Optimization of Growth Conditions to Enhance PHA Production by Cupriavidus necator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Production Organism
2.3. Cultivation of Cupriavidus necator DMS 545 Cells
2.4. Screening the Components Affecting Biomass Production and PHB Yield
2.5. Optimization of the Propagation Medium
2.6. Analytical Methods
2.7. Statistical Analysis
3. Results and Discussion
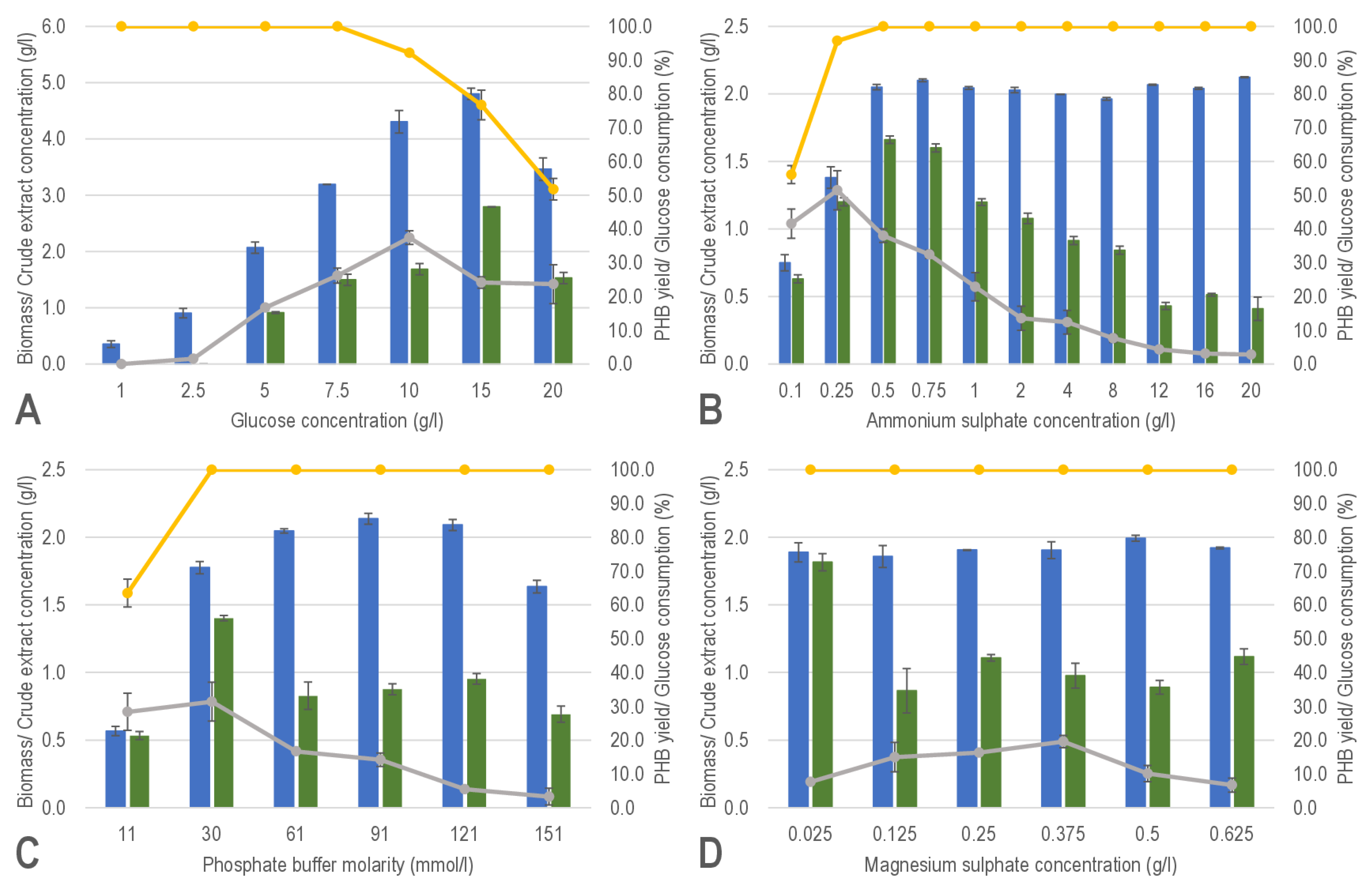
3.1. Screening the Variable Components Affecting Biomass Production and PHB Production
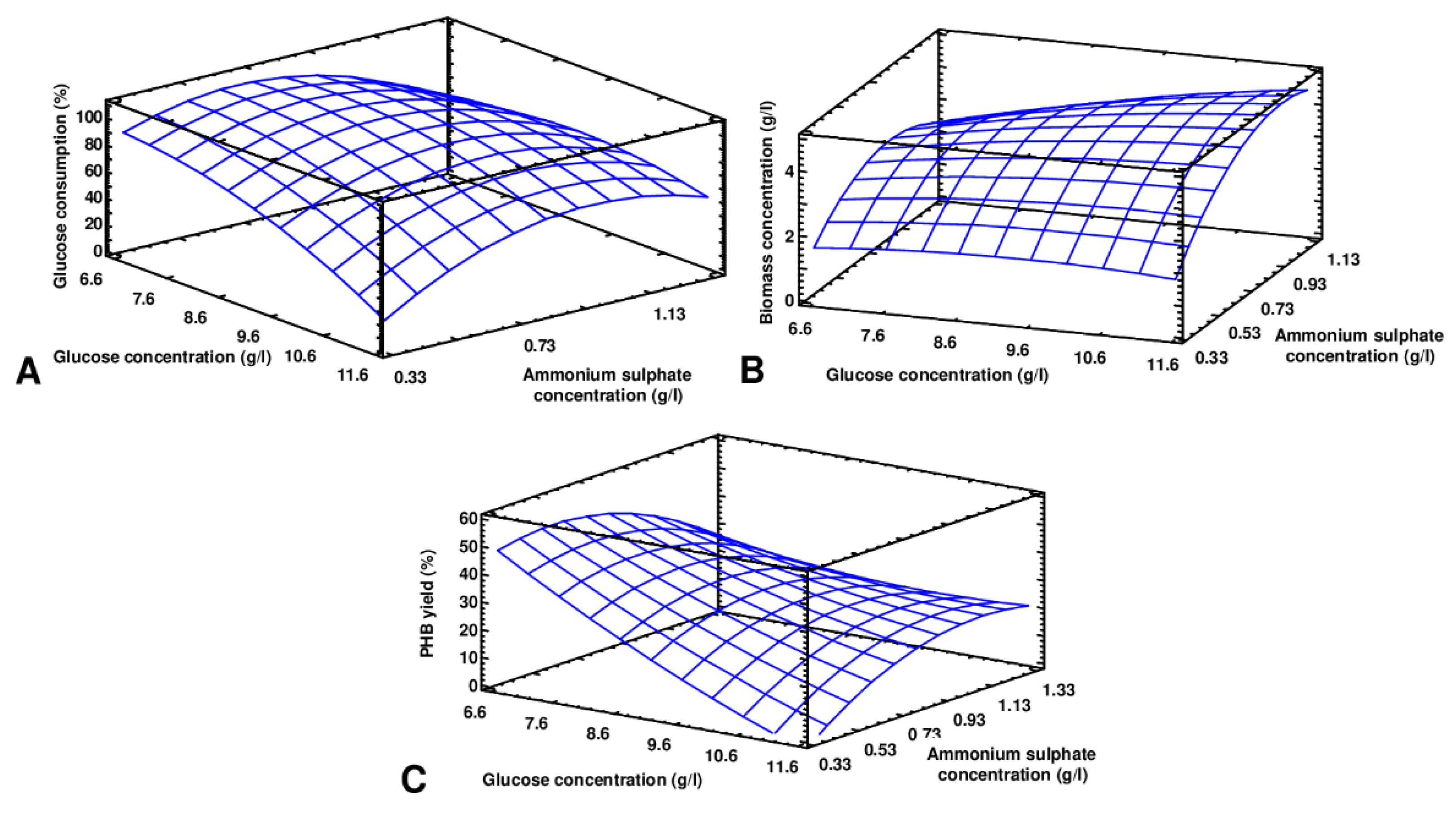
3.2. RSM Optimization
3.3. Optimization and Verification of the Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kumar, M.; Gupta, A.; Thakur, I.S. Carbon dioxide sequestration by chemolithotrophic oleaginous bacteria for production and optimization of polyhydroxyalkanoate. Bioresour. Technol. 2016, 213, 249–256. [Google Scholar] [CrossRef]
- Maheshwari, N.; Kumar, M.; Thakur, I.S.; Srivastava, S. Production, process optimization and molecular characterization of polyhydroxyalkanoate (PHA) by CO2 sequestering B. cereus SS105. Bioresour. Technol. 2018, 254, 75–82. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Chen, X.-Y.; Wu, F.Q.; Chen, J.-C. Polyhydroxyalkanoates (PHA) toward cost competitiveness and functionality. Adv. Ind. Eng. Polym. Res. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Chmelová, D.; Legerská, B.; Ondrejovič, M. Recombinant DNS technology as a tool for improving production of polyhydroxyalkanoates by the natural producers. Nova Biotechnol. Chim. 2020, 19, 124–137. [Google Scholar] [CrossRef]
- Santolin, L.; Waldburger, S.; Neubauer, P.; Riedel, S.L. Substrate-flexible two-stage fed-batch cultivations for the production of the PHA copolymer P(HB-co-HHx) with Cupriavidus necator Re2058/pCB113. Front. Bioeng. Biotechnol. 2021, 9, 623890. [Google Scholar] [CrossRef]
- Araceli, F.S.; Ramsay, J.A.; Berenice, V.-P.; Fermin, P.-G.; Ramsay, B.A. High amounts of medium-chain-length polyhydroxyalkanoates subunits can be accumulated in recombinant Cupriavidus necator with wild-type synthase. J. Biotechnol. 2022, 349, 25–31. [Google Scholar] [CrossRef]
- Chmelová, D.; Legerská, B.; Ondrejovič, M.; Miertuš, S. Optimization of propagation medium for enhanced polyhydroxyalkanoate production by Pseudomonas oleovorans. Fermentation 2022, 8, 16. [Google Scholar] [CrossRef]
