Chemical Composition and In Situ Degradability of Desmanthus spp. Forage Harvested at Different Maturity Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment 1
2.2. Experiment 2
2.3. Animal Management
2.4. In Situ Incubations and Chemical Analysis
2.5. Data Analysis
2.5.1. Calculations
2.5.2. Statistical Analysis
3. Results
3.1. Experiment 1: L/S, DM and Chemical Composition
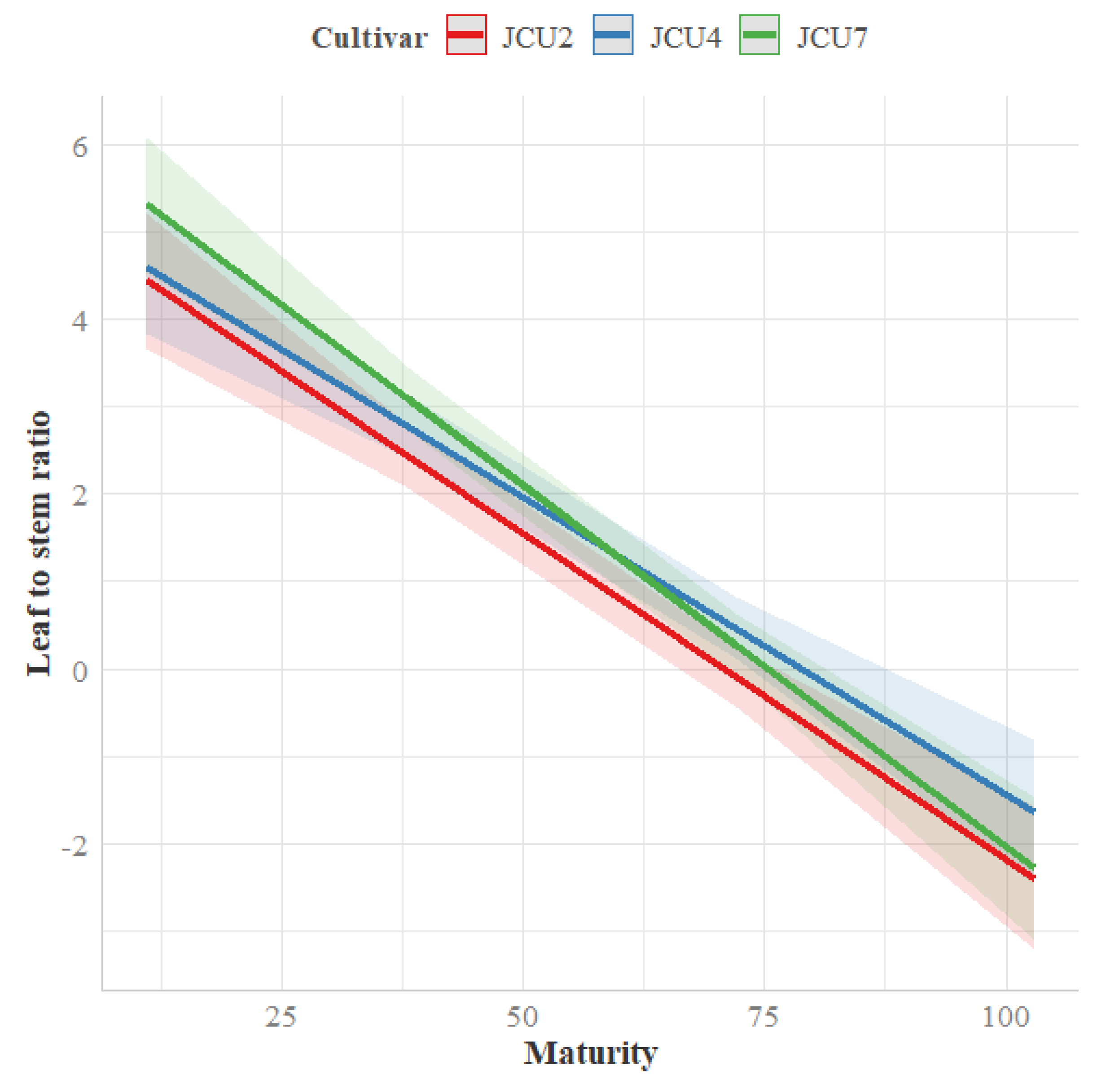
3.1.1. Leaf to Stem Mass Ratio
3.1.2. Dry Matter Concentration
3.1.3. Crude Protein Composition
3.1.4. Fibre Composition
3.2. Experiment 2: Forage DM and Chemical Composition
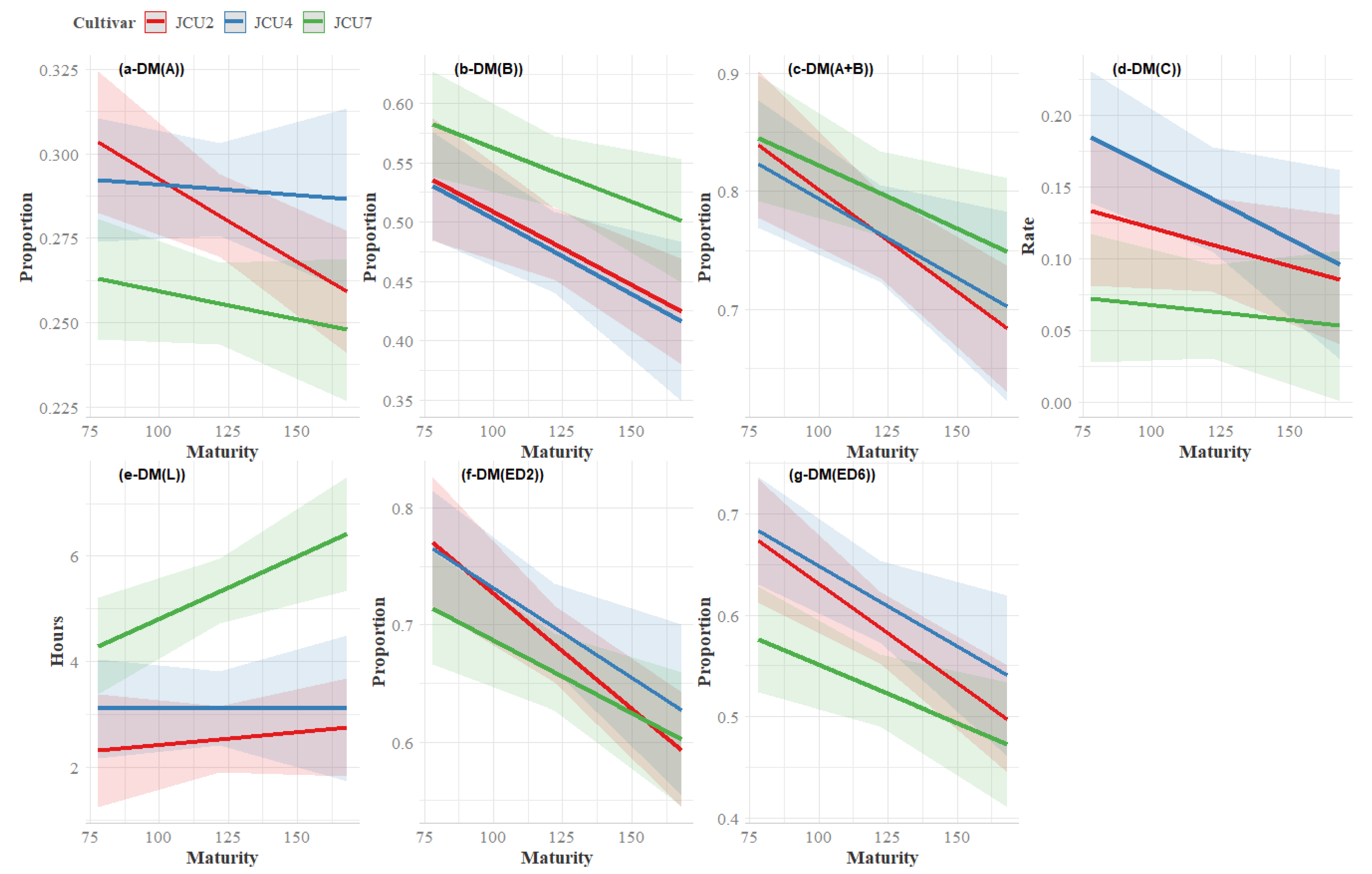
3.3. Dry Matter Degradation
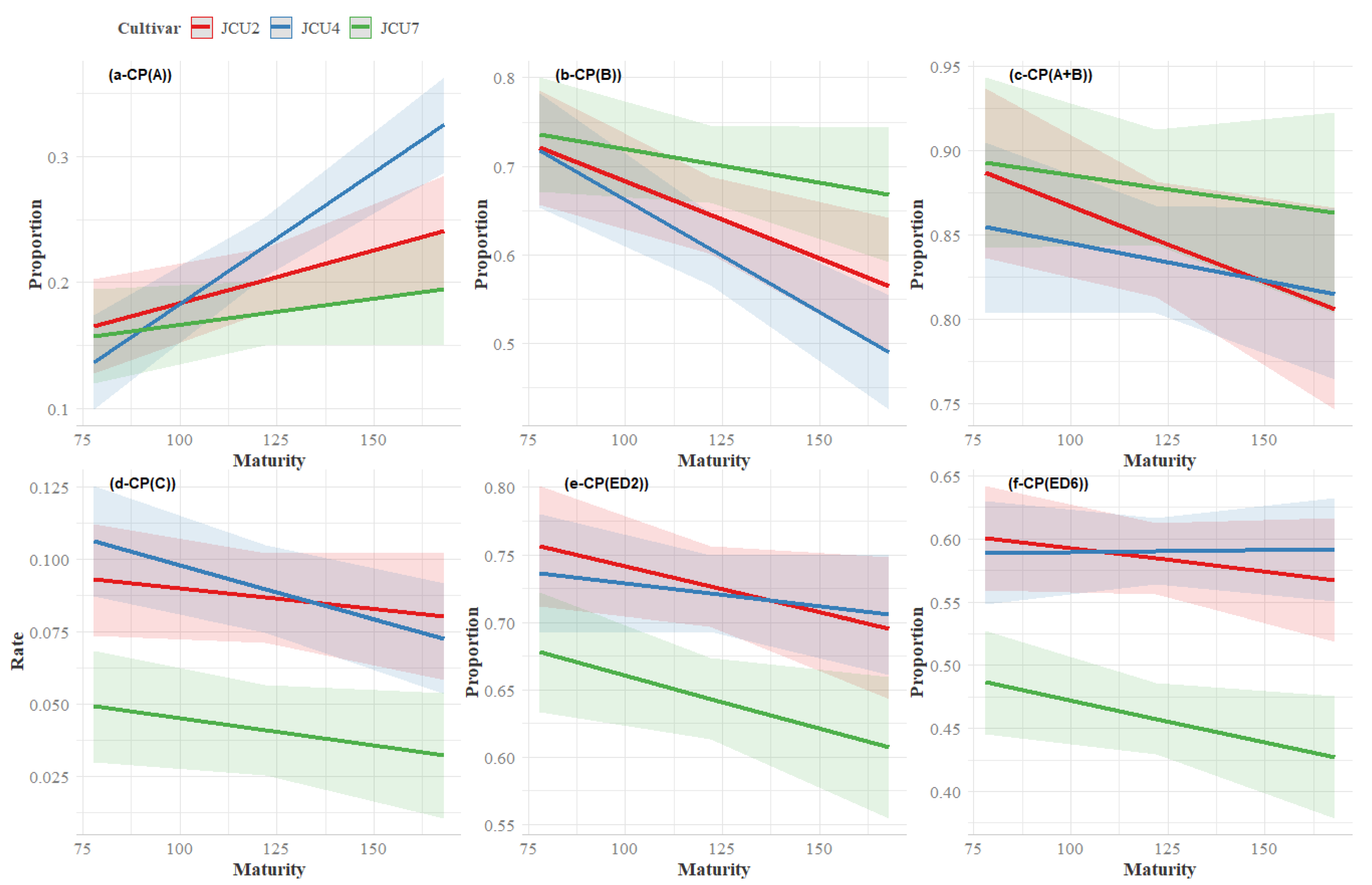
3.4. Crude Protein Degradation
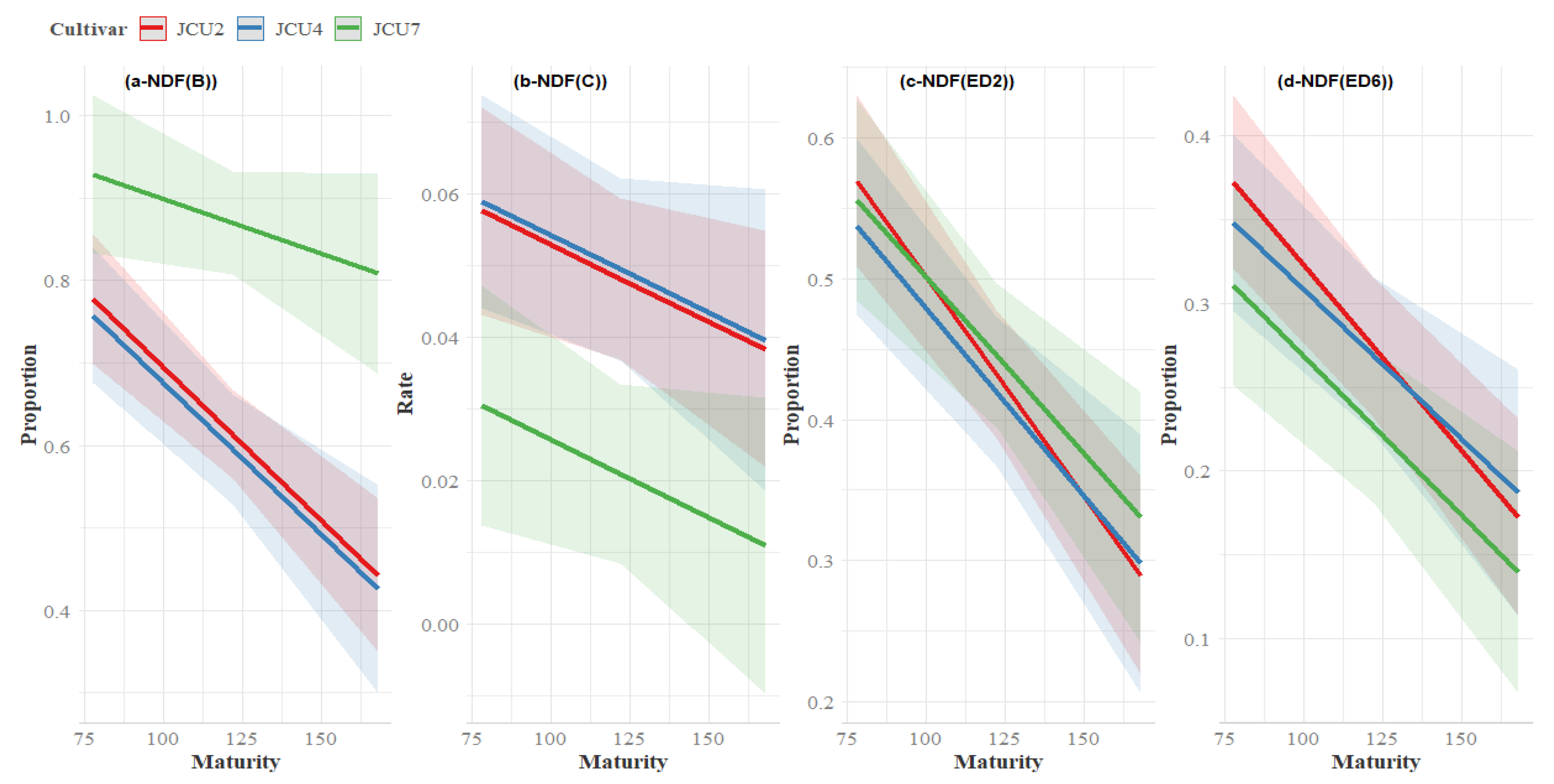
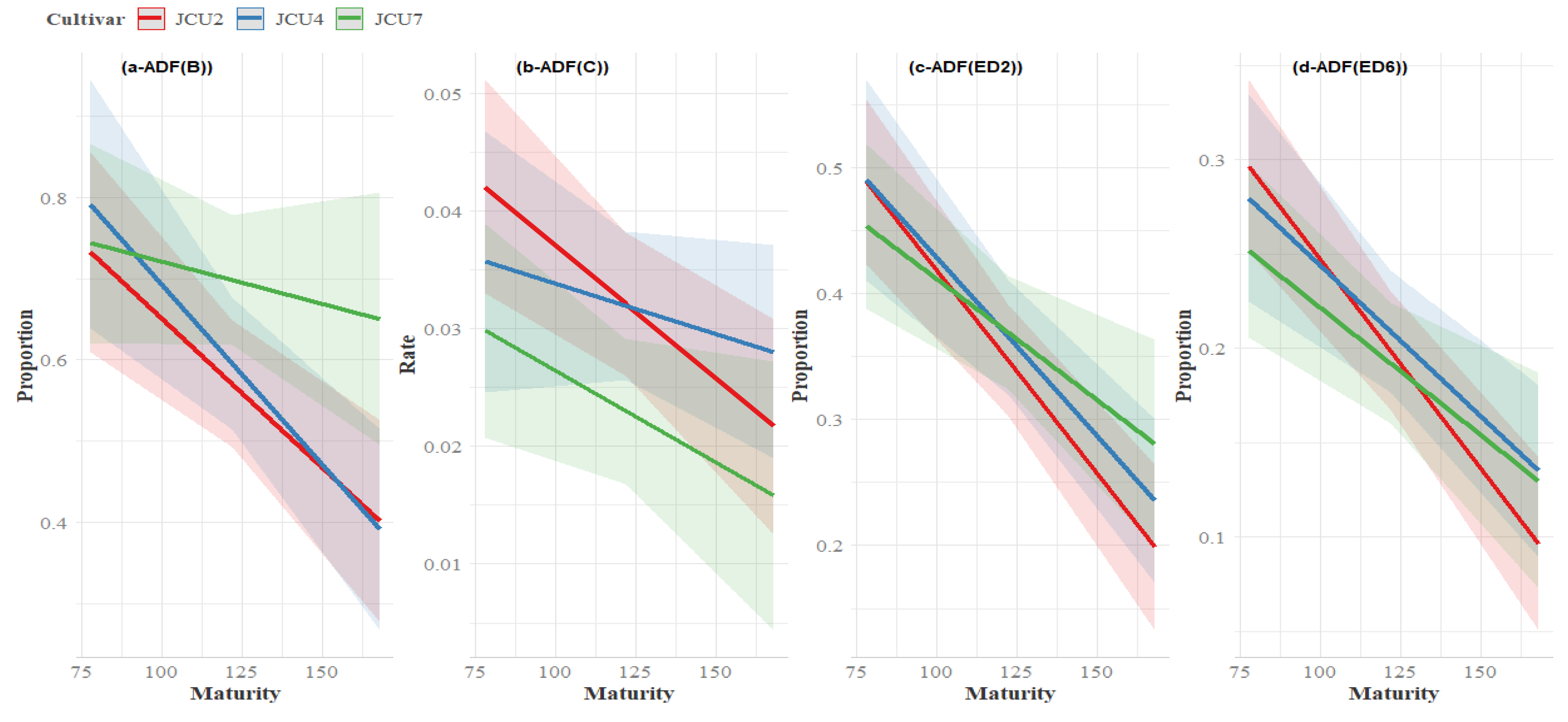
3.5. Fibre Degradation
4. Discussion
4.1. Leaf to Stem Mass Ratio
4.2. Chemical Composition
4.3. Dry Matter Degradability
4.4. Fibre Degradability
4.5. Crude Protein Degradability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coates, D.B.; Miller, C.P.; Hendricksen, R.E.; Jones, R.J. Stability and productivity of stylosanthes pastures in Australia. II. Animal production from stylosanthes pastures. Trop. Grassl. 1997, 31, 494–502. [Google Scholar]
- Leng, R.A. Factors affecting the utilization of ‘poor-quality’ forages by ruminants particularly under tropical conditions. Nutr. Res. Rev. 1990, 3, 277–303. [Google Scholar] [CrossRef]
- McCown, R.L. The climatic potential for beef cattle production in tropical Australia: Part I-Simulating the annual cycle of liveweight change. Agric. Syst. 1981, 6, 303–317. [Google Scholar] [CrossRef]
