Screening and Probiotic Properties of Lactic Acid Bacteria with Potential Immunostimulatory Activity Isolated from Kimchi
Abstract
1. Introduction
2. Materials and Methods
2.1. LAB Strains and Cultivation Conditions
2.2. Cell-Free Supernatant (CFS) Preparation
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Nitric Oxide Assay
2.6. Cytokine Assay
2.7. Phagocytosis Assay
2.8. Western Blot Analysis
2.9. Probiotic Properties
2.9.1. Resistance to Simulated Gastrointestinal Conditions
2.9.2. Adhesion to HT-29 Cell Line
2.9.3. Hemolytic Activity
2.9.4. Antimicrobial Susceptibility Assay
2.10. Statistical Analysis
3. Results
3.1. LAB Strains Induced NO Production in RAW264.7 Cells
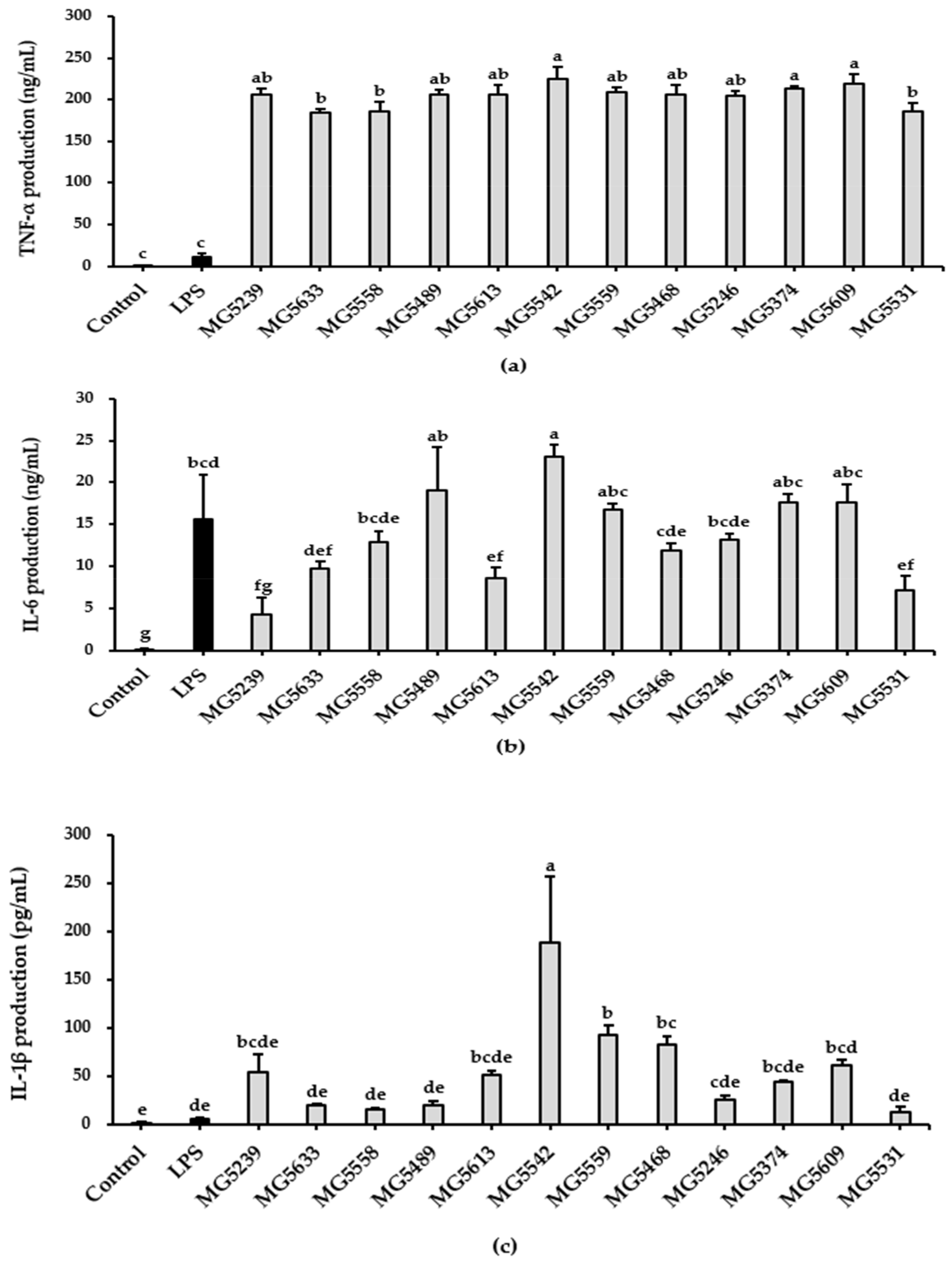
3.2. LAB Strains Induced Pro-Inflammatory Cytokines Production in RAW264.7 Cells
3.3. The Selected Strains Induced Enhancement of Phagocytic Activity in RAW264.7 Cells
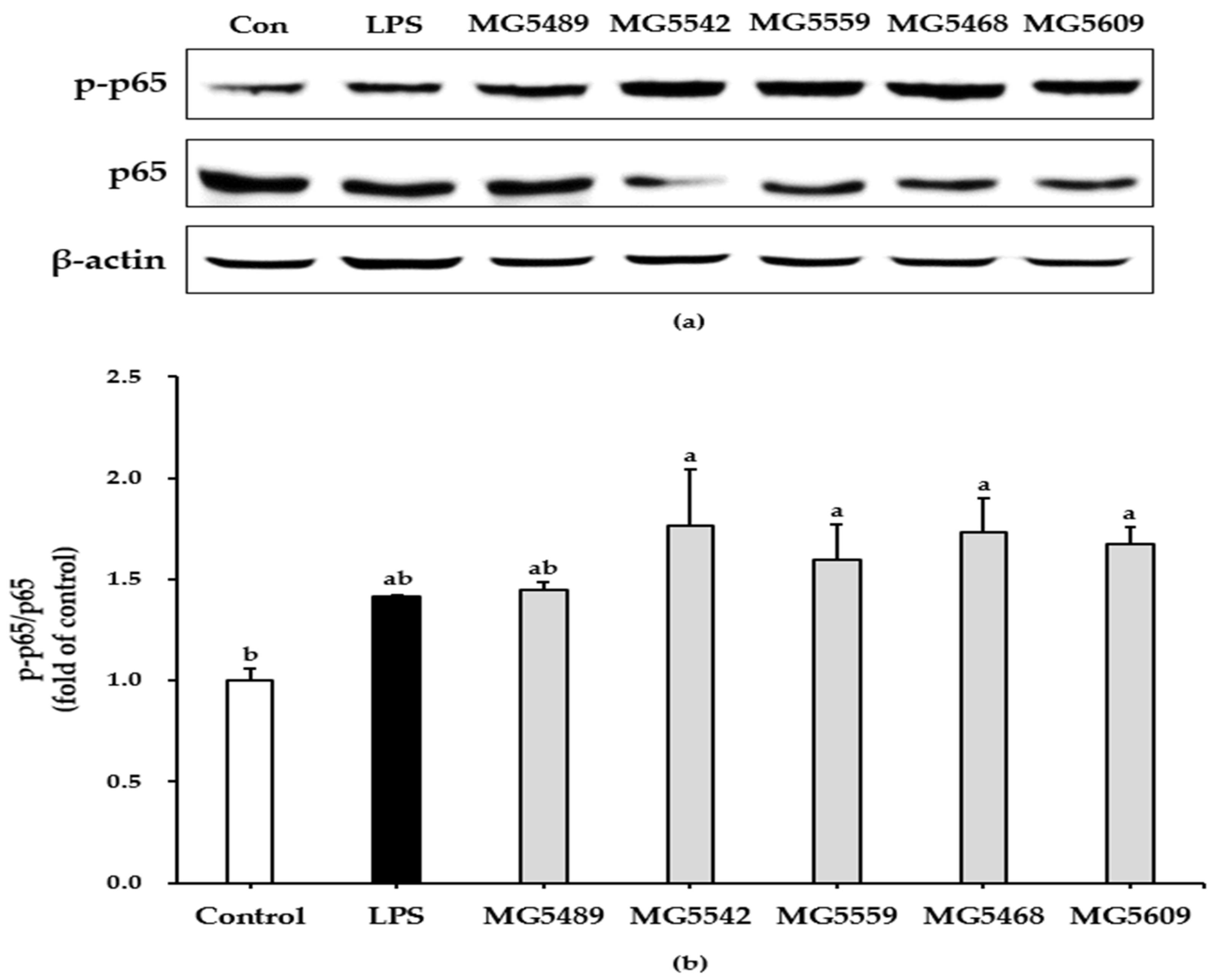
3.4. The Selected Strains Induced NF-κB Activation in RAW 264.7 Cells
3.5. Probiotic Properties of the Selected Strains
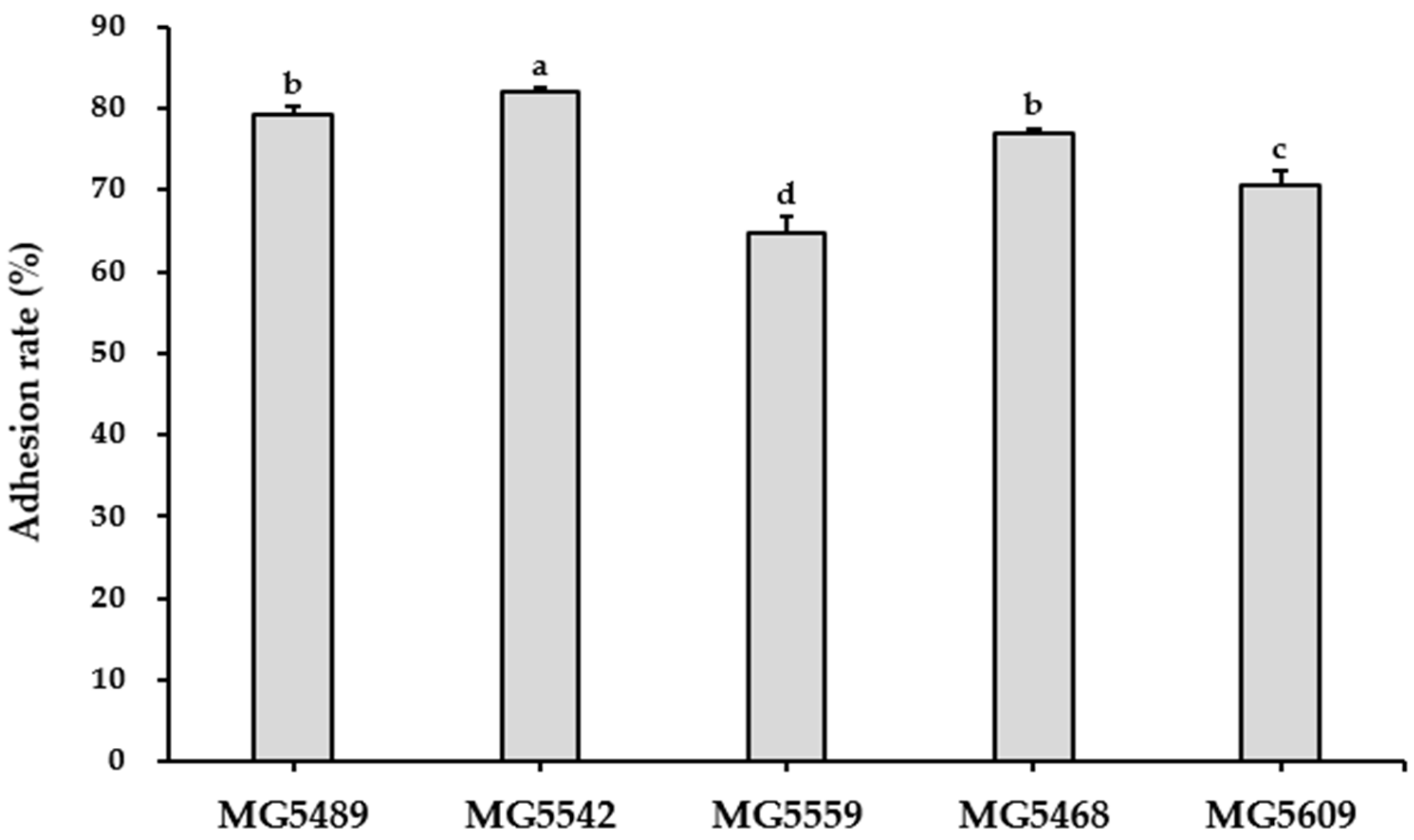
3.5.1. GIT Stability and Adhesion Ability of the Selected Strains
3.5.2. Safety of the Selected Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Liu, H.; Zhao, J. Macrophage Polarization Induced by Probiotic Bacteria: A Concise Review. Probiotics Antimicrob. Proteins 2020, 12, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, S. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front. Immunol. 2018, 9, 1930. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Camille, N.; Dealtry, G. Regulation of M1/M2 macrophage polarization by Sutherlandia frutescens via NFkB and MAPK signaling pathways. South Afr. J. Bot. 2018, 116, 42–51. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Plavec, T.V.; Berlec, A. Safety Aspects of Genetically Modified Lactic Acid Bacteria. Microorganisms 2020, 8, 297. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Update of the List of QPS-Recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA 13: Suitability of Taxonomic Units Notified to EFSA until September 2020. EFSA J. 2021, 19, e06377. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011, 27, 496. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef]