- Brojanigo, S.; Parro, E.; Cazzorla, T.; Favaro, L.; Basaglia, M.; Casella, S. Conversion of starchy waste streams into polyhydroxyalkanoates using Cupriavidus necator DSM 545. Polymers 2020, 12, 1496. [Google Scholar] [CrossRef]
- Rayasam, V.; Chavan, P.; Kumar, T. Polyhydroxyalkanoate synthesis by bacteria isolated from landfill and ETP with pomegranate peels as carbon source. Arch. Microbiol. 2020, 202, 2799–2808. [Google Scholar] [CrossRef]
- Arias-Roblero, M.; Mora-Villalobos, V.; Velazquez-Carrillo, C. Evaluation of fed-batch fermentation for production of polyhydroxybutyrate with a banana pulp juice substrate from an agro industrial by-product. Front. Sustain. Food Syst. 2021, 5, 681596. [Google Scholar] [CrossRef]
- Koller, M.; Obruča, S. Biotechnological production of polyhydroxyalkanoates from glycerol: A review. Biocatal. Agric. Biotechnol. 2022, 42, 102333. [Google Scholar] [CrossRef]
- Koller, M. Advances in polyhydroxyalkanoate (PHA) production, volume 3. Bioengineering 2022, 9, 328. [Google Scholar] [CrossRef]
- Obruča, S.; Dvořák, P.; Sedláček, P.; Koller, M.; Sedlář, K.; Pernicová, I.; Šafránek, D. Polyhydroxyalkanoates synthesis by halophiles and thermophiles: Towards sustainable production of microbial bioplastics. Biotechnol. Adv. 2022, 58, 107906. [Google Scholar] [CrossRef]
- Passanha, P.; Esteves, S.R.; Kedia, G.; Dinsdale, R.M.; Guwy, A.J. Increasing polyhydroxyalkanoate (PHA) yields from Cupriavidus necator by using filtered digestate liquors. Bioresour. Technol. 2013, 147, 345–352. [Google Scholar] [CrossRef]
- Aljuraifani, A.A.; Berekaa, M.M.; Ghazwani, A.A. Bacterial biopolymer (polyhydroxyalkanoate) production from low-cost sustainable sources. MicrobiologyOpen 2019, 8, e00755. [Google Scholar] [CrossRef]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial polyhydroxyalkanoates: Still fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef]
- Madhusoodanan, G.; Hariharapura, R.C.; Somashekara, D. Dissolved oxygen as a propulsive parameter for polyhydroxyalkanoate production using Bacillus endophyticus cultures. Environ. Dev. Sustain. 2022, 24, 4641–4658. [Google Scholar] [CrossRef]
- Basak, B.; Ince, O.; Artan, N.; Yagci, N.; Ince, B.K. Effect of nitrogen limitation on enrichment of activated sludge for PHA production. Bioprocess Biosyst. Eng. 2011, 34, 1007–1016. [Google Scholar] [CrossRef]
