Abstract
Utilising ‘wastes’ as ‘resources’ is key to a circular economy. While there are multiple routes to waste valorisation, anaerobic digestion (AD)—a biochemical means to breakdown organic wastes in the absence of oxygen—is favoured due to its capacity to handle a variety of feedstocks. Traditional AD focuses on the production of biogas and fertiliser as products; however, such low-value products combined with longer residence times and slow kinetics have paved the way to explore alternative product platforms. The intermediate steps in conventional AD—acidogenesis and acetogenesis—have the capability to produce biohydrogen and volatile fatty acids (VFA) which are gaining increased attention due to the higher energy density (than biogas) and higher market value, respectively. This review hence focusses specifically on the production of biohydrogen and VFAs from organic wastes. With the revived interest in these products, a critical analysis of recent literature is needed to establish the current status. Therefore, intensification strategies in this area involving three main streams: substrate pre-treatment, digestion parameters and product recovery are discussed in detail based on literature reported in the last decade. The techno-economic aspects and future pointers are clearly highlighted to drive research forward in relevant areas.
1. Introduction
There is a need to address the ever-increasing energy and materials demand sustainably. Simultaneously, growing anthropogenic activities have led to an increase in global CO2 levels, and there is, therefore, a pressing need to reduce emissions to control the global warming potential. Cumulative global CO2 emissions have risen by ~64% over the past three decades [1]. The major contributors (>60%) to global emissions have been the electricity, heat and transportation sectors. While this has been the global trend, national emissions vary significantly between countries due to the difference in implementation of environmental policies, population density, per capita income and per capita emissions. For instance, in the UK, the cumulative CO2 emissions have fallen by ~38% in the past three decades [1]. In particular, the electricity and heat sectors have recently managed to curb their CO2 emissions significantly. With the implementation of the UK Net Zero strategy to achieve zero CO2 emission targets by 2050, the cumulative emissions are expected to decrease more rapidly in the coming years. However, to achieve such stringent targets, it is important that emissions in all sectors are mitigated appropriately. For instance, the major contributor to emissions in the UK currently is the transportation sector (~35% of national emissions) (Figure 1). To address this issue directly, the use of sustainable and cleaner fuels is required in the transportation sector. This includes the use of both gaseous and liquid biofuels, such as biogas, biohydrogen and bioethanol. In addition to biofuels, electric vehicles also have a significant role to play in reducing emissions. The source of electricity will however be influential in determining the emission potential.

Figure 1.
Global and UK CO2 emissions by sector in 2019. Data obtained from International Energy Agency, Data and Statistics website [1].
Beyond the use of sustainable renewable energy as a means to mitigate CO2 emissions, the sustainable production of chemicals and materials of high value is also necessary. Currently, the production of platform and commodity chemicals is highly reliant on fossil fuels, and whilst being an economically favourable route, it does not achieve the triple bottom line performance of being socio-economically and environmentally beneficial when produced from these materials. The production of these chemicals from biomass however offers extensive prospects where both renewable energy and high-value platform chemicals may be produced either simultaneously or sequentially in ‘biorefineries’. The production of multiple products from a feedstock would also lead to approaching a circular bioeconomy which is critical to achieving the Net Zero targets.
1.1. ‘Waste’ to ‘Value’ for Approaching a Circular Economy
The backbone of a circular economy is “to generate, utilise and recycle”, ensuring that wastes generated do not exit the loop. In this context, the utilisation of ‘wastes’ as ‘resources’ is critical to minimise reverting back to a linear economy framework. The utilisation of waste biomass is of particular interest to this perspective. All ‘waste’ biomasses are second-generation feedstocks, which neither interfere with the food chain nor compete for space with agricultural land. Examples of such ‘waste’ biomasses include agri and forest residues, food waste, paper and pulp industry wastes, distillery waste, wastewater and sludge. All these organic-matter-rich streams are originally ‘waste streams’ that have the potential to be valorised to biofuels and high-value chemicals. Utilising these ‘wastes’ as ‘resources’ would ensure that the feedstock-dependent end product pricing is reduced while ensuring sustainability and process circularity. It is however crucial to ensure that the yield of desired product per unit mass of the waste is sufficiently high to minimise net emissions.
Multiple routes to biomass valorisation are currently available (Figure 2). Typically, biomass streams can be valorised either via biochemical pathways or thermochemical pathways [2]. Biochemical pathways include anaerobic digestion (AD) to produce biogas, biohydrogen, volatile fatty acids (VFAs) and fertilisers, fermentation for the production of solvents and biofuels (e.g., bioethanol, acetone, butanol), and value-added chemicals (e.g., succinic acid, citric acid, lactic acid). Thermochemical valorisation routes mentioned in Figure 2 are typically used to produce bio-oil, bio-coal, biochar and syngas. Physico-chemical valorisation routes have also gained attention recently [3,4,5,6,7]. These routes often utilise biomass and its derivates as sacrificial electron donors for the production of renewable hydrogen or oxidised products such as sugars and short chain acids [4,8].
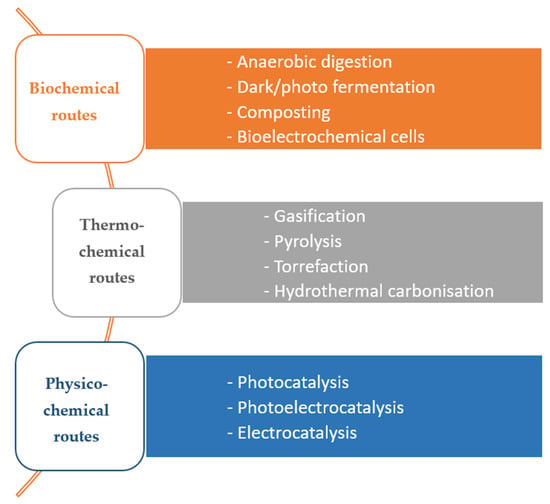
Figure 2.
Biomass valorisation routes.
AD is a well-established technology widely used in the secondary stage of wastewater treatment. Its popularity in this process is due to its ability to remediate waste streams whilst generating energy vectors in the form of biogas [9]. While the technology is mature, limitations such as long residence times (>4 weeks), leading to large reactor volumes in the order of thousands of m3, slower digestion kinetics, and sub-optimal carbon conversion leads to process inefficiencies and high capital expenditure [2]. In terms of revenue generation, biomethane (upgraded from biogas) is a low-value product (~EUR 0.5/kg [10]), and hence, allied products such as concentrated fertilisers from digestate are required to generate additional revenue [11]. Intensification strategies such as optimising operating parameters, and biomass pre-treatment to address feedstock complexity, have been proposed as effective routes to overcome these inefficiencies and maximise biomass conversion [2] and hence remain as the future perspectives for progressing the field. Thermochemical biomass conversion routes have similarly been extensively discussed in the literature [12,13,14] and the future direction for this route remains clear in maximising the techno-economics and understanding the life cycle impacts of the process. While AD and thermochemical routes are already commercially exploited, physico-chemical routes are on the lower end of the technology readiness level (TRL) spectrum (<TRL 3). This is primarily due to the heterogeneity of the biomass, with both the catalyst and biomass being solids suspended in a liquid phase, leading to mass transfer limitations [15], non-specificity of biomass breakdown [4,8] and lower biomass conversion rates [6]. While current literature has extensive information on the conversion of biomass derivatives to value-added compounds [16,17,18], information on direct conversion is limited. Biomass derivatives and ‘whole’ biomass are different in nature due to their structure, physico-chemical bonding, solubility and reactivity; therefore, physico-chemical valorisation routes investigating the valorisation of biomass derivatives and whole biomass are not comparable. The research direction in this area is therefore clear in identifying novel routes for direct valorisation leading to higher TRL applications.
Most of the aforementioned biomass valorisation routes have clear pathways and future directions. There has been renewed focus on biohydrogen and VFA production from AD, as opposed to biomethane and fertiliser, and so, a critical analysis of this recent work is required. In addition, when biohydrogen or VFAs are produced via AD, the fertiliser potential of the digestate is not compromised and can still yield additional revenue. Increased recent interest in hydrogen is mainly due to its potential to decarbonise a variety of sectors that are generally hard to ‘electrify’ and its capability in the accelerated achievement of Net Zero goals. VFAs, on the other hand, are platform chemicals which find their use in a variety of industries, including food and beverages, cosmetics, chemicals and pharmaceutical industries [19]. The current fossil-based route for VFA synthesis is unsustainable, and therefore, a waste valorisation technology for the production of VFAs is ideal to decarbonise a number of these end-use sectors.
The UK recently devised a ten-point plan for a green industrial revolution to achieve net neutrality by 2050 [20] and to meet the Carbon Budget Six (CB6) targets. One of the key aspects of the plan is to enhance the production of low carbon hydrogen. It is expected that the low carbon hydrogen capacity of the UK will reach 1 GW in 2025 and 5 GW by 2030, leading to savings of ~41 MtCO2e (equivalent to ~9% of UK emissions in 2018). With the projected enhanced capacity, it is expected that 20–35% of the total energy consumption in the UK will be based on low-carbon hydrogen by 2050 [21].
To align with the Net Zero targets, the UK was the first country to develop an industrial decarbonisation strategy and aimed to reduce industrial carbon emissions by over 90% of 2018 levels [22]. Moreover, resource efficiency has also been a focus, as per the 25-year environment plan to mitigate the amount of waste generated [23]. Therefore, it is vital that a circular economy model incorporating intersectoral integration is established. Since most wastes are organic in nature (with cellulose in biomass being the world’s most abundant organic material), biochemical valorisation routes are promising options to minimise waste and maximise value via such intersectoral integration approaches with a possibility of developing a multi-product biorefinery platform. An example is the valorisation of waste biomass to biohydrogen and VFAs which are discussed in this review.
1.2. Green Hydrogen and VFAs—Need for Process Intensification
The majority of global hydrogen is produced from steam methane reforming (SMR), termed grey hydrogen, or from coal gasification, known as brown hydrogen, due to the low cost of production and high efficiency. SMR, however, has a high carbon footprint of 9–12 kg CO2/kg H2 [24,25,26] and requires a consistent supply of methane that is often derived from fossil fuels. Alternatively, SMR using biomethane (derived from conventional AD) is being pursued as a renewable and much cleaner option [27]. Biomethane-based SMR can fulfil the hydrogen generation needs intermittently until a cost-competitive complementary technology to electrolysis is established. The transition from grey and brown hydrogen is of utmost importance to achieve net neutrality and therefore, blue hydrogen (with carbon capture) and green hydrogen (renewables based) are gaining more interest. Grey or brown hydrogen coupled with carbon capture and storage (CCS) results in the production of blue hydrogen which has the potential to reduce carbon emissions by 35–85% [28]. The issues surrounding blue hydrogen however are the intensive capital requirement and potential CO2 leakage [29]. Carbon capture storage and utilisation (CCSU) has therefore been proposed as an alternative strategy for blue hydrogen production [29]. In this case, the captured CO2 may be used as a secondary feedstock for the production of high-value compounds (e.g., gas fermentation, microbial electrosynthesis) [30].
Green hydrogen, analogous to low carbon hydrogen, can be produced from water electrolysis or biomass electrolysis powered by renewable electricity. It has a near-zero carbon footprint (<0.6 kg CO2/kg H2) [31] and can greatly boost the acceleration towards achieving net neutrality. With water electrolysis largely favoured due to its higher TRL levels, the problems at scale depend on the consistent supply of renewable electricity as well as the use of critical raw materials as catalysts. Global green hydrogen trends (based on electrolysis) are currently shifting focus towards the use of non-critical, earth-abundant raw materials to de-stress the supply chain.
Other promising routes to produce green hydrogen include dark fermentation (via AD) and photo fermentation of biomass. Photo fermentation is an attractive pathway; however, it suffers from low hydrogen production rates and stringent reactor design to maximise light distribution within the bioreactors [32]. Photo fermentation is especially limited when wastewater or lignocellulosic biomass is used as a feedstock due to the light scattering, shielding and loss of photons caused by the selective absorption of light by coloured wastewater, thereby limiting the light harvesting efficiencies and metabolic rates. Dark fermentation can overcome these challenges. The biochemical pathway leading to the production of hydrogen in dark fermentation is an intermediate step (acidogenesis) in the conventional AD pathway. While the product of interest in conventional AD is biomethane, the methanogenic activity needs to be suppressed to ensure that biohydrogen is derived as the end product in acidogenic fermentation. Assuming C6H12O6 (hexose) as the model molecular formula of the biomass and accounting for fractional biomass utilisation for its growth and energetic needs, ~0.1 kg H2/kg biomass could be produced stoichiometrically. This corresponds to ~12 MJ energy recovered from 1 kg of biomass. While the green hydrogen productivity, especially via the AD route, is attractive, it is currently not cost competitive compared to grey hydrogen. One of the primary reasons is that hydrogen production in AD is affected by simultaneous VFA production. While both VFAs and hydrogen can be produced via methanogenesis suppressed AD, it is only possible to produce either VFAs or biohydrogen with higher yields at a given point of time in the digester. This is mainly because the yields of VFAs and hydrogen are interlinked, and often, their concentrations are inversely proportional to each other. The acidogenic and acetogenic stages of AD lead to the production of VFAs. In addition, homoacetogens present in the microbial consortia can further utilise hydrogen for acetic acid production. The VFA product mixture in AD typically includes acetic acid, propionic acid, butyric acid and valeric acid in varying proportions. Therefore, understanding the digestion kinetics and optimising the system for the production of desired products is important when acidogenic fermentation is the focus.
There are similarities between VFA and biohydrogen production in terms of biochemical pathways utilising waste organic matter as feedstock, mode of operation and scale-up. Furthermore, the prospect of retrofitting existing infrastructure to support hydrogen storage and transport as well as high VFA productivities are major advantages of the acidogenic fermentation route for biomass valorisation. These can directly address the challenges such as ‘technological uncertainty’ and ‘enabling infrastructure’ [21]; however, challenges around ‘affordable costs’ still exist. This is predominantly due to the productivity that is linked to two aspects, namely feedstock heterogeneity (complexity due to recalcitrant inter and intramolecular bonding) and product selectivity (type of VFA or choice between biohydrogen, VFA and biomethane). These are the two main components that influence the carbon footprint as well as the techno-economics of the process.
This review therefore aims to bring together the intensification strategies that can predominantly enhance the productivities of desired products, namely biohydrogen and VFAs. While a number of challenges exist in improving the product yields, the scope of this review is restricted to three main areas that specifically influence the process scale up, namely:
- Pre-digestion—strategies to address feedstock heterogeneity and improve the bioavailability of the biomass;
- Anaerobic digestion—strategies to improve the bioconversion of biomass to desired products;
- Product recovery—strategies to maximise recovery and purity of desired products.
The influence of these strategies on the techno-economics and the life cycle of the process are also pointed out. Finally, the future research focus in this particular area of AD is also discussed from our point of view.
2. Pre-Digestion
Pre-digestion in this context refers to the steps involved in preparing the feedstock for acidogenic fermentation. Predominantly, with the feedstock type and composition being the detrimental factors influencing the productivity of the desired product, it is important to ensure that the organic matter in the feedstock is highly bioavailable to the microbial consortia for digestion. In conventional AD, the rate limiting steps could either be the hydrolysis step or the methanogenic stage [33]. The former is linked to feedstock complexity, whereas the latter is linked to the growth rate of the methanogenic archaea. Therefore, once the substrate is readily bioavailable, the biochemical pathways will be initiated toward the desired product formation. It is therefore critical to ensure that the feedstock complexity is addressed to speed up the hydrolysis stage. Whether the desired product is biohydrogen or VFAs, the hydrolysis stage is the common precursory step. The branching out of biochemical pathways happens after the hydrolysis stage, so the discussion around addressing feedstock complexity in this section is common to both these fermentative pathways.
Overlapping with conventional AD, acidogenic fermentation can utilise any organic feedstock (e.g., wastewater, sewage sludge, agri-forest residue, food waste). The availability of the feedstock (quantity availability and frequency of feedstock production) and its composition are detrimental factors that can impact the digestion process. Irrespective of these factors, it is first important to assess the biochemical hydrogen potential (BHP) and the VFA potential (VFAP) of each feedstock separately. While BHP can be analogous to conventional biochemical methanation potential (BMP) tests, VFAP has never been performed before. Therefore, there is immense scope to develop standardised tests for BHP and VFAP via AD. This needs to be performed stoichiometrically first to determine the theoretical potential of the feedstock (similar to the Buswell–Muller methane yields for conventional AD). While the theoretical limits are indicators of the digestion ability of the feedstock, these can never be experimentally achieved due to the utilisation of a fraction of the feedstock for microbial growth and metabolism as well as the recalcitrance posed by a fraction of the feedstock (e.g., lignin). It is however paramount that the maximum achievable yield of the desired product is targeted by intensifying the digestion process. If the feedstock has high soft suspended solid content, such as sewage sludge or food waste, improving the degree of disintegration leading to an increased soluble COD concentration is required to have a positive impact on the digestion process. If the feedstock is lignocellulosic in nature such as agricultural or forest residues, improving the bioavailability of holocellulose (and/or delignification) to enhance microbial hydrolysis is important. As lignin provides structural integrity to the biomass, delignification or at least the exposure of the holocellulose to hydrolytic bacteria is critical. With either of the feedstock categories, inhibitor formation as a result of pre-treatment should be suppressed to prevent negatively influencing anaerobic digestion. In addition, the preferred pre-treatment should also be net positive (energetically and economically) and scalable if high TRL hydrogen and VFA production are targeted.
A number of researchers have investigated a wide range of pre-treatment methods for acidogenic fermentation, falling broadly under four categories: physical, chemical, biological and physico-chemical [34,35,36]. The mode of action of each pre-treatment on lignocellulosic biomass is shown in Figure 3. The most utilised methods in the past decade are however exclusively discussed in this review to restrict the scope purposefully to recent work (Table 1 and Table 2).
Physical pre-treatment methods often comprise of techniques such as shredding, comminution or homogenisation, which target the reduction in particle size and increase in specific surface area. It has also been shown to affect the crystallinity index of the cellulose upon milling [37]. The reduced particle size of the feedstock favours faster hydrolysis rates and, in turn, higher desired product yields. While physical pre-treatment is known to generate no microbial inhibitors, it is often limited by its high specific energy consumption (thereby high OPEX). The specific energy consumption is directly related to the comminution ratio (i.e., the ratio of final particle size to the initial particle size) and the moisture content of the feedstock. The milling energy is reduced considerably when the moisture content in the biomass is reduced. To achieve this, an additional drying step (consuming more energy) is required. For instance, Miao et al. [38] compared the milling of a variety of lignocellulosic biomass by hammer mills and knife mills. To achieve the same final particle size of 1 mm with Miscanthus, the hammer mill required ~200 kJ/kg TS which is ~3.5 fold lower than a knife mill. Similarly, the dried biomass consumed nearly 50% less energy (~950 kJ/kg TS) as compared to biomass with 15% moisture. When valorisation via anaerobic digestion is desired, the moisture content in the feedstock is required, which helps with the mass transfer and hydrodynamics of the digester. Even with dry digestion, a significant amount of moisture is still retained in the feed slurry (~15–20% TS). Therefore, drying the feedstock prior to milling and then rehydrating the feedstock is neither resource nor energy efficient. Thus, when opting for physical treatment, it is of utmost importance to consider the type of biomass, moisture content and the comminution ratio required.