- Kanani, J.; Lukefahr, S.D.; Stanko, R.L. Evaluation of tropical forage legumes (Medicago sativa, Dolichos lablab, Leucaena leucocephala and Desmanthus bicornutus) for growing goats. Small Rumin. Res. 2006, 65, 1–7. [Google Scholar] [CrossRef]
- Johnson, D.E.; Johnson, K.A. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Hill, J.O.; Coates, D.B.; Whitbread, A.M.; Clem, R.L.; Robertson, M.J.; Pengelly, B.C. Seasonal changes in pasture quality and diet selection and their relationship with liveweight gain of steers grazing tropical grass and grass-legume pastures in northern Australia. Anim. Prod. Sci. 2009, 49, 983–993. [Google Scholar] [CrossRef]
- Schultze-Kraft, R.; Rao, I.M.; Peters, M.; Clements, R.J.; Bai, C.; Liu, G. Tropical forage legumes for environmental benefits: An overview. Trop. Grassl. Forrajes Trop. 2018, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gardiner, C.P.; Burt, R.L. Performance characteristics of Desmanthus virgatus in contrasting tropical environments. Trop. Grassl. 1995, 29, 183–187. [Google Scholar]
- Jones, R.M.; Brandon, N.J. Persistence and productivity of eight accessions of Desmanthus virgatus under a range of grazing pressures in subtropical Queensland. Trop. Grassl. 1998, 32, 145–152. [Google Scholar]
- Pengelly, B.C.; Conway, M.J. Pastures on cropping soils: Which tropical pasture legume to use? Trop. Grassl. 2000, 34, 162–168. [Google Scholar]
- Hall, T.J.; Walker, R.W. Pasture legume adaptation to six environments of the seasonally dry tropics of north Queensland. Trop. Grassl. 2005, 39, 182–196. [Google Scholar]
- Gardiner, C.; Wright, C.; Coventry, M. The germination, passage and viability of Desmanthus virgatus (L.) Willenow seed through sheep and its implication for dispersal in tropical rangelands. In Proceedings of the 16th Australian Society of Agronomy Conference, Armidale, NSW, Australia, 14–18 October 2012; pp. 16–19. [Google Scholar]