- Ntaikou, I.; Koumelis, I.; Kamilari, M.; Iatridi, Z.; Tsitsilianis, C.; Lyberatos, G. Effect of nitrogen limitation on polyhydroxyalkanoates production efficiency, properties and microbial dynamics using a soil-derived mixed continuous culture. Int. J. Biobased Plast. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Papapostolou, A.; Karawavvas, E.; Chatzidoukas, C. Oxygen mass transfer limitations set the performance boundaries of microbial PHA production processes—A model-based problem investigation supporting scale-up studies. Biochem. Eng. J. 2019, 148, 224–238. [Google Scholar] [CrossRef]
- Quintanar-Gómez, S.; Abreu-Corona, A.; Zamudio-Pérez, E.; Vargas-Hérnanez, G.; Téllez-Jurado, A.; Gracida-Rodríguez, J. Production of medium chain length polyhydroxyalkanoates from Cupriavidus necator with beeswax hydrolyzates as carbon source. Rev. Int. Contam. Ambient. 2018, 34, 467–474. [Google Scholar] [CrossRef]
- Nygaard, D.; Yasshuk, O.; Hermida, É.B. Evaluation of culture medium on poly(3-hydroxybutyrate) production by Cupriavidus necator ATCC 17697: Application of the response surface methodology. Heliyon 2019, 5, e01374. [Google Scholar] [CrossRef]
- Nehra, K.; Lathwal, P.; Gupta, S.; Sidhu, P.K.; Rana, J.S. Agro-industrial byproducts as alternate cost-effective medium components for production of polyhydroxybutyrate. Indian J. Exp. Biol. 2020, 58, 631–642. [Google Scholar]
- Nygaard, D.; Yaschuk, O.; Noseda, D.G.; Araoz, B.; Hermida, É.B. Improved fermentation strategies in a bioreactor for enhancing poly(3-hydroxybutyrate) (PHB) production by wild type Cupriavidus necator from fructose. Heliyon 2021, 7, e05979. [Google Scholar] [CrossRef]
- Zhila, N.O.; Sapozhnikova, K.Y.; Kiselev, E.G.; Vasiliev, A.D.; Nemstev, I.V.; Shishatskaya, E.I.; Volova, T.G. Properties of degradable polyhydroxyalkanoates (phas) synthesized by a new strain, Cupriavidus necator IBP/SFU-1, from various carbon sources. Polymers 2021, 13, 3142. [Google Scholar] [CrossRef]
- Ballard, D.G.H.; Holmes, P.A.; Senior, P.J. Formation of polymers of β-hydroxybutyric acid in bacterial cells and a comparison of the morphology of growth with the formation of polyethylene in the solid state. Recent Adv. Mech. Synth. Asp. Polym. 1987, 215, 293–314. [Google Scholar]
- Anderson, A.J.; Dawes, E.A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 1990, 54, 450–472. [Google Scholar] [CrossRef]
