Optimization and Implementation of Fed-Batch Strategy to Produce Ligninolytic Enzyme from the White-Rot Basidiomycete Pycnoporus sanguineus in Bubble Column Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Basidiomycete and Growth Medium
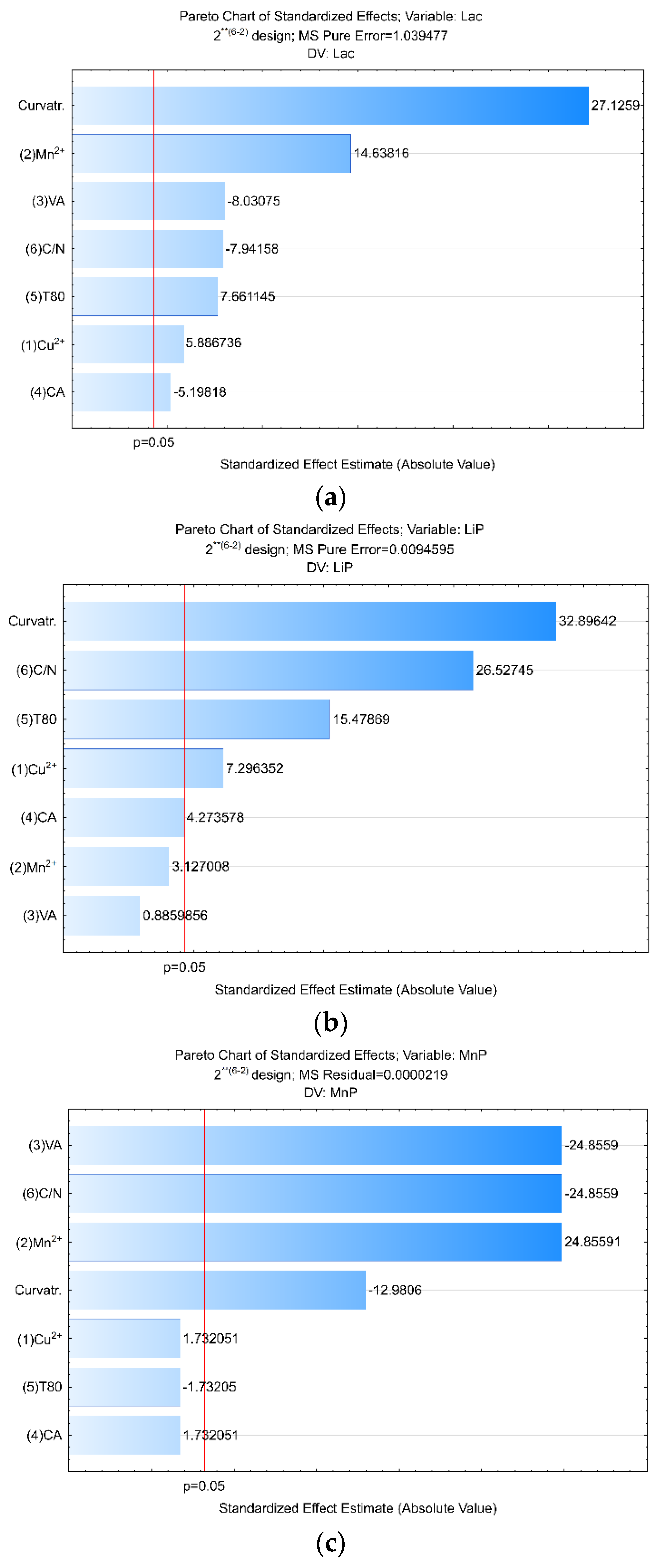
2.2. Experimental Design and Optimization for Maximum Production of Ligninolytic Enzymes by P. sanguineus 2512
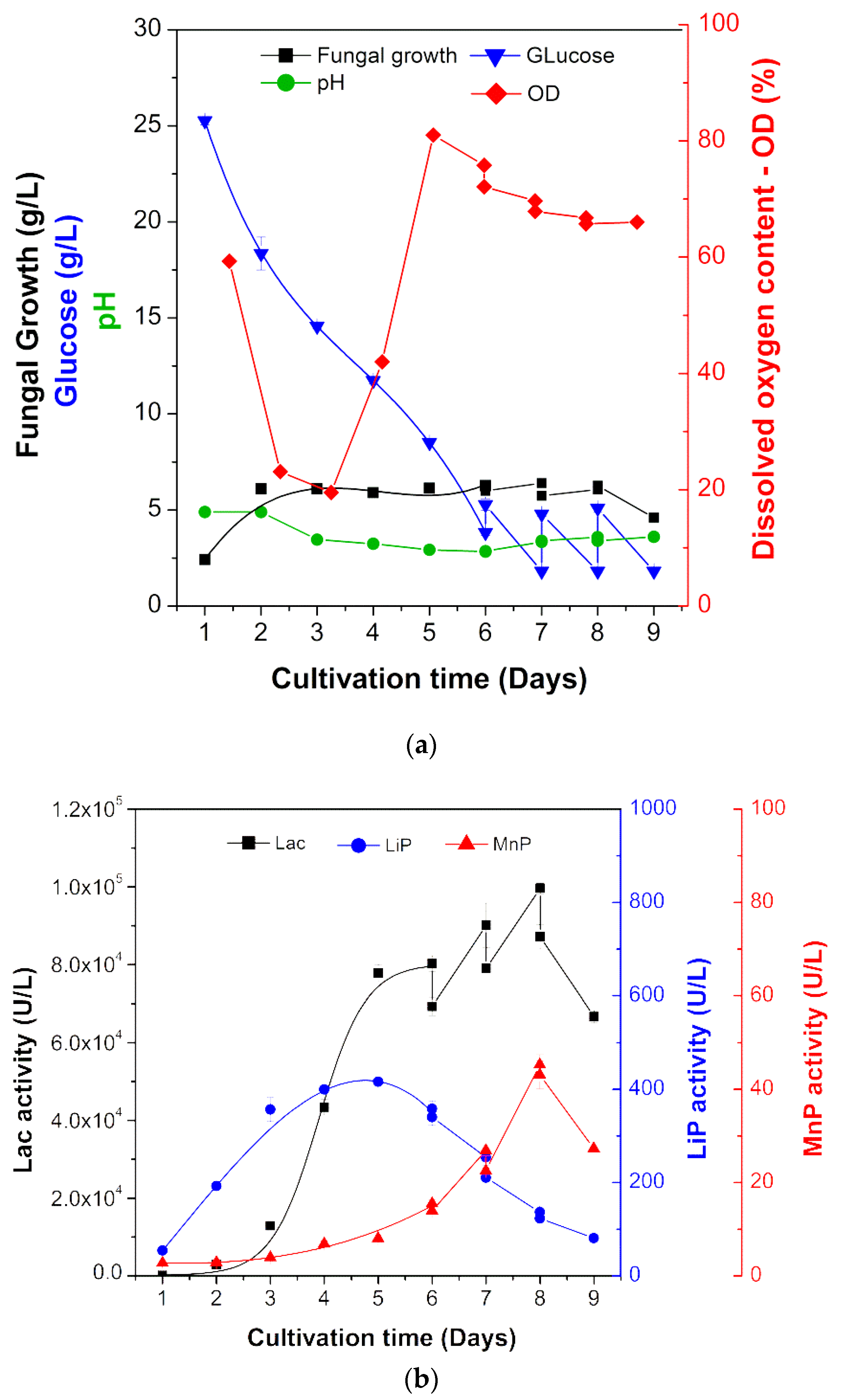
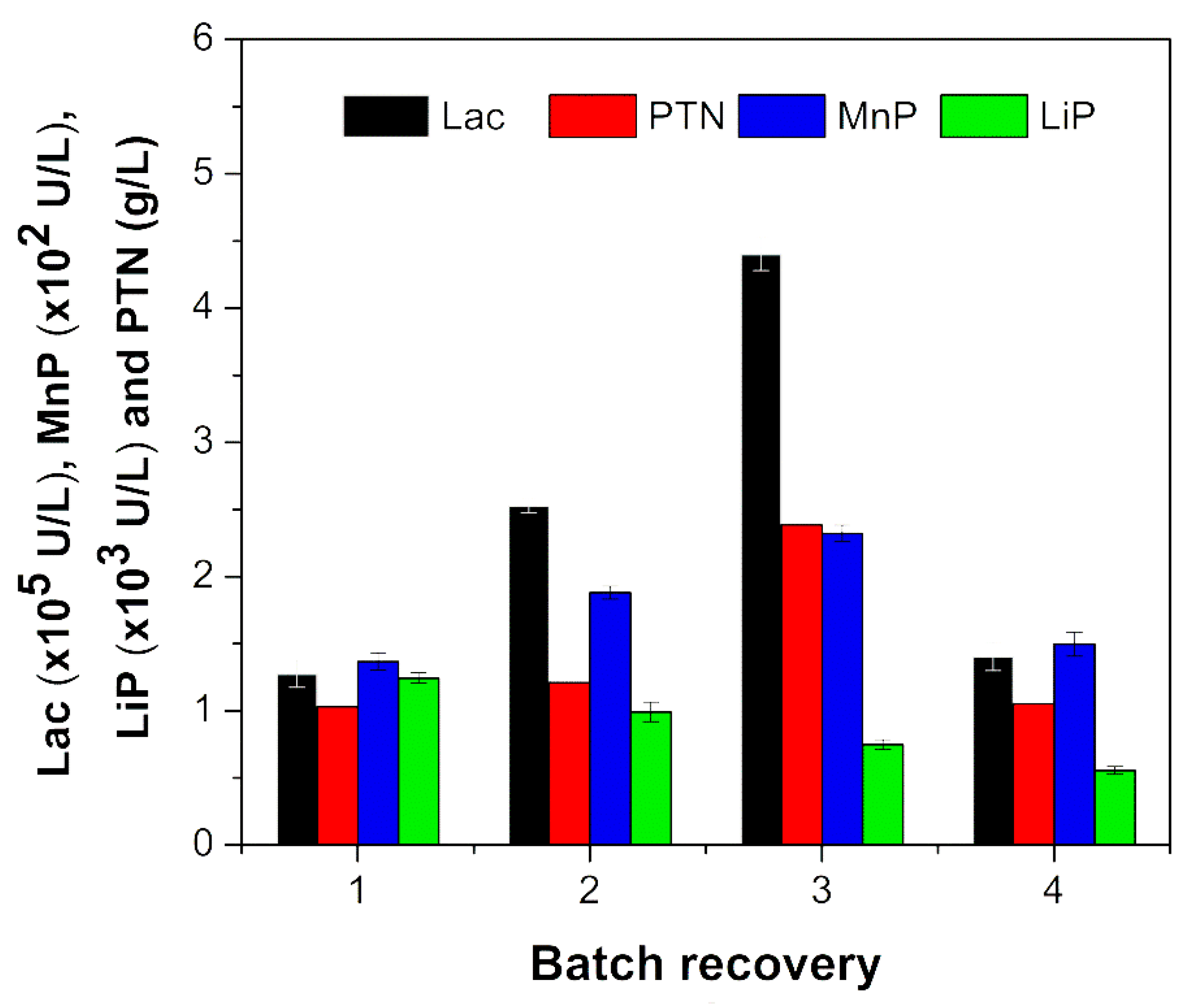
2.3. Fed-Batch Cultivation in a Bubble Column and Tangential-Flow Filtration
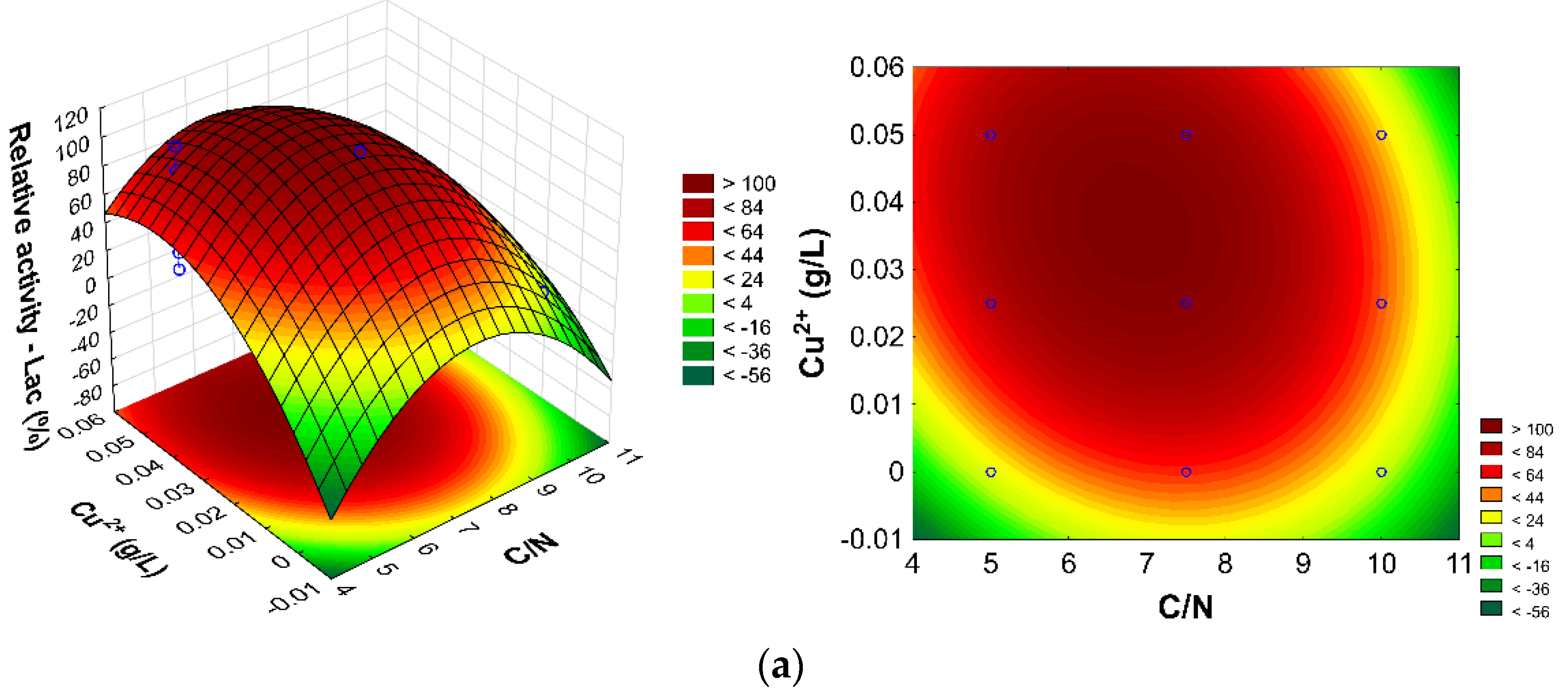
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Couto, S.R.; Toca-Herrera, J.L. Laccase production at reactor scale by filamentous fungi. Biotechnol. Adv. 2007, 25, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Plácido, J.; Capareda, S. Ligninolytic enzymes: A biotechnological alternative for bioethanol production. Bioresour. Bioprocess. 2015, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cagide, C.; Castro-Sowinski, S. Technological and biochemical features of lignin-degrading enzymes: A brief review. Environ. Sustain. 2020, 3, 371–389. [Google Scholar] [CrossRef]
- Alessandra, P.; Cinzia, P.; Paola, G.; Vincenza, F.; Sannia, G. Heterologous laccase production and its role in industrial applications. Bioeng. Bugs. 2010, 1, 252–262. [Google Scholar]
