student’s t-test was used to determine the statistical significance of the differences between mean values at p ≤ 0.05 []. All statistical analyses were carried out using the SPSS 19.0 statistical software (IBM Inc. Chicago, IL, USA).
3. Results
3.1. Analysis of Changes in Growth
3.1.1. The Effect of Glucose Concentration on the Growth of Parachlorella kessleri TY
The changes recorded in the growth of P. kessleri TY cultivated in autotrophic, heterotrophic, and mixotrophic media supplemented with different concentrations of glucose are shown in Figure 1. Regardless of whether autotrophic, heterotrophic, or mixotrophic cultivation was used, the dry weight of the resulting algae solution exhibited an increasing trend, and all samples entered a stable period after experiencing the logarithmic phase. In the full experiment cycle, the initial dry reset under all conditions was set to be consistent at about 0.07 g·L−1. At 0–1 d of cultivation, the algae strains were in an adaptive state, and the biomass of the algae solution did not change greatly under all conditions. The dry weight was measured at about 0.1 g·L−1. After 1 d, the algal culture, under all conditions, entered the logarithmic phase. When the algae solution was supplemented with glucose concentrations under heterotrophic conditions, its growth rate was lower than that of the control group (0 g·L−1). In heterotrophic conditions, the liquid phase of algae that was supplemented with a glucose concentration of 10 g·L−1 showed the best growth (Figure 1a). The growth of algal strains receiving sufficient light under mixotrophic conditions was very different from that seen under heterotrophic conditions. Except for the alga culture supplemented with 40 g·L−1 glucose, where growth was slightly lower than that of the control group, all alga cultures supplemented with various glucose concentrations grew well, and growth was higher than that in the control group (Figure 1b). Regardless of whether the algae were grown in heterotrophic conditions or mixotrophic conditions, the best growth was seen in the alga culture supplemented with 10 g·L−1 glucose, followed by the alga culture supplemented with 15 g·L−1, 20 g·L−1, 5 g·L−1 and 40 g·L−1 glucose. In summary, the medium supplemented with 10 g·L−1 glucose was selected for subsequent experiments regarding the heterotrophic and mixotrophic cultivation of P. kessleri TY.
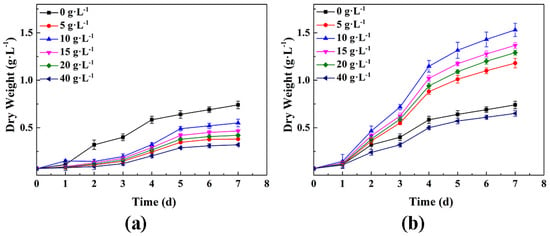
Figure 1.
Changes in the dry weight of Parachlorella kessleri TY under conditions with the supplementation of different concentrations of glucose over 0−7 days; (a) heterotrophic cultivation in dark conditions; (b) mixotrophic cultivation in light conditions. (Data are shown as +/− standard deviation).
3.1.2. The Effect of Heterotrophic Culture and Mixotrophic Culture on the Growth of P. kessleri TY
The dry weight of P. kessleri TY grown via autotrophic culture, heterotrophic culture, and mixotrophic culture was consistent with biomass productivity, which was at a maximum when grown via mixotrophic cultivation (Figure 2 and Table 1). The changing trend of the three growth conditions was consistent in the experiment cycle; all trends were rising, and all trends entered a stable period after reaching the logarithmic phase. The growth rate was the fastest in the autotrophic group, followed by the heterotrophic group. The initial dry reset of the three groups was consistent, at about 0.07 g·L−1. After 1 d, the cultures all entered the logarithmic phase, and the growth of the mixotrophic group was significantly faster than that of the autotrophic group and heterotrophic group. The autotrophic group and the mixotrophic group entered a stable period on Day 4, while the heterotrophic group entered a stable period on Day 5. The growth of the mixotrophic group after Day 4 was relatively slow compared with the previous days, but the group still maintained sustained growth. By Day 7, all groups had reached the maximum dry weight and biomass productivity. The dry weight and biomass productivity of autotrophically grown algae were 0.74 g·L−1 and 105.81 mg·L−1d−1, respectively. The dry weight of algae from the heterotrophic group was 0.74 times that of the autotrophic group (0.55 g·L−1), and the biomass productivity was 78.57 mg·L−1d−1. The dry weight of algae in the mixotrophic group was the highest, reaching 1.53 g·L−1, which was about 2.07 times that of the autotrophic group, and its biomass productivity was 218.57 mg·L−1d−1. The results indicate that heterotrophic cultivation conditions were not conducive to the growth of P. kessleri TY. However, the mixotrophic culture conditions were favorable to the growth of P. kessleri TY. At the end of the experimental period, the dry weight and biomass productivity of the mixotrophic group were both higher than the sum of that of the autotrophic group and the heterotrophic group. This result was similar to those reported by Yan [].
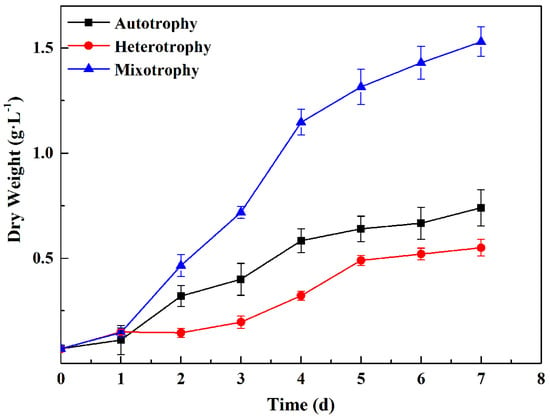
Figure 2.
Changes in the dry weight of P. kessleri TY grown under different trophic modes over 0−7 days. (Data are shown as +/− standard deviation).

Table 1.
Changes in the biomass productivity of Parachlorella kessleri TY grown under different trophic modes on Day 7.
3.2. Analysis of Pigment Content
The changes in the periodic pigment content of P. kessleri TY under different trophic modes are shown in Figure 3. The pigment contents of the autotrophic group and mixotrophic group were higher than those of the heterotrophic group. Likewise, the contents of chlorophyll a, chlorophyll b, chlorophyll (a + b), and carotenoids in the various alga cultures increased under the three conditions. However, the pigment content in the heterotrophic group cultivated in dark conditions changed more slowly. The pigment content of the three trophic modes indicated significant differences from the first day of testing. The autotrophic group and the mixotrophic group first entered the logarithmic phase and then entered the stable phase after 4 d. The pigment content in the autotrophic group was markedly higher than that in the other two groups throughout the whole experimental cycle. The content of chlorophyll a in the autotrophic group at Day 7 was 2 times and 8.5 times of that seen in the mixotrophic group and heterotrophic group, respectively. The carotenoid contents were 1.3 times and 3.6 times that in the mixotrophic and heterotrophic groups, respectively; these results are consistent with those reported by Li et al. []. The above results indicate that autotrophy was more beneficial to the synthesis of chlorophyll and carotenoid contents than heterotrophic and mixotrophic cultivation.