- Cramm, R. Genomic view of energy metabolism in Ralstonia eutropha H16. J. Mol. Microbiol. Biotechnol. 2009, 16, 38–52. [Google Scholar] [CrossRef]
- Jendrossek, D.; Pfeiffer, D. New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate). Environ. Microbiol. 2014, 16, 2357–2373. [Google Scholar] [CrossRef]
- Tajima, K.; Han, X.; Hashimoto, Y.; Satoh, Y.; Satoh, T.; Taguchi, S. In vitro synthesis of polyhydroxyalkanoates using thermostable acetyl-CoA synthase, CoA transferase, and PHA synthase from thermotolerant bacteria. J. Biosci. Bioeng. 2016, 12, 660–665. [Google Scholar] [CrossRef]
- Novackova, I.; Hrabalova, V.; Slaninova, E.; Sedlacek, P.; Samek, O.; Koller, M.; Krzyzanek, V.; Hrubanova, K.; Mrazova, K.; Nebesarova, J.; et al. The role of polyhydroxyalkanoates in adaptation of Cupriavidus necator to osmotic pressure and high concentration of copper ions. Int. J. Biol. Macromol. 2022, 206, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Karasavvasa, E.; Chatzidoukas, C. Model-based dynamic optimization of the fermentative production of polyhydroxyalkanoates (PHAs) in fed-batch and sequence of continuously operating bioreactors. Biochem. Eng. J. 2020, 162, 107702. [Google Scholar] [CrossRef]
- Norhafini, H.; Huong, K.-H.; Amirul, A.A. High PHA density fed-batch cultivation strategies for 4HB-rich P(3HB-co-4HB) copolymer production by transformant Cupriavidus malaysiensis USMAA1020. Int. J. Biol. Macromol. 2019, 125, 1024–1032. [Google Scholar] [CrossRef]
- Koller, M.; Braunegg, G. Potential and prospects of continuous polyhydroxyalkanoate (PHA) production. Bioengineering 2015, 2, 94–121. [Google Scholar] [CrossRef] [PubMed]
- Byrom, D. Polymer synthesis by microorganisms: Technology and economics. Trends Biotechnol. 1987, 5, 246–250. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lanham, A.B.; Ricardo, A.R.; Albuquerque, M.G.E.; Pardelha, F.; Carvalheira, M.; Coma, M.; Frandinho, J.; Carvalho, G.; Oehmen, A.; Reis, M.A.M. Determination of the extraction kinetics for the quantification of polyhydroxyalkanoate monomers in mixed microbial systems. Process Biochem. 2013, 48, 1626–1634. [Google Scholar] [CrossRef]