- Mandels, M.; Reese, E.T. Induction of cellulase in fungi by cellobiose. J. Bacteriol. 1960, 79, 816–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfenden, B.S.; Willson, R.L. Radical-cations as reference chromogens in kinetic studies of ono-electron transfer reactions: Pulse radiolysis studies of 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate). J. Chem. Soc. Perkin Trans. 2 1982, 7, 805–812. [Google Scholar] [CrossRef]
- Kuwahara, M.; Glenn, J.K.; Morgan, M.A.; Gold, M.H. Separation and characterization of two extracelluar H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete Chrysosporium. FEBS Lett. 1984, 169, 247–250. [Google Scholar] [CrossRef] [Green Version]
- Tien, M.; Kirk, T.K. Lignin-degrading enzyme from Phanerochaete Chrysosporium: Purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc. Natl. Acad. Sci. USA 1984, 81, 2280–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Schneider, W.D.H.; Fontana, R.C.; Mendonça, S.; de Siqueira, F.G.; Dillon, A.J.P.; Camassola, M. High level production of laccases and peroxidases from the newly isolated white-rot basidiomycete Marasmiellus Palmivorus VE111 in a stirred-tank bioreactor in response to different carbon and nitrogen sources. Process. Biochem. 2018, 69, 1–11. [Google Scholar] [CrossRef]
- Majeau, J.A.; Brar, S.K.; Tyagi, R.D. Laccases for removal of recalcitrant and emerging pollutants. Bioresour. Technol. 2010, 101, 2331–2350. [Google Scholar] [CrossRef] [PubMed]