- Christoffersen, T.E.; Hult, L.T.O.; Kuczkowska, K.; Moe, K.M.; Skeie, S.; Lea, T.; Kleiveland, C.R. In vitro comparison of the effects of probiotic, commensal and pathogenic strains on macrophage polarization. Probiotics Antimicrob. Proteins 2014, 6, 1–10. [Google Scholar] [CrossRef]
- Guha, D.; Banerjee, A.; Mukherjee, R.; Pradhan, B.; Peneva, M.; Aleksandrov, G.; Suklabaidya, S.; Senapati, S.; Aich, P. A probiotic formulation containing Lactobacillus bulgaricus DWT1 inhibits tumor growth by activating pro-inflammatory responses in macrophages. J. Funct. Foods 2019, 56, 232–245. [Google Scholar] [CrossRef]
- Jang, S.E.; Hyam, S.R.; Han, M.J.; Kim, S.Y.; Lee, B.G.; Kim, D.H. Lactobacillus brevis G-101 ameliorates colitis in mice by inhibiting NF-κB, MAPK and AKT pathways and by polarizing M1 macrophages to M2-like macrophages. J. Appl. Microbiol. 2013, 115, 888–896. [Google Scholar] [CrossRef]
- Jang, S.E.; Han, M.J.; Kim, S.Y.; Kim, D.H. Lactobacillus plantarum CLP-0611 ameliorates colitis in mice by polarizing M1 to M2-like macrophages. Int. Immunopharmacol. 2014, 21, 186–192. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, J.H.; Jung, Y.R.; Kang, C.H. Lactobacillus gasseri MG4247 and Lacticaseibacillus paracasei MG4272 and MG4577 Modulate Allergic Inflammatory Response in RAW 264.7 and RBL-2H3 cells. Probiotics Antimicrob. Proteins 2022, 1, 1–10. [Google Scholar] [CrossRef]
- Kang, C.H.; Kim, J.S.; Kim, H.; Park, H.M.; Paek, N.S. Heat-Killed Lactic Acid Bacteria Inhibit Nitric Oxide Production via Inducible Nitric Oxide Synthase and Cyclooxygenase-2 in RAW 264.7 Cells. Probiotics Antimicrob. Proteins 2021, 13, 1530–1538. [Google Scholar] [CrossRef]
- Geum, N.G.; Eo, H.J.; Kim, H.J.; Park, G.H.; Son, H.J.; Jeong, J.B. Immune-enhancing activity of Hydrangea macrophylla subsp. serrata leaves through TLR4/ROS-dependent activation of JNK and NF-κB in RAW264.7 cells and immunosuppressed mice. J. Funct. Foods 2020, 73, 104139. [Google Scholar] [CrossRef]