Figure 3.
An overview of lignocellulosic biomass pre-treatment methods. Reprinted with permission from Konde et al. [39], Copyright Royal Society of Chemistry.
Chemical pre-treatment utilises acid, alkali or oxidising agents to depolymerise, hydrolyse or delignify the biomass [40]. Acid hydrolysis, when employed as a lignocellulosic biomass pre-treatment method often generates fermentation inhibitors such as furans and furfurals, while alkali hydrolysis results in partial delignification, leading to the increase in the concentration of soluble phenolics. Oxidative pre-treatment using O3 or H2O2 are non-specific pre-treatment methods and tend to depolymerise any fraction of the biomass but have the tendency to delignify the biomass predominantly. These compounds may be inhibitory to the metabolism of the microbial consortium and can reduce the desired product yield [33,41]. It has been reported that furans are more inhibitory to acidogenic bacteria than soluble phenolics [33]. The inhibition of the metabolic activity of these classes of bacteria may result in the increased abundance of non-hydrogen/VFA producers and divert the digester towards the production of lactate or ethanol [33]. When the end product of digestion is conventional biogas, these alternative end products may be beneficial; however, when acidogenic fermentation is in focus, care has to be taken to ensure such inhibition is avoided. Additionally, from an engineering perspective, equipment corrosion can also occur and impede the process operation when acids are used in the system. This can indirectly lead to an increased concentration of heavy metals in the solution, thereby further reducing the desired product yields.
Biological pre-treatment often consists of the fungal or enzymatic pre-treatment of feedstock. White rot fungi or brown rot fungi are the commonly deployed species due to their ability to secrete extracellular ligninolytic and cellulolytic enzymes or perform hemicellulose hydrolysis, respectively [41]. Another filamentous fungus, Trichoderma reesei, has also been reported to pre-treat lignocellulosic biomass due to its ability to hydrolyse cellulose [42]. While biological substrate pre-treatment is effective in enhancing product yields, slow kinetics of pre-treatment (ranging from days to weeks) requiring large reactor volumes (incapable of efficient scale up) and its lack of capacity to continuously pre-treat the feedstock are seen as limitations. Alternatively, enzymatic hydrolysis of the feedstock has been proposed as a targeted biological pre-treatment strategy, but the cost of enzyme production/recovery still has to be considered prior to scale up.
The final category of pre-treatment consists of physico-chemical methods. In this kind of pre-treatment, the biomass is pre-treated to achieve the combined effect of both the physical as well as chemical methods, i.e., particle size reduction or increase in the surface area along with partial hydrolysis of polymers. Physico-chemical methods overcome the disadvantages posed by physical or chemical pre-treatment methods; they consume considerably less energy as compared to physical methods and generate insignificant quantities of inhibitors. For instance, steam explosion works on the basis of applying compressed steam to the biomass slurry followed by rapid depressurisation and consumes ~70% less energy than physical pre-treatment methods [43]. To favour enhanced product yield by avoiding inhibitors, liquid hot water pre-treatment has been suggested as an alternative to steam explosion [44]. Another physico-chemical pre-treatment method that is gaining attention in the area of anaerobic digestion is hydrodynamic cavitation [2,45,46]. Cavitation is the phenomenon of generation, growth and implosion of vaporous cavities. Acoustic cavitation, commonly known as ultrasonication, has been reported extensively in the literature for the pre-treatment of biomass; however, due to the handling volumes being limited to mL scale and high specific energy inputs (at times, higher than physical pre-treatment), they cannot be scaled up. Hydrodynamic cavitation, on the other hand, is less energy intensive and has been reported to be scaled up [45,47] with low specific energy inputs and high net energy gains. For instance, Nagarajan and Ranade [48] reported that the specific energy required to pre-treat sugarcane bagasse (at a low solid loading of 1%) was 0.5 MJ/kg TS; however, the net energy gain as a result of enhanced biomethane generation was reported to be ~1.4 MJ/kg TS. Overtreatment can however result in the generation of inhibitors, higher energy consumption and reduced desired product yield. Therefore, it is important to optimise the process to maximise the product yields and energy efficiency. The use of physico-chemical pre-treatment seems to be a promising method; however, with limited literature in the area of acidogenic fermentation, it needs more research. While a general overview of pre-treatment was presented so far, the following sections will discuss specific examples from literature in the past decade that has reported an impact on intensifying acidogenic fermentation.
2.1. Substrate Pre-Treatment to Enhance Biohydrogen Production
Table 1 shows an overview of reported data on various pre-treatment methods used to enhance biohydrogen yield. Physical pre-treatment is the most common conventional pre-treatment to enhance digestion yields. In the last decade, however, the use of milling-based methods to intensify biohydrogen production has dwindled. It is understandable that this decrease could have been due to the inability of these methods to achieve a net positive energy gain. One of the few papers that reported the use of physical pre-treatment was by Yukesh Kannah et al. [49], who used chopped rice straw as the feedstock and high-speed dispersion as the pre-treatment methodology. A 2 L batch pre-treatment was performed with a 2% straw concentration. The gap between the rotor and stator was 0.3 mm, which enabled the effective disintegration of straw at an optimum speed of 12,000 rpm. At a specific energy consumption of ~1.47 MJ/kg TS, a degree of disintegration of ~9.5% was observed. Upon mesophilic batch digestion, this corresponded to a 7.3-fold increase in hydrogen yield as compared to the untreated straw that generated 8 mL H2/g COD. While an increase in hydrogen yield was observed, a net positive energy gain could not be achieved with this kind of pre-treatment.
Deng et al. [50] reported the use of 2% sulphuric acid pre-treatment on grass silage (2%) at an elevated optimum temperature of 135 °C for 15 min. At these conditions, a hydrolysis efficiency of ~50% was observed and resulted in a three-fold increase in hydrogen yield. Amongst the reducing sugars formed due to acid hydrolysis, xylose dominated the hydrolysate with ~70% concentration, suggesting that hemicellulose hydrolysis was predominantly achieved with acid pre-treatment leaving behind a cellulolignin solid residue. Reilly et al. [51], on the other hand, utilised alkali pre-treatment of wheat straw as a strategy to enhance hydrogen production. The substrate was soaked in 80 mM lime for 2 days, and it was determined that ~36% of the hemicellulose and minimal lignin were solubilised, whilst cellulose remained unaffected. The pre-treated solid residue upon neutralisation was subjected to digestion with the bioreactors supplemented with an Accelerase enzyme cocktail. The optimal conditions resulted in a biohydrogen yield, which was ~29-fold higher (59 mL H2/g VS) than the untreated straw. The hydrolysate, when added to the solid residue for digestion, resulted in the inhibition of hydrogen production due to the presence of enhanced concentrations of CaCO3. Instead of lime, a stronger alkali, NaOH was used to pre-treat milled corn cobs by Kucharska et al. [52]. They also supplemented the pre-treated slurry with an enzymatic cocktail to intensify the hydrolysis process and enhance the biohydrogen yields by >5 fold. Unlike acid and alkali pre-treatment, oxidative degradation pre-treatment has also been reported to enhance biohydrogen production. For example, Wu et al. [53] reported the use of ozone pre-treatment to intensify biohydrogen production from milled wheat straw. Since oxidative pre-treatments are non-specific in nature, they tend to degrade polymers in their vicinity. With lignin present in the cell wall of the biomass structure, it tends to be degraded first via oxidative pre-treatments. Accordingly, at optimal conditions, nearly 40% delignification was reported, corresponding to a 2.5-fold increase in biohydrogen yield.
Biological pre-treatment in its current state, while being efficient, is not scalable. With conventional enzymatic hydrolysis systems, this is related to the enzyme recovery costs. With the cost of enzyme production becoming relatively cheaper, there is still potential to explore this area. For instance, Leaño and Babel [54] reported the enzymatic hydrolysis of cassava wastewater using various commercially available enzymes. They used OPTIMASH BG©, which is commonly used in the bioethanol industry and α-amylase in separate experiments to determine the effect on biohydrogen production. In addition to an increased yield of biohydrogen (~50%), a reduced lag time in hydrogen production was also observed, suggesting that the complexity of the wastewater was reduced during enzymatic hydrolysis. To improve the effectiveness of the biological pre-treatment systems, novel and innovative strategies have also been reported. For example, Chandrasekhar and Venkata Mohan [55] reported the use of bioelectrochemical hydrolysis as an unconventional means of biological pre-treatment to intensify biohydrogen production. With a 10 h HRT and graphite electrodes (without an external voltage supply), they pre-treated blended food waste. The overflow from the bioelectrochemical cell was used as the feedstock for the fed-batch mesophilic digester that operated with an HRT of 72 h. At optimum conditions, ~35% increase in biohydrogen yields was observed. The fed-batch operation of pre-treatment is a promising strategy and can pave the way for a step-wise, modular scale up; however, intensive research is required to achieve this.
Physico-chemical pre-treatment is a promising strategy to enhance biohydrogen yields due to its ability to process wet feedstock and scalability. For instance, hot compressed water was used to pre-treat sake brewery waste (sake lees). Compressed hot water at a high temperature of 130 °C and pressure of 3 bars was used to treat 10% biomass at a holding time of 1 h [56]. A reduction in lag time for biohydrogen generation was reported as a result of pre-treatment. Asadi and Zilouei [57] reported the use of an organosolv pre-treatment of rice straw to enhance biohydrogen production. In their case, the biomass was blended with an ethanol–water mix (45% v/v). One percent sulphuric acid was used to catalyse the hydrolysis process at an optimal temperature of 180 °C at a 30 min holding time. Upon treatment, a sequential enzymatic hydrolysis step was also carried out using 5% Cellic CTec2 to further increase the reducing sugar yield. At these conditions, the glucose concentration was enhanced by >4-fold and positively influenced the biohydrogen yield. Other researchers have also reported such complex and sequential pre-treatment processes [58,59] to improve hydrogen yield. While such processes may be beneficial in enhancing biohydrogen yields, the need to use a complex process requires justification both economically and environmentally. Alternatively, other researchers have reported simpler processes involving heat/irradiation and mild acid to achieve similar, if not higher, biohydrogen yields [60,61]. Cavitation, mainly sonication, is another physico-chemical method that has been reported to enhance biohydrogen yields. For instance, Hu et al. [62] reported the use of sonication followed by alkali treatment of antibiotic fermentation residue to enhance biohydrogen yields by 79%. Enhanced soluble carbohydrate release, resulting in reduced lag time, was attributed to the increase in biohydrogen yields. A similar increase in biohydrogen was also reported by Gadhe et al. [63], who sonicated food waste at an optimum, but high specific energy input of 13.5 MJ/kg TS.

Table 1.
Pre-treatment methods reported to enhance biohydrogen production.
Table 1.
Pre-treatment methods reported to enhance biohydrogen production.
| Feedstock | Pre-Treatment Conditions | Digestion Conditions | Influence on H2 Yield | Reference |
|---|---|---|---|---|
| Physical Pre-treatment | ||||
| Chopped dried rice straw | 20 g/L straw, 2 L, high-speed disperser at 12,000 rpm, 30 min, 0.3 mm gap between rotor and stator | 1 L batch, 25% inoculum, 70% straw slurry, 37 °C, 100 rpm, 10 days | 7.3-fold increase in specific H2 yield | [49] |
| Chemical Pre-treatment | ||||
| Air-dried and milled corn cobs | 5 g biomass, 0.1 L pH 11.5 NaOH, 25 °C, 6 h, followed by enzymatic hydrolysis with Viscozyme L and glucosidase (0.001 L/g biomass), 42 °C, 24 h | 1 L batch, 10% v/v inoculum, pH 7, 37 °C, 320 rpm, 116 h | >5-fold increase in H2 yield | [52] |
| Grass silage | 2% silage, 0.1 L, 2% H2SO4, 135 °C, 15 min | 1% silage, 0.2 L batch, 0.02 L inoculum, pH 7, 4 days (1st stage of a 2-stage system) | 3-fold increase in H2 yield | [50] |
| Milled wheat straw | 5 g straw, 40% water, 0.75 bars, 0.63 LPM O3, 45 min | 0.08 L, 2 g TS, pH 6, 1.9% inoculum (v/v), 1 mL hydrolytic enzyme mix, 35 °C, 60 rpm, 8 days | ~2.5-fold increase in cumulative H2 yield | [53] |
| Milled wheat straw | 5 g VS, 62.5 mL 80 mM Ca(OH)2, 20 °C, 2 days | 0.5 L, 8% w/v inoculum, 1 mL Accelerase-1500, pH 6.25, 35 °C, 16 days | ~29-fold increase in specific H2 yield | [51] |
| Biological Pre-treatment | ||||
| Blended food waste | 0.5 L, bioelectrochemical hydrolysis, open to air graphite cathode, graphite anode, 0.075 L inoculum, 20 g COD/L, pH 7, 10 h HRT, 29 °C | 0.25 L fed-batch, 0.075 L inoculum, 10 g/L, pH 6, 72 h HRT, 29 °C | ~35% increase in cumulative H2 yield | [55] |
| Cassava wastewater | 0.2% OPTIMASH BG® enzyme, 0.22 L wastewater, pH 4, 60 °C, 45 rpm | 0.06 L, substrate to inoculum ratio 5 (v/v basis), pH 7, 37 °C, 90 rpm, 10 days | Reduced lag time, 51% increase in specific H2 yield | [54] |
| 0.2% α-amylase enzyme, 0.22 L wastewater, 37 °C, 45 rpm | Reduced lag time, 49% increase in specific H2 yield | |||
| Physico-chemical Pre-treatment | ||||
| Commercial Sake Lees | 10% biomass, 0.1 L, 130 °C, 3 bars, 1 h | 0.11 L batch, 9% biomass, substrate to inoculum ratio of 1:1 v/v, pH 6, 75 rpm, 37 °C, 5 days | Reduction in lag time observed after pre-treatment | [56] |
| Marine macroalgae Ulva reticulate | Acidic H2O2 induced microwave, 0.5 L, 2% biomass, 0.024 g H2O2/g TS, 0.1 N H2SO4, pH 5, 40% microwave power, 10 min, 10.8 MJ/kg TS | 0.15 L batch, 70% substrate, 25% inoculum, pH 5.5, 130 rpm, 37 °C, | 7.7-fold increase in specific H2 yield | [59] |
| Waste-activated sludge | 0.15 L sludge, 0.3 g sodium citrate/g sludge, 1 h, 150 rpm, followed by 121 °C, 30 min | 0.2 L batch, substrate to inoculum ratio 3 (v/v basis), pH 7, 100 rpm, 37 °C | 4.4-fold increase in specific H2 yield | [58] |
| Antibiotic fermentation residue | 0.2 L, 6 mm sonication probe, 30 min, 4 s ON 6 s OFF, followed by 5 M NaOH addition to reach pH 10, mixed for 24 h | 0.2 L batch, substrate to inoculum ratio 3 (v/v basis), pH 7, 37 °C | 79% increase in specific H2 yield | [62] |
2.2. Substrate Pre-Treatment to Enhance VFA Production
VFAs are platform chemicals and value-added products that are of growing interest due to their applicability in a variety of chemical and process industries. Intensification of VFA production via effective pre-treatment is therefore also gaining significant attention, especially if the feedstock of interest is a ‘waste’. Similar to biohydrogen production, studies focusing on physical pre-treatment have moved away from energy-intensive milling methods. More recently, freezing and thawing as a pre-treatment was reported by She et al. [64] to intensify VFA production from waste-activated sludge. A higher degree of disintegration can be possible with such a pre-treatment strategy. Furthermore, it has been claimed that the formation of intracellular crystals during the freezing stage can lead to the breakage of cell membranes, leading to an enhanced soluble COD content upon pre-treatment. She et al. [64] performed five cycles of freezing and thawing (one cycle = −24 °C freezing for 8 h, 35 °C thawing for 2 h) with a 0.45 L batch of sludge and followed it up with fed-batch mesophilic digestion to achieve a 35% increase in VFA concentration compared to the controls. Zeng et al. [65], on the other hand, utilised waste-activated sludge in a bioelectrochemical cell with graphite electrodes at an applied potential of 12 V for 30 min, which suppressed biomethane production and improved the VFA yield by ~100-fold. The gradual shift of microbial communities upon pre-treatment showed that the digestion favoured VFA accumulation rather than methanogenesis.
Conventional alkali pre-treatment has been reported to enhance VFA yields. For instance, Pham et al. [66] pre-treated seaweed (40% TS) with 0.5 N NaOH to enhance the VFA yield by two-fold. Unconventional treatment possibilities have however also been explored, such as using alkaline ferrate [67], carbide slag [68] or tetrakis hydroxymethyl phosphonium sulphate [69]. At pH 10 (2 M NaOH) and 0.5 g/g VSS K2FeO4, increased solubilisation of waste-activated sludge coupled with extracellular polymeric substance release resulted in a 2.4-fold increase in VFA concentration [67]. Acetic acid was found to be the predominant product in the VFA mixture. In a first-of-its-kind work, Tao et al. [68] reported the use of carbide slag to pre-treat grass and intensify VFA yields. Carbide slag is an alkaline waste that is generated as a by-product of calcium carbide hydrolysis [70]. It may be used to produce cement; however, it has a high potential to pollute the atmosphere (dust) and water bodies (leaching). Due to its chemical composition and alkalinity, it may be used to pre-treat biomass [68]. In this study, 5% grass was pre-treated with 1.75% slag at 120 °C for 40 min. Upon treatment, the solid residue was separated, washed until a neutral pH was reached and subjected to enzymatic hydrolysis prior to mesophilic acidogenic digestion. Similar to most alkali treatment methods, the hemicellulose and lignin were solubilised to an extent, leaving behind a cellulose-rich solid residue. Enzymatic hydrolysis of the pre-treated residue resulted in a >6-fold increase in reducing sugars, thereby leading to an enhanced VFA production of up to 2.4-fold. Acetate dominated the VFA mixture, followed by butyrate and propionate. Another unconventional chemical pre-treatment that was reported was the use of a biocide tetrakis hydroxymethyl phosphonium sulphate on sludge [69]. In total, 20 mg/g of biocide was found to be optimum at room temperature; however, a 2-day treatment time was required. A 49% increase in soluble COD content was observed, leading to a four-fold increase in VFA concentration. Higher molecular weight fatty acids dominated the VFA mixture obtained from the pre-treated feedstock.