- Vrsanska, M.; Voberkova, S.; Langer, V.; Palovcikova, D.; Moulick, A.; Adam, V.; Kopel, P. Induction of laccase, lignin peroxidase and manganese peroxidase activities in white-rot fungi using copper complexes. Molecules 2016, 21, 1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildén, K.; Martinez, A.T.; Hatakka, A.; Lundell, T. The two manganese peroxidases Pr-MnP2 and Pr-MnP3 of Phlebia radiata, a lignin-degrading basidiomycete, are phylogenetically and structurally divergent. Fungal Genet. Biol. 2005, 42, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Bettin, F.; Montanari, Q.; Calloni, R.; Gaio, T.A.; Silveira, M.M.; Dillon, A.J.P. Production of laccases in submerged process by Pleurotus Sajor-Caju PS-2001 in relation to carbon and organic nitrogen sources, antifoams and tween 80. J. Ind. Microbiol. Biotechnol. 2009, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Antecka, A.; Blatkiewicz, M.; Głuszcz, P.; Ledakowicz, S. Improvement of laccase biosynthesis by various feeding strategies and in situ integration of biomass separation. Chem. Eng. Process. 2021, 159, 108237. [Google Scholar] [CrossRef]
- Souza, E.F.; Passos, D.F.; Souto, F.; Calado, V.M.A.; Pereira, N. Enhancement of kraft lignin molecular relaxation based on Laccases from Pycnoporus Sanguineus produced in instrumented bioreactors. Biomass Convers. Biorefin. 2021, 1, 1–12. [Google Scholar] [CrossRef]