- Wei, J.; Wang, B.; Chen, Y.; Wang, Q.; Ahmed, A.F.; Zhang, Y.; Kang, W. The Immunomodulatory Effects of Active Ingredients From Nigella sativa in RAW264.7 Cells Through NF-κB/MAPK Signaling Pathways. Front. Nutr. 2022, 9, 899797. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Zavala, L.; Carasi, P.; Trejo, S.A.; Bronsoms, S.; De los Ángeles Serradell, M.; Garrote, G.L.; Abraham, A.G. Simulated gastrointestinal conditions increase adhesion ability of Lactobacillus paracasei strains isolated from kefir to Caco-2 cells and mucin. Food Res. Int. 2018, 103, 462–467. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, H.; Jeong, Y.; Kang, C.H. Lactic Acid Bacteria Exert a Hepatoprotective Effect against Ethanol-Induced Liver Injury in HepG2 Cells. Microorganisms 2021, 9, 1844. [Google Scholar] [CrossRef]
- Buxton, R. Blood Agar Plates and Hemolysis Protocols. Am. Soc. Microbiol. 2005, 30, 1–9. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; De Lourdes Bastos, M.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; et al. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, e05206. [Google Scholar] [CrossRef]
- Shreshtha, S.; Sharma, P.; Kumar, P.; Sharma, R.; Singh, S.P. Nitric Oxide: It’s Role in Immunity. J. Clin. Diagn. Res. 2018, 12, BE01–BE05. [Google Scholar] [CrossRef]
- Strieter, R.M.; Belperio, J.A.; Keane, M.P. Cytokines in innate host defense in the lung. J. Clin. Investig. 2002, 109, 699. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S. The Role of the Immune System Beyond the Fight Against Infection. Adv. Exp. Med. Biol. 2017, 1003, 3–14. [Google Scholar] [CrossRef]
- Mowat, A.M.I.; Viney, J.L. The anatomical basis of intestinal immunity. Immunol. Rev. 1997, 156, 145–166. [Google Scholar] [CrossRef]
- Dargahi, N.; Johnson, J.; Donkor, O.; Vasiljevic, T.; Apostolopoulos, V. Immunomodulatory effects of probiotics: Can they be used to treat allergies and autoimmune diseases? Maturitas 2019, 119, 25–38. [Google Scholar] [CrossRef]