- Arumugam, A.; Senthamizhan, S.G.; Ponnusami, V.; Sudalai, S. Production and optimization of polyhydroxyalkanoates from non-edible Calophyllum inophyllum oil using Cupriavidus necator. Int. J. Biol. Macromol. 2018, 112, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Fu, J.; Spicer, V.; Krokhin, O.V.; Cicek, N.; Sparling, R.; Levin, D.B. Global changes in the proteome of Cupriavidus necator H16 during poly-(3-hydroxybutyrate) synthesis from various biodiesel by-product substrates. AMB Express 2016, 6, 36. [Google Scholar] [CrossRef]
- Khanna, S.; Srivastava, A.K. Statistical media optimization studies for growth and PHB production by Rastonia eutropha. Process Biochem. 2005, 40, 2173–2182. [Google Scholar] [CrossRef]
- Zahari, M.A.K.M.; Ariffin, H.; Mokhtar, M.N.; Salihon, J.; Shirai, Y.; Hassan, M.A. Factors affecting poly(3-hydroxybutyrate) production from oil palm frond juice by Cupriavidus necator (CCUG52238(T)). J. Biomed. Biotechnol. 2012, 2012, 125865. [Google Scholar]
- Bhatia, S.K.; Otari, S.V.; Jeon, J.-M.; Gurav, R.; Choi, Y.K.; Bhatia, R.K.; Pugazhendhi, A.; Kumar, V.; Banu, J.R.; Yoon, J.-J.; et al. Biowaste-to-bioplastic (polyhydroxyalkanoates): Conversion technologies, strategies, challenges, and perspective. Bioresour. Technol. 2021, 326, 124733. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Reddy, M.V. Optimization of critical factors to enhance polyhydroxyalkanoates (PHA) synthesis by mixed culture using Taguchi design of experimental methodology. Bioresour. Technol. 2013, 128, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Trakunjae, C.; Boondaeng, A.; Apiwatanapiwat, W.; Kosugi, A.; Arai, T.; Sudesh, K.; Vaithanomsat, P. Enhanced polyhydroxybutyrate (PHB) production by newly isolated rare actinomycetes Rhodococcus sp. strain BSRT1-1 using response surface methodology. Sci. Rep. 2021, 11, 1896. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, E.G.; Demidendko, A.V.; Zhila, N.O.; Shishatskaya, E.I.; Volova, T.G. Sugar beet molasses as a potential c-substrate for PHA production by Cupriavidus necator. Bioengineering 2022, 9, 154. [Google Scholar] [CrossRef]
- Biglari, N.; Dashti, M.G.; Abdeshahian, P.; Orita, I.; Fukui, T.; Sudesh, K. Enhancement of bioplastic polyhydroxybutyrate P(3HB) production from glucose by newly engineered strain Cupriavidus necator NSDG-GG using response surface methodology. 3 Biotech 2018, 8, 330. [Google Scholar] [CrossRef]