- Luckow, M. Monograph of Desmanthus (Leguminosae-Mimosoideae). In Systematic Botany Monographs; American Society of Plant Taxonomists: Laramie, WY, USA, 1993; Volume 38, pp. 1–166. [Google Scholar]
- Suybeng, B.; Charmley, E.; Gardiner, C.P.; Malau-Aduli, B.S.; Malau-Aduli, A.E.O. Methane emissions and the use of desmanthus in beef cattle production in Northern Australia. Animals 2019, 9, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, B.; Pengelly, B.; Brown, S.; Donnelly, J.; Eagles, D.; Franco, A.; Hanson, J.; Mullen, B.; Partridge, I.; Peters, M.; et al. Tropical Forages: An Interactive Selection Tool. Available online: https://www.tropicalforages.info/text/intro/index.html (accessed on 2 February 2022).
- Gardiner, C.P.; Swan, S.J. Abandoned pasture legumes offer potential economic and environmental benefits in semiarid clay soil rangelands. In Proceedings of the Australian Rangeland Society 15th Biennial Conference Proceeding, Charters Towers, QLD, Australia, 28 September–2 October 2008; p. 93. [Google Scholar]
- Suybeng, B.; Charmley, E.; Gardiner, C.P.; Malau-Aduli, B.S.; Malau-Aduli, A.E.O. Supplementing Northern Australian beef cattle with desmanthus tropical legume reduces in-vivo methane emissions. Animals 2020, 10, 2097. [Google Scholar] [CrossRef] [PubMed]
- Vandermeulen, S.; Singh, S.; Ramírez-Restrepo, C.A.; Kinley, R.D.; Gardiner, C.P.; Holtum, J.A.M.; Hannah, I.; Bindelle, J. In vitro assessment of ruminal fermentation, digestibility and methane production of three species of Desmanthus for application in northern Australian grazing systems. Crop Pasture Sci. 2018, 69, 797–807. [Google Scholar] [CrossRef]