- Georis, J.; Lomascolo, A.; Camarero, S.; Dorgeo, V.; Herpoël, I.; Asther, M.; Martinez, A.T.; Dauvrin, T. Pycnoporus cinnabarinus laccases: An interesting tool for food or non-food applications. Commun. Agric. Appl. Biol. Sci. 2003, 68, 263–266. [Google Scholar] [PubMed]
- Galhaup, C.; Goller, S.; Peterbauer, C.K.; Strauss, J.; Haltrich, D. Characterization of the major laccase isoenzyme from Trametes Pubescens and regulation of its synthesis by metal ions. Microbiology 2002, 148, 2159–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Factors | Minimum | Central Point | Maximum |
|---|---|---|---|
| −1 | 0 | +1 | |
| Cu2+ (g/L) | 0 | 0.025 | 0.05 |
| Mn2+ (mM) | 0 | 1.5 | 3.0 |
| VA (mM) | 0 | 1.5 | 3.0 |
| CA (g/L) | 0 | 1.0 | 2.0 |
| T80 % (v/v) | 0 | 0.025 | 0.05 |
| C/N ratio | 5 | 7.5 | 10.0 |
| Factors | Relative Lac (%) R² = 0.8788; R-Adj. = 0.81416 | Relative LiP (%) R² = 0.68323; R-Adj. = 0.47959 | Relative MnP (%) R² =0.77506; R-Adj. = 0.6432 |
|---|---|---|---|
| C/N | (L) and (Q) p ≤ 0.05 | (L) and (Q) p ≤ 0.05 | (L) and (Q) p ≤ 0.05 |
| Cu2+ | (L) and (Q) p ≤ 0.05 | (L) and (Q) p ≤ 0.05 | (L) and (Q) p ≤ 0.05 |
| Mn2+ | (L) and (Q) p ≤ 0.05 | (L) p ≤ 0.05 | (L) p ≤ 0.05 |
| VA | (L) and (Q) p ≤ 0.05 | (L) and (Q) p > 0.05 | (L) p > 0.05 and (Q) p ≤ 0.05 |
| T80 | (L) p > 0.05 and (Q) p ≤ 0.05 | (L) and (Q) p ≤ 0.05 | (L) p ≤ 0.05 and (Q) p > 0.05 |
| Factors | Confidence Limit | Predicted Value | Experimental Result | ||