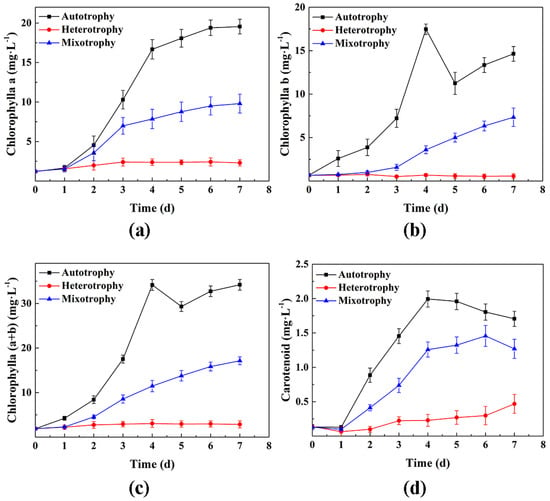
Figure 3.
Changes in the pigment content of P. kessleri TY under different trophic modes of cultivation over 0−7 days; (a) the contents of chlorophyll a; (b) the contents of chlorophyll b; (c) the contents of chlorophyll (a + b); (d) the carotenoid contents. (Data are shown as +/− standard deviation).
3.3. Analysis of Chlorophyll Fluorescence Characteristics
As shown in Figure 4, Fv/Fm, Fv/Fo, and PIABS all showed a downward trend in chlorophyll fluorescence under autotrophic, heterotrophic, and mixotrophic culture conditions. However, levels in the heterotrophic group were significantly lower than those in the autotrophic and mixotrophic groups. After 0–1 d, the chlorophyll fluorescence parameters of the three groups under different conditions began to differ; the chlorophyll fluorescence values for the autotrophic group were significantly higher than those of the heterotrophic group. After 7 d of cultivation, the values for the autotrophic group were the highest, about twice that of the heterotrophic group. However, the values of Fv/Fm, Fv/Fo, and PIABS in the autotrophic group were similar to those in the mixotrophic group (albeit slightly higher in the autotrophic group). The experiment indicated that both the dark reaction conditions and glucose supplementation inhibited the photosynthetic capacity and PSII activity of P. kessleri TY, with the dark reaction having a more serious effect.
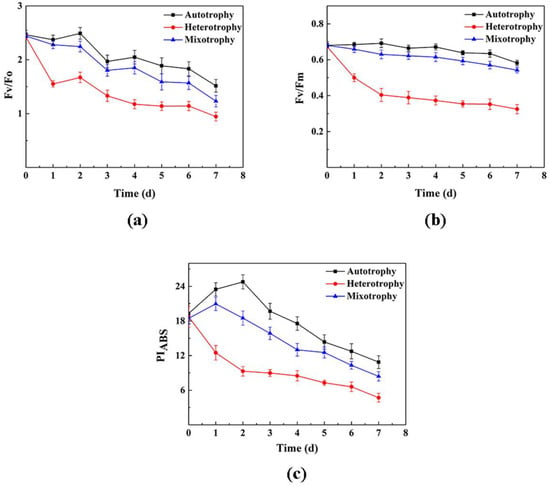
Figure 4.
Changes in the chlorophyll fluorescence parameter of P. kessleri TY under different trophic modes over 0−7 days; (a) Fv/Fo (the potential activity of photosystem II); (b) Fv/Fm (the maximum quantum yield of photosystem II photochemistry); (c) PIABS (the maximum quantum yield of photosystem II photochemistry). (Data are shown as +/− standard deviation).
3.4. Analysis of Total Carbohydrate Content
As shown in Figure 5, the carbohydrate content of P. kessleri TY under different trophic modes was consistent with the results for its total lipid content. The mixotrophic group had the highest carbohydrate content of about 27.45%, followed by the heterotrophic group, the carbohydrate content of which was 24.95%. In comparison, the autotrophic group had the lowest carbohydrate content of 22.5%. The above results indicated that the addition of glucose as an organic carbon source could promote the total carbohydrate content of P. kessleri TY, and they also show that the presence of light was more conducive to the increase in total carbohydrate content than growth in dark conditions.
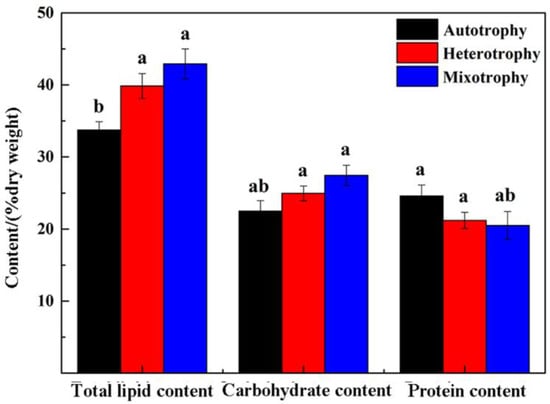
Figure 5.
Total lipid content, carbohydrate content, and protein content of P. kessleri TY grown under different trophic modes on Day 7. (Data are shown as +/− standard deviation. Values with different letters are significantly different within the group (p < 0.05), values with the same letters are not significantly different within the group (p > 0.05) [], and letters are sorted according to the average size of the data).
3.5. Analysis of Total Protein Content
The total protein content from the alga cultures grown using three different trophic modes showed an opposite trend to the total lipid and total carbohydrate content (Figure 5). The total protein contents of the heterotrophic group and mixotrophic group were lower than those of the autotrophic group, which was 24.6%. In comparison, those of the heterotrophic group and mixotrophic group were 21.21% and 20.5%, respectively. These results demonstrate that the medium used for P. kessleri TY was significantly affected by glucose.
3.6. Analysis of Neutral Lipid Content
As shown in Figure 6, the neutral lipid content increased over time under all three conditions. The neutral lipid content in the heterotrophic group and the mixotrophic group was significantly higher than that in the autotrophic group, and the neutral lipid content in the heterotrophic group and the mixotrophic group increased logarithmically at 1–4 d, while in contrast, the autotrophic group showed lower levels. On Day 7, the neutral lipid content in the mixotrophic group was the highest, at about 2.7 times that in the autotrophic group, followed by the heterotrophic group, about 2.5-fold of that seen in the autotrophic group (Figure 6). The above results clarified that adding glucose as an organic carbon source is beneficial to the accumulation of neutral lipids in P. kessleri TY, which was also one of the factors affecting the accumulation of neutral lipids.