- Ahn, J.; Jho, E.H.; Nam, K. Effect of C/N ratio on polyhydroxyalkanoates (PHA) accumulation by Cupriavidus necator and its implication on the use of rice straw hydrolysates. Environ. Eng. Res. 2015, 20, 246–253. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, B.S. Production of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by Ralstonia eutropha from soybean oil. New Biotechnol. 2011, 28, 719–724. [Google Scholar] [CrossRef]
- Amini, M.; Yousefi-Massumabad, H.; Younesi, H.; Abyar, H.; Bahramifar, N. Production of the polyhydroxyalkanoate biopolymer by Cupriavidus necator using beer brewery wastewater containing maltose as a primary carbon source. J. Environ. Chem. Eng. 2020, 8, 102588. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mohan, S.V. Effect of substrate load and nutrients concentration on the polyhydroxyalkanoates (PHA) production using mixed consortia through wastewater treatment. Bioresour. Technol. 2012, 114, 573–582. [Google Scholar] [CrossRef]
| Variables | Unit | Code Levels | ||||
|---|---|---|---|---|---|---|
| −1.682 | −1 | 0 | 1 | 1.682 | ||
| glucose concentration | (g/L) | 6.66 | 7.50 | 8.75 | 10.00 | 10.84 |
| ammonium sulfate concentration | (g/L) | 0.33 | 0.50 | 0.75 | 1.00 | 1.17 |
| phosphate buffer molarity | (mmol/L) | 20.00 | 30.00 | 45.00 | 60.00 | 70.00 |
| Run Number | Glucose Concentration (g/L) | Ammonium Sulfate Concentration (g/L) | Phosphate Buffer Molarity (mmol/L) | Biomass Concentration (g/L) | Glucose Consumption (%) | PHB Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 7.50 (−1) | 0.50 (−1) | 30 (−1) | 2.91 ± 0.05 | 91.9 ± 0.4 | 44.9 ± 2.1 |
| 2 | 10.00 (1) | 1.00 (1) | 30 (−1) | 3.25 ± 0.00 | 78.2 ± 0.9 | 27.6 ± 1.2 |
| 3 | 10.00 (1) | 0.50 (−1) | 60 (1) | 3.06 ± 0.03 | 75.5 ± 0.2 | 40.2 ± 1.5 |
| 4 | 8.75 (0) | 0.75 (0) | 45 (0) | 3.84 ± 0.09 | 97.4 ± 0.2 | 39.1 ± 0.2 |
| 5 | 7.50 (−1) | 1.00 (1) | 60 (1) | 3.30 ± 0.13 | 99.6 ± 0.2 | 22.8 ± 2.1 |
| 6 | 7.50 (−1) | 1.00 (1) | 30 (−1) | 2.53 ± 0.06 | 85.6 ± 1.5 | 44.8 ± 1.8 |
| 7 | 10.00 (1) | 1.00 (1) | 60 (1) | 4.25 ± 0.13 | 87.7 ± 0.9 | 41.9 ± 5.4 |
| 8 | 8.75 (0) | 0.75 (0) | 45 (0) | 3.84 ± 0.03 | 96.6 ± 0.5 | 41.0 ± 4.0 |
| 9 | 7.50 (−1) | 0,50 (−1) | 60 (1) | 2.80 ± 0.08 | 96.6 ± 2.5 | 49.4 ± 0.8 |
| 10 | 10.00 (1) | 0.50 (−1) | 30 (−1) | 3.06 ± 0.19 | 59.7 ± 5.6 | 32.2 ± 1.1 |
| 11 | 10.84 (1.682) | 0.75 (0) | 45 (0) | 4.13 ± 0.10 | 70.0 ± 1.0 | 35.1 ± 1.8 |
| 12 | 8.75 (0) | 0.75 (0) | 20 (−1.682) | 2.36 ± 0.10 | 68.7 ± 5.3 | 23.4 ± 1.4 |
| 13 | 6.66 (−1.682) | 0.75 (0) | 45 (0) | 3.02 ± 0.14 | 97.9 ± 0.8 | 35.1 ± 1.1 |