|---|---|---|---|---|---|
| −95% | +95% | Optimized Medium | Non-Optimized Medium | ||
| Relative Lac (%) | 60.93 | 81.58 | 71.22 | 79.01 | 7.43 |
| Relative MnP (%) | 26.11 | 37.65 | 31.88 | 34.15 | 23.06 |
| Relative LiP (%) | 20.10 | 29.96 | 25.03 | 22.42 | 12.45 |
| Kinetic Parameters | 1 SB | 2 FB 1 | FB 2 | FB 3 |
|---|---|---|---|---|
| Lac activity (× 104 U/ L) | 8.02 | 9.01 | 9.96 | 6.67 |
| MnP activity (U/L) | 15.00 | 26.00 | 45.00 | 27.00 |
| LiP activity (U/L) | 358.00 | 254.00 | 136.00 | 81.00 |
| Time (days) | 6.00 | 7.00 | 8.00 | 9.00 |
| Residual glucose (g/L) | 3.80 | 1.80 | 1.80 | 1.80 |
| Mycelial biomass X (g/L) | 6.66 | 6.00 | 6.06 | 4.60 |
| Lac volumetric productivity (× 103 U/L∙d) | 13.37 | 12.87 | 12.45 | 7.41 |
| MnP volumetric productivity (U/L∙d) | 2.50 | 3.71 | 5.62 | 3.00 |
| LiP volumetric productivity (U/L∙d) | 59.67 | 36.28 | 17.00 | 9.00 |
| Substrate yield on Lac YE/S (× 104 U/g) | 2.11 | 5.00 | 5.53 | 3.70 |
| Substrate yield on MnP YE/S (U/g) | 3.95 | 14.44 | 25.00 | 15.00 |
| Substrate yield on LiP YE/S (U/g) | 94.21 | 141.11 | 75.55 | 45.00 |
| Substrate yield on biomass YX/S (g/g) | 0.57 | 0.30 | 0.30 | 0.39 |
| Biomass volumetric productivity PX (g/L∙d) | 1.11 | 0.85 | 0.75 | 0.51 |
| Lac specific activity SALac (× 104 U/g of biomass) | 1.20 | 1.50 | 1.64 | 1.45 |
| MnP specific activity SAMnP (U/g of biomass) | 2.25 | 4.33 | 7.42 | 5.86 |