- Gardiner, C.; Parker, A. Steer liveweight gains on ProgardesTM desmanthus/buffel pastures in Queensland. In Proceedings of the 2nd Australian and New Zealand Societies of Animal Production Joint Conference, Lincoln, New Zealand, 2–5 July 2012. [Google Scholar]
- Rangel, J.H.D.A.; Gardiner, C.P. Stimulation of wool growth by Desmanthus spp. as a supplement to a diet of Mitchell grass hay. Trop. Grassl. 2009, 43, 106–111. [Google Scholar]
- Marsetyo, D.R.; Rusiyantono, Y.; Syukur, S.H. The effect of supplementation of different legume leaves on feed intake, digestion and growth of Kacang goats given Mulato grass. J. Agric. Sci. Technol. 2017, 7, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Aoetpah, A.; Gardiner, C.; Gummow, B.; Walker, G. Growth and eye muscle area of cross-bred Boer goats fed desmanthus cultivar JCU 1 hay. In Proceedings of the 32nd Biennial Conference of the Australian Society of Animal Production, Wagga Wagga, NSW, Australia, 2–6 July 2018. [Google Scholar]
- Mwangi, F.W.; Suybeng, B.; Gardiner, C.P.; Kinobe, R.T.; Charmley, E.; Malau-Aduli, B.S.; Malau-Aduli, A.E.O. Effect of incremental proportions of Desmanthus spp. in isonitrogenous forage diets on growth performance, rumen fermentation and plasma metabolites of pen-fed growing Brahman, Charbray and Droughtmaster crossbred beef steers. PLoS ONE 2022, 17, e0260918. [Google Scholar] [CrossRef]
- Norman, H.C.; Hulm, E.; Humphries, A.W.; Hughes, S.J.; Vercoe, P.E. Broad near-infrared spectroscopy calibrations can predict the nutritional value of >100 forage species within the Australian feedbase. Anim. Prod. Sci. 2020, 60, 1111–1122. [Google Scholar] [CrossRef]
- Australian Government Bureau of Meteorology. Climate Statistics for Australian Locations. Available online: http://www.bom.gov.au/climate/averages/tables/cw_032040.shtml (accessed on 10 June 2022).