| 14 | 8.75 (0) | 0.75 (0) | 70 (1.682) | 3.63 ± 0.11 | 96.7 ± 0.7 | 27.9 ± 0.9 |
| 15 | 8.75 (0) | 1.17 (1.682) | 45 (0) | 2.86 ± 0.15 | 77.8 ± 5.5 | 17.9 ± 0.5 |
| 16 | 8.75 (0) | 0.75 (0) | 45 (0) | 3.88 ± 0.00 | 97.0 ± 0.7 | 29.9 ± 3.7 |
| 17 | 8.75 (0) | 0.33 (−1.682) | 45 (0) | 2.08 ± 0.00 | 67.2 ± 2.1 | 23.5 ± 1.5 |
| Effect | Factor | Biomass Concentration (g/L) | Glucose Consumption (%) | PHB Yield (%) |
|---|---|---|---|---|
| Constant | −2.47246 | −25.4555 | 258.859 | |
| Linear | A | 0.377065 * | 18.0657 * | −43.417 |
| B | 4.40674 * | 67.7699 * | 9.06373 * | |
| C | 0.0569158 * | 1.49479 * | −1.2257 | |
| Quadratic | AA | −0.0357402 | −2.12928 * | 1.32338 |
| BB | −7.20779 * | −118.663 * | −49.2582 * | |
| CC | −0.0011688 * | −0.016771 * | −0.005873 | |
| Interaction | AB | 0.504 * | 13.6 * | 9.552 * |
| AC | 0.00226667 | 0.044 | 0.2648 * | |
| BC | 0.0626667 * | 0.1 | −0.67067 |
| Independent Variable | |||||
|---|---|---|---|---|---|
| Dependent Variable | Glucose Concentration (g/L) | Ammonium Sulfate Concentration (g/L) | Phosphate Buffer Molarity (mmol/L) | Predicted Value | Experimental Value |
| biomass concentration | 10.8 | 0.95 | 60.2 | 4.54 g/L | 4.84 ± 0.00 g/L |
| glucose consumption | 7.1 | 0.72 | 56.0 | 100% | 100 ± 0.14% |
| PHB yield | 6.7 | 0.60 | 20.0 | 54.7% | 49.0 ± 1.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronďošová, S.; Legerská, B.; Chmelová, D.; Ondrejovič, M.; Miertuš, S. Optimization of Growth Conditions to Enhance PHA Production by Cupriavidus necator. Fermentation 2022, 8, 451. https://doi.org/10.3390/fermentation8090451
Ronďošová S, Legerská B, Chmelová D, Ondrejovič M, Miertuš S. Optimization of Growth Conditions to Enhance PHA Production by Cupriavidus necator. Fermentation. 2022; 8(9):451. https://doi.org/10.3390/fermentation8090451
Chicago/Turabian StyleRonďošová, Soňa, Barbora Legerská, Daniela Chmelová, Miroslav Ondrejovič, and Stanislav Miertuš. 2022. "Optimization of Growth Conditions to Enhance PHA Production by Cupriavidus necator" Fermentation 8, no. 9: 451. https://doi.org/10.3390/fermentation8090451
APA StyleRonďošová, S., Legerská, B., Chmelová, D., Ondrejovič, M., & Miertuš, S. (2022). Optimization of Growth Conditions to Enhance PHA Production by Cupriavidus necator. Fermentation, 8(9), 451. https://doi.org/10.3390/fermentation8090451