Fang et al. [71] reported the use of white rot fungi to pre-treat autoclaved solid digestate (obtained from a biogas plant digesting agricultural, fruit and vegetable residues). A 6-week pre-treatment period was required to increase the VFA concentration by 1.2-folds. This is a typical example of the long pre-treatment times taken by biological methods in breaking down lignocellulosic materials. Furthermore, they use dried, chopped and autoclaved substrates, all of which might have an impact on the biomass structure and composition. Therefore, this could be classified under combined pre-treatment methods rather than just ‘biological’ pre-treatment. They performed a similar exercise with mushroom residue and achieved a >70% increase in VFA yield [72]. Unlike traditional biological methods, Pham et al. [66] reported the use of Vibrio spp. to pre-treat seaweed samples. Seaweed lacks (or contain in negligible quantities) lignin in its cell wall; however, the complexity in digestion arises due to the presence of other polymers, such as alginate. The alginate lyase activity of the bacteria was effective in pre-treating the seaweed prior to VFA production, as reported by the authors. Bacterial treatment was found to be more effective than alkali pre-treatment in this case, and a 2.5-fold increase in VFA concentration was observed with the pre-treated seaweed. Acetate (53%) followed by propionate (27%) and butyrate (15%) dominated the mixture. Despite being effective, the bacterial- or fungal-based methods require a long time to hydrolyse the substrate. Enzymatic treatment can be an alternative if costs are not inhibitory. Bahreini et al. [73] reported the use of Novozym 50199 to pre-treat (10 min) primary sludge and enhance the maximum VFA concentration by 56% in a fed-batch digester. Similar results with a VFA increase of up to 39% were reported by Owusu-Agyeman et al. [74], who used an enzyme cocktail of α-amylase, lipase, cellulase, dextranase and protease to pre-treat primary sludge.
The use of physico-chemical pre-treatment methods for intensifying VFA production has been growing in the recent decade. Conventional hydrothermal treatment of thickened activated sludge at 190 °C, 12.5 bars and 10 min was reported to increase the maximum VFA concentration by three-fold [75]. Hydrothermal pre-treatment was effective in increasing the soluble COD content by almost 10-fold compared to the untreated sludge with a soluble COD of ~2 g/L. This corresponded to a decreased total suspended solid concentration of the sludge with a reduced particle size distribution. The specific energy consumption for this pre-treatment was reported to be 481 kJ/kg sludge. Another conventional method is a thermo-chemical pre-treatment method, namely autoclaving in the presence of alkali to enhance digestion efficiency. Suresh et al. [76] autoclaved lipid extracted 5% microalgal slurry in the presence of 1% NaOH and subjected the samples to mesophilic digestion and observed a 20% increase in VFA concentration with the pre-treated sample. They also pre-treated the lipid-extracted microalgae using a microwave-based method in the presence of 1% NaOH [76]. They achieved >50% solubilisation of the substrate; however, the increase in the maximum VFA concentration was only 10% and significantly less than the NaOH-autoclave pre-treatment. Microwave-assisted ionic-liquid-based pre-treatment of straw was found to produce VFA with a five-fold increase as compared to the untreated straw [77]. While the combined effect helped in enhancing the VFA yield, the microwave assistance helped to lower the required ionic liquid loading needed for pre-treatment. Suresh et al. [76] also investigated the use of sonication as a pre-treatment in the presence of alkali and reported that although the degree of solubilisation was similar to microwave-alkali pre-treatment but less than autoclave-alkali pre-treatment (~80%), the enhancement in the maximum VFA concentration was 30% when compared to the untreated substrate as well as higher than the other two reported methods. Sonication has also been used to pre-treat crushed food waste by Guo et al. [78], who, at an optimal specific energy input of 1.2 kJ/mL (37.7 kJ/g TS), achieved >55% degree of disintegration corresponding to a 4.3-fold increase in maximum VFA concentration. Liu et al. [79] however observed a 63% increase in VFA concentration from sonicated food waste at an optimal energy input of 1.8 kJ/mL (18 kJ/g TS). Beyond food waste, sonication has also been investigated for lignocellulosic biomass such as grass. Wang et al. [80] sonicated 2% dried and milled grass slurry in 0.75% lime solution with a specific energy input of 1.5 kJ/mL (7.8 kJ/g TS) in pulsed mode (5 s ON 5 s OFF). The solids and liquids were separated, neutralised and subjected to mesophilic digestion. Compared to the digestion of the slurry, the cumulative VFAs produced from the solid and liquid fractions were significantly higher and were found to be >2 fold higher than the untreated feedstock.
While sonication has been reported extensively to pre-treat biomass to enhance digestion efficiency by improving COD solubilisation and increasing the degree of disintegration, it is limited by its volume of operation and high specific energy requirements. To overcome these limitations, hydrodynamic cavitation is a suitable alternative. For the first time, Lanfranchi et al. [46] reported the use of hydrodynamic cavitation for pre-treating mixed organic waste (waste-activated sludge, vegetable and fruit waste) to improve VFA yields. They used a rotor-stator device at an inlet pressure of 2 bar, an inflow rate in the range of 80–100 LPM and a rotor speed in the range of 1450–1550 rpm. An optimum pre-treatment time of 50 min was found to be beneficial in increasing the soluble COD by 83%, corresponding to a nine-fold increase in maximum VFA concentration. The specific energy consumption was reported to be 3.7 MJ/kg TS, which is significantly lower than the acoustic cavitation-based pre-treatment methods. Nevertheless, due to the presence of moving parts, there are issues pertaining to clogging and maintenance at an industrial scale. Furthermore, compared to other hydrodynamic cavitation devices, rotor-stator devices are known to be relatively energy intensive [2]. Nonetheless, it is promising to see that a scalable biomass pre-treatment is being exploited for intensifying digestion yields.
A promising aspect of the recently reported pre-treatment methods is that despite the kind of pre-treatment used, desired product type or enhancement achieved, researchers are moving in the right direction of not only understanding the fundamentals of pre-treatment but also the interaction of the pre-treated substrate and the microbial consortia. Most of the discussed papers in this section have also reported omics studies, looking into the abundance and diversity of specific genus and their shifts as a result of pre-treatment. Such investigation will help to better understand the pre-treatment and digestion processes and lead to devising effective monitoring tools and scale-up strategies.

Table 2.
Pre-treatment methods reported to enhance VFA production.
Table 2.
Pre-treatment methods reported to enhance VFA production.
| Feedstock | Pre-Treatment Conditions | Digestion Conditions | Influence on VFA Yield | Reference |
|---|---|---|---|---|
| Physical Pre-treatment | ||||
| Waste-activated sludge | 0.45 L, 5 cycles of freezing and thawing, −24 °C freezing for 8 h, 35 °C thawing for 2 h | 1 L fed-batch, sludge-to-inoculum ratio of 2 (w/w), 80 rpm, 25 days retention time, 35 °C | 35% increase in maximum VFA concentration | [64] |
| Waste-activated sludge | 0.5 L, graphite electrodes, 15 V, pH 6.7, 30 min, 25 °C | 0.06 L sludge, 0.02 L inoculum, 35 °C, 60 rpm, 35 days | Suppressed CH4 production, ~100-fold increase in specific VFA yield | [65] |
| Chemical Pre-treatment | ||||
| Waste-activated sludge | 0.8 L feedstock, pH 10 (2 M NaOH), 0.5 g/g VSS K2FeO4, 120 rpm, 60 min | 0.4 L batch, 10% v/v inoculum, 160 rpm, 35 °C, 12 days | ~2.4-fold increase in maximum VFA concentration | [67] |
| Air-dried and chopped macroalgae | 40% TS, 0.5 N NaOH, 18 h | 0.1 L batch, 4% TS feedstock, 10% inoculum, 35 °C, 150 rpm, 4 days | 2-fold increase in maximum VFA concentration | [66] |
| Grass waste | 0.2 L, 5% grass, 1.75% carbide slag, 120 °C, 40 min | 0.25 L batch, substrate-to-inoculum ratio 2 (VS basis), pH 7, 100 rpm, 35 °C, 14 days | 0.6–2.4-fold increase in maximum VFA concentration | [68] |
| Sludge | 0.5 L sludge, 20 mg/g tetrakis hydroxymethyl phosphonium sulphate, 2 days, 150 rpm, 30 °C | 0.35 L sludge batch, 0.03 L inoculum, pH 6, 2 days, 150 rpm, 30 °C | 4-fold increase in maximum VFA concentration | [69] |
| Biological Pre-treatment | ||||
| Autoclaved solid digestate | 100 g TS, 10 g white rot fungi Pleurotus Sajor-Caju, 25 °C, 70% relative humidity, 6 weeks | 0.4 L batch, 15% TS, inoculum-to-substrate ratio 2 (TS basis), 30 °C, 18 days | 1.2-fold increase in maximum VFA concentration | [71] |
| Air-dried and chopped macroalgae | 4% TS, 0.09 L, Vibrio spp., 26–30 °C, 2 days | 0.1 L batch, 4% TS feedstock, 10% inoculum, 35 °C, 150 rpm, 4 days | 2.5-fold increase in maximum VFA concentration | [66] |
| Primary sludge | 1% Novozym 50199 to biomass, 300 rpm, 10 min | 0.5 L fed-batch, 2-day retention time, 25 °C | 56% increase in maximum VFA concentration | [73] |
| Physico-chemical Pre-treatment | ||||
| Crushed food waste | 0.3 L feedstock, 8 mm 20 kHz sonication probe, 1 W/mL, 20 min | 0.18 L batch, substrate to inoculum ratio 6 (VS basis), 180 rpm, 35 °C, 5 days | ~4.3-fold increase in maximum VFA concentration | [78] |
| Lipid-extracted microalgae Ettlia sp. | 10 mL of 5% microalgal slurry, 1% NaOH, 25% amplitude sonication | 0.1 L batch, 3% TS, 20% v/v inoculum, pH 7.2, 150 rpm, 35 °C, 7 days | 30% increase in maximum VFA concentration | [76] |
| 10 mL of 5% microalgal slurry, 1% NaOH, microwave | 10% increase in maximum VFA concentration | |||
| 10 mL of 5% microalgal slurry, 1% NaOH, autoclave 121 °C, 1 h, 1 bar | 20% increase in maximum VFA concentration | |||
| Thickened waste-activated sludge | 1 L sludge, 190 °C, 10 min, 12.5 bars | 0.3 L batch, 1 gTCOD/gVSS substrate to inoculum ratio, pH 5.5, 120 rpm, 37 °C, 3 days | 3-fold increase in maximum VFA concentration | [75] |
| Waste-activated sludge | 0.2 L sludge, 0.01 g sodium dodecylbenzene sulfonate/g TS, 70 °C, 1 h, 400 rpm | 0.2 L batch, 150 rpm, 37 °C, 7 days | 4-fold increase in maximum VFA concentration | [81] |
| Grass clippings | 0.1 L, 2% grass, 0.75% Ca(OH)2, sonication at 2.5 W/mL for 10 min (5 s ON 5 s OFF pulse) | Solids and liquids were separated and fermented, 0.2 L batch, pH 7, 120 rpm, 35 °C, 12 days | ~2.1-fold increase in maximum VFA concentration | [80] |
| Waste-activated sludge and vegetable/fruit waste | Rotor-stator hydrodynamic cavitation, 2 bars inlet pressure, 80–100 L/min inflow rate, 1450–1550 rpm rotor speed, 50 min | 4 L batch, substrate-to-inoculum ratio 6–7 (VS basis), 37 °C, 14 rpm | ~9-fold increase in maximum VFA concentration | [46] |
3. Anaerobic Digestion for the Production of Biohydrogen or VFAs
AD shows promise as an industrially viable method for the production of biohydrogen and VFAs, due to its feasibility of utilising various organic wastes as potential feedstocks. Carbohydrates are the preferred carbon source for fermentation; however, the use of carbohydrate-rich substrates such as glucose, sucrose and starch are associated with high commercial costs and competition with human-food requirements [82]. There are however numerous waste streams that contain a wide spectrum of carbohydrates that can be obtained from industrial, agricultural and municipal sources that are available at little to no cost [83].
Conventional AD broadly proceeds through four main stages: hydrolysis, acidogenesis, acetogenesis and methanogenesis (Figure 4). During hydrolysis, complex organic macromolecules, such as carbohydrates, proteins and lipids, are broken down into their respective monomers (monosaccharides, amino acids and fatty acids) by hydrolytic bacteria.
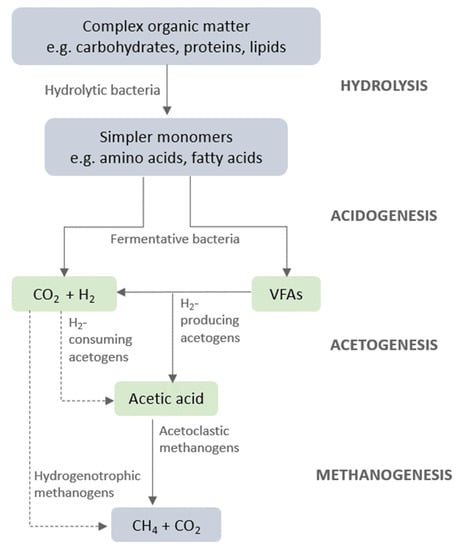
Figure 4.
The four main stages of AD.
The products of hydrolysis are then fermented by acidogenic bacteria, which facilitate the formation of VFAs and hydrogen (Figure 5). Acidogenesis largely proceeds through the acetic (1) and butyric acid (3) pathways [84], resulting in hydrogen as a by-product. However, propionic acid is another common VFA produced in the AD of organic wastes, and this process is hydrogen-consuming (2).
C6H12O6 + 2H2O → 2CH3COOH + 4H2 + 2CO2
C6H12O6 + 2H2 → 2CH3CH2COOH + 2H2O
C6H12O6 + 2H2O → C3H7COOH + 2H2 + 2CO2

Figure 5.
The metabolic pathways during acidogenesis that lead to hydrogen and VFAs from hexose. (NAD: Nicotinamide adenine dinucleotide; Fd: Ferredoxin; ATP: Adenosine triphosphate; ADP: Adenosine diphosphate).
The preferred pathway for acidogenic bacteria is the production of acetic acid since it provides the biggest energy yield for growth. However, as the partial pressure of hydrogen increases, the process that allows for the conversion of pyruvate to acetate becomes energetically unfavourable. The metabolic pathways therefore shift to produce VFAs that are more reduced than acetic acid, such as propionic and butyric acid [85]. Pyruvate is often the pivotal intermediate which can be converted into a spectrum of products, such as acetate, propionate, butyrate, lactate, propanol, butanol, hydrogen and carbon dioxide. The proportions of pyruvate directed to each pathway depend on several factors, including substrates, environmental conditions and microbial populations [86].
Hydrogen and acetate are produced during the acetogenic phase through the oxidation of the longer chain fatty acids. However, these catabolic reactions are endergonic and depend on low concentrations of acetate and hydrogen to drive the oxidation pathway forward. Acetogenesis also includes a hydrogen consumption process, known as homoacetogenesis, which is utilised to fix carbon dioxide into more acetic acid. Both hydrogen and VFAs are consumed in the final methanogenic stage, whereby acetoclastic and hydrogenotrophic methanogens either convert acetic acid into carbon dioxide and methane or oxidise hydrogen to reduce carbon dioxide to methane, respectively [87].
The yields of VFAs and hydrogen produced through AD are largely dependent on bioreactor conditions. Bioprocesses that favour the formation of either hydrogen or a VFA mixture will often produce the other as a by-product. In fact, single-stage fermentation for the co-production of hydrogen and VFAs have achieved bioconversion efficiencies of up to 64% [88]. Increased VFA concentrations however can negatively interfere with hydrogen production, either as a result of hydrogen consumption by homoacetogens or inhibition by undissociated acid molecules [89]. Higher partial pressures of hydrogen within bioreactors can also alter the proportions of VFAs obtained [90].
While the metabolic pathways leading to either H2 or VFAs are similar, enhanced production of either of the products requires process optimisation. This is largely achieved by optimising operational conditions and bioreactor design to ensure the inhibition of methanogenic activity and that conditions are favourable for either hydrogen- or VFA-producing microorganisms. Process parameters that influence the acidogenic fermentation yield are discussed in detail in this section. Each of the subsections will discuss the influence of the parameter on both biohydrogen and VFA production.
3.1. Hydraulic Retention Time (HRT)
HRT is an important engineering parameter and is influenced by the reactor volume, V and the mass inflow rate, Q of the feedstock (4).
HRT has been reported to play an important role in maximising hydrogen yield when fermenting organic wastes. Shorter HRTs can suppress homoacetogenesis and methanogenesis, which are both hydrogen-consuming pathways [91]. If HRTs are too short, however, biomass washout can occur [92]. HRTs as low as 6 h have proven successful in enhancing hydrogen yields of a galactose reactor [93]. However, this study only investigated three short HRTs (2 h, 3 h and 6 h), so it is unclear whether longer HRTs would have elicited higher yields. For instance, another study using a waste sugar feedstock discovered that an HRT between 14–15 h was optimal for biohydrogen production [84]. In a study investigating hydrogen production from three feedstocks at three different HRTs, Salem et al. [60] discovered that the optimum HRT for hydrogen yield using bean wastewater was 24 h (80 mL H2/g VS), and for potato wastewater, it was 18 h (150 mL H2/g VS). These results are consistent with a study by Massanet-Nicolau et al. [94] that utilised sewage biosolids as the feedstock and found that a 24 h HRT resulted in the most stable hydrogen producing period during which a hydrogen yield of 21.9 mL H2/g VS was achieved. A 24 h HRT was also found to be optimum for a food waste reactor, producing a yield of 255.4 ± 33.6 mL H2/g VS/d [95].
Several studies have also analysed the effect that HRT has on VFA production, with results varying depending on the feedstock. For instance, studies using synthetic and low-strength wastewater have found that HRTs as low as 6–8 h produce the maximum total VFA concentration [96,97]. Although with more recalcitrant feedstocks, particularly those utilised at an industrial scale, longer HRTs are generally more beneficial for VFA production. A 6-day HRT increased total VFA concentration from 2.4 g COD/L to over 50 g COD/L in a reactor fed with urban biowastes [98]. Using other organic waste streams, Jankowska et al. [99] found that for cheese whey, the optimum HRT was 20 days, producing a total VFA concentration of 16.3 g/L, and for mixed sludge fermentation, it was 12 days, producing a total VFA concentration of 12.5 g/L. Overall, the literature suggests that HRTs in the order of hours are beneficial for H2 production, whereas in the order of days is required to obtain VFAs.