- National Health and Medical Research Council. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 8th ed.; National Health and Medical Research Council: Canberra, ACT, Australia, 2013; ISBN 1864965975. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Sweeney, R.A.; Rexroad, P.R. Comparison of LECO FP-228 “nitrogen determinator” with AOAC copper catalyst Kjeldahl method for crude protein. J. Assoc. Off. Anal. Chem. 1987, 70, 1028–1030. [Google Scholar] [CrossRef]
- McDonald, I. Short Note: A revised model for the estimation of protein degradability in the rumen. J. Agric. Sci. 1981, 96, 251–252. [Google Scholar] [CrossRef]
- Ørskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef] [Green Version]
- Buxton, D.R.; Mertens, D.R.; Moore, K.J. Forage quality for ruminants: Plant and animal considerations. Prof. Anim. Sci. 1995, 11, 121–131. [Google Scholar] [CrossRef]
- Huhtanen, P.; Ahvenjärvi, S.; Weisbjerg, M.R.; Norgaard, P. Digestion and passage of fibre in ruminants. In Ruminant Physiology. Digestion, Metabolism and Impact of Nutrition on Gene Expression, Immunology and Stress; Sejrsen, K., Hvelplund, T., Nielson, M.O., Eds.; Wageningen Academic: Wageningen, The Netherlands, 2006; pp. 87–138. [Google Scholar]
- Olowu, O.O.; Firincioğlu, S.Y. Feed evaluation methods: Performance, economy and environment. Eurasian J. Agric. Res. 2019, 3, 48–57. [Google Scholar]
- Ørskov, E.R. The in situ technique for the estimation of forage degradability in ruminants. In Forage Evaluation in Ruminant Nutrition; Givens, D.I., Owen, E., Axford, R.F.E., Omed, H.M., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 175–188. ISBN 0851993443. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. Nutrient Requirements of Beef Cattle, 8th ed.; National Academies Press: Washington, DC, USA, 2016; ISBN 978-0-309-31702-3. [Google Scholar]
- Huhtanen, P.; Ahvenjärvi, S.; Broderick, G.A.; Reynal, S.M.; Shingfield, K.J. Quantifying ruminal digestion of organic matter and neutral detergent fiber using the omasal sampling technique in cattle-A meta-analysis. J. Dairy Sci. 2010, 93, 3203–3215. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academies Press: Washington, DC, USA, 2001; ISBN 978-0-309-06997-7. [Google Scholar]
- Lemàire, G.; Bélanger, G. Allometries in plants as drivers of forage nutritive value: A review. Agriculture 2020, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Calado, T.B.; Da Cunha, M.V.; Teixeira, V.I.; Dos Santos, M.V.F.; Cavalcanti, H.S.; Lira, C.C. Morphology and productivity of “Jureminha” genotypes (Desmanthus spp.) under different cutting intensities. Rev. Caatinga 2016, 29, 742–752. [Google Scholar] [CrossRef] [Green Version]
- Wilman, D.; Moghaddam, P.R. In vitro digestibility and neutral detergent fibre and lignin contents of plant parts of nine forage species. J. Agric. Sci. 1998, 131, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Grev, A.M.; Wells, M.S.; Catalano, D.N.; Martinson, K.L.; Jungers, J.M.; Sheaffer, C.C. Stem and leaf forage nutritive value and morphology of reduced lignin alfalfa. Agron. J. 2020, 112, 406–417. [Google Scholar] [CrossRef]
- Suksombat, W.; Buakeeree, K. Effect of cutting interval and cutting height on yield and chemical composition of hedge lucerne (Desmanthus virgatus). Asian-Australas. J. Anim. Sci. 2006, 19, 31–34. [Google Scholar] [CrossRef]
- Sheaffer, C.C.; Martin, N.P.; Lamb, J.A.F.S.; Cuomo, G.R.; Grimsbo Jewett, J.; Quering, S.R. Leaf and stem properties of alfalfa entries. Agron. J. 2000, 92, 733–739. [Google Scholar] [CrossRef]
- Durmic, Z.; Ramírez-Restrepo, C.A.; Gardiner, C.; O’Neill, C.J.; Hussein, E.; Vercoe, P.E. Differences in the nutrient concentrations, in vitro methanogenic potential and other fermentative traits of tropical grasses and legumes for beef production systems in northern Australia. J. Sci. Food Agric. 2017, 97, 4075–4086. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, X.; Anrique, R. In situ rumen degradation kinetics of high-protein forage crops in temperate climates. Chil. J. Agric. Res. 2011, 71, 572–577. [Google Scholar] [CrossRef] [Green Version]
- Gierus, M. Changes in crude protein fractions of forage legumes during the spring growth and summer regrowth period. J. Agric. Sci. 2013, 151, 72–90. [Google Scholar]
- Brandao, V.L.N.; Faciola, A.P. Unveiling the relationships between diet composition and fermentation parameters response in dual-flow continuous culture system: A meta-analytical approach. Transl. Anim. Sci. 2019, 3, 1064–1075. [Google Scholar] [CrossRef] [Green Version]