| LiP specific activity SALiP (U/g of biomass) | 53.75 | 42.33 | 22.44 | 17.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, E.F., Jr.; Santos, I.M.T.S.; Souto, F.; Calado, V.; Pereira, N., Jr. Optimization and Implementation of Fed-Batch Strategy to Produce Ligninolytic Enzyme from the White-Rot Basidiomycete Pycnoporus sanguineus in Bubble Column Reactor. Fermentation 2022, 8, 418. https://doi.org/10.3390/fermentation8090418
de Souza EF Jr., Santos IMTS, Souto F, Calado V, Pereira N Jr. Optimization and Implementation of Fed-Batch Strategy to Produce Ligninolytic Enzyme from the White-Rot Basidiomycete Pycnoporus sanguineus in Bubble Column Reactor. Fermentation. 2022; 8(9):418. https://doi.org/10.3390/fermentation8090418
Chicago/Turabian Stylede Souza, Evanildo F., Jr., Isabella M. T. S. Santos, Felipe Souto, Verônica Calado, and Nei Pereira, Jr. 2022. "Optimization and Implementation of Fed-Batch Strategy to Produce Ligninolytic Enzyme from the White-Rot Basidiomycete Pycnoporus sanguineus in Bubble Column Reactor" Fermentation 8, no. 9: 418. https://doi.org/10.3390/fermentation8090418
APA Stylede Souza, E. F., Jr., Santos, I. M. T. S., Souto, F., Calado, V., & Pereira, N., Jr. (2022). Optimization and Implementation of Fed-Batch Strategy to Produce Ligninolytic Enzyme from the White-Rot Basidiomycete Pycnoporus sanguineus in Bubble Column Reactor. Fermentation, 8(9), 418. https://doi.org/10.3390/fermentation8090418