3.2. Organic Loading Rate (OLR)
The OLR within a bioreactor can aid in maximising production performance in a continuous system and is often altered by adjusting the HRT. Kumar et al. [93] reported a peak hydrogen production rate at a 3 h HRT and an OLR of 120 g galactose/L/d. However, when the HRT was adjusted to 6 h, the OLR was halved to 60 g galactose/L/d, and this resulted in the maximum hydrogen yield of 2.21 mol H2/mol galactose. This study determined that at higher OLRs under lower HRTs, more H2-producing bacteria, such as Clostridia, become dominant. Operating at a higher HRT, however, resulted in a stronger ability to retain active biomass in the system, leading to greater overall hydrogen yields. A similar hydrogen yield (2.1 mol H2/mol glucose) was achieved at an OLR of 6.5 g COD/L/d in a glucose reactor at an HRT of 8 h [100]. In a different study, using industrial wastewater feedstock, Ferraz Júnior et al. [100] reported a maximum hydrogen yield (1.4 mol H2/mol total carbohydrates) at an OLR of 72.4 g COD/L/d and HRT of 12 h. This study also reported that applying OLRs in excess of 100 g COD/L/d can result in significant reductions in biohydrogen yield and production rate, and such organic overloads can cause biomass washout in suspended-growth systems, e.g., continuously stirred tank reactors (CSTRs). Therefore, higher OLRs may only be suitable in immobilised-cell reactors, such as packed bed reactors, where the washout of active biomass is hindered [101].
Varying the OLR to enhance VFA production has also recently been investigated. Tang et al. [102] studied the impact of three separate OLRs on the VFA concentration in a food waste reactor (at a 5-day fixed HRT) and found higher VFA yields when the OLR was increased from 14 to 22 g TS/L/d. Iglesias-Iglesias et al. [103] also reported increases in VFA production at higher OLRs using sewage sludge as the substrate. Similarly, a stepwise increase in OLR from 3 to 12 g COD/L/d at an 8-day HRT enhanced VFA production in a microalgae biomass fermentation experiment [104]. These results are consistent with another study that found an optimum OLR value of 12.9 g COD/L/d at a 12-day HRT using olive mill solid residue as the feedstock [105,106]. Some recent studies have also reported the effect that OLR has on VFA composition, with results indicating that higher OLRs produce greater yields of longer chain VFAs, such as butyric, valeric and caproic acids [96,103,107,108].
3.3. pH
The pH level within a reactor is a key parameter that can influence the metabolic pathways of AD. Methanogens are most active between the pH range of 6.5–8.2 [109], and although the optimum pH for hydrolytic and acidogenic bacteria has been suggested as 5.4–6.5, pH levels as low as 4.0 and as high as 11.0 have been employed with various substrates [110].
For hydrogen production, it has been reported that a pH of 5.5 can be most beneficial due to the inhibition of both methanogenesis and homoacetogenesis [111]. Indeed, many studies have reported an optimum pH for hydrogen production from food waste within the range of 5.0–6.0 [112,113,114], whereas a pH value within the range of 6.0–7.0 has proven most successful with crop residues and agricultural wastes [115,116,117]. It is clear from the literature that the optimum pH for hydrogen production is largely dependent on the substrate used. For instance, a recent study by Tsigkou et al. [118] found that the optimum pH for hydrogen production when using a fruit/vegetable mixture was 6.5, but when using a mixed waste substrate, it was 7.5. Generally, the optimum pH for biohydrogen production from organic wastes is within the range of 5.0–7.0 since this favours the activity of hydrogenases and is also suitable for microbial growth and metabolism [119].
For VFA production, recent studies suggest a higher pH level is more beneficial. A more alkaline pH has been shown to not only improve hydrolysis efficiency but also enhance VFA yield when using complex feedstocks [120,121,122]. Cabrera et al. [120] reported an increased acetic acid concentration from 1.08 g/L to 3.14 g/L when the pH was increased from 5.0 to 9.0. A maintained pH level of 10.0 was also optimum for VFA production in a waste-activated sludge reactor [123], which is consistent with [124], which also reported a peak VFA production efficiency at pH 10.0. The pH level also plays a critical role in determining the VFA composition. Acidic conditions often result in a higher acetic acid concentration, and alkaline conditions result in a butyrate-dominant product mixture [124,125].
3.4. Temperature
Fermentation temperature is an important factor that can impact microbial metabolisms and the efficiency of substrate conversion to desired products [126]. Mesophilic fermentation often takes place within the range of 30–40 °C, whilst the thermophilic range is typically 50–60 °C. Recent research also suggests the use of psychrophilic temperatures (<20 °C) within anaerobic digesters, which could be particularly useful in countries with colder climates. Studies have shown that process parameters, including COD removal and biogas production, are comparable in a psychrophilic and mesophilic reactor [127]. The biotechnological potential of psychrophilic reactors is still under-utilised since certain disadvantages limit their use on a larger scale. These include the alteration of physical and chemical properties within biomass, thereby reducing substrate availability, inhibition of important cellular processes and the requirement to modify existing digester designs and, in some cases, use acclimated microbial biomass [128]. As a result, studies using mesophilic and thermophilic bioreactors still dominate the literature. A temperature of 55 °C has been reported as optimum for biohydrogen production from the fermentation of rice straw [129], food waste and manure [130] and sewage sludge [131]. Conversely, other studies have observed higher hydrogen yields at mesophilic temperatures. Ziara et al. [132] tested four different temperatures (35, 45, 50 and 55 °C) on a digester fed with lactate wastewater and found that biohydrogen production only occurred at 35 and 45 °C. These results are consistent with [126], who reported a maximum hydrogen yield (492.3 ± 5.1 mL/g TS) at a temperature of 36.6 °C.
Similar findings have been reported for VFA yields at various temperatures. Huang et al. [133] investigated the effect of eight temperatures between the range 25–65 °C on the AD of waste-activated sludge. They reported that the average acetate concentration increased with temperature until a peak at 40 °C, producing a yield of 0.29 g/L, beyond which the accumulation decreased. A study by Moretto et al. [98] also reported an optimum temperature of 37 °C, which resulted in a maximum total VFA concentration of 65 g/L. Mesophilic temperatures have also proven preferential for VFA production from sewage sludge [134] and the organic fraction of municipal solid waste (OFMSW) [135].
Although thermophilic conditions present advantages, including an increased rate of hydrolysis, enhanced pathogen destruction and a higher rate of organic matter destruction, mesophilic conditions are most promising for larger-scale digesters due to the lower energy requirements and more stable operation [129,136].
3.5. Operational Mode and Reactor Configuration
Batch bioreactors have been extensively used for both biohydrogen and VFA production to evaluate the viability of feedstocks and the effect of various process conditions. Batch experiments are widely used to optimise process parameters at the lab scale, and the results often indicate an initial increase in desired products, followed by a rapid decline as the feedstock is used up [137]. While the initial insight provided by batch systems is useful for understanding the digestion process, it is important to understand digester behaviour in (semi)continuous systems to aid scale up.
With respect to acidogenic digestion, various bioreactor configurations have been investigated at a laboratory scale, both under batch and (semi)continuous modes. The most extensively studied is the continuously stirred tank reactor (CSTR); however, other methods include anaerobic fluidised bed reactors (AFBRs), anaerobic sequencing batch reactors (ASBRs), anaerobic packed bed reactors (APBRs) and up flow anaerobic sludge blanket reactors (UASBRs). There is also a strong correlation between production efficiencies and the size of the microbial population present within the reactor. Therefore, cell retention strategies such as granulation and immobilisation systems have also been investigated to enhance overall product yields [138]. An overview of the typical reactor configurations used for biohydrogen and VFA production is shown in Table 3 and discussed in detail in this section.

Table 3.
Advantages and disadvantages of typical reactor configurations used for biohydrogen and VFA production.
3.5.1. CSTR
In a CSTR, the microbes and substrates are constantly suspended and mixed, which facilitates effective contact and higher mass transfer [139]. Their benefits include simple design, easy maintenance, homogenous mixing, and a well-maintained HRT. CSTRs are also the preferred reactor configuration when no differentiation between solids and liquid retention times are required. CSTRs are widely considered an effective and economical approach for the production of both biohydrogen [140,141,142,143] and VFAs [98,144,145] from organic waste streams. Although this kind of reactor is sensitive to operational parameters, including pH, temperature and HRT, limitations in mass transfer have proven a critical parameter for optimum performance. Research indicates that the concentration of desired products increases when the mixing speed increases until an optimum; exceeding this can result in shear strain that can damage floc particles and relevant microbial populations [146]. Their main drawback however is biomass washout at lower HRTs [92]. As a result of this, some studies have combined CSTRs with immobilised systems to retain more active biomass in the reactor. Keskin et al. [147] reported higher hydrogen yields and greater resistance to biomass washout in an immobilised bioreactor configuration compared to a conventional CSTR, particularly at higher OLRs.
3.5.2. AFBR
In AFBRs, a fluidisation medium (liquid or gas) is passed through the digester containing the feedstock, usually of high solid content, to ensure suspension. This enhances microbial activity via enhanced mass transfer and can cause greater degradation of wastewaters [148]. In comparison with CSTRs, the likelihood of biomass washout is lower, but more energy is required for constant fluidisation. Often, a support material is also required for biomass adhesion; examples from the literature include shredded tires [149], activated carbon [150], polystyrene and expanded clay [151]. The literature indicates the broader use of AFBRs for hydrogen production in comparison to studies focused on VFA production. This could be due to the fact that they can operate at shorter HRTs and higher OLRs which favours biohydrogen production (Section 3.2). For instance, Amorim et al. [152] reported an increase in hydrogen yield from 0.13 to 1.91 mol H2/mol glucose when the HRT decreased from 8 to 2 h in an AFBR utilising cassava wastewater.
3.5.3. ASBR
This reactor process involves cycling through the stages of feeding, reaction, settling and decanting. Within this semi-batch process, the use of a settling stage allows for greater solid retention within the reactor, meaning that the solid retention time (SRT) becomes independent of HRT. Recent studies have obtained high hydrogen yields using ASBR systems. Maaroff et al. [153] tested a two-stage ASBR system for biohydrogen production from palm oil mill effluent and achieved yields as high as 2.52 mol H2/mol sugar at an optimum HRT of 12 h. A similar HRT was utilised in a study by Santiago et al. [154], who reported a 16 h HRT and 55 h SRT as optimum for biohydrogen yields from organic waste. This study also examined the effect of SRT and HRT on VFA production and found that a similar SRT (60 h) but a longer HRT (48 h) was optimum for VFA yields. The ability to adjust the SRT in an ASBR provides an additional mechanism to manipulate microbial communities, which in turn can be used to enhance desired metabolic pathways. Through analysis of population dynamics within ASBRs, it is reported that hydrolytic bacteria are dominant at shorter SRTs, whilst acidogenic and acetogenic bacteria become more dominant at longer SRTs [155].
3.5.4. APBR
These reactors are often used with feedstocks of high organic content, and the beds can contain granules or biofilms to improve function at lower HRTs and increase tolerance to high OLRs [156]. APBRs are easy to operate and require lower construction costs when compared with other reactors [139]. Additionally, due to their ability to retain high feedstock concentrations within the reactor, high conversion rates can be achieved. Despite this, some studies have reported unstable operation, mainly attributed to excess biomass accumulation in the bed, which can lead to the proliferation of H2- and VFA-consuming microbes [157]. Since there is no continuous mixing, such as in a CSTR, a recirculation loop is often implemented to improve mass transfer and enhance product yields [158]. Various support materials have been tested for their ability to immobilise relevant microbes within APBRs and therefore impact production performance. Muri et al. [159] analysed the impact of three different support materials (Mutag BioChip™, expanded clay and activated carbon) on hydrogen yields within an APBR fed with synthetic wastewater and reported the highest yield (1.80 mol H2/mol glucose) when the reactor was packed with Mutag BioChip™.
3.5.5. UASBR
UASBRs are another reactor configuration that aims to retain microbes within the reactor. They do not use biofilms or support materials and instead rely on the formation of biological granules. UASBRs are best utilised with medium–high strength wastewaters since the feedstocks need to have good settling characteristics. They are a simple and reliable method for wastewater treatment, and many large-scale plants have been successfully operated [160]. They have proven successful for biohydrogen and VFA production from various organic waste streams [161,162,163,164,165]; however, some disadvantages include an extended start-up time and excess pathogen, nutrient and overall biomass content in the effluent.
3.6. Additives
The impact that additional chemicals and nutrients have on the production yields of hydrogen and VFAs has been extensively investigated. Adjusting the carbon to nitrogen (C/N) ratio of feedstocks is a common parameter used to enhance digester performance. Ratios that are too high (>30) can lead to insufficient nitrogen available to maintain microbial biomass, whilst ratios that are too low can increase ammonia production, which can inhibit microbial activity (Section 3.6) [166]. Studies suggest that increasing C/N ratios can enhance biohydrogen yield with ratios as high as 137 [157] and 173 [167], resulting in maximum hydrogen yields from reactors fed with sucrose and wheat powder solution, respectively. Argun et al. also reported a maximum VFA yield at the same C/N ratio, producing 11 g/L of total VFAs. A C/N ratio of 47 was reported as optimum for biohydrogen production in a sewage sludge reactor, whilst the same study determined a C/N ratio of 130 resulted in the maximum VFA yield [168].
Metals have been among the most widely employed additives in AD systems. The addition of iron and nickel (in ion or nanoparticle form) have shown significant enhancements in biohydrogen yields due to their ability to facilitate the acceleration of the electron transfer between ferredoxin and hydrogenase, which, in turn, drives hydrogen generation [169]. The addition of nickel ion and Ni0 nanoparticles has been shown to effectively enhance biohydrogen yields [170], and the use of biologically synthesised iron nanoparticles improved biohydrogen yields by up to 44% when compared with no addition [171]. In contrast, the use of metal additives in studies focused on VFA production have produced varying results. Zhang et al. [172] reported higher VFA yields when adding iron to a cadmium-containing system compared to adding nickel. The addition of Co2+ and Zn2+ [173] lowered total VFA yields in comparison to the control, but yields of propionic acid were significantly increased (from 28.71 to 317 mg of propionic acid/g of COD).
Biochar addition has also recently been investigated in various anaerobic digesters. A 15 g/L biochar addition produced a maximum hydrogen yield of 3990 mL/L in Zhao et al. [174]. Sugiarto et al. [175] found that biochar addition not only increased hydrogen yields by 107% but also the primary elements present in biochar (Fe, K and Ca) were responsible for increasing both acetic acid and butyric acid concentrations. Similarly, acetic acid concentration increased from 0.18 to 0.36 g/L in response to a 0.6 g/L biochar addition in a study by Lu et al. [176].
The influence of salinity on biohydrogen and VFA yields has produced contrasting results in the recent literature. Taheri et al. [177] found that increasing NaCl concentration from 0.5 g/L to 30 g/L had a negative effect on hydrogen yield, decreasing it from 1.1 mol H2/mol glucose to 0.3 mol H2/mol glucose. However, Sarkar et al. [178] reported that a NaCl concentration as high as 40 g/L resulted in maximum hydrogen yields from a food waste reactor. This study also found that a 40 g/L NaCl addition improved total VFA yields by 1.35 times, producing 6.58 g/L compared with 4.84 g/L from the control experiment. He et al. [179] examined the impact of 4 NaCl concentrations (10, 30, 50 and 70 g/L) on VFA production, and although the highest yield was achieved at 10 g/L (0.542 g/g dry weight of food waste), yields remained high at 70 g/L (0.441 g/g dry weight).
The addition of antibiotics is associated with the suppressions of methanogenesis and therefore has been studied as a method to increase biohydrogen and VFA yields. Recent research indicates that certain antibiotics can have an inhibitory effect on each stage of AD; however, the more severe inhibition impacts acetogenesis and methanogenesis [180,181]. The addition of roxithromycin to a waste-activated sludge fermenter had the most severe inhibitory effect on methanogenesis, so the VFA yield more than doubled when the antibiotic concentration increased from 0 to 100 mg/kg TSS [182]. Huang et al. [181] reported maximum VFA yields when concentrations of clarithromycin reached 1000 mg/kg TSS. Contrastingly, Tao et al. [183] found a negative correlation between the concentration of thiosulfate and yields of both VFAs and hydrogen. This study inferred that thiosulfate inhibited several metabolic pathways of acidogenesis and in particular restricted the activity of key enzymes, including butyryl CoA and NADH.
Although the addition of some chemicals and nutrients may have a positive impact on hydrogen and VFA yields, most of the studies reported yields from small-scale digesters (<1 L). It is therefore unclear how beneficial the addition of chemicals and antibiotics would be on a larger scale, particularly from an economic point of view.
3.7. Undesired By-Products and Inhibitors
The accumulation of hydrogen and VFAs within reactor systems can make it thermodynamically unfavourable for their continued production and result in inhibited digestion with reduced yields. Studies have shown that the continuous recovery of hydrogen and VFAs from fermentation broths can enhance the overall yields of both products. Jones et al. [184] utilised proton exchange membranes to remove hydrogen and electrodialysis to remove VFAs from a sucrose reactor to effectively enhance hydrogen yields by a factor of 3.75. In Hassan et al.’s work [185], the in situ recovery of VFAs from a food waste reactor almost doubled hydrogen yields and increased VFA concentration from 1.9 to 4.7 g/L. Further studies operating electrodialysis on reactors fed with grass waste [87] and food waste [145] were also effective in enhancing VFAs yield. Methods for hydrogen and VFA extraction are discussed further in Section 4.
While end-product inhibition is commonly observed with acidogenic digestion, inhibition due to the formation of undesired by-products should not be ignored. The formation of undesired by-products is influenced by the type of substrate, operating conditions (temperature and pH) and the diversity and abundance of the microbial community present in the digester. The most common inhibitor encountered in digesters is ammonia. The presence of excess ammonia, both in free (NH3) and ionic (NH4+) form, can induce inhibition. Ammonia is able to pass through cell walls and react with protons to produce NH4+, which can alter intracellular pH and inhibit microbial activity [186]. Whilst methanogenesis is considered the most severely inhibited AD stage, excess ammonia can also have an inhibitory effect on fermentative bacteria [187]. The severity of ammonia inhibition is dependent on operational conditions, and studies reveal that thermophilic temperatures and a higher pH (>7) can increase the percentage of total ammonia present in digesters [188]. Methods to limit ammonia inhibition in reactors include adsorption using zeolites [189] or biochars [190], pH reduction [191] and adjustments in C/N ratios [192]. Hydrogen yields were increased by 10–26% in a study by [193] utilising zeolites for ammonia stripping. The use of nitrogen sparging and sulfuric acid absorption was used by Ye et al. [123] to remove ammonia from a waste-activated sludge digester, which enhanced total VFA yields by 21.7%.
Many other inhibitory substances and contaminants, such as sulphides, phenols and furans, may already be present in organic waste streams and methods to limit their inhibition include dilution, adsorption, acclimatisation and precipitation. Sulphate derivates, such as sulphides, can be toxic to fermentative microbes, and sulphate-reducing bacteria have been reported to outcompete hydrogen-producing acetogens through the consumption of substrates, such as butyrate and propionate [194,195]. It is therefore critical to control the levels of sulphates (and sulphides) within bioreactors. Desulphurisation techniques, including the use of bio scrubbers [196] and biofilms [197], have shown promise in large-scale systems. Additionally, Dhar et al. [198] reported that the Fe2+ addition was successful in limiting biohydrogen inhibition caused by sulphide in excess of 0.025 g/L. Metal ion addition has also been used to limit the inhibition effect of humic acid in a sludge anaerobic digester [199].