- Hristov, A.N.; Ropp, J.K.; Grandeen, K.L.; Abedi, S.; Etter, R.P.; Melgar, A.; Foley, A.E. Effect of carbohydrate source on ammonia utilization in lactating dairy cows. J. Anim. Sci. 2005, 83, 408–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, D.W.; Brown, J.R. Rotational grazing systems and livestock grazing behavior in shrub-dominated semi-arid and arid rangelands. Rangel. Ecol. Manag. 2011, 64, 1–9. [Google Scholar] [CrossRef]
- Buxton, D.R. Quality-related characteristics of forages as influenced by plant environment and agronomic factors. Anim. Feed Sci. Technol. 1996, 59, 37–49. [Google Scholar] [CrossRef]
- Suybeng, B.; Mwangi, F.W.; McSweeney, C.S.; Charmley, E.; Gardiner, C.P.; Malau-Aduli, B.S.; Malau-Aduli, A.E.O. Response to climate change: Evaluation of methane emissions in northern Australian beef cattle on a high quality diet supplemented with desmanthus using open-circuit respiration chambers and GreenFeed emission monitoring systems. Biology 2021, 10, 943. [Google Scholar] [CrossRef]
- Amben, S.; Gardiner, C.; Walker, G. Desmanthus silage: A potential feed resource for the Northern Australian cattle industry. In Proceedings of the 20th Agronomy Australia Conference, Toowoomba, QLD, Australia, 18–22 September 2022; pp. 3–6. [Google Scholar]
- Saylor, B.A.; Min, D.; Bradford, B.J. Effects of cultivar and harvest days after planting on dry matter yield and nutritive value of teff. J. Anim. Sci. Technol. 2021, 63, 510–519. [Google Scholar] [CrossRef]
- Bowman, J.G.P.; Sowell, B.F.; Paterson, J.A. Liquid supplementation for ruminants fed low-quality forage diets: A review. Anim. Feed Sci. Technol. 1995, 55, 105–138. [Google Scholar] [CrossRef]
- Ayyadurai, P.; Priya, R.S.; Jegathjothi, N.; Gokila, J. A review on optimizing forage quality through management. Int. J. Agric. Sci. Res. 2013, 3, 173–180. [Google Scholar]
- Gusha, J.; Ngongoni, N.T.; Halimani, T.E. Nutritional composition and effective degradability of four forage trees grown for protein supplementation. Online J. Anim. Feed Res. 2013, 3, 170–175. [Google Scholar]
- Mupangwa, J.F.; Ngongoni, N.T.; Topps, J.H.; Acamovic, T.; Hamudikuwanda, H. Rumen degradability and post-ruminal digestion of dry matter, nitrogen and amino acids in three tropical forage legumes estimated by the mobile nylon bag technique. Livest. Prod. Sci. 2003, 79, 37–46. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ribeiro, G.O.; Ran, T.; Marami Milani, M.R.; Yang, W.Z.; Khanaki, H.; Gruninger, R.; Tsang, A.; McAllister, T.A. Recombinant fibrolytic feed enzymes and ammonia fibre expansion (AFEX) pretreatment of crop residues to improve fibre degradability in cattle. Anim. Feed Sci. Technol. 2019, 256, 114260. [Google Scholar] [CrossRef]
- Krause, D.O.; Denman, S.E.; Mackie, R.I.; Morrison, M.; Rae, A.L.; Attwood, G.T.; Mcsweeney, C.S. Opportunities to improve fiber degradation in the rumen: Microbiology, ecology and genomics. FEMS Microbiol. Rev. 2003, 27, 663–693. [Google Scholar] [CrossRef] [Green Version]
- Martineau, R.; Lapierre, H.; Ouellet, D.R.; Pellerin, D.; Berthiaume, R. In situ degradation of timothy conserved as restrictively or extensively fermented silage or as hay. Can. J. Anim. Sci. 2006, 86, 299–306. [Google Scholar] [CrossRef]
- Jung, H.G.; Mertens, D.R.; Payne, A.J. Correlation of acid detergent lignin and Klason lignin with digestibility of forage dry matter and neutral detergent fiber. J. Dairy Sci. 1997, 80, 1622–1628. [Google Scholar] [CrossRef]
- Bowen, M.K.; Poppi, D.P.; McLennan, S.R. Ruminal protein degradability of a range of tropical pastures. Aust. J. Exp. Agric. 2008, 48, 806–810. [Google Scholar] [CrossRef] [Green Version]
- Flachowsky, G.; Lebzien, P. Possibilities for reduction of nitrogen (N) excretion from ruminants and the need for further research—A review. Landbauforsch. Volkenrode 2006, 56, 19–30. [Google Scholar]
- Waghorn, G.C.; Shelton, I.D. Effect of condensed tannins in Lotus corniculatus on the nutritive value of pasture for sheep. J. Agric. Sci. Camb. 1997, 128, 365–372. [Google Scholar] [CrossRef]
- Hook, S.E.; Wright, A.D.G.; McBride, B.W. Methanogens: Methane producers of the rumen and mitigation strategies. Archaea 2010, 2010, 50–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Castro-Montoya, J.M.; Dickhoefer, U. The nutritional value of tropical legume forages fed to ruminants as affected by their growth habit and fed form: A systematic review. Anim. Feed Sci. Technol. 2020, 269, 114641. [Google Scholar] [CrossRef]
- Trevin, J.; Gil, A. Crude protein fractions in common vetch (Vicia sativa L.) fresh forage during pod filling. J. Anim. Sci. 2001, 79, 2449–2455. [Google Scholar]