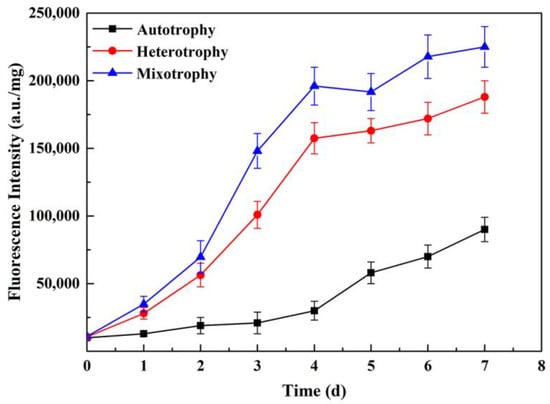
Figure 6.
Changes in the fluorescence intensity of P. kessleri TY under different trophic modes over 0−7 days. (The content of neutral lipids in the cells is shown in fluorescence intensity. Data are shown as +/− standard deviation).
3.7. Analysis of Total Lipid Content and Fatty Acid Composition
3.7.1. Analysis of Total Lipid Content
As shown in Figure 5, on Day 7, the total lipid content in both the heterotrophic group and the mixotrophic group was higher than that in the autotrophic group; the total lipid content in the mixotrophic group was the highest (up to 42.92%), and the content in the heterotrophic group was 39.85%, while that in the autotrophic group was 33.75%. Moreover, the contents were determined for the autotrophic, heterotrophic, and mixotrophic groups on day 7 of lipid productivity, and the contents were 35.69 mg·L−1d−1, 31 mg·L−1d−1 and 93.61 mg·L−1d−1, respectively. It was clear that the lipid productivity of the mixotrophic group was obviously higher than that of the other two groups; the lipid productivity of the heterotrophic group was the lowest, while its total lipid content was higher than that of the autotrophic group. This is because of its low biomass productivity, leading to low lipid productivity (Table 2). In conclusion, the addition of glucose as an organic carbon source could promote the total lipid content of P. kessleri TY. It was found that cultivation in the light was more advantageous than in the dark in terms of lipid accumulation. In addition, the dry weight of biomass, total lipid content, and lipid productivity were all at their highest under conditions of mixotrophic cultivation, which identifies it as most conducive to the larger-scale accumulation and application of P. kessleri TY.

Table 2.
Lipid productivity in P. kessleri TY under different trophic modes on Day 7.
3.7.2. Analysis of Fatty Acid Composition
The content of each type of fatty acid in P. kessleri TY that was cultured under different trophic modes is listed in Table 3. In total, 7 fatty acids were detected in the samples cultured under autotrophic conditions, and 6 fatty acids were detected in the samples grown under heterotrophic and mixotrophic conditions. They were mainly composed of C16 and C18 fatty acids with high contents, namely 87.56% in the autotrophic group, 90.6% in the heterotrophic group, and 91.12% in the mixotrophic group. All fatty acids contained both saturated fatty acids (SFA) and unsaturated fatty acids (UFA); there was no aromatic ring heterogeneous structure to the fatty acids, and the unsaturated double bonds were no more than four. Although the contents were slightly different for the different trophic modes regarding the main fatty acids in the samples, they mainly contained three fatty acids: palmitic acid (C16:0, autotrophic group: 22%, heterotrophic group: 23.6%, and mixotrophic group: 24%), linoleic acid (C18:2, autotrophic group: 14.64%, heterotrophic group: 20.5%, and mixotrophic group: 21.1%) and linolenic acid (C18:3, autotrophic group: 28.79%, heterotrophic group: 19.7%, and mixotrophic group: 17.95%). In addition to the three main fatty acids mentioned above, oleic acid (C18:1) also accounted for a large proportion of fatty acids (15.51% and 15.84%, respectively) in the heterotrophic group and the mixotrophic group. According to the literature, C16:0, C18:1, C18:2, and C18:3 are the main fatty acids used in biodiesel preparation [].

Table 3.
Fatty acids composition in P. kessleri TY under different trophic modes on Day 7. (Data are shown as +/− standard deviation).
It can be seen from Table 3 that the composition of fatty acids and the content of each component in the heterotrophic group and the mixotrophic group were similar but were still slightly different. The sum of C16 and C18 in the heterotrophic group and the mixotrophic group was basically the same (as shown in the table, the difference is not significant), and their components were simpler than those in the autotrophic group. In addition, the contents of oleic acid (C18:1), SFA, and monounsaturated fatty acids (MUFA) in the heterotrophic group and the mixotrophic group were higher than those in the autotrophic group. However, the content of polyunsaturated fatty acid (PUFA) was significantly higher in the autotrophic group (57.38%) than in the other groups (heterotrophic group—44.37%, mixotrophic group—42.85%). These results were basically consistent with those reported in the study by Li et al. [].
4. Discussion
At present, most of the research on microalgae is aimed at autotrophic culture methods. However, due to the limitations of light, algal photosynthesis is fixed, and it is difficult to increase its biomass to a high level. Conversely, organic compounds are absorbed via aerobic respiration through exposure to light conditions; therefore, organic carbon could be fully utilized and, thus, encourage better growth []. Heterotrophy involves cultivation under dark conditions without light and depends on the addition of organic compounds. However, the ability to achieve heterotrophy is very weak, meaning that the energy provided by respiration is not enough to sustain the growth of the microalgae. Therefore, not all algal strains are suitable for heterotrophic culture. Mixotrophic cultivation could supplement autotrophy with organic compounds, therefore obtaining higher biomass and greater biomass productivity at a lower cost than heterotrophic cultivation []. According to some studies, the light sensitivity of microalgae that were cultured concurrently was lower than that of microalgae grown under autotrophy or heterotrophy. For example, Yan [] reported that the growth of Scenedesmus obliquus grown using the mixotrophic system was superior to that in samples grown using the autotrophic and heterotrophic systems, and its biomass productivity was 4 times that seen in samples grown under autotrophic conditions and 3.2 times that in samples grown under heterotrophic conditions, respectively. Ma et al. [] found that the biomass of Chlorella sp. Grown under mixotrophic conditions was 2.4 times and 2.6 times that of samples grown under autotrophic and heterotrophic conditions, respectively, while the biomass of Dunaliella alina that had been grown under mixotrophic conditions was 1.9 times and 1.3 times that of samples grown under autotrophic and heterotrophic conditions, respectively. Kabir et al. [] found that the biomass of Selenastrum sp. samples grown under mixotrophic conditions was 1.6 times and 1.36 times that of samples grown under autotrophic and heterotrophic conditions. Because the research focuses on different species of algae, the relationship between photosynthesis and respiration was different in each study, meaning that the growth rates that were reported were different for autotrophy, heterotrophy, and mixotrophy. For example, Zhang et al. [] reported that the growth rate of C. zofingiensis samples grown under mixotrophic cultivation conditions was the same as that of samples grown under autotrophic and heterotrophic conditions. Yan [] reported that the growth rate of S. obliquus was higher under mixotrophic culture conditions than that seen under autotrophic culture and heterotrophic culture conditions. However, Kabir et al. [] reported that the growth rate of C. vulgaris cultivated under mixotrophic conditions was lower than the sum of those grown under autotrophy and heterotrophy. The results showed that the photoinhibition of algae growth under an autotrophic system could be eliminated due to the protective effect of the organic carbon source, and the presence of an organic carbon source also promotes autotrophic growth.