The presence of phenols and furans within bioreactors can negatively impact the growth and cell membrane function of fermentative bacteria [200], and hence, methods to extract them from bioreactors, as well as limit their production, have been investigated. An adsorption resin was used by Trujillo-Reyes et al. [201] to successfully extract phenolic compounds from a raspberry waste stream, accumulating up to 2402 mg gallic acid equivalents/kg of feedstock which significantly enhanced biogas yields. Adsorption using activated carbon supported with a nano zero-valent iron material was also effective in removing phenolic compounds from organic wastewater, which improved the efficiency of hydrogen-producing bacteria and increased the conversion rate of organic matter [202]. As mentioned in Section 2, the choice of pre-treatment method is often one of the most important parameters that impact the presence of both phenolic and furan compounds. For instance, Kim and Karthikeyan [203] found that carbohydrate degradation to furan-derived compounds, in particular furfural, was much greater when applying an acid pre-treatment (873 ppm) compared with an alkali pre-treatment (375 ppm).
While the most common parameters influencing acidogenic fermentation were discussed in this section, it is worth noting that pre-digestion parameters such as inoculum treatment (to limit methanogenesis) and substrate pre-treatment (to enhance its bioavailability) as well as downstream processing (product recovery) are also key to enhance product yields. It is therefore important to approach acidogenic fermentation with a holistic approach with a specific focus on each of these aspects.
4. Product Recovery
Global hydrogen demand increased from 19.2 Mt in 1975 to 73.9 Mt in 2018 [204]. Increasing the production of hydrogen sustainably via low carbon technologies is therefore critical to meet the growing demand. While much focus has been given to biohydrogen production, equal attention has also been given to its recovery. It is important to advance on both these fronts to maximise the desired product yields. A range of recovery options for biohydrogen and VFAs are shown in Table 4.

Table 4.
Examples of biohydrogen and VFA recovery strategies employed in the literature.
Recent work has reported prolonging thermodynamically favourable fermentation conditions for continued and enhanced hydrogen production without subsequent methanogenesis [205]. In this study, a proton exchange membrane was used to recover hydrogen from the gas phase of a sucrose bioreactor in conjunction with carbon dioxide scrubbing. The in situ recovery of these gasses had two effects. The first was that homoacetogenesis was arrested by making the substrates (hydrogen and carbon dioxide) unavailable for homoacetogens to consume. The second effect was to arrest end-product inhibition by preventing hydrogen accumulation in the reactor headspace. This maintained thermodynamically favourable conditions for continued hydrogen production whilst continually recovering purified hydrogen as it was being produced. Hydrogen yields in a 4 L sucrose CSTR were increased from 0.07 to 1.79 mol H2/mol hexose using this methodology over three 24 h HRTs. A similar methodology was employed by Jones et al. [184]; however, in addition to electrochemical hydrogen recovery and carbon dioxide scrubbing, electrodialysis was also used to alleviate end-product inhibition, increasing hydrogen yields almost four-fold from 0.24 to 0.90 mol H2/mol hexose. Whilst no techno-economical work was carried out in this study, the hydraulic retention times were increased from a matter of hours to 2 days, resulting in greater substrate utilisation rates. This is of particular importance to waste remediation industries since, currently, the fermentation of organic wastes to hydrogen is not viable due to low hydraulic retention times resulting in either poor substrate utilisation rates or the requirement to add sparging gases such as nitrogen at economic and environmental costs. Shortening hydraulic retention times may also become problematic for waste treatment facilities, and the contaminated nature of waste streams means that it would be difficult to maintain conditions in which a targeted microbial consortium could thrive. The use of electrochemical means to recover hydrogen from the gas phase of bioreactors would be relatively straightforward to retrofit to existing infrastructure could be powered using excess renewables and increase hydraulic retention times.
In addition to the biological production of hydrogen and methane, the targeted biological production and recovery of VFAs in preference to these biogases have been investigated. Whilst the majority of research into the recovery of VFAs from fermentation broths is at the millilitre scale and could not yet be considered to be industrially applicable, there has been some research, as reported in more detail in Jones et al. [207] in which fermentations at larger scales using real waste, and waste analogues, have been carried out with subsequent VFA recovery. For example, acetic acid, propionic acid, butyric acid, and valeric acid were recovered from a 4 L fermentation of olive mill waste via a combination of filtration and electrodialysis into a solution with total VFA concentrations over 14 g/L [208]. Jones et al. [87,145,206] showed that the targeted recovery of VFAs from the liquid phase of 100 L bioreactors arrests methanogenesis and increases the yields of VFAs. In one of these studies, a combination of filtration and electrodialysis was used to recover a VFA solution of up to 4 g/L from a 100 L continuously fed food waste digester [145]. This VFA recovery also had the effect of increasing substrate utilisation rates and increased the rate of production of VFAs from 4 to 35 mg VFA/g VS d−1 by alleviating end-product inhibition. In the other two studies, similar methodologies were used to recover VFAs from continuously-fed 100 L grass fermentations [87,206]. In one, a combination of mechanical sieving, filtration and electrodialysis recovered a VFA solution of up to 4.8 g/L and increased yields from 287 to 404 mg VFA/g VS. In the other, the addition of a pervaporation stage to exclude non-volatile compounds from recovery, a VFA solution of 4.5 g/L was produced, and yields as high as 875 mg VFA/g VS were achieved. These sorts of results are promising when considering VFAs as a valorisation route for wastes since the methodologies employed would be easily retrofitted to existing infrastructure and are scalable. No techno-economic considerations were made in these bodies of work, and so more research to clarify this aspect of the process would be useful.
5. Techno-Economics and Process Life Cycle
Broadly, biohydrogen production can be split into photo and dark fermentation. Producing hydrogen in these ways has CO2 emissions of 0.1–4.0 kg CO2/kg H2, compared with up to 38 kg CO2/kg H2 for fossil fuel derived hydrogen [204]. For comparison, power to hydrogen via electrolysis is carbon neutral at the point of use; however, the whole life carbon emissions of hydrogen as an energy vector are contingent upon its production pathway [209] (Figure 6).

Figure 6.
Carbon footprint of different pathways to hydrogen. * At point of use when powered by excess renewable energy.
According to IEA [210], coal, natural gas, LPG, gasoline, and diesel have kg CO2 equivalents per kWh of 1.05, 0.55, 0.62, 0.69 and 0.73, respectively. These are still, globally, the most commonly used energy vectors [211], and renewable energy sources contribute to just 10% of global energy consumption, excluding transport, so it would be necessary to increase the global installed capacity of renewables from 2500 GW in 2020 [212] by a factor of six to meet Paris Agreement 2050 targets [213]. The intermittent nature of installed renewable energy sources, 90% of which is solar and wind, requires reliable and sustainable energy storage [211], and hydrogen is an obvious candidate for such storage. In Europe, it is reported that the production costs of hydrogen could fall to less than EUR 2.00/kg by 2050 [214]; however, this relies upon the continued decline of the cost of renewable energy.
Techno-economic analyses for photo fermentative biohydrogen production have briefly been investigated in recent articles. In one [215], a combination of biohydrogen production from 140 ha open pond and 14 ha photobioreactor sources yielded 1.2 million GJ/year of hydrogen, with an initial investment of USD 55 m. Whilst they posit that biohydrogen production units greater than 30 m3 could be profitable, approximately 14% of that profit is derived from the co-production of CO2. Fu et al. [216], however, note that photo fermentation is not without its own issues, including the costly nature of constant illumination and the opacity of the fermentation broth.
When considering dark-fermentation, modelling of biohydrogen production from the co-digestion of food and beverage wastewater found that a plant with a biohydrogen production rate of 63,000 m3/year could have a production cost of USD 1/m3 [217]. In the same article, it was reported that when agricultural residue was used as the substrate, hydrogen could be produced at USD 2.57 and 2.83/kg with dark and photo fermentation, respectively. Encouragingly, payback periods of between 5 and 10 years have been reported for hydrogen production from the fermentation, depending on scale, comparing favourably with gasification and steam methane reforming, which can have payback periods as high as 20 years [218].
Despite these promising production data, other researchers [219] conclude that the lack of synergy between academic research and industrial implementation makes a current jump to market unachievable for biohydrogen, especially where biohydrogen at the lab scale has been produced under very strict and steady fermentation conditions. Further, an economic feasibility threshold of USD 1.50/kg has been reported in other research [220], suggesting that the technology is not yet mature enough for commercial success. Other studies [221] also report costs as high as USD 6.98/kg for hydrogen production from dark fermentation, so there is considerable disparity amongst the literature.
When considering hydrogen production from wastewaters only, dark fermentation is shown to be the best economical path to hydrogen when compared with photo fermentation, electrolysis, electrodialysis, photocatalysis, photoelectrochemical methods and super water gasification [220]. There is however room for considerable improvements in biohydrogen production. A number of bottlenecks to biological hydrogen production have been established, and there is a growing body of lab-scale work to overcome these problems. Fu et al. [216] review anaerobic biohydrogen production from waste-activated sludge and note that whilst the literature widely reports high theoretical maxima for hydrogen yields from waste-activated sludge, real-world yields are always significantly lower. This is mostly due to hydrogen-producing microorganisms mostly metabolising soluble carbohydrates in favour of proteins, humic matter, VFAs and alcohols, all of which are typically abundant in industrial wastewaters. Further, the hydrogen produced is typically consumed rapidly by methanogens, acetogens and sulphate reducers to produce H2S.
As mentioned in Section 2 and Section 3, strategies to improve hydrogen production have long been employed. The techno-economic applicability of these strategies to industrial-scale hydrogen production however has yet to be investigated, and more work is called for. A recent study [222] notes that because these limitations of hydrogen production have yet to be addressed and tested on large scales, the techno-economics of biohydrogen production are still less attractive than producing hydrogen from even non-renewable sources such as fossil fuels. This is exacerbated by higher costs per unit of hydrogen at smaller scales, which may be reduced by up to eight times upon scale-up [204]. Similarly, Lepage et al. [223] state that whilst biohydrogen is a promising future possibility, it is currently not mature enough to compete with established thermochemical pathways despite the unsustainable nature of the latter. This highlights the need for greater work to demonstrate larger-scale hydrogen production utilising industrially applicable engineering solutions.
Another issue for the economic feasibility of biohydrogen is competition from the other sustainable pathways to hydrogen, notably electrolysis driven by excess wind, solar and hydropower. The decreasing costs of renewable energy as a means through which to power electrolysis, especially considering solar PV costs less than USD 1/W, thanks to falling silicon prices [224]. Competitive levelized costs of energy from hydrogen have been demonstrated in several studies. Off-grid hybrid systems of solar PV, wind and hydrogen fuel cells were simulated in various configurations for regularly and seasonally occupied households and found energy costs as low as USD 0.309/kWh [225], taking into account the capital expenditure of the technologies required to achieve such an energy scenario. On larger scales, similar and self-sufficient energy scenarios have been reported with energy costs of USD 0.394/kWh [226] and USD 0.334/kWh. It is also reported however that energy management systems and methods to integrate and coordinate sustainable hydrogen production systems need to be optimised before they can no longer be considered economic drawbacks to larger-scale projects [211].
A common theme in the literature is that the cost of electrolysis is the main economic limiting factor when considering hydrogen as a sustainably derived energy vector [214,227]. Storage has been posited as a means through which to make better use of existing electrolysis capacity without the installation of further electrolysis apparatus, especially when that hydrogen can be stored in major geological features such as salt caves [227]. In some European countries, the hydrogen capacity in such formations exceeds 10 EJ of hydrogen [214].
As alluded to above, storing hydrogen is becoming less of an unknown, especially with the emergence of solid-state storage in stand-alone use cases. It is reported however that more work is required to establish the availability, mass and degeneration of storage facilities [224]. It is also reported that storing hydrogen in its compressed form is already commercially viable [228], provided it is transported by road, and that liquefied hydrogen with a gravimetric energy density of 2.0 kWh/kg can be achieved at a cost of USD 6.00/kWh, lower than the USA Department of Energy’s goal of USD 8.00/kWh.
In shifting from a carbon-based energy economy to a hydrogen-based one, changes to infrastructure need to be considered from a techno-economic point of view. The majority of the reported literature excludes calculations surrounding vehicular hydrogen use, which is of concern because transportation is currently the least diverse energy use sector, with 90% of its energy demand met by fossil fuels [229]. By 2050 however, the transportation sector is predicted to be the single greatest demand case for hydrogen as an energy vector [229]. To make this affordable for consumers however high transportation and refuelling station costs will need to be addressed [230]. The purity required for vehicular hydrogen will pose a problem for biological hydrogen, which will either need to be purified downstream or extracted from biogas mixtures in such a way that makes it inherently high-purity as per the electrochemical approach employed by Massanet-Nicolau et al. [205].
Another bottleneck for large-scale and industrial hydrogen production is likely to be international cooperation. Nazir et al. [228] summarise that a critical and often overlooked issue for any future zero-carbon economy is international cooperation and the requirement for legislation and regulations to be harmonious and transnational. Modelling has been undertaken [231] investigating the hypothetical outcomes of the implementation of a renewable hydrogen quota imposed upon European gas and electricity markets. This would increase electricity prices by 12%, with renewable energy producers being the biggest beneficiaries. Gas prices however would fall by 3% for the consumer; however, there would be a net benefit to energy producers. Policy recommendations for European hydrogen implementation have been suggested [232], including coordinated incentivisation of hydrogen vehicle purchases and refuelling infrastructure; harmonising the blending concentrations for hydrogen and natural gas; implementing certificates and obligations for products produced and obtained using low-carbon hydrogen; and promoting the production of low-carbon hydrogen by penalising polluting activities further.
As mentioned in the previous section, biological hydrogen production is similar in methodology to biological VFA production, and both can be produced from common substrates. As such, there will be direct competition for these substrates, and the likelihood is that the most economically viable product will be successful, especially considering there are competing, alternative, renewable pathways to hydrogen. According to the IEA [210], the demand for hydrogen is approximately 120 megatons per year, with a global market value of USD 130 bn. The demand for VFAs is 16,820–18,500 kilotons per year, with an approximate market size of USD 17.1 bn per year [87,233]; smaller in terms of both mass and market size. There is also a growing case for the economically viable production of VFAs from waste. The wholesale price for VFAs such as propionic acid is currently USD 6.00/kg, and assuming sympathetic government incentives and favourable conditions, it can be derived from industrial wastewater at USD 3.80/kg [233]. Bahreini et al. [234] also found that although VFA extraction inherently reduces biogas yields, the cost of this reduction is offset and exceeded by the income generated by the recovered VFAs, resulting in short payback periods ranging from 1.6 to 6.3 years. There is even now an emerging case for the utilisation of cash crops from which to derive VFAs from anaerobic digestion. Moscariello et al. [235] carried out millilitre scale batch digestions of pre-treated hemp biomass residue and calculated that with alkaline pre-treatment, revenues of EUR 9364/ha/year could be achieved. It is not clear however how appropriate this sort of pre-treatment would be at industrial scales, especially from an environmental standpoint. The versatility of VFAs as a platform chemical with which to valorise biowastes is highlighted in Huq et al. [236], who make the techno-economic case for the use of food-waste-derived VFAs in the synthesis of aviation fuel. In this case, production costs are as low as USD 0.30/kg, with a wholesale price of USD 2.50/gallon. This compares favourably with the cost of producing hydrogen from biomass, being several orders of magnitude less costly than the above-reported costs of biohydrogen production.
As it stands, the least costly means through which to produce hydrogen sustainably remains to use excess renewable energy, potentially leaving room for VFA production from bioremediation of wastes in preference to methane. This is especially true when considering the diverse, growing markets for VFAs, including disinfection, herbicides, bleaching, the food industry, the textile industry, pharmaceuticals, fungicides, polymer production, cosmetics and lubricants [207]. Given the increasing demand for bioplastic production and the finite nature of fossil fuels, PHA production is of particular interest here with recent research [237], demonstrating through life cycle analyses that the cumulative energy demand of PHAs produced using VFAs from an early stage, non-optimised grass-fed bioreactors are in the same order of magnitude as deriving polymers from fossil fuels.
Further work is called for to optimise the VFA production and recovery processes to fulfil the promise shown by this sustainable pathway to PHAs, framed in the context of decreasing demand for grass as a grazing crop but with little scope for viable alternative uses for that land.
6. Future Perspectives
There is an immense potential to utilise ‘wastes’ as ‘resources’ to generate value and contribute to a circular economy framework. Biorefineries are seen as one of the promising routes to contribute to sustainability. A multiple-product platform is attractive techno-economically; however, the challenges in achieving desired product yields to break even are not yet established at an industrial scale. With biogas from AD being a low-value product, research attention has been diverted towards the production of biohydrogen and VFAs as promising energy carriers and platform chemicals. While the biochemical pathways to produce biohydrogen and VFAs via AD overlap, there is a possibility of establishing a biorefinery where biohydrogen can be the primary product. The VFA mixture in the liquid phase could be recovered (as a mixture as opposed to a specific VFA with high purity) and valorised further to produce polyhydroxyalkanoates (PHAs) that can be used to make bioplastics [238]. Moreover, coupling allied processes such as photo fermentation and dark fermentation [239] or biochemical and bioelectrochemical systems [240] can pave the way for maximising product recovery via biorefineries.
A number of strategies relating to the intensification of AD for biohydrogen or VFA production were discussed throughout this review; however, there are gaps in several areas that still need more attention. For instance, with the feedstock composition and heterogeneity largely influencing the end product productivity and yields, focused research on scalable pre-treatment methods is needed. The general consensus is that physico-chemical methods are energetically and environmentally favourable when compared to their counterparts, and while this is a generality, evidence to support this is limited. Hydrodynamic cavitation has been utilised effectively as a pre-treatment method for enhancing biogas yields [2], and the preliminary evidence on positively influencing acidogenic fermentation yields is promising [46]. Similarly, thermal hydrolysis has shown immense potential for enhancing biogas yields (e.g., the CAMBI thermal hydrolysis process) from sewage sludge at a commercial level; however, its influence on acidogenic fermentation is unclear.
The choice of bioreactor and its configuration is also important in maximising product yields. While CSTRs have always been the first-choice reactor, alternative configurations with better offerings must be explored to improve the fluid dynamics and mass transfer within the system (Table 3). Another aspect that requires deep focus is the selection of appropriate product recovery methodologies. While there are established product recovery strategies for recovering biohydrogen and VFAs from bioreactors, the combination of technology, scale and mode of operation and productivity must not lead to detrimental techno-economics. Furthermore, to achieve a triple bottom line performance of social-economic-environmental benefits, in addition to techno-economic feasibility studies, an LCA investigation is also required. In particular, with the market demand for sustainable VFAs being high, a limited investigation at scale and LCA is needed.