- Wyffels, S.A.; Boss, D.L.; Sowell, B.F.; DelCurto, T.; Bowman, J.G.P.; McNew, L.B. Dormant season grazing on northern mixed grass prairie agroecosystems: Does protein supplement intake, cow age, weight and body condition impact beef cattle resource use and residual vegetation cover? PLoS ONE 2020, 15, e0240629. [Google Scholar] [CrossRef]



| Harvest Day | Cultivar | ||
|---|---|---|---|
| JCU2 | JCU4 | JCU7 | |
| Experiment 1 | |||
| 11 | Vegetative | Vegetative | Vegetative |
| 38 | Pre-bloom | Pre-bloom | Vegetative |
| 72 | Developing seeds | Developing seeds | Vegetative |
| 103 | Mid-mature seeds | Mid-mature seeds | Full bloom |
| Experiment 2 | |||
| 78 | Vegetative | Pre-bloom | Vegetative |
| 122 | Full bloom | Full bloom | Vegetative |
| 168 | Fully mature seeds | Fully mature seeds | Early bloom |
| Variable 1 | Cultivar 2 | Maturity at Harvest | Mean | SEM 3 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 11 | 38 | 72 | 103 | C | M | C*M | ||||
| L/S | JCU2 | 2.2 | 0.60 | 0.74 | 0.89 | 1.1 a | 0.09 | <0.01 | <0.01 | 0.04 |
| JCU4 | 2.4 | 0.77 | 1.7 | 1.5 | 1.6 b | |||||
| JCU7 | 3.5 | 0.82 | 1.1 | 1.2 | 1.7 b | |||||
| Mean | 2.7 | 0.73 | 1.2 | 1.2 | ||||||
| Leaf | ||||||||||
| DM | JCU2 | 22.8 | 28.5 | 33.5 | 42.4 | 31.8 | 0.99 | 0.14 | <0.01 | 0.01 |
| JCU4 | 24.0 | 27.8 | 31.0 | 53.6 | 34.1 | |||||
| JCU7 | 25.9 | 26.3 | 31.9 | 44.2 | 32.1 | |||||
| Mean | 24.2 | 27.5 | 32.1 | 46.7 | ||||||
| CP | JCU2 | 28.1 | 23.5 | 23.7 | 19.5 | 23.7 | 0.57 | 0.12 | <0.01 | <0.01 |
| JCU4 | 29.0 | 27.2 | 19.9 | 16.9 | 23.3 | |||||
| JCU7 | 24.3 | 23.4 | 23.0 | 18.7 | 22.4 | |||||
| Mean | 27.1 | 24.7 | 22.2 | 18.4 | ||||||
| NDF | JCU2 | 47.1 | 49.6 | 51.5 | 38.2 | 46.6 b | 0.74 | <0.01 | 0.01 | 0.27 |
| JCU4 | 45.4 | 39.2 | 37.9 | 41.4 | 41.0 a | |||||
| JCU7 | 46.5 | 44.6 | 51.0 | 43.1 | 46.3 b | |||||
| Mean | 46.3 | 44.5 | 46.8 | 40.9 | ||||||
| ADF | JCU2 | 14.6 | 24.5 | 27.1 | 25.1 | 22.8 a | 0.79 | 0.02 | <0.01 | 0.03 |
| JCU4 | 16.1 | 23.3 | 27.8 | 32.3 | 24.9 b | |||||
| JCU7 | 18.0 | 27.7 | 26.5 | 29.6 | 25.5 b | |||||
| Mean | 16.2 | 25.2 | 27.1 | 29.0 | ||||||
| Stem | ||||||||||
| DM | JCU2 | 19.5 | 31.2 | 41.0 | 42.2 | 33.5 a | 1.2 | 0.03 | <0.01 | 0.02 |
| JCU4 | 17.8 | 33.6 | 44.3 | 51.1 | 36.7 b | |||||
| JCU7 | 18.9 | 32.3 | 35.8 | 48.9 | 34.0 a | |||||
| Mean | 18.7 | 32.4 | 40.4 | 47.4 | ||||||
| CP | JCU2 | 12.5 | 7.2 | 6.1 | 6.7 | 8.1 | 0.41 | 0.63 | <0.01 | 0.29 |
| JCU4 | 13.1 | 6.8 | 4.6 | 7.2 | 7.9 | |||||
| JCU7 | 11.6 | 6.2 | 8.4 | 7.9 | 8.5 | |||||
| Mean | 12.4 | 6.7 | 6.4 | 7.3 | ||||||
| NDF | JCU2 | 54.2 | 65.6 | 68.8 | 67.1 | 63.9 | 0.92 | 0.88 | 0.01 | <0.01 |
| JCU4 | 53.9 | 67.3 | 72.8 | 64.2 | 64.6 | |||||
| JCU7 | 63.4 | 69.1 | 63.1 | 59.6 | 63.8 | |||||
| Mean | 57.2 | 67.3 | 68.2 | 63.6 | ||||||
| ADF | JCU2 | 40.0 | 52.8 | 54.3 | 50.7 | 49.5 | 0.85 | 0.94 | 0.01 | 0.02 |
| JCU4 | 40.4 | 52.9 | 56.3 | 49.3 | 49.7 | |||||
| JCU7 | 46.7 | 55.5 | 50.3 | 44.8 | 49.3 | |||||
| Mean | 42.4 | 53.7 | 53.6 | 48.3 | ||||||
| Variable 1 | JCU2 | JCU4 | JCU7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 78 | 122 | 168 | Mean | 78 | 122 | 168 | Mean | 78 | 122 | 168 | Mean | |
| DM | 26.1 | 29.4 | 33.2 | 29.6 | 27.4 | 26.6 | 34.3 | 29.4 | 28.3 | 28.1 | 30.3 | 28.9 |
| CP | 25.2 | 20.4 | 16.9 | 20.8 | 23.0 | 26.4 | 17.3 | 22.2 | 23.8 | 22.0 | 20.8 | 22.2 |
| NDF | 25.4 | 34.2 | 38.7 | 32.8 | 25.6 | 27.4 | 47.9 | 33.6 | 29.3 | 31.2 | 35.2 | 31.9 |
| ADF | 14.0 | 19.3 | 24.5 | 19.3 | 14.0 | 15.9 | 32.4 | 20.8 | 13.0 | 18.2 | 21.2 | 17.5 |
| Variable 1 | Cultivar 2 | Maturity at Harvest | p-Value 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 78 | 122 | 168 | Mean | SEM 3 | C | M | C*M | ||
| A | JCU2 | 0.30 | 0.27 | 0.26 | 0.28 b | 0.00 | <0.01 | 0.01 | 0.22 |
| JCU4 | 0.28 | 0.30 | 0.26 | 0.28 b | |||||
| JCU7 | 0.25 | 0.26 | 0.24 | 0.25 a | |||||
| Mean | 0.28 | 0.28 | 0.25 | ||||||
| B | JCU2 | 0.53 | 0.48 | 0.42 | 0.48 b | 0.01 | <0.01 | <0.01 | 0.51 |
| JCU4 | 0.49 | 0.54 | 0.31 | 0.45 a | |||||
| JCU7 | 0.59 | 0.51 | 0.52 | 0.54 c | |||||
| Mean | 0.54 | 0.51 | 0.42 | ||||||
| A + B | JCU2 | 0.84 | 0.75 | 0.68 | 0.76 | 0.01 | 0.30 | <0.01 | 0.66 |
| JCU4 | 0.78 | 0.84 | 0.58 | 0.73 | |||||
| JCU7 | 0.85 | 0.78 | 0.76 | 0.79 | |||||
| Mean | 0.82 | 0.79 | 0.67 | ||||||
| C (/h) | JCU2 | 0.13 | 0.11 | 0.08 | 0.11 ab | 0.01 | <0.01 | 0.03 | 0.48 |
| JCU4 | 0.17 | 0.15 | 0.07 | 0.13 b | |||||
| JCU7 | 0.06 | 0.07 | 0.04 | 0.06 a | |||||
| Mean | 0.12 | 0.11 | 0.06 | ||||||
| Lag (h) | JCU2 | 2.1 | 2.7 | 2.6 | 2.5 a | 0.31 | <0.01 | 0.04 | 0.17 |
| JCU4 | 3.0 | 3.1 | 3.0 | 3.0 a | |||||
| JCU7 | 4.0 | 5.7 | 6.1 | 5.3 b | |||||
| Mean | 3.0 | 3.8 | 3.9 | ||||||
| ED2 | JCU2 | 0.77 | 0.68 | 0.59 | 0.68 | 0.01 | 0.25 | <0.01 | 0.55 |
| JCU4 | 0.72 | 0.77 | 0.51 | 0.67 | |||||
| JCU7 | 0.71 | 0.66 | 0.59 | 0.65 | |||||