However, not all kinds of algae are suitable for heterotrophic culture and mixotrophic culture. In this study, the identified species of algae P. kessleri TY was supplemented with an appropriate concentration of glucose. It was found that although its total lipid content was high, its lipid productivity was also low due to its low biomass; that is, the growth of the algal strain was poor in dark conditions. However, the biomass and biomass productivity of P. kessleri TY that was grown under mixotrophic culture conditions were both high, and the values were higher than the sum of the two other cultivation methods. The results showed that species of microalgae that were suitable for mixotrophic cultivation could convert light energy and organic carbon into biomass energy via photosynthesis and respiration, and their dry weight and biomass productivity could also be significantly improved []. This could be because most of the glucose metabolism in healthy microalgae cells occurs through glycolysis and pentose phosphate pathways, even when operating under photoautotrophic metabolism. Therefore, under photoautotrophic conditions, the addition of glucose to the medium provides the cells with immediate energy to a certain extent and significantly accelerates growth, which is mainly due to the assimilation of organic carbon, which is a more energy-efficient process for algal cells than assimilation through photosynthesis (the Calvin–Benson cycle) []. In addition, the microalgae grown in heterotrophic culture conditions could only obtain carbon and energy from the reducing organic substrate. In contrast, microalgae in mixotrophic culture conditions could be collected from all available sources (organic carbon, CO2, and light) present in the medium at the same time. Moreover, mixotrophic cultivation is not simply a combination of autotrophic and heterotrophic growth techniques. The two processes interact with each other and have a synergistic effect that can increase the biomass dry weight and biomass yield, which will be higher than the sum of the two metabolisms at the same time, as well as independently [,].
El-sheekh et al. [] reported that the pigment content in C. vulgaris grown under both mixotrophic and heterotrophic conditions was lower than that under autotrophic conditions (the pigment content under mixotrophic conditions was higher than that in samples grown under heterotrophic conditions). The results indicated that grown under light conditions had a positive effect on pigment content, while glucose had an inhibitory effect on pigment content. The reports made by Lewitus et al. [] and Oliveira [] both indicated that mixotrophic and heterotrophic cultures were not conducive to chlorophyll accumulation in microalgae; these findings are consistent with the results of this study. In general, the amount of pigment synthesized through the photo collection process is lower in samples from mixotrophic cultures compared to those cultured in autotrophic growth conditions, possibly because organic carbon is thought to cause major changes in the photosynthetic system. Under mixotrophic conditions, microalgae could not only use light energy to synthesize organic matter but also use organic carbon sources for glucose metabolism, therefore enriching the biomass. In addition, in the presence of glucose, cell growth is almost independent of photosynthetic carbon assimilation []. As expected, heterotrophically grown samples produce negligible pigment levels because, in these cultivation methods, by definition, the only source of energy is chemicals. When the photosynthetic system is inactive, the cells do not have access to light []. Furthermore, chlorophyll is an important photosynthetic pigment of microalgae; however, heterotrophy is characterized by a decrease in chlorophyll content. Since darkness is not conducive to chlorophyll synthesis, cell division leads to chlorophyll dilution. In microalgae, most of the chlorophyll is involved in the formation of light-harvesting complex II, and the regulation of its size mainly occurs at the level of chlorophyll synthesis. Heterotrophic cultivation is carried out under dark conditions, meaning that the algae cannot synthesize chlorophyll, and cultivation is accompanied by the rapid degradation of light-harvesting complex II, resulting in a more pronounced decrease in chlorophyll b relative to chlorophyll []. At the same time, carotenoids are also related to photosynthesis. They are important in secondary cell metabolism (mainly in photoprotection and allelopathy) and are mainly regulated by light. Therefore, the carotenoid content is lower in the absence of light, which is consistent with the results of this study []. As a result, the demand for chlorophyll in samples grown under heterotrophic cultivation and mixotrophic cultivation is reduced, resulting in a continuous decline in chlorophyll content.
In addition, this study also found that the photosynthetic capacity under heterotrophic and mixotrophic conditions was similar to that in samples grown under autotrophic conditions, both being lower than that in samples grown under autotrophic conditions. The results showed that the introduction of organic carbon sources exhibited different degrees of inhibition on the photosynthetic system activity of microalgae. Vidotti et al. [] found that after Chlorella sp. was switched from autotrophic conditions to mixotrophic conditions, the activity and efficiency of photosynthetic system II and photosynthetic system I decreased, and the photosynthetic pigment content decreased. Liu [] studied the effects of different trophic modes on Phaeodactylum tricornutum and found that after the addition of organic carbon sources, the contents of photosynthetic pigments in the cells decreased, along with the activity of the PS II system/photosynthetic oxygen release rate, i.e., organic carbon reduced the photosynthetic capacity of P. tricornutum. Exogenous glucose provides energy and a small molecular carbon skeleton for the growth of the cells through catabolism, therefore reducing the dependence of cells on light energy. As a result, the photosynthetic capacity of cells under mixotrophic conditions was lower than the level in the autotrophic samples. The results showed that both dark reaction conditions and the presence of glucose inhibited the photosynthetic capacity and PSII activity of microalgae [].
Moreover, carbohydrates and proteins are two other important storage substances occurring alongside the lipids found in microalgae cells. Studying the changes in carbohydrate and protein contents in microalgae is helpful when analyzing the flow direction of carbon in the three culture systems. However, there are few reports detailing the carbohydrate and protein contents of microalgae grown under heterotrophic and mixotrophic conditions. Although this experiment was carried out in the current study, the results showed that carbohydrates in the heterotrophic group and the mixotrophic group were also higher than those in the autotrophic group, but that their total protein contents were lower than those in the autotrophic group, meaning that the production of algal proteins depended on the use of both a carbon source and a light source. These findings concur with those reported by Ogbonna and Tanaka []; they also found that the protein content of Chlorella during the process of glucose addition in heterotrophic and mixotrophic conditions continued to fall (maintained under conditions where the carbohydrate and protein contents were higher than in samples grown under heterotrophic conditions). The reason for this might be due to the decrease in protein content in samples grown under mixotrophic conditions, which is related to the tricarboxylic acid cycle. The TCA cycle, which causes reduced power in the cells, is also involved in the amino acid synthesis pathway. The assimilation of organic carbon sources through the TCA cycle provided the necessary conditions for microalga cultivation. Therefore, NADH and amino acid metabolism could both occur. As a result, in mixotrophic culture conditions, microalgae cells obtain reduced power from other sources, resulting in the down-regulation of the TCA cycle and a decreased protein content []. Moreover, during the growth and metabolism process of algal cells, carbon, and nitrogen will form a competitive relationship because the participation of carbon will convert the proteins, requiring higher nitrogen levels for their conversion into saccharide fatty acids [,], leading to a decrease in protein content. In this scenario, protein synthesis decreases while carbohydrate and lipid contents increase, in particular inducing the accumulation of triacylglycerol (TAG) in algal cells; that is, the algal cells convert more carbon and energy for lipid synthesis [,,,].