Besides these aspects that require extensive focus, some of the emerging areas in AD include two-stage digestion for biohythane production (maximising energy recovery), on-demand production of VFAs or biohydrogen (feeding the supply chain depending on market needs) and intersectoral coupling to enable efficient waste management (unconventional wastes as resources). Collective efforts in multiple innovative areas as required in addition to intensifying the existing promising options to meet the goals of net-zero emissions by 2050.
7. Conclusions
Shifting focus from conventional biogas production towards biohydrogen or VFAs has been receiving renewed attention recently. Advancements in technology and policy support have paved the way for initiating this revived interest. This presented a need to analyse the literature critically and establish the current status of acidogenic fermentation. This review has specifically addressed this in three main areas; pre-digestion focusing on substrate pre-treatment as a measure to address feedstock heterogeneity, parameter optimisation for improving product yields and product recovery. The techno-economic aspects of biohydrogen and VFA production were also discussed as appropriate. Finally, gaps in these areas were highlighted and the future research direction required was identified. Amongst the areas discussed, three main conclusions were drawn: a scalable pre-treatment that is both effective and energetically favourable is needed, reactor designs specific to handling the feedstock of interest must be exploited and energy-efficient and scalable product recovery options need to be integrated downstream of the fermenter to maximise product yields and economics.
Author Contributions
Conceptualization, S.N.; writing—original draft preparation, S.N., R.J.J. and L.O.; writing—review and editing, S.N., J.M.-N. and A.G.; funding acquisition, J.M.-N. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sêr Cymru II—WEFO ERDF Programme 80761 for the VFA Factory project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge the support of Maria Ramos-Suarez for providing her comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- International Energy Agency. Data and Statistics. Available online: https://www.iea.org/data-and-statistics/data-browser?country=UK&fuel=CO2%20emissions&indicator=CO2BySector (accessed on 3 July 2022).
- Nagarajan, S.; Ranade, V.V. Valorizing Waste Biomass via Hydrodynamic Cavitation and Anaerobic Digestion. Ind. Eng. Chem. Res. 2021, 60, 16577–16598. [Google Scholar] [CrossRef]
- Demir, M.E.; Chehade, G.; Dincer, I.; Yuzer, B.; Selcuk, H. Design and analysis of a new system for photoelectrochemical hydrogen production from wastewater. Energy Convers. Manag. 2019, 199, 111903. [Google Scholar] [CrossRef]
- Nagarajan, S.; Skillen, N.; Robertson, P.; Lawton, L. Cellulose Photocatalysis for Renewable Energy Production. In Metal, Metal-Oxides and Metal Sulfides for Batteries, Fuel Cells, Solar Cells, Photocatalysis and Health Sensors; Rajendran, S., Karimi-Maleh, H., Qin, J., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–34. [Google Scholar]
- Nagarajan, S.; Skillen, N.C.; Irvine, J.T.S.; Lawton, L.A.; Robertson, P.K.J. Cellulose II as bioethanol feedstock and its advantages over native cellulose. Renew. Sustain. Energy Rev. 2017, 77, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Skillen, N.; Nagarajan, S.; Ralphs, K.; Irvine, J.T.S.; Lawton, L.; Robertson, P.K.J. Using cellulose polymorphs for enhanced hydrogen production from photocatalytic reforming. Sustain. Energy Fuels 2019, 3, 1971–1975. [Google Scholar] [CrossRef]
- Wakerley, D.W.; Kuehnel, M.F.; Orchard, K.L.; Ly, K.H.; Rosser, T.E.; Reisner, E. Solar-driven reforming of lignocellulose to H2 with a CdS/CdOx photocatalyst. Nat. Energy 2017, 2, 17021. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, S. Development of Photocatalytic Reactor Technology for the Production of Fermentable Sugars. Ph.D. Thesis, Queen’s University, Belfast, Ireland, 2017. [Google Scholar]
- Meynell, P.J. Methane: Planning a Digester; Schocken Books: New York, NY, USA, 1976. [Google Scholar]
- NNFCC. Biomethane RTFC Calculator. Available online: https://www.nnfcc.co.uk/publications/tool-biomethane-rtfc-calculator (accessed on 6 April 2022).
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Nutrient Recovery from Digestate: Systematic Technology Review and Product Classification. Waste Biomass Valorization 2017, 8, 21–40. [Google Scholar] [CrossRef] [Green Version]
- Pang, S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef]
- Kang, K.; Klinghoffer, N.B.; ElGhamrawy, I.; Berruti, F. Thermochemical conversion of agroforestry biomass and solid waste using decentralized and mobile systems for renewable energy and products. Renew. Sustain. Energy Rev. 2021, 149, 111372. [Google Scholar] [CrossRef]
- Ong, H.C.; Chen, W.-H.; Farooq, A.; Gan, Y.Y.; Lee, K.T.; Ashokkumar, V. Catalytic thermochemical conversion of biomass for biofuel production: A comprehensive review. Renew. Sustain. Energy Rev. 2019, 113, 109266. [Google Scholar] [CrossRef]
- Nagarajan, S.; Stella, L.; Lawton, L.A.; Irvine, J.T.S.; Robertson, P.K.J. Mixing regime simulation and cellulose particle tracing in a stacked frame photocatalytic reactor. Chem. Eng. J. 2017, 313, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-W.; Nguyen, B.-S.; Wu, J.C.S.; Nguyen, V.-H. A current perspective for photocatalysis towards the hydrogen production from biomass-derived organic substances and water. Int. J. Hydrogen Energy 2020, 45, 18144–18159. [Google Scholar] [CrossRef]
- Lu, X.; Xie, S.; Yang, H.; Tong, Y.; Ji, H. Photoelectrochemical hydrogen production from biomass derivatives and water. Chem. Soc. Rev. 2014, 43, 7581–7593. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Kamarudin, S.K.; Minggu, L.J. Biofuel from biomass via photo-electrochemical reactions: An overview. J. Power Sources 2014, 259, 33–42. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current perspectives on acidogenic fermentation to produce volatile fatty acids from waste. Rev. Environ. Sci. Bio/Technol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- UK BEIS. The Ten Point Plan for a Green Industrial Revolution. 2020; Volume 38. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/936567/10_POINT_PLAN_BOOKLET.pdf (accessed on 7 April 2022).
- UK BEIS. UK Hydrogen Strategy. 2021. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1011283/UK-Hydrogen-Strategy_web.pdf (accessed on 7 April 2022).
- UK BEIS. Industrial Decarbonisation Strategy. 2021. Available online: https://www.gov.uk/government/publications/industrial-decarbonisation-strategy (accessed on 7 April 2022).
- UK DEFRA. A Green Future: Our 25 Year Plan to Improve the Environment. 2018. Available online: https://www.gov.uk/government/publications/25-year-environment-plan (accessed on 7 April 2022).
- European Commission. A Hydrogen Strategy for a Climate-Neutral Europe. 2020. Available online: https://ec.europa.eu/energy/sites/ener/files/hydrogen_strategy.pdf (accessed on 8 April 2022).
- Woodward, E.; Han, O.; Forbes, C. Enabling the European Hydrogen Economy; Aurora Energy Research: Oxford, UK, 2021. [Google Scholar]
- International Energy Agency. Global Hydrogen Review; IEA: Paris, France, 2021; p. 224. [Google Scholar]
- Braga, L.B.; Silveira, J.L.; da Silva, M.E.; Tuna, C.E.; Machin, E.B.; Pedroso, D.T. Hydrogen production by biogas steam reforming: A technical, economic and ecological analysis. Renew. Sustain. Energy Rev. 2013, 28, 166–173. [Google Scholar] [CrossRef]
- Gorski, J.; Jutt, T.; Tam Wu, K. Carbon intensity of blue hydrogen production. Pembin. Inst. 2021. Available online: https://www.pembina.org/reports/carbon-intensity-of-blue-hydrogen-revised.pdf (accessed on 16 June 2022).
- Ali Khan, M.H.; Daiyan, R.; Neal, P.; Haque, N.; MacGill, I.; Amal, R. A framework for assessing economics of blue hydrogen production from steam methane reforming using carbon capture storage & utilisation. Int. J. Hydrogen Energy 2021, 46, 22685–22706. [Google Scholar]
- Sun, X.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. Syngas fermentation process development for production of biofuels and chemicals: A review. Bioresour. Technol. Rep. 2019, 7, 100279. [Google Scholar]
- Ewing, M.; Israel, B.; Jutt, T.; Talebian, H.; Stepanik, L. Hydrogen on the Path to Net-Zero Emissions–Costs and Climate Benefits; Pembina Institute: Toronto, ON, Canada, 2020; p. 6. [Google Scholar]
- Hitam, C.N.C.; Jalil, A.A. A review on biohydrogen production through photo-fermentation of lignocellulosic biomass. Biomass Convers. Biorefin. 2020, 1–19. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar]
- Bundhoo, Z.M.A. Potential of bio-hydrogen production from dark fermentation of crop residues: A review. Int. J. Hydrogen Energy 2019, 44, 17346–17362. [Google Scholar]
- Rajesh Banu, J.; Merrylin, J.; Mohamed Usman, T.M.; Yukesh Kannah, R.; Gunasekaran, M.; Kim, S.-H.; Kumar, G. Impact of pretreatment on food waste for biohydrogen production: A review. Int. J. Hydrogen Energy 2020, 45, 18211–18225. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Loh, K.-C. Review and perspectives of enhanced volatile fatty acids production from acidogenic fermentation of lignocellulosic biomass wastes. Bioresour. Bioprocess. 2021, 8, 68. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Q.; Zhang, Z.; Jing, Y.; Hu, J.; He, C.; Lu, C. A review on biological recycling in agricultural waste-based biohydrogen production: Recent developments. Bioresour. Technol. 2022, 347, 126595. [Google Scholar] [PubMed]
- Miao, Z.; Grift, T.E.; Hansen, A.C.; Ting, K.C. Energy requirement for comminution of biomass in relation to particle physical properties. Ind. Crops Prod. 2011, 33, 504–513. [Google Scholar] [CrossRef]
- Konde, K.S.; Nagarajan, S.; Kumar, V.; Patil, S.V.; Ranade, V.V. Sugarcane bagasse based biorefineries in India: Potential and challenges. Sustain. Energy Fuels 2021, 5, 52–78. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Bundhoo, M.A.Z.; Mohee, R.; Hassan, M.A. Effects of pre-treatment technologies on dark fermentative biohydrogen production: A review. J. Environ. Manag. 2015, 157, 20–48. [Google Scholar] [CrossRef]
- Cheng, X.-Y.; Liu, C.-Z. Fungal pretreatment enhances hydrogen production via thermophilic fermentation of cornstalk. Appl. Energy 2012, 91, 1–6. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Garuti, M.; Langone, M.; Fabbri, C.; Piccinini, S. Monitoring of full-scale hydrodynamic cavitation pretreatment in agricultural biogas plant. Bioresour. Technol. 2018, 247, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Lanfranchi, A.; Tassinato, G.; Valentino, F.; Martinez, G.A.; Jones, E.; Gioia, C.; Bertin, L.; Cavinato, C. Hydrodynamic cavitation pre-treatment of urban waste: Integration with acidogenic fermentation, PHAs synthesis and anaerobic digestion processes. Chemosphere 2022, 301, 134624. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Ranade, V.V. Pre-treatment of distillery spent wash (vinasse) with vortex based cavitation and its influence on biogas generation. Bioresour. Technol. Rep. 2020, 11, 100480. [Google Scholar] [CrossRef]
- Nagarajan, S.; Ranade, V.V. Pretreatment of Lignocellulosic Biomass Using Vortex-Based Devices for Cavitation: Influence on Biomethane Potential. Ind. Eng. Chem. Res. 2019, 58, 15975–15988. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Kavitha, S.; Sivashanmugham, P.; Kumar, G.; Nguyen, D.D.; Chang, S.W.; Rajesh Banu, J. Biohydrogen production from rice straw: Effect of combinative pretreatment, modelling assessment and energy balance consideration. Int. J. Hydrogen Energy 2019, 44, 2203–2215. [Google Scholar] [CrossRef]
- Deng, C.; Lin, R.; Cheng, J.; Murphy, J.D. Can acid pre-treatment enhance biohydrogen and biomethane production from grass silage in single-stage and two-stage fermentation processes? Energy Convers. Manag. 2019, 195, 738–747. [Google Scholar] [CrossRef]
- Reilly, M.; Dinsdale, R.; Guwy, A. Mesophilic biohydrogen production from calcium hydroxide treated wheat straw. Int. J. Hydrogen Energy 2014, 39, 16891–16901. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Konopacka-Łyskawa, D.; Słupek, E.; Makoś, P.; Cieśliński, H.; Kamiński, M. Influence of alkaline and oxidative pre-treatment of waste corn cobs on biohydrogen generation efficiency via dark fermentation. Biomass Bioenergy 2020, 141, 105691. [Google Scholar] [CrossRef]
- Wu, J.; Upreti, S.; Ein-Mozaffari, F. Ozone pretreatment of wheat straw for enhanced biohydrogen production. Int. J. Hydrogen Energy 2013, 38, 10270–10276. [Google Scholar] [CrossRef]
- Leaño, E.P.; Babel, S. Effects of pretreatment methods on cassava wastewater for biohydrogen production optimization. Renew. Energy 2012, 39, 339–346. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Venkata Mohan, S. Bio-electrohydrolysis as a pretreatment strategy to catabolize complex food waste in closed circuitry: Function of electron flux to enhance acidogenic biohydrogen production. Int. J. Hydrogen Energy 2014, 39, 11411–11422. [Google Scholar] [CrossRef]
- Choiron, M.; Tojo, S.; Chosa, T. Biohydrogen production improvement using hot compressed water pretreatment on sake brewery waste. Int. J. Hydrogen Energy 2020, 45, 17220–17232. [Google Scholar] [CrossRef]
- Asadi, N.; Zilouei, H. Optimization of organosolv pretreatment of rice straw for enhanced biohydrogen production using Enterobacter aerogenes. Bioresour. Technol. 2017, 227, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, J. Enhancing biohydrogen production from disintegrated sewage sludge by combined sodium citrate-thermal pretreatment. J. Cleaner Prod. 2021, 312, 127756. [Google Scholar] [CrossRef]
- Dinesh Kumar, M.; Kaliappan, S.; Gopikumar, S.; Zhen, G.; Rajesh Banu, J. Synergetic pretreatment of algal biomass through H2O2 induced microwave in acidic condition for biohydrogen production. Fuel 2019, 253, 833–839. [Google Scholar] [CrossRef]
- Salem, A.H.; Brunstermann, R.; Mietzel, T.; Widmann, R. Effect of pre-treatment and hydraulic retention time on biohydrogen production from organic wastes. Int. J. Hydrogen Energy 2018, 43, 4856–4865. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J. Pretreatment of macroalgal Laminaria japonica by combined microwave-acid method for biohydrogen production. Bioresour. Technol. 2018, 268, 52–59. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, Y.; Wang, J. Pretreatment of antibiotic fermentation residues by combined ultrasound and alkali for enhancing biohydrogen production. J. Clean. Prod. 2020, 268, 122190. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Ultrasonic pretreatment for an enhancement of biohydrogen production from complex food waste. Int. J. Hydrogen Energy 2014, 39, 7721–7729. [Google Scholar] [CrossRef]
- She, Y.; Hong, J.; Zhang, Q.; Chen, B.-Y.; Wei, W.; Xin, X. Revealing microbial mechanism associated with volatile fatty acids production in anaerobic acidogenesis of waste activated sludge enhanced by freezing/thawing pretreatment. Bioresour. Technol. 2020, 302, 122869. [Google Scholar] [CrossRef]
- Zeng, Q.; Zan, F.; Hao, T.; Khanal, S.K.; Chen, G. Sewage sludge digestion beyond biogas: Electrochemical pretreatment for biochemicals. Water Res. 2022, 208, 117839. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; Um, Y.; Yoon, H.H. Pretreatment of macroalgae for volatile fatty acid production. Bioresour. Technol. 2013, 146, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, J.; Wang, M.; Xin, X.; Xu, J.; Zhang, J. Efficient Volatile Fatty Acids Production from Waste Activated Sludge after Ferrate Pretreatment with Alkaline Environment and the Responding Microbial Community Shift. ACS Sustain. Chem. Eng. 2018, 6, 16819–16827. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, P.; Zhang, G.; Nabi, M.; Wang, S.; Ye, J.; Bao, S.; Zhang, Q.; Chen, N. Carbide slag pretreatment enhances volatile fatty acid production in anaerobic fermentation of four grass biomasses. Energy Convers. Manag. 2019, 199, 112009. [Google Scholar] [CrossRef]
- Wu, Q.-L.; Guo, W.-Q.; Bao, X.; Yin, R.-L.; Feng, X.-C.; Zheng, H.-S.; Luo, H.-C.; Ren, N.-Q. Enhancing sludge biodegradability and volatile fatty acid production by tetrakis hydroxymethyl phosphonium sulfate pretreatment. Bioresour. Technol. 2017, 239, 518–522. [Google Scholar] [CrossRef]
- Han, F.; Wu, L. Comprehensive Utilization of Carbide Slag. In Industrial Solid Waste Recycling in Western China; Han, F., Wu, L.E., Eds.; Springer: Singapore, 2019; pp. 357–391. [Google Scholar]
- Fang, W.; Zhang, P.; Zhang, X.; Zhu, X.; van Lier, J.B.; Spanjers, H. White rot fungi pretreatment to advance volatile fatty acid production from solid-state fermentation of solid digestate: Efficiency and mechanisms. Energy 2018, 162, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Zhang, X.; Zhang, P.; Carol Morera, X.; van Lier, J.B.; Spanjers, H. Evaluation of white rot fungi pretreatment of mushroom residues for volatile fatty acid production by anaerobic fermentation: Feedstock applicability and fungal function. Bioresour. Technol. 2020, 297, 122447. [Google Scholar] [CrossRef]