- Ren, C.; Cheng, L.; Sun, Y.; Zhang, Q.; De Haan, B.J.; Zhang, H.; Faas, M.M.; De Vos, P. Lactic acid bacteria secrete toll like receptor 2 stimulating and macrophage immunomodulating bioactive factors. J. Funct. Foods 2020, 66, 103783. [Google Scholar] [CrossRef]
- Yang, S.Y.; Chae, S.A.; Bang, W.Y.; Lee, M.; Ban, O.H.; Kim, S.J.; Jung, Y.H.; Yang, J. Anti-inflammatory potential of Lactiplantibacillus plantarum IDCC 3501 and its safety evaluation. Braz. J. Microbiol. 2021, 52, 2299–2306. [Google Scholar] [CrossRef]
- Vincenti, J.E.; Wright, D.A.; Sarker, M. The influence of cell-free Lactobacillus rhamnosus GG supernatant on the phagocytic activity of macrophages. Biosci. Horizons Int. J. Student Res. 2010, 3, 105–112. [Google Scholar] [CrossRef][Green Version]
- Chang, C.K.; Wang, S.C.; Chiu, C.K.; Chen, S.Y.; Chen, Z.T.; Duh, P. Der Effect of lactic acid bacteria isolated from fermented mustard on immunopotentiating activity. Asian Pac. J. Trop. Biomed. 2015, 5, 281–286. [Google Scholar] [CrossRef]
- Quinteiro-Filho, W.M.; Brisbin, J.T.; Hodgins, D.C.; Sharif, S. Lactobacillus and Lactobacillus cell-free culture supernatants modulate chicken macrophage activities. Res. Vet. Sci. 2015, 103, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yang, L.; Wang, C.; Ma, S.; Cui, L.; Huang, S.; Sheng, X.; Weng, Q.; Xu, M. Immunostimulatory activity of protein hydrolysate from oviductus ranae on macrophage in vitro. Evid.-Based Complement. Alternat. Med. 2014, 2014, 180234. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204. [Google Scholar] [CrossRef]
- Cavaillon, J.M. Cytokines and macrophages. Biomed. Pharmacother. 1994, 48, 445–453. [Google Scholar] [CrossRef]
- Aderem, A.; Underhill, D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef]
- Xiu, L.; Zhang, H.; Hu, Z.; Liang, Y.; Guo, S.; Yang, M.; Du, R.; Wang, X. Immunostimulatory activity of exopolysaccharides from probiotic Lactobacillus casei WXD030 strain as a novel adjuvant in vitro and in vivo. Food Agric. Immunol. 2018, 29, 1086–1105. [Google Scholar] [CrossRef]