Furthermore, the study by Yun et al. [] clarified that the lipid content of Chlorella sp. cultivated under mixotrophic and heterotrophic conditions, along with additional carbon sources, was significantly higher than that found in samples grown under autotrophic conditions. Mixotrophic culture has certain advantages, not only in light sensitivity and biomass productivity but also in lipid content and lipid productivity. In addition, the study by El-sheekh et al. [] illustrated that glucose had a positive effect on the lipid production of Chlorella grown under mixotrophic culture conditions. Liu et al. [] also believed that glucose could improve the biomass and lipid contents of microalgae (to a level significantly better than that under autotrophic conditions). In this study, regardless of the neutral lipid content, the total lipid content, or lipid productivity, all values for algae in the mixotrophic culture group were significantly higher than those in the autotrophic group; its lipid content and lipid productivity reached 42.92% and 93.61 mg·L−1d−1, respectively. The above results indicate that the lipid content in algae cultivated under mixotrophic conditions was significantly higher than that found in algae grown under autotrophic conditions, which may be due to the addition of organic substrates, contributing to lipid accumulation as a means of energy storage []. Moreover, under mixotrophic cultivation, part of the energy is used for growth. In contrast, the rest of the energy is stored as carbohydrates and lipids, which increases the volume of microalgae and, therefore, increases the lipid content. However, under both mixotrophic and heterotrophic conditions, the total lipid content was higher than the carbohydrate content, while the carbohydrate content was lower than the lipid content. This may be related to the carbohydrate content because lipid synthesis depends, to a significant extent, on starch degradation []. Furthermore, lipid production in microalgae depends on the utilization of acetyl-CoA and NADPH. Due to the presence of organic matter and inorganic carbon in mixotrophic cultivation, carbon dioxide was released and then fixed through the interaction of autotrophic and heterotrophic metabolism. This led to further electron flows between PSI and PSII, meaning that more energy and NADPH were produced under mixotrophic conditions [].
In this study, the fatty acid composition and content of P. kessleri TY samples grown under different trophic modes were determined and analyzed. It was found that the cells in the heterotrophic group and the mixotrophic group exhibited a similar composition and content of fatty acids. In contrast, their contents of the C16 and C18 fatty acids were higher than those in the autotrophic group. The difference between the values for the heterotrophic group and the mixotrophic group was not significant; the values were 90.6% and 91.12%, respectively. This may be due to the balance of ATP and the reducing agent (NADPH or NADH) between fatty acid synthesis and glucose oxidation []. Furthermore, the biosynthesis pathway of heterotrophic and mixotrophic metabolism could up-regulate the production of fatty acids and inhibit the enzymes involved in fatty acid degradation. Glycolysis/gluconeogenesis, the tricarboxylic acid cycle, and pyruvate metabolism are all required for fatty acid biosynthesis to provide the required ATP and acetyl-CoA. Under heterotrophic culture conditions, in addition to the above metabolism process, a pentose phosphate pathway was also present; however, metabolism could only take place under dark conditions, therefore promoting the biosynthesis of fatty acids []. Moreover, the presence of glucose changed its metabolic pathway. Glucose leads to metabolic pathways that are conducive to glycolysis, pathways in which the intermediate product (dihydroxyacetone phosphate) is the substrate required for lipid synthesis. Therefore, the contents of fatty acids were higher under heterotrophic and mixotrophic conditions []. In addition, compared with the autotrophic group, the contents of C18:1 and C18:2 in the heterotrophic group and the mixotrophic group increased, while the content of C18:3 decreased. Furthermore, the levels of saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA) were highest in the mixotrophic group, followed by the heterotrophic group, and were the lowest in the autotrophic group. The results showed significant differences, but the polyunsaturated fatty acid (PUFA) contents of the various samples were contrary. SFA and MUFA are beneficial to the production of biodiesel, which finding is basically consistent with the results published by Li et al. []. According to the relevant standards for biodiesel, the main fatty acids are palmitic acid (C16:0), oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18:3). The fatty acids in this study did not contain an aromatic cyclic isomerism structure; most of the carbon chain length comprised units of 14 to 20 carbons, while the unsaturated double bonds did not exceed 4 carbons [,,]. Moreover, the SFA exhibited antioxidant properties, which would be conducive to the long-term storage of biodiesel. The MUFA demonstrated better stability at low temperatures and, thus, would not degrade due to high levels of oxidation []. In the determination of fatty acids performed in this study, P. kessleri TY demonstrated the maximum values of C16:0, C18:1, and C18:2 when grown under mixotrophic culture conditions. At the same time, the contents of SFA and MUFA were also higher than those of the other two groups. The enhancement of SFA and the saturation of unsaturated fatty acids increase the value of biological resources as industrial fatty acids. In contrast, the increase in carbon chains indicates the possibility of producing high-value lipids. In conclusion, concurrent culture techniques increased the SFA content of P. kessleri TY and saturated its unsaturated fatty acids (UFA) []. Therefore, the contents of fatty acids when using mixotrophic and heterotrophic culture methods were higher. In comparison, the contents of polyunsaturated fatty acids were lower than those in the samples grown under autotrophic culture conditions. The possible reasons for this finding are as follows: (1) most of the saturated fatty acids and monounsaturated fatty acids accumulated under mixotrophic and heterotrophic culture conditions; (2) photosynthesis and lipid synthase would be more fully utilized under autotrophic conditions; (3) the desaturation process involved in the biosynthesis of polyunsaturated fatty acids requires molecular oxygen, and the high levels that developed under autotrophic conditions increased the unsaturation of fatty acids []. Therefore, P. kessleri TY, when cultured under mixotrophic conditions, is quite suitable for use as biodiesel. This study lays a foundation for the improvement and development of biological resources to produce high-value lipids.