| Mean | 0.73 | 0.70 | 0.57 | ||||||
| ED6 | JCU2 | 0.67 | 0.58 | 0.49 | 0.58 ab | 0.02 | <0.01 | <0.01 | 0.53 |
| JCU4 | 0.65 | 0.67 | 0.44 | 0.59 b | |||||
| JCU7 | 0.56 | 0.54 | 0.46 | 0.52 a | |||||
| Mean | 0.63 | 0.60 | 0.46 | ||||||
| Variable 1 | Cultivar 2 | Maturity at Harvest | p-Value 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 78 | 122 | 168 | Mean | SEM 3 | C | M | C*M | ||
| A | JCU2 | 0.16 | 0.20 | 0.23 | 0.20 ab | 0.01 | 0.01 | <0.01 | <0.01 |
| JCU4 | 0.13 | 0.22 | 0.32 | 0.23 b | |||||
| JCU7 | 0.15 | 0.17 | 0.19 | 0.17 a | |||||
| Mean | 0.15 | 0.20 | 0.25 | ||||||
| B | JCU2 | 0.72 | 0.63 | 0.57 | 0.64 ab | 0.01 | 0.01 | <0.01 | 0.11 |
| JCU4 | 0.66 | 0.70 | 0.44 | 0.60 a | |||||
| JCU7 | 0.72 | 0.71 | 0.65 | 0.70 b | |||||
| Mean | 0.70 | 0.68 | 0.55 | ||||||
| A + B | JCU2 | 0.89 | 0.83 | 0.81 | 0.84 | 0.01 | 0.20 | 0.04 | <0.01 |
| JCU4 | 0.80 | 0.93 | 0.76 | 0.83 | |||||
| JCU7 | 0.88 | 0.89 | 0.85 | 0.87 | |||||
| Mean | 0.86 | 0.88 | 0.81 | ||||||
| C (/h) | JCU2 | 0.09 | 0.08 | 0.08 | 0.08 b | 0.00 | <0.01 | <0.01 | 0.47 |
| JCU4 | 0.10 | 0.09 | 0.06 | 0.08 b | |||||
| JCU7 | 0.05 | 0.03 | 0.03 | 0.04 a | |||||
| Mean | 0.08 | 0.07 | 0.06 | ||||||
| ED2 | JCU2 | 0.76 | 0.71 | 0.70 | 0.72 b | 0.01 | <0.01 | 0.01 | 0.71 |
| JCU4 | 0.69 | 0.80 | 0.66 | 0.72 b | |||||
| JCU7 | 0.67 | 0.64 | 0.60 | 0.64 a | |||||
| Mean | 0.71 | 0.72 | 0.65 | ||||||
| ED6 | JCU2 | 0.60 | 0.57 | 0.57 | 0.58 b | 0.01 | <0.01 | 0.16 | 0.43 |
| JCU4 | 0.55 | 0.65 | 0.56 | 0.59 b | |||||
| JCU7 | 0.48 | 0.45 | 0.42 | 0.45 a | |||||
| Mean | 0.55 | 0.56 | 0.52 | ||||||
| Variable 1 | Cultivar 2 | Maturity at Harvest | p-Value 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 78 | 122 | 168 | Mean | SEM 3 | C | M | C*M | ||
| NDF | |||||||||
| B | JCU2 | 0.77 | 0.62 | 0.43 | 0.61 a | 0.03 | <0.01 | <0.01 | 0.12 |
| JCU4 | 0.71 | 0.71 | 0.31 | 0.58 a | |||||
| JCU7 | 0.96 | 0.82 | 0.88 | 0.89 b | |||||
| Mean | 0.81 | 0.71 | 0.54 | ||||||
| C (/h) | JCU2 | 0.05 | 0.04 | 0.03 | 0.04 b | 0.00 | <0.01 | <0.01 | 0.99 |
| JCU4 | 0.05 | 0.05 | 0.03 | 0.04 b | |||||
| JCU7 | 0.03 | 0.02 | 0.01 | 0.02 a | |||||
| Mean | 0.04 | 0.04 | 0.02 | ||||||
| ED2 | JCU2 | 0.56 | 0.43 | 0.27 | 0.42 | 0.02 | 0.70 | <0.01 | 0.75 |
| JCU4 | 0.50 | 0.51 | 0.19 | 0.40 | |||||
| JCU7 | 0.57 | 0.41 | 0.39 | 0.46 | |||||
| Mean | 0.55 | 0.45 | 0.28 | ||||||
| ED6 | JCU2 | 0.37 | 0.27 | 0.16 | 0.27 | 0.01 | 0.66 | <0.01 | 0.77 |
| JCU4 | 0.33 | 0.33 | 0.10 | 0.25 | |||||
| JCU7 | 0.32 | 0.20 | 0.18 | 0.23 | |||||
| Mean | 0.34 | 0.27 | 0.15 | ||||||
| ADF | |||||||||
| B | JCU2 | 0.71 | 0.60 | 0.38 | 0.56 | 0.03 | 0.09 | <0.01 | 0.14 |
| JCU4 | 0.64 | 0.69 | 0.32 | 0.55 | |||||
| JCU7 | 0.77 | 0.65 | 0.71 | 0.71 | |||||
| Mean | 0.71 | 0.64 | 0.47 | ||||||
| C (/h) | JCU2 | 0.04 | 0.02 | 0.02 | 0.03 b | 0.00 | 0.02 | <0.01 | 0.47 |
| JCU4 | 0.03 | 0.03 | 0.02 | 0.03 b | |||||
| JCU7 | 0.03 | 0.02 | 0.01 | 0.02 a | |||||
| Mean | 0.03 | 0.02 | 0.02 | ||||||
| ED2 | JCU2 | 0.49 | 0.31 | 0.21 | 0.34 | 0.02 | 0.77 | <0.01 | 0.29 |
| JCU4 | 0.39 | 0.42 | 0.19 | 0.33 | |||||
| JCU7 | 0.46 | 0.34 | 0.31 | 0.37 | |||||
| Mean | 0.45 | 0.36 | 0.24 | ||||||
| ED6 | JCU2 | 0.30 | 0.16 | 0.11 | 0.19 | 0.01 | 0.55 | <0.01 | 0.28 |
| JCU4 | 0.22 | 0.24 | 0.10 | 0.18 | |||||
| JCU7 | 0.26 | 0.17 | 0.15 | 0.19 | |||||
| Mean | 0.26 | 0.19 | 0.12 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwangi, F.W.; Charmley, E.; Adegboye, O.A.; Gardiner, C.P.; Malau-Aduli, B.S.; Kinobe, R.T.; Malau-Aduli, A.E.O. Chemical Composition and In Situ Degradability of Desmanthus spp. Forage Harvested at Different Maturity Stages. Fermentation 2022, 8, 377. https://doi.org/10.3390/fermentation8080377
Mwangi FW, Charmley E, Adegboye OA, Gardiner CP, Malau-Aduli BS, Kinobe RT, Malau-Aduli AEO. Chemical Composition and In Situ Degradability of Desmanthus spp. Forage Harvested at Different Maturity Stages. Fermentation. 2022; 8(8):377. https://doi.org/10.3390/fermentation8080377
Chicago/Turabian StyleMwangi, Felista W., Edward Charmley, Oyelola A. Adegboye, Christopher P. Gardiner, Bunmi S. Malau-Aduli, Robert T. Kinobe, and Aduli E. O. Malau-Aduli. 2022. "Chemical Composition and In Situ Degradability of Desmanthus spp. Forage Harvested at Different Maturity Stages" Fermentation 8, no. 8: 377. https://doi.org/10.3390/fermentation8080377
APA StyleMwangi, F. W., Charmley, E., Adegboye, O. A., Gardiner, C. P., Malau-Aduli, B. S., Kinobe, R. T., & Malau-Aduli, A. E. O. (2022). Chemical Composition and In Situ Degradability of Desmanthus spp. Forage Harvested at Different Maturity Stages. Fermentation, 8(8), 377. https://doi.org/10.3390/fermentation8080377