- Yang, F.; Tang, E.; Guan, K.; Wang, C.-Y. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J. Immunol. 2003, 170, 5630–5635. [Google Scholar] [CrossRef]
- Jang, S.E.; Joh, E.H.; Lee, H.Y.; Ahn, Y.T.; Lee, J.H.; Huh, C.S.; Han, M.J.; Kim, D.H. Lactobacillus plantarum HY7712 ameliorates cyclophosphamide-induced immunosuppression in mice. J. Microbiol. Biotechnol. 2013, 23, 414–421. [Google Scholar] [CrossRef]
- Jang, H.J.; Yu, H.S.; Lee, N.K.; Paik, H.D. Immune-stimulating Effect of Lactobacillus plantarum Ln1 Isolated from the Traditional Korean Fermented Food, Kimchi. J. Microbiol. Biotechnol. 2020, 30, 926–929. [Google Scholar] [CrossRef]
- Naissinger da Silva, M.; Tagliapietra, B.L.; Flores, V.D.A.; Pereira dos Santos Richards, N.S. In vitro test to evaluate survival in the gastrointestinal tract of commercial probiotics. Curr. Res. Food Sci. 2021, 4, 320–325. [Google Scholar] [CrossRef]
- Reid, G. The scientific basis for probiotic strains of Lactobacillus. Appl. Environ. Microbiol. 1999, 65, 3763–3766. [Google Scholar] [CrossRef]
- Mantzourani, I.; Chondrou, P.; Bontsidis, C.; Karolidou, K.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Galanis, A.; Plessas, S. Assessment of the probiotic potential of lactic acid bacteria isolated from kefir grains: Evaluation of adhesion and antiproliferative properties in in vitro experimental systems. Ann. Microbiol. 2019, 69, 751–763. [Google Scholar] [CrossRef]
- Georgieva, R.; Yocheva, L.; Tserovska, L.; Zhelezova, G.; Stefanova, N.; Atanasova, A.; Danguleva, A.; Ivanova, G.; Karapetkov, N.; Rumyan, N.; et al. Antimicrobial activity and antibiotic susceptibility of Lactobacillus and Bifidobacterium spp. intended for use as starter and probiotic cultures. Biotechnol. Biotechnol. Equip. 2015, 29, 84–91. [Google Scholar] [CrossRef]
- Sharma, P.; Tomar, S.K.; Goswami, P.; Sangwan, V.; Singh, R. Antibiotic resistance among commercially available probiotics. Food Res. Int. 2014, 57, 176–195. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sánchez, B.; De los Reyes-Gavilán, C.G.; Margolles, A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013, 4, 202. [Google Scholar] [CrossRef]