5. Conclusions
In this study, it was found that Parachlorella kessleri TY grew fastest under mixotrophic culture conditions but grew more slowly under heterotrophic culture conditions. Both the heterotrophic and mixotrophic methods of culture could encourage the accumulation of the grease present in P. kessleri TY; the contents of neutral lipids, in particular, notably increased, and the contents of C16:0, C18:1, and C18:2 (especially C18:1), which are suitable for use as biodiesel raw materials, were significantly higher than those found in samples grown under autotrophic culture conditions The contents of SFA and MUFA were also high. Because the biomass productivity was too low when using heterotrophic culture methods, the lipid content after heterotrophic culture was also significantly reduced, which was not conducive to the production of biodiesel; conversely, the lipid productivity when using co-culture methods was higher. To sum up, when combined with an analysis of two very important indexes of lipid productivity and fatty acid composition, this study shows that it is beneficial to increase the biomass productivity and lipid productivity of P. kessleri TY using mixotrophic culture methods. It also indicates that P. kessleri TY could convert the absorbed organic carbon into lipids more efficiently when grown using this culture system. This result is of great significance for the production of biodiesel.
Author Contributions
Conceptualization, L.J. and S.X.; methodology, Y.G. and Y.L.; software, Y.L. and Y.Y.; validation, Y.G., Y.L., Y.Y., and J.F.; formal analysis, L.J. and S.X.; investigation, Y.G. and Y.L.; resources, Y.G. and Y.Y.; data curation, Y.Y. and J.F.; writing—original draft preparation, Y.G.; writing—review and editing, L.J. and S.X.; visualization, Y.G., L.J., and S.X.; supervision, L.J. and S.X.; project administration, L.J. and S.X.; funding acquisition, Y.G., Y.L., and L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by: 1. the National Natural Science Foundation of China, grant number 42177057 (by Li Ji) and 42207528 (by Yuan Li); 2. Key Research and Development Project in Shanxi Province, grant number 202202140601019 (by Li Ji); 3. Fundamental Research Program of Shanxi Province, grant number 202103021223266 (by Yifan Gao) and 202203021212305 (by Yuan Li); 4. Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi, grant number 2021L310 (by Yifan Gao); 5. the Research Award Fund for Outstanding Doctoral Coming to Work in Shanxi, grant number 20212023 (by Yifan Gao); 6. and the Doctoral Scientific Research Foundation for Taiyuan University of Science and Technology, grant number 20202069 (by Yifan Gao).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thank other laboratory members for algal culture and technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parakh, S.K.; Tian, Z.; Wong, J.Z.E.; Tong, Y.W.F. From microalgae to bioenergy: Recent advances in biochemical conversion processes. Fermentation 2023, 9, 529–576. [Google Scholar] [CrossRef]
- Khan, S.; Das, P.; Quadir, A.M.; Thaher, M.I.; Mahata, C.; Sayadi, S.; Al-Jabri, H. Microalgal feedstock for biofuel production: Recent advances, challenges, and future perspective. Fermentation 2023, 9, 281–315. [Google Scholar] [CrossRef]
- Cunha, A.E.P.; Satiro, J.R.; Escobar, B.P.; Simoes, R.M. Chlorella vulgaris growth, pigment and lipid accumulation: Effect of progressive light and hydrogen peroxide exposure. J. Chem. Technol. Biotechnol. 2023, 98, 442–450. [Google Scholar] [CrossRef]
- Touliabah, E.S.; Almutairi, A.W. Effect of phytohormones supplementation under nitrogen depletion on biomass and lipid production of Nannochloropsis oceanica for integrated application in nutrition and biodiesel. Sustainability 2021, 13, 592. [Google Scholar] [CrossRef]
- Trivedi, J.; Atray, N.; Agrawal, D.; Ray, A. Enhanced lipid production in Scenedesmus obliquus via nitrogen starvation in a two-stage cultivation process and evaluation for biodiesel production. Fuel 2022, 316, 123418. [Google Scholar] [CrossRef]
- Pugliese, A.; Biondi, L.; Bartocci, P.; Fantozzi, F. Selenastrum capricornutum a new strain of algae for biodiesel production. Fermentation 2020, 6, 46–58. [Google Scholar] [CrossRef]
- Gao, Y.F.; Lv, J.P.; Feng, J.; Liu, Q.; Xie, S.L. Morphology, phylogeny and lipid components of an oil-rich microalgal strain. J. Appl. Bot. Food Qual. 2017, 90, 298–305. [Google Scholar] [CrossRef]
- Kalantaryan, N.K.; Harutyunyan, B.; Minasyan, E.V.; Goginyan, V. Comparative assessment of brewery wastewater treatment potential by microalgae Parachlorella kessleri and Chlorella vulgaris. Biol. J. Armen. 2021, 4, 72–79. [Google Scholar]
- Hasegawa, M.; Yoshida, T.; Yabuta, M.; Terazima, M.; Kumazaki, S. Anti-stokes fluorescence spectra of chloroplasts in Parachlorella kessleri and maize at room temperature as characterized by near-infrared continuous-wave laser fluorescence microscopy and absorption microscopy. J. Phys. Chem. B 2011, 115, 4184–4194. [Google Scholar] [CrossRef]
- Turmel, M.; Otis, C.; Lemieux, C. The chloroplast genomes of the green algae Pedinomonas minor, Parachlorella kessleri, and Oocystis solitaria reveal a shared ancestry between the Pedinomonadales and Chlorellales. Mol. Biol. Evol. 2009, 26, 2317–2331. [Google Scholar] [CrossRef]
- Beigbeder, J.B.; Sanglier, M.; Medeiros Dantas, J.M.; Lavoie, J.M. CO2 capture and inorganic carbon assimilation of gaseous fermentation effluents using Parachlorella kessleri microalgae. J. CO2 Util. 2021, 50, 101581. [Google Scholar] [CrossRef]
- Bauenova, M.O.; Sadvakasova, A.K.; Mustapayeva, Z.O.; Kokociński, M.; Zayadan, B.K.; Wojciechowicz, M.K.; Balouch, H.; Akmukhanova, N.R.; Alwasel, S.; Allakhverdiev, S.I. Potential of microalgae Parachlorella kessleri Bh-2 as bioremediation agent of heavy metals cadmium and chromium. Algal Res. 2021, 59, 102463. [Google Scholar] [CrossRef]
- Kadukova, J. Surface sorption and nanoparticle production as a silver detoxification mechanism of the freshwater alga Parachlorella kessleri. Bioresour. Technol. 2016, 216, 406–413. [Google Scholar] [CrossRef]