- Bahreini, G.; Nazari, L.; Ho, D.; Flannery, C.C.; Elbeshbishy, E.; Santoro, D.; Nakhla, G. Enzymatic pre-treatment for enhancement of primary sludge fermentation. Bioresour. Technol. 2020, 305, 123071. [Google Scholar] [CrossRef]
- Owusu-Agyeman, I.; Balachandran, S.; Plaza, E.; Cetecioglu, Z. Co-fermentation of municipal waste streams: Effects of pretreatment methods on volatile fatty acids production. Biomass Bioenergy 2021, 145, 105950. [Google Scholar] [CrossRef]
- Kakar, F.l.; Koupaie, E.H.; Razavi, A.S.; Hafez, H.; Elbeshbishy, E. Effect of Hydrothermal Pretreatment on Volatile Fatty Acids Production from Thickened Waste Activated Sludge. BioEnergy Res. 2020, 13, 591–604. [Google Scholar] [CrossRef]
- Suresh, A.; Seo, C.; Chang, H.N.; Kim, Y.-C. Improved volatile fatty acid and biomethane production from lipid removed microalgal residue (LRμAR) through pretreatment. Bioresour. Technol. 2013, 149, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ma, H.; Boer, E.d.; Wu, W.; Wang, Q.; Gao, M.; Vo, D.-V.N.; Guo, M.; Xia, C. Effect of microwave/hydrothermal combined ionic liquid pretreatment on straw: Rumen anaerobic fermentation and enzyme hydrolysis. Environ. Res. 2022, 205, 112453. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wu, Q.; Yang, S.; Luo, H.; Peng, S.; Ren, N. Optimization of ultrasonic pretreatment and substrate/inoculum ratio to enhance hydrolysis and volatile fatty acid production from food waste. RSC Adv. 2014, 4, 53321–53326. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, J.; Yan, F.; Gao, Y.; Meng, Y.; Aihemaiti, A.; Ju, T. Enhancement of volatile fatty acid production and biogas yield from food waste following sonication pretreatment. J. Environ. Manag. 2018, 217, 797–804. [Google Scholar] [CrossRef]
- Wang, S.; Tao, X.; Zhang, G.; Zhang, P.; Wang, H.; Ye, J.; Li, F.; Zhang, Q.; Nabi, M. Benefit of solid-liquid separation on volatile fatty acid production from grass clipping with ultrasound-calcium hydroxide pretreatment. Bioresour. Technol. 2019, 274, 97–104. [Google Scholar] [CrossRef]
- Wan, J.; Fang, W.; Zhang, T.; Wen, G. Enhancement of fermentative volatile fatty acids production from waste activated sludge by combining sodium dodecylbenzene sulfonate and low-thermal pretreatment. Bioresour. Technol. 2020, 308, 123291. [Google Scholar] [CrossRef]
- Baeyens, J.; Zhang, H.; Nie, J.; Appels, L.; Dewil, R.; Ansart, R.; Deng, Y. Reviewing the potential of bio-hydrogen production by fermentation. Renew. Sustain. Energy Rev. 2020, 131, 110023. [Google Scholar] [CrossRef]
- Elsharnouby, O.; Hafez, H.; Nakhla, G.; El Naggar, M.H. A critical literature review on biohydrogen production by pure cultures. Int. J. Hydrogen Energy 2013, 38, 4945–4966. [Google Scholar] [CrossRef]
- Krupp, M.; Widmann, R. Biohydrogen production by dark fermentation: Experiences of continuous operation in large lab scale. Int. J. Hydrogen Energy 2009, 34, 4509–4516. [Google Scholar] [CrossRef]
- Mosey, F.E. Mathematical Modelling of the Anaerobic Digestion Process: Regulatory Mechanisms for the Forma Tion of Short-Chain Volatile Acids From Glucose; IWA: Nanjing, China, 1983; pp. 209–232. [Google Scholar]
- Zhou, M.; Yan, B.; Wong, J.W.C.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Fernandez-Feito, R.; Dinsdale, R.M.; Guwy, A.J. Recovery and enhanced yields of volatile fatty acids from a grass fermentation via in-situ solids separation and electrodialysis. J. Clean. Prod. 2021, 296, 126430. [Google Scholar] [CrossRef]
- Greses, S.; Tomás-Pejó, E.; González-Fernández, C. Short-chain fatty acids and hydrogen production in one single anaerobic fermentation stage using carbohydrate-rich food waste. J. Clean. Prod. 2021, 284, 124727. [Google Scholar] [CrossRef]
- Van Ginkel, S.; Logan, B.E. Inhibition of biohydrogen production by undissociated acetic and butyric acids. Environ. Sci. Technol. 2005, 39, 9351–9356. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, Y.; Dai, K.; Shen, N.; Zeng, R.J. The glucose metabolic distribution in thermophilic (55 °C) mixed culture fermentation: A chemostat study. Int. J. Hydrogen Energy 2015, 40, 919–926. [Google Scholar] [CrossRef]
- Kirli, B.; Karapinar, I. The effect of HRT on biohydrogen production from acid hydrolyzed waste wheat in a continuously operated packed bed reactor. Int. J. Hydrogen Energy 2018, 43, 10678–10685. [Google Scholar] [CrossRef]
- Nagarajan, S.; Prasad Sarvothaman, V.; Knörich, M.; Ranade, V.V. A simplified model for simulating anaerobic digesters: Application to valorisation of bagasse and distillery spent wash. Bioresour. Technol. 2021, 337, 125395. [Google Scholar] [CrossRef]
- Kumar, G.; Sivagurunathan, P.; Park, J.H.; Park, J.H.; Park, H.D.; Yoon, J.J.; Kim, S.H. HRT dependent performance and bacterial community population of granular hydrogen-producing mixed cultures fed with galactose. Bioresour. Technol. 2016, 206, 188–194. [Google Scholar] [CrossRef]
- Massanet-Nicolau, J.; Dinsdale, R.; Guwy, A. Hydrogen production from sewage sludge using mixed microflora inoculum: Effect of pH and enzymatic pretreatment. Bioresour. Technol. 2008, 99, 6325–6331. [Google Scholar] [CrossRef]
- Moreno-Andrade, I.; Carrillo-Reyes, J.; Santiago, S.G.; Bujanos-Adame, M.C. Biohydrogen from food waste in a discontinuous process: Effect of HRT and microbial community analysis. J. Hydrogen Energy 2015, 30, 17246–17252. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.; Liu, Y.; Nghiem, L.D.; Chang, S.W.; Nguyen, D.D.; Zhang, S.; Luo, G.; Jia, H. Optimization of hydraulic retention time and organic loading rate for volatile fatty acid production from low strength wastewater in an anaerobic membrane bioreactor. Bioresour. Technol. 2019, 271, 100–108. [Google Scholar] [CrossRef]
- Lv, N.; Cai, G.; Pan, X.; Li, Y.; Wang, R.; Li, J.; Li, C.; Zhu, G. pH and hydraulic retention time regulation for anaerobic fermentation: Focus on volatile fatty acids production/distribution, microbial community succession and interactive correlation. Bioresour. Technol. 2022, 347, 126310. [Google Scholar] [CrossRef] [PubMed]
- Moretto, G.; Valentino, F.; Pavan, P.; Majone, M.; Bolzonella, D. Optimization of urban waste fermentation for volatile fatty acids production. Waste Manag. 2019, 92, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.; Duber, A.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Conversion of organic waste into volatile fatty acids–The influence of process operating parameters. Chem. Eng. J. 2018, 345, 395–403. [Google Scholar] [CrossRef]
- Ferraz Júnior, A.Ô.D.N.; Zaiat, M.; Gupta, M.; Elbeshbishy, E.; Hafez, H.; Nakhla, G. Impact of organic loading rate on biohydrogen production in an up-flow anaerobic packed bed reactor (UAnPBR). Bioresour. Technol. 2014, 164, 371–379. [Google Scholar] [CrossRef]
- Fuess, L.T.; Mazine Kiyuna, L.S.; Garcia, M.L.; Zaiat, M. Operational strategies for long-term biohydrogen production from sugarcane stillage in a continuous acidogenic packed-bed reactor. Int. J. Hydrogen Energy 2016, 41, 8132–8145. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Wang, X.; Hu, Y.; Zhang, Y.; Li, Y. Lactic acid fermentation from food waste with indigenous microbiota: Effects of pH, temperature and high OLR. Waste Manag. 2016, 52, 278–285. [Google Scholar] [CrossRef]
- Iglesias-Iglesias, R.; Campanaro, S.; Treu, L.; Kennes, C.; Veiga, M.C. Valorization of sewage sludge for volatile fatty acids production and role of microbiome on acidogenic fermentation. Bioresour. Technol. 2019, 291, 121817. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Greses, S.; González-Fernández, C. Impact of Organic Loading Rate in Volatile Fatty Acids Production and Population Dynamics Using Microalgae Biomass as Substrate. Sci. Rep. 2019, 9, 18374. [Google Scholar] [CrossRef]
- Zulkhairi, M.; Yusoff, M.; Rahman, N.a.A.; Abd-Aziz, S.; Ling, C.M.; Hassan, M.A.; Shirai, Y. The Effect of Hydraulic Retention Time and Volatile Fatty Acids on Biohydrogen Production from Palm Oil Mill Effluent under Non-Sterile Condition. Aust. J. Basic Appl. Sci. 2010, 4, 577–587. [Google Scholar]
- Rincón, B.; Sánchez, E.; Raposo, F.; Borja, R.; Travieso, L.; Martín, M.A.; Martín, A. Effect of the organic loading rate on the performance of anaerobic acidogenic fermentation of two-phase olive mill solid residue. Waste Manag. 2008, 28, 870–877. [Google Scholar] [CrossRef]
- Malinowsky, C.; Nadaleti, W.; Debiasi, L.R.; Gonçalves Moreira, A.J.; Bayard, R.; Borges de Castilhos Junior, A. Start-up phase optimization of two-phase anaerobic digestion of food waste: Effects of organic loading rate and hydraulic retention time. J. Environ. Manag. 2021, 296, 113064. [Google Scholar] [CrossRef] [PubMed]
- De Groof, V.; Coma, M.; Arnot, T.C.; Leak, D.J.; Lanham, A.B. Adjusting Organic Load as a Strategy to Direct Single-Stage Food Waste Fermentation from Anaerobic Digestion to Chain Elongation. Processes 2020, 8, 1487. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; Karakashev, D.; Xie, L.; Zhou, Q.; Angelidaki, I. Long-term effect of inoculum pretreatment on fermentative hydrogen production by repeated batch cultivations: Homoacetogenesis and methanogenesis as competitors to hydrogen production. Biotechnol. Bioeng. 2011, 108, 1816–1827. [Google Scholar] [CrossRef]
- Chinellato, G.; Cavinato, C.; Bolzonella, D.; Heaven, S.; Banks, C.J. Biohydrogen production from food waste in batch and semi-continuous conditions: Evaluation of a two-phase approach with digestate recirculation for pH control. Int. J. Hydrogen Energy 2013, 38, 4351–4360. [Google Scholar] [CrossRef] [Green Version]
- Doi, T.; Matsumoto, H.; Abe, J.; Morita, S. Feasibility study on the application of rhizosphere microflora of rice for the biohydrogen production from wasted bread. Int. J. Hydrogen Energy 2009, 34, 1735–1743. [Google Scholar] [CrossRef]
- Kim, S.H.; Han, S.K.; Shin, H.S. Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int. J. Hydrogen Energy 2004, 29, 1607–1616. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Kornaros, M. Effect of pH on the anaerobic acidogenesis of agroindustrial wastewaters for maximization of bio-hydrogen production: A lab-scale evaluation using batch tests. Bioresour. Technol. 2014, 162, 218–227. [Google Scholar] [CrossRef]
- Yokoyama, H.; Waki, M.; Moriya, N.; Yasuda, T.; Tanaka, Y.; Haga, K. Effect of fermentation temperature on hydrogen production from cow waste slurry by using anaerobic microflora within the slurry. Appl. Microbiol. Biotechnol. 2007, 74, 474–483. [Google Scholar] [CrossRef]
- Zhang, M.L.; Fan, Y.T.; Xing, Y.; Pan, C.M.; Zhang, G.S.; Lay, J.J. Enhanced biohydrogen production from cornstalk wastes with acidification pretreatment by mixed anaerobic cultures. Biomass Bioenergy 2007, 31, 250–254. [Google Scholar] [CrossRef]
- Tsigkou, K.; Tsafrakidou, P.; Athanasopoulou, S.; Zafiri, C.; Kornaros, M. Effect of pH on the Anaerobic Fermentation of Fruit/Vegetables and Disposable Nappies Hydrolysate for Bio-hydrogen Production. Waste Biomass Valorization 2020, 11, 539–551. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrre, H.; Steyer, J.P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Cabrera, F.; Serrano, A.; Torres, Á.; Rodriguez-Gutierrez, G.; Jeison, D.; Fermoso, F.G. The accumulation of volatile fatty acids and phenols through a pH-controlled fermentation of olive mill solid waste. Sci. Total Environ. 2019, 657, 1501–1507. [Google Scholar] [CrossRef]
- Jankowska, E.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Volatile fatty acids production during mixed culture fermentation–The impact of substrate complexity and pH. Chem. Eng. J. 2017, 326, 901–910. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Q.; Wang, X.; Zhou, X.; Zhu, J. Effect of pH on volatile fatty acid production from anaerobic digestion of potato peel waste. Bioresour. Technol. 2020, 316, 123851. [Google Scholar] [CrossRef]
- Ye, M.; Luo, J.; Zhang, S.; Yang, H.; Li, Y.-Y.; Liu, J. In-situ ammonia stripping with alkaline fermentation of waste activated sludge to improve short-chain fatty acids production and carbon source availability. Bioresour. Technol. 2020, 301, 122782. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, O.; Schnürer, A.; Cetecioglu, Z. Volatile fatty acids production via mixed culture fermentation: Revealing the link between pH, inoculum type and bacterial composition. Bioresour. Technol. 2019, 292, 121889. [Google Scholar] [CrossRef]
- Begum, S.; Anupoju, G.R.; Sridhar, S.; Bhargava, S.K.; Jegatheesan, V.; Eshtiaghi, N. Evaluation of single and two stage anaerobic digestion of landfill leachate: Effect of pH and initial organic loading rate on volatile fatty acid (VFA) and biogas production. Bioresour. Technol. 2018, 251, 364–373. [Google Scholar] [CrossRef]
- Nguyen, P.K.T.; Kim, J.; Das, G.; Yoon, H.H.; Lee, D.H. Optimization of simultaneous dark fermentation and microbial electrolysis cell for hydrogen production from macroalgae using response surface methodology. Biochem. Eng. J. 2021, 171, 108029. [Google Scholar] [CrossRef]
- Connaughton, S.; Collins, G.; O’Flaherty, V. Psychrophilic and mesophilic anaerobic digestion of brewery effluent: A comparative study. Water Res. 2006, 40, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.R.; Rouissi, T.; Brar, S.K.; Surampalli, R.Y. Critical insights into psychrophilic anaerobic digestion: Novel strategies for improving biogas production. Waste Manag. 2021, 131, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, J.; Huang, R.; Zhang, W.; He, W.; Deng, Z.; Han, Y.; Xiao, B.; Luo, H.; Qu, W. Effects of temperature and total solid content on biohydrogen production from dark fermentation of rice straw: Performance and microbial community characteristics. Chemosphere 2022, 286, 131655. [Google Scholar] [CrossRef]
- Karlsson, A.; Vallin, L.; Ejlertsson, J. Effects of temperature, hydraulic retention time and hydrogen extraction rate on hydrogen production from the fermentation of food industry residues and manure. Int. J. Hydrogen Energy 2008, 33, 953–962. [Google Scholar] [CrossRef]
- Zheng, H.-S.; Guo, W.-Q.; Yang, S.-S.; Feng, X.-C.; Du, J.-S.; Zhou, X.-J.; Chang, J.-S.; Ren, N.-Q. Thermophilic hydrogen production from sludge pretreated by thermophilic bacteria: Analysis of the advantages of microbial community and metabolism. Bioresour. Technol. 2014, 172, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Ziara, R.M.M.; Miller, D.N.; Subbiah, J.; Dvorak, B.I. Lactate wastewater dark fermentation: The effect of temperature and initial pH on biohydrogen production and microbial community. Int. J. Hydrogen Energy 2019, 44, 661–673. [Google Scholar] [CrossRef]
- Huang, C.; Wang, W.; Sun, X.; Shen, J.; Wang, L. A novel acetogenic bacteria isolated from waste activated sludge and its potential application for enhancing anaerobic digestion performance. J. Environ. Manag. 2020, 255, 109842. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Hjort, M.; Cirne, D.; Gérardin, F.; Lacroix, S.; Gaval, G.; Karabegovic, L.; Alexandersson, T.; Johansson, P.; Karlsson, A.; et al. Integrated production of polyhydroxyalkanoates (PHAs) with municipal wastewater and sludge treatment at pilot scale. Bioresour. Technol. 2015, 181, 78–89. [Google Scholar] [CrossRef]
- Garcia-Aguirre, J.; Aymerich, E.; de Goñi, J.G.-M.; Esteban-Gutiérrez, M. Selective VFA production potential from organic waste streams: Assessing temperature and pH influence. Bioresour. Technol. 2017, 244, 1081–1088. [Google Scholar] [CrossRef]
- Xiao, B.; Qin, Y.; Wu, J.; Chen, H.; Yu, P.; Liu, J.; Li, Y.Y. Comparison of single-stage and two-stage thermophilic anaerobic digestion of food waste: Performance, energy balance and reaction process. Energy Convers. Manag. 2018, 156, 215–223. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Tan, T. Batch and semi-continuous anaerobic digestion of food waste in a dual solid-liquid system. Bioresour. Technol. 2013, 145, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Show, K.Y.; Tay, J.H.; Liang, D.T.; Lee, D.J. Biohydrogen production with anaerobic fluidized bed reactors-A comparison of biofilm-based and granule-based systems. Int. J. Hydrogen Energy 2008, 33, 1559–1564. [Google Scholar] [CrossRef]
- Brar, K.K.; Cortez, A.A.; Pellegrini, V.O.A.; Amulya, K.; Polikarpov, I.; Magdouli, S.; Kumar, M.; Yang, Y.-H.; Bhatia, S.K.; Brar, S.K. An overview on progress, advances, and future outlook for biohydrogen production technology. Int. J. Hydrogen Energy 2022, in press. [Google Scholar] [CrossRef]
- Castillo-Hernández, A.; Mar-Alvarez, I.; Moreno-Andrade, I. Start-up and operation of continuous stirred-tank reactor for biohydrogen production from restaurant organic solid waste. Int. J. Hydrogen Energy 2015, 2015, 17239–17245. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y. Coproduction of hydrogen and methane in a CSTR-IC two-stage anaerobic digestion system from molasses wastewater. Water Sci. Technol. 2019, 79, 270–277. [Google Scholar] [CrossRef]