| Species | Strains | Cell Viability (%) | NO (μM) |
|---|---|---|---|
| Untreated | 100.00 ± 6.44 | 0.65 ± 0.11 | |
| LPS (10 ng/mL) | 101.99 ± 5.47 | 9.08 ± 0.86 ** | |
| Lactiplantibacillus plantarum | MG5239 | 107.69 ± 12.24 | 19.64 ± 1.24 *** |
| MG5363 | 103.29 ± 9.13 | 5.18 ± 1.11 | |
| MG5420 | 101.72 ± 2.66 | 4.04 ± 0.29 | |
| MG5633 | 100.11 ± 1.53 | 18.07 ± 0.66 *** | |
| MG5558 | 101.75 ± 3.18 | 18.07 ± 1.94 *** | |
| Limosilactobacillus fermentum | MG5489 | 103.91 ± 7.27 | 29.96 ± 2.51 *** |
| MG5613 | 103.84 ± 4.94 | 21.21 ± 6.88 *** | |
| Lacticaseibacillus paracasei | MG5219 | 101.86 ± 5.85 | 8.26 ± 0.39 * |
| MG5559 | 101.74 ± 6.20 | 15.55 ± 1.91 *** | |
| Latilactobacillus sakei | MG5218 | 99.94 ± 1.97 | 8.13 ± 0.86 * |
| MG5235 | 102.02 ± 2.32 | 4.55 ± 1.32 | |
| MG5468 | 101.38 ± 5.12 | 12.60 ± 0.76 *** | |
| MG5496 | 101.03 ± 3.07 | 7.57 ± 1.15 | |
| MG5557 | 100.88 ± 1.25 | 4.23 ± 2.40 | |
| Latilactobacillus curvatus | MG5246 | 100.24 ± 4.72 | 22.35 ± 1.04 *** |
| MG5374 | 100.35 ± 4.86 | 24.11 ± 1.28 *** | |
| MG5609 | 101.74 ± 3.20 | 23.92 ± 1.53 *** | |
| Levilactobacillus brevis | MG5250 | 100.55 ± 3.18 | 2.85 ± 0.82 |
| MG5264 | 100.59 ± 2.22 | 1.97 ± 0.58 | |
| MG5291 | 100.36 ± 6.45 | 1.72 ± 0.19 | |
| MG5426 | 100.49 ± 4.40 | 1.47 ± 0.71 | |
| MG5531 | 100.23 ± 1.95 | 10.14 ± 0.76 ** | |
| Lactococcus lactis | MG5542 | 101.72 ± 1.51 | 16.37 ± 1.11 *** |
| Weissella cibaria | MG5223 | 100.10 ± 4.83 | 2.28 ± 0.82 |
| MG5234 | 103.89 ± 5.40 | 1.97 ± 0.11 | |
| MG5285 | 104.11 ± 6.01 | 2.03 ± 0.66 | |
| MG5362 | 100.15 ± 6.44 | 2.22 ± 0.39 | |
| MG5569 | 102.56 ± 5.58 | 2.22 ± 0.11 | |
| Strains | Initial Counts (log CFU/mL) | SGF (log CFU/mL) | SIF (log CFU/mL) | Survival Rate (%) |
|---|---|---|---|---|
| L. fermentum MG5489 | 8.82 ± 0.00 | 7.68 ± 0.01 | 7.67 ± 0.03 | 86.99 ± 0.29 |
| Lc. lactis MG5542 | 7.84 ± 0.03 | 4.61 ± 0.08 | 4.20 ± 0.19 | 53.63 ± 2.44 |