- Velgosova, O.; Anna, M.; Elena, C.; Jaroslav, M. Green synthesis of Ag nanoparticles: Effect of algae life cycle on Ag nanoparticle production and long-term stability. Trans. Nonferr. Metal. Soc. 2018, 28, 974–979. [Google Scholar] [CrossRef]
- Najafabadi, H.A.; Malekzadeh, M.; Jalilian, F.; Vossoughi, M.; Pazuki, G. Effect of various carbon sources on biomass and lipid production of Chlorella vulgaris during nutrient sufficient and nitrogen starvation conditions. Bioresour. Technol. 2015, 180, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Ananthi, V.; Raja, R.; Carvalho, I.S.; Brindhadevi, K.; Pugazhendhi, A.; Arun, A. A realistic scenario on microalgae based biodiesel production: Third generation biofuel. Fuel 2021, 284, 118965. [Google Scholar] [CrossRef]
- He, Y.; Hong, Y.; Liu, X.; Zhang, Q.; Liu, P.; Wang, S. Influences of carbon and nitrogen sources and metal ions on the heterotrophic culture of Scenedesmus sp. LX1. Environ. Sci. Pollut. R 2019, 26, 13381–13389. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.F.; Hu, H.; Ma, L.L. FAMEs production from Scenedesmus obliquus in autotrophic, heterotrophic and mixotrophic cultures under different nitrogen conditions. Environ. Sci. Wat. Res. 2018, 4, 461–468. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; D’Alessandro, E.B.; Filho, N.R.A.; Lopes, R.G.; Derner, R.B. Synergistic effect of growth conditions and organic carbon sources for improving biomass production and biodiesel quality by the microalga Choricystis minor var. minor. Sci. Total Environ. 2021, 759, 143476. [Google Scholar] [CrossRef]
- Leon-Vaz, J.R.S. Using agro-industrial wastes for mixotrophic growth and lipids production by the green microalga Chlorella sorokiniana. N. Biotechnol. 2019, 51, 31–38. [Google Scholar] [CrossRef]
- Ende, S.S.W.; Noke, A. Heterotrophic microalgae production on food waste and by-products. J. Appl. Phycol. 2019, 31, 1565–1571. [Google Scholar] [CrossRef]
- Katiyar, R.; Gurjar, B.R.; Bharti, R.K.; Kumar, A.; Biswas, S.; Pruthi, V. Heterotrophic cultivation of microalgae in photobioreactor using low cost crude glycerol for enhanced biodiesel production. Renew. Energy 2018, 113, 1359–1365. [Google Scholar] [CrossRef]
- Lin, T.S.; Wu, J.Y. Effect of carbon sources on growth and lipid accumulation of newly isolated microalgae cultured under mixotrophic condition. Bioresour. Technol. 2015, 184, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.H.; Tsai, C.C. Enhancing oil accumulation of a mixed culture of Chlorella sp. and Saccharomyces cerevisiae using fish waste hydrolysate. J. Taiwan Inst. Chem. E 2016, 67, 377–384. [Google Scholar] [CrossRef]
- Silva Benavides, A.M.; Campos Rudin, M.; Villalobos, N.; Touloupakis, E.; Torzillo, G. Growth and hydrogen production by three Chlamydomonas strains cultivated in a commercial fertilizer. Int. J. Hydrogen. Energ. 2019, 44, 9849–9855. [Google Scholar] [CrossRef]
- Lv, J.P.; Guo, J.Y.; Feng, J.; Liu, Q.; Xie, S.L. Effect of sulfate ions on growth and pollutants removal of self-flocculating microalga Chlorococcum sp. GD in synthetic municipal wastewater. Bioresour. Technol. 2017, 234, 289–296. [Google Scholar] [CrossRef]
- Mera, R.; Torres, E.; Abalde, J. Effects of sodium sulfate on the freshwater microalga Chlamydomonas moewusii: Implications for the optimization of algal culture media. J. Phycol. 2016, 52, 75–88. [Google Scholar] [CrossRef]
- Markou, G.; Muylaert, K. Effect of light intensity on the degree of ammonia toxicity on PSII activity of Arthrospira platensis and Chlorella vulgaris. Bioresour. Technol. 2016, 216, 453–461. [Google Scholar] [CrossRef]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000; pp. 184–199. [Google Scholar]
- Prajapati, S.K.; Kaushik, P.; Malik, A.; Vijay, V.K. Phycoremediation and biogas potential of native algal isolates from soil and wastewater. Bioresour. Technol. 2013, 135, 232–238. [Google Scholar] [CrossRef]
- He, Q.N.; Yang, H.J.; Hu, C.X. Effects of temperature and its combination with high light intensity on lipid production of Monoraphidium dybowskii Y2 from semi-arid desert areas. Bioresour. Technol. 2018, 265, 407–414. [Google Scholar] [CrossRef]
- Guo, Y.N. Optimizing Cultivation and Mechanism of Lipid Accumulation Research in Chlorococcum sphacosum GD. Master’s Thesis, Shanxi University, Taiyuan, China, 2017. [Google Scholar]
- Chen, W.; Zhang, C.; Song, L.; Sommerfeld, M.; Hua, Q. A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Meth. 2015, 77, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Karima, A.; Silalahi, M.D.; Rinanti, A. Increasing content of lipid in tropical microalgae Chlorella sorokiniana and Closterium sp. with variation of nitrogen content and extraction temperature. Appl. Sci. Eng. Conf. 2018, 197, 13019. [Google Scholar] [CrossRef]
- Thang, D.V.; Faruq, A.; Thomas-Hall, S.R.; Simon, Q.; Ekaterina, N.; Schenk, P.M. High protein and high lipid-producing microalgae from northern Australia as potential feedstock for animal feed and biodiesel. Front. Bioeng. Biotech. 2015, 3, 53–60. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Sun, Z.; Zhu, Y.J. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: Assessment of algal oils for biodiesel production. Bioresour. Technol. 2011, 102, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.X.; Deng, D.Y.; Liu, J.P.; Cheng, K.; Xu, Y.J. Field trial multiple comparison results alphabetic difference counting method and its letter abbreviation. Seeds 2018, 5, 131–132. [Google Scholar] [CrossRef]
- Yan, W.J. Characteristics of Coccomyxa subellipsoidea C-169 in Trophic Growth and Evaluation of Efficiency on Yeast Wastewater Treatment. Master’s Thesis, South China University of Technology, Guangzhou, China, 2016. [Google Scholar]
- Li, C.L.; Yang, H.L.; Li, Y.J.; Wang, W. Effect of culture models on metabolism and protein components of microalgae Chlorella vulgaris. J. Food Sci. Biotechnol. 2014, 33, 56–62. [Google Scholar] [CrossRef]
- Shokravi, Z.; Shokravi, H.; Atabani, A.E.; Lau, W.J.; Chyuan, O.H.; Ismail, A.F. Impacts of the harvesting process on microalgae fatty acid profiles and lipid yields: Implications for biodiesel production. Renew. Sust. Energy Rev. 2022, 161, 112410. [Google Scholar] [CrossRef]