- Ri, P.C.; Kim, J.S.; Kim, T.R.; Pang, C.H.; Mun, H.G.; Pak, G.C.; Ren, N.Q. Effect of hydraulic retention time on the hydrogen production in a horizontal and vertical continuous stirred-tank reactor. Int. J. Hydrogen Energy 2019, 44, 17742–17749. [Google Scholar] [CrossRef]
- Tena, M.; Perez, M.; Solera, R. Effect of hydraulic retention time on hydrogen production from sewage sludge and wine vinasse in a thermophilic acidogenic CSTR: A promising approach for hydrogen production within the biorefinery concept. Int. J. Hydrogen Energy 2021, 46, 7810–7820. [Google Scholar] [CrossRef]
- Greses, S.; Tomás-Pejó, E.; Gónzalez-Fernández, C. Agroindustrial waste as a resource for volatile fatty acids production via anaerobic fermentation. Bioresour. Technol. 2020, 297, 122486. [Google Scholar] [CrossRef]
- Jones, R.J.; Fernández-feito, R.; Massanet-nicolau, J.; Dinsdale, R.; Guwy, A. Continuous recovery and enhanced yields of volatile fatty acids from a continually-fed 100 L food waste bioreactor by filtration and electrodialysis. Waste Manag. 2021, 122, 81–88. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Shanmuganathan, R.; Pugazhendhi, A.; Gunasekar, P.; Manigandan, S. Biohydrogen production using horizontal and vertical continuous stirred tank reactor- a numerical optimization. Int. J. Hydrogen Energy 2021, 46, 11305–11312. [Google Scholar] [CrossRef]
- Keskin, T.; Giusti, L.; Azbar, N. Continuous biohydrogen production in immobilized biofilm system versus suspended cell culture. Int. J. Hydrogen Energy 2012, 37, 1418–1424. [Google Scholar] [CrossRef]
- Anjana Anand, A.S.; Adish Kumar, S.; Rajesh Banu, J.; Ginni, G. The performance of fluidized bed solar photo Fenton oxidation in the removal of COD from hospital wastewaters. Desalin. Water Treat. 2016, 57, 8236–8242. [Google Scholar] [CrossRef]
- Chaves, T.C.; Gois, G.N.S.B.; Peiter, F.S.; Vich, D.V.; de Amorim, E.L.C. Biohydrogen production in an AFBR using sugarcane molasses. Bioprocess. Biosyst. Eng. 2021, 44, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tay, J.; Show, K.; Yan, R.; Teeliang, D.; Lee, D.; Jiang, W. Biohydrogen production in a granular activated carbon anaerobic fluidized bed reactor. Int. J. Hydrogen Energy 2007, 32, 185–191. [Google Scholar] [CrossRef]
- Barros, A.R.; Cavalcante de Amorim, E.L.; Reis, C.M.; Shida, G.M.; Silva, E.L. Biohydrogen production in anaerobic fluidized bed reactors: Effect of support material and hydraulic retention time. Int. J. Hydrogen Energy 2010, 35, 3379–3388. [Google Scholar] [CrossRef]
- Amorim, N.C.S.; Alves, I.; Martins, J.S.; Amorim, E.L.C. Biohydrogen production from cassava wastewater in an anaerobic fluidized bed reactor. Braz. J. Chem. Eng. 2014, 31, 603–612. [Google Scholar] [CrossRef]
- Maaroff, R.M.; Md Jahim, J.; Azahar, A.M.; Abdul, P.M.; Masdar, M.S.; Nordin, D.; Abd Nasir, M.A. Biohydrogen production from palm oil mill effluent (POME) by two stage anaerobic sequencing batch reactor (ASBR) system for better utilization of carbon sources in POME. Int. J. Hydrogen Energy 2019, 44, 3395–3406. [Google Scholar] [CrossRef]
- Santiago, S.G.; Morgan-Sagastume, J.M.; Monroy, O.; Moreno-Andrade, I. Biohydrogen production from organic solid waste in a sequencing batch reactor: An optimization of the hydraulic and solids retention time. Int. J. Hydrogen Energy 2020, 45, 25681–25688. [Google Scholar] [CrossRef]
- Lagoa-Costa, B.; Kennes, C.; Veiga, M.C. Cheese whey fermentation into volatile fatty acids in an anaerobic sequencing batch reactor. Bioresour. Technol. 2020, 308, 123226. [Google Scholar] [CrossRef]
- Show, K.Y.; Lee, D.J.; Tay, J.H.; Lin, C.Y.; Chang, J.S. Biohydrogen production: Current perspectives and the way forward. Int. J. Hydrogen Energy 2012, 37, 15616–15631. [Google Scholar] [CrossRef]
- Anzola-Rojas, M.d.P.; Gonçalves da Fonseca, S.; Canedo da Silva, C.; Maia de Oliveira, V.; Zaiat, M. The use of the carbon/nitrogen ratio and specific organic loading rate as tools for improving biohydrogen production in fixed-bed reactors. Biotechnol. Rep. 2015, 5, 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomczak, W.; Ferrasse, J.-H.; Giudici-Orticoni, M.-T.; Soric, A. Effect of hydraulic retention time on a continuous biohydrogen production in a packed bed biofilm reactor with recirculation flow of the liquid phase. Int. J. Hydrogen Energy 2018, 43, 18883–18895. [Google Scholar] [CrossRef] [Green Version]
- Muri, P.; Marinšek-Logar, R.; Djinović, P.; Pintar, A. Influence of support materials on continuous hydrogen production in anaerobic packed-bed reactor with immobilized hydrogen producing bacteria at acidic conditions. Enzyme Microb. Technol. 2018, 111, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.A.; Ghufran, R.; Wahid, Z.A.; Ahmad, A. Integrated application of upflow anaerobic sludge blanket reactor for the treatment of wastewaters. Water Res. 2011, 45, 4683–4699. [Google Scholar] [CrossRef]
- Castelló, E.; García y Santos, C.; Iglesias, T.; Paolino, G.; Wenzel, J.; Borzacconi, L.; Etchebehere, C. Feasibility of biohydrogen production from cheese whey using a UASB reactor: Links between microbial community and reactor performance. Int. J. Hydrogen Energy 2009, 34, 5674–5682. [Google Scholar] [CrossRef]
- Eregowda, T.; Kokko, M.E.; Rene, E.R.; Rintala, J.; Lens, P.N.L. Volatile fatty acid production from Kraft mill foul condensate in upflow anaerobic sludge blanket reactors. Environ. Technol. 2021, 42, 2447–2460. [Google Scholar] [CrossRef]
- Intanoo, P.; Chaimongkol, P.; Chavadej, S. Hydrogen and methane production from cassava wastewater using two-stage upflow anaerobic sludge blanket reactors (UASB) with an emphasis on maximum hydrogen production. Int. J. Hydrogen Energy 2016, 41, 6107–6114. [Google Scholar] [CrossRef]
- Jung, K.-W.; Kim, D.-H.; Shin, H.-S. Continuous fermentative hydrogen production from coffee drink manufacturing wastewater by applying UASB reactor. Int. J. Hydrogen Energy 2010, 35, 13370–13378. [Google Scholar] [CrossRef]
- Yang, H.; Shao, P.; Lu, T.; Shen, J.; Wang, D.; Xu, Z.; Yuan, X. Continuous bio-hydrogen production from citric acid wastewater via facultative anaerobic bacteria. Int. J. Hydrogen Energy 2006, 31, 1306–1313. [Google Scholar] [CrossRef]
- Estevez, M.M.; Linjordet, R.; Morken, J. Effects of steam explosion and co-digestion in the methane production from Salix by mesophilic batch assays. Bioresour. Technol. 2012, 104, 749–756. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F.; Kapdan, I.K.; Oztekin, R. Biohydrogen production by dark fermentation of wheat powder solution: Effects of C/N and C/P ratio on hydrogen yield and formation rate. Int. J. Hydrogen Energy 2008, 33, 1813–1819. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lay, C.H. Carbon/nitrogen-ratio effect on fermentative hydrogen production by mixed microflora. Int. J. Hydrogen Energy 2004, 29, 41–45. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Yang, G.; Sun, G.; Sage, V. A Review of the Enhancement of Bio-Hydrogen Generation by Chemicals Addition. Catalysts 2019, 9, 353. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, Y.; Yang, G.; Sun, Z. Optimization of biohydrogen production using acid pretreated corn stover hydrolysate followed by nickel nanoparticle addition. Int. J. Energy Res. 2020, 44, 1843–1857. [Google Scholar] [CrossRef]
- Sinharoy, A.; Pakshirajan, K. A novel application of biologically synthesized nanoparticles for enhanced biohydrogen production and carbon monoxide bioconversion. Renew. Energy 2020, 147, 864–873. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Tian, Y.; Zheng, L.; Hao, H.; Huang, H. Impact of Fe and Ni Addition on the VFAs’ Generation and Process Stability of Anaerobic Fermentation Containing Cd. Int. J. Environ. Res. Public Health 2019, 16, 4066. [Google Scholar] [CrossRef] [Green Version]
- Dahiya, S.; Lakshminarayanan, S.; Venkata Mohan, S. Steering acidogenesis towards selective propionic acid production using co-factors and evaluating environmental sustainability. Chem. Eng. J. 2020, 379, 122135. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, C.; Ren, H.-Y.; Wu, J.-T.; Meng, J.; Nan, J.; Cao, G.-L.; Yang, S.-S.; Ren, N.-Q. Feasibility of enhancing hydrogen production from cornstalk hydrolysate anaerobic fermentation by RCPH-biochar. Bioresour. Technol. 2020, 297, 122505. [Google Scholar] [CrossRef]
- Sugiarto, Y.; Sunyoto, N.M.S.; Zhu, M.; Jones, I.; Zhang, D. Effect of biochar in enhancing hydrogen production by mesophilic anaerobic digestion of food wastes: The role of minerals. Int. J. Hydrogen Energy 2021, 46, 3695–3703. [Google Scholar] [CrossRef]
- Lu, J.-H.; Chen, C.; Huang, C.; Zhuang, H.; Leu, S.-Y.; Lee, D.-J. Dark fermentation production of volatile fatty acids from glucose with biochar amended biological consortium. Bioresour. Technol. 2020, 303, 122921. [Google Scholar] [CrossRef]
- Taheri, E.; Amin, M.M.; Fatehizadeh, A.; Pourzamani, H.; Bina, B.; Spanjers, H. Biohydrogen production under hyper salinity stress by an anaerobic sequencing batch reactor with mixed culture. J. Environ. Health Sci. Eng. 2018, 16, 159–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, O.; Kiran Katari, J.; Chatterjee, S.; Venkata Mohan, S. Salinity induced acidogenic fermentation of food waste regulates biohydrogen production and volatile fatty acids profile. Fuel 2020, 276, 117794. [Google Scholar] [CrossRef]
- He, X.; Yin, J.; Liu, J.; Chen, T.; Shen, D. Characteristics of acidogenic fermentation for volatile fatty acid production from food waste at high concentrations of NaCl. Bioresour. Technol. 2019, 271, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, X.; Chen, F.; Wang, Y.; Li, X.; Wang, D.; Tao, Z.; Xu, D.; Xue, W.; Geng, M.; et al. Clarithromycin affect methane production from anaerobic digestion of waste activated sludge. J. Clean. Prod. 2020, 255, 120321. [Google Scholar] [CrossRef]
- Huang, X.; Xu, Q.; Wu, Y.; Wang, D.; Yang, Q.; Chen, F.; Wu, Y.; Pi, Z.; Chen, Z.; Li, X.; et al. Effect of clarithromycin on the production of volatile fatty acids from waste activated sludge anaerobic fermentation. Bioresour. Technol. 2019, 288, 121598. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, X.; Zhou, Y.; Yang, X.; Lam, S.S.; Wang, D. Influence of roxithromycin as antibiotic residue on volatile fatty acids recovery in anaerobic fermentation of waste activated sludge. J. Hazard. Mater. 2020, 394, 122570. [Google Scholar] [CrossRef]
- Tao, Z.; Yang, Q.; Yao, F.; Huang, X.; Wu, Y.; Du, M.; Chen, S.; Liu, X.; Li, X.; Wang, D. The inhibitory effect of thiosulfinate on volatile fatty acid and hydrogen production from anaerobic co-fermentation of food waste and waste activated sludge. Bioresour. Technol. 2020, 297, 122428. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Mulder, M.J.J.; Premier, G.; Dinsdale, R.; Guwy, A. Increased biohydrogen yields, volatile fatty acid production and substrate utilisation rates via the electrodialysis of a continually fed sucrose fermenter. Bioresour. Technol. 2017, 229, 46–52. [Google Scholar] [CrossRef]
- Hassan, G.K.; Jones, R.J.; Massanet-Nicolau, J.; Dinsdale, R.; Abo-Aly, M.M.; El-Gohary, F.A.; Guwy, A. Increasing 2 -Bio- (H2 and CH4) production from food waste by combining two-stage anaerobic digestion and electrodialysis for continuous volatile fatty acids removal. Waste Manag. 2021, 129, 20–25. [Google Scholar] [CrossRef]
- Bundhoo, M.A.Z.; Mohee, R. Inhibition of dark fermentative bio-hydrogen production: A review. Int. J. Hydrogen Energy 2016, 41, 6713–6733. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Astals, S.; Robles, Á.; Steyer, J.P. Unraveling the literature chaos around free ammonia inhibition in anaerobic digestion. Renew. Sustain. Energy Rev. 2020, 117, 109487. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Kotoulas, A.; Agathou, D.; Triantaphyllidou, I.; Tatoulis, T.; Akratos, C.; Tekerlekopoulou, A.; Vayenas, D. Zeolite as a Potential Medium for Ammonium Recovery and Second Cheese Whey Treatment. Water 2019, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Chen, F.; Xue, S.; Pan, J.; Khoshnevisan, B.; Yang, Y.; Liu, H.; Qiu, L. Improving anaerobic digestion of chicken manure under optimized biochar supplementation strategies. Bioresour. Technol. 2021, 325, 124697. [Google Scholar] [CrossRef]
- Ho, L.; Ho, G. Mitigating ammonia inhibition of thermophilic anaerobic treatment of digested piggery wastewater: Use of pH reduction, zeolite, biomass and humic acid. Water Res. 2012, 46, 4339–4350. [Google Scholar] [CrossRef] [Green Version]
- Quintana-Najera, J.; Blacker, A.J.; Fletcher, L.A.; Ross, A.B. Influence of augmentation of biochar during anaerobic co-digestion of Chlorella vulgaris and cellulose. Bioresour. Technol. 2022, 343, 126086. [Google Scholar] [CrossRef]
- Silva, R.M.; Abreu, A.A.; Salvador, A.F.; Alves, M.M.; Neves, I.C.; Pereira, M.A. Zeolite addition to improve biohydrogen production from dark fermentation of C5/C6-sugars and Sargassum sp. biomass. Sci. Rep. 2021, 11, 16350. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Choi, J.-A.; Oh, Y.-K.; Abou-Shanab, R.A.I.; Song, H.; Min, B.; Cho, Y.; Na, J.-G.; Koo, J.; Jeon, B.-H. Hydrogen production from sulfate- and ferrous-enriched wastewater. Int. J. Hydrogen Energy 2011, 36, 13984–13990. [Google Scholar] [CrossRef]
- Mizuno, O.; Li, Y.Y.; Noike, T. The behavior of sulfate-reducing bacteria in acidogenic phase of anaerobic digestion. Water Res. 1998, 32, 1626–1634. [Google Scholar] [CrossRef]
- Haosagul, S.; Prommeenate, P.; Hobbs, G.; Pisutpaisal, N. Sulfide-oxidizing bacteria community in full-scale bioscrubber treating H2S in biogas from swine anaerobic digester. Renew. Energy 2020, 150, 973–980. [Google Scholar] [CrossRef]
- Kobayashi, T.; Li, Y.-Y.; Kubota, K.; Harada, H.; Maeda, T.; Yu, H.-Q. Characterization of sulfide-oxidizing microbial mats developed inside a full-scale anaerobic digester employing biological desulfurization. Appl. Microbiol. Biotechnol. 2012, 93, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Dhar, B.R.; Elbeshbishy, E.; Nakhla, G. Influence of iron on sulfide inhibition in dark biohydrogen fermentation. Bioresour. Technol. 2012, 126, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hao, X.; van Loosdrecht, M.C.M.; Liu, R. Relieving the inhibition of humic acid on anaerobic digestion of excess sludge by metal ions. Water Res. 2021, 188, 116541. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Cheng, J.; Ding, L.; Song, W.; Zhou, J.; Cen, K. Inhibitory effects of furan derivatives and phenolic compounds on dark hydrogen fermentation. Bioresour. Technol. 2015, 196, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Reyes, Á.; Cubero-Cardoso, J.; Rodríguez-Gutiérrez, G.; García-Martín, J.F.; Rodríguez-Galán, M.; Borja, R.; Serrano, A.; Fermoso, F.G. Extraction of phenolic compounds and production of biomethane from strawberry and raspberry extrudates. Biochem. Eng. J. 2019, 147, 11–19. [Google Scholar] [CrossRef]
- Dong, D.; Wang, R.; Geng, P.; Li, C.; Zhao, Z. Enhancing effects of activated carbon supported nano zero-valent iron on anaerobic digestion of phenol-containing organic wastewater. J. Environ. Manag. 2019, 244, 1–12. [Google Scholar] [CrossRef]
- Kim, J.R.; Karthikeyan, K.G. Effects of severe pretreatment conditions and lignocellulose-derived furan byproducts on anaerobic digestion of dairy manure. Bioresour. Technol. 2021, 340, 125632. [Google Scholar] [CrossRef]
- Wijayasekera, S.C.; Hewage, K.; Siddiqui, O.; Hettiaratchi, P.; Sadiq, R. Waste-to-hydrogen technologies: A critical review of techno-economic and socio-environmental sustainability. Int. J. Hydrogen Energy 2022, 47, 5842–5870. [Google Scholar] [CrossRef]
- Massanet-Nicolau, J.; Jones, R.J.; Guwy, A.; Dinsdale, R.; Premier, G.; Mulder, M.J.J. Maximising Biohydrogen Yields via Continuous Electrochemical Hydrogen Removal and Carbon Dioxide Scrubbing. Bioresour. Technol. 2016, 218, 512–517. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Fernandez–Feito, R.; Dinsdale, R.M.; Guwy, A.J. Fermentative volatile fatty acid production and recovery from grass using a novel combination of solids separation, pervaporation, and electrodialysis technologies. Bioresour. Technol. 2021, 342, 125926. [Google Scholar] [CrossRef]
- Jones, R.; Massanet-Nicolau, J.; Guwy, A. A review of carboxylate production and recovery from organic wastes. Bioresour. Technol. Rep. 2021, 16, 100826. [Google Scholar]
- Scoma, A.; Varela-Corredor, F.; Bertin, L.; Gostoli, C.; Bandini, S. Recovery of VFAs from anaerobic digestion of dephenolized Olive Mill Wastewaters by Electrodialysis. Sep. Purif. Technol. 2016, 159, 81–91. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- IEA. The Future of Hydrogen; IEA: Paris, France, 2019. [Google Scholar]
- Maestre, V.M.; Ortiz, A.; Ortiz, I. Challenges and prospects of renewable hydrogen-based strategies for full decarbonization of stationary power applications. Renew. Sustain. Energy Rev. 2021, 152, 111628. [Google Scholar] [CrossRef]
- International Renewable Energy Agency. Renewable Capacity Statistics