| L. paracasei MG5559 | 7.61 ± 0.04 | 5.96 ± 0.10 | 5.69 ± 0.04 | 74.75 ± 0.47 |
| L. sakei MG5468 | 8.33 ± 0.01 | 6.96 ± 0.01 | 6.87 ± 0.02 | 82.57 ± 0.28 |
| L. curvatus MG5609 | 7.44 ± 0.06 | 5.42 ± 0.04 | 4.54 ± 0.11 | 61.01 ± 1.43 |
| Antimicrobials | Susceptibility (S/R) | ||||
|---|---|---|---|---|---|
| MG5489 | MG5542 | MG5559 | MG5468 | MG5609 | |
| Ampicillin | S | S | S | S | S |
| (0.19) | (0.5) | (0.75) | (0.5) | (1) | |
| Gentamicin | S | S | S | S | S |
| (0.25) | (2) | (2) | (2) | (0.75) | |
| Kanamycin | S | S | S | S | S |
| (16) | (8) | (32) | (8) | (2) | |
| Streptomycin | S | S | S | S | S |
| (6) | (24) | (24) | (32) | (6) | |
| Tetracycline | S | S | S | S | S |
| (1.5) | (0.25) | (1) | (3) | (1.5) | |
| Chloramphenicol | S | S | R | S | S |
| (3) | (6) | (6) | (3) | (2) | |
| Erythromycin | S | S | S | S | S |
| (0.75) | (0.38) | (0.094) | (0.125) | (0.094) | |
| Vancomycin | - | S | - | - | - |
| (n.r) | (0.25) | (n.r) | (n.r) | (n.r) | |
| Clindamycin | S | S | S | S | S |
| (0.125) | (0.094) | (0.25) | (0.125) | (0.016) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kim, S.; Kang, C.-H. Screening and Probiotic Properties of Lactic Acid Bacteria with Potential Immunostimulatory Activity Isolated from Kimchi. Fermentation 2023, 9, 4. https://doi.org/10.3390/fermentation9010004
Lee J, Kim S, Kang C-H. Screening and Probiotic Properties of Lactic Acid Bacteria with Potential Immunostimulatory Activity Isolated from Kimchi. Fermentation. 2023; 9(1):4. https://doi.org/10.3390/fermentation9010004
Chicago/Turabian StyleLee, Jaekoo, Seonyoung Kim, and Chang-Ho Kang. 2023. "Screening and Probiotic Properties of Lactic Acid Bacteria with Potential Immunostimulatory Activity Isolated from Kimchi" Fermentation 9, no. 1: 4. https://doi.org/10.3390/fermentation9010004
APA StyleLee, J., Kim, S., & Kang, C.-H. (2023). Screening and Probiotic Properties of Lactic Acid Bacteria with Potential Immunostimulatory Activity Isolated from Kimchi. Fermentation, 9(1), 4. https://doi.org/10.3390/fermentation9010004