- Li, T.T.; Zheng, Y.; Yu, L.; Chen, S.L. Mixotrophic cultivation of a Chlorella sorokiniana strain for enhanced biomass and lipid production. Biomass Bioenergy 2014, 66, 204–213. [Google Scholar] [CrossRef]
- Abreu, A.P.; Morais, R.C.; Kazmerski, L. A comparison between microalgal autotrophic growth and metabolite accumulation with heterotrophic, mixotrophic and photoheterotrophic cultivation modes. Renew. Sust. Energy Rev. 2022, 159, 112247. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Wang, F. Mixotrophic cultivation of microalgae for biodiesel production: Status and prospects. Appl. Biochem. Biotechnol. 2014, 172, 3307–3329. [Google Scholar] [CrossRef]
- Yan, S.K. The Effect of Nitrogen and Phosphorus Conditions and Culture Modes on the Growth and Lipid Content of Microalgae. Master’s Thesis, Anhui Normal University, Hefei, China, 2019. [Google Scholar]
- Ma, D.D.; Li, Y.F. Heterotrophic culture of Chlorella sp. and Dunaliella salina using alginate oligosaccharide and its growth promoting mechanism. Period. Ocean. Univ. China 2020, 50, 40–45. [Google Scholar] [CrossRef]
- Kabir, F.; Gulfraz, M.; Raja, G.K.; Inam-Ul-Haq, M.; Shadloo, M.S. Screening of native hyper-lipid producing microalgae strains for biomass and lipid production. Renew. Energy 2020, 160, 1295–1307. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, D.; Wu, T.; Lo, Y.; Lee, Y.; Liu, J. The synergistic energy and carbon metabolism under mixotrophic cultivation reveals the coordination between photosynthesis and aerobic respiration in Chlorella zofingiensis. Algal Res. 2017, 25, 109–116. [Google Scholar] [CrossRef]
- Vidotti, A.D.S.; Riano-Pachon, D.M.; Mattiello, L.; Giraldi, L.A.; Franco, T.T. Analysis of autotrophic, mixotrophic and heterotrophic phenotypes in the microalgae Chlorella vulgaris using time-resolved proteomics and transcriptomics approaches. Algal Res. 2020, 51, 102060. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Bedaiwy, M.Y.; Osman, M.E.; Ismail, M.M. Mixotrophic and heterotrophic growth of some microalgae using extract of fungal-treated wheat bran. Int. J. Recycl. Org. Waste Agric. 2012, 1, 12. [Google Scholar] [CrossRef]
- Lewitus, A.J.; Kana, T.M. Light respiration in six estuarine phytoplankton species: Contrasts under photoautotrophic and mixotrophic growth conditions. J. Phycol. 2010, 31, 754–761. [Google Scholar] [CrossRef]
- Huang, Y.; Li, P.; Huang, Y.; Xia, A.; Zhu, X.; Liao, Q. A synchronous photoautotrophic-heterotrophic biofilm cultivation mode for chlorella vulgaris biomass and lipid simultaneous accumulation. J. Clean. Prod. 2022, 336, 130453. [Google Scholar] [CrossRef]
- Karimian, A.; Mahdavi, M.A.; Gheshlaghi, R. Algal cultivation strategies for enhancing production of Chlorella sorokiniana IG-W-96 biomass and bioproducts. Algal Res. 2022, 62, 102630. [Google Scholar] [CrossRef]
- Liu, X.J. Charaeteristics of Photoautotrophy, Mixotrophy and Heterotrophy of Phaeodactylum tricornutum. Ph.D. Thesis, Jinan University, Guangzhou, China, 2008; pp. 58–88. [Google Scholar]
- Pleissner, D.; Eriksen, N.T. Effects of phosphorous, nitrogen, and carbon limitation on biomass composition in batch and continuous flow cultures of the heterotrophic dinoflagellate Crypthecodinium cohnii. Biotechnol. Bioeng. 2012, 109, 2005–2016. [Google Scholar] [CrossRef]
- Ogbonna, J.C.; Tanaka, H. Production of pure photosynthetic cell biomass for environmental biosensors. Mat. Sci. Eng. 2000, 12, 9–15. [Google Scholar] [CrossRef]
- Pereira, M.I.B.; Chagas, B.M.E.; Sassi, R.; Medeiros, G.F.; Rangel, A.H.N. Mixotrophic cultivation of Spirulina platensis in dairy wastewater: Effects on the production of biomass, biochemical composition and antioxidant capacity. PLoS ONE 2019, 14, e0224294. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Sahoo, P.K.; Singhal, S.; Patel, A. Impact of various media and organic carbon sources on biofuel production potential from Chlorella spp. 3 Biotech 2016, 6, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Pancha, I.; Chokshi, K.; George, B.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2014, 156, 146–154. [Google Scholar] [CrossRef]
- Rios, L.F.; Klein, B.C.; Luz, L.F.J.; Filho, R.M.; Maciel, M.R.F. Nitrogen starvation for lipid accumulation in the microalga species Desmodesmus sp. Appl. Biochem. Biotechnol. 2015, 175, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Siaut, M.; Cuine, S.; Cagnon, C.; Fessler, B.; Nguyen, M.; Carrier, P.; Beyly, A.; Beisson, F. Oil accumulation in the model green alga Chlamydomonas reinhardtii: Characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol. 2011, 11, 7. [Google Scholar] [CrossRef]
- Zhu, S.N.; Huang, W.; Xu, J.; Wang, Z.M.; Xu, J.L.; Yuan, Z.H. Metabolic changes of starch and lipid triggered by nitrogen starvation in the microalga Chlorella zofingiensis. Bioresour. Technol. 2014, 152, 292–298. [Google Scholar] [CrossRef]
- Yun, H.S.; Kim, Y.S.; Yoon, H.S. Effect of different cultivation modes (photoautotrophic, mixotrophic, and heterotrophic) on the growth of Chlorella sp. and biocompositions. Front. Bioeng. Biotechnol. 2021, 9, 774143. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Fan, K.W.; Jiang, Y.; Zhong, Y.; Sun, Z.; Chen, F. Production potential of Chlorella zofingienesis as a feedstock for biodiesel. Bioresour. Technol. 2010, 101, 8658–8663. [Google Scholar] [CrossRef]
- Cheng, P.F.; Huang, J.; Song, X.; Yao, T.; Jiang, J.; Zhou, C.; Yan, X.J.; Ruan, R. Heterotrophic and mixotrophic cultivation of microalgae to simultaneously achieve furfural wastewater treatment and lipid production. Bioresour. Technol. 2022, 349, 126888. [Google Scholar] [CrossRef]
- Leong, W.H.; Saman, N.A.M.; Kiatkittipong, W.; Assabumrungrat, S.; Najdanovic-Visak, V.; Wang, J. Photoperiod-induced mixotrophic metabolism in Chlorella vulgaris for high biomass and lipid to biodiesel productions using municipal wastewater medium. Fuel J. Fuel Sci. 2022, 313, 123052. [Google Scholar] [CrossRef]
- Ahmad, F.T.; Seyed, K.M.; Meisam, T.; Masoud, T.; Abdolreza, B.; Mehrshad, Z.; Hossein, H.M.; Mehrdad, M.; Saeid, M.; Shiva, B. Fatty acids profiling: A selective criterion for screening microalgae strains for biodiesel production. Algal Res. 2013, 2, 258–267. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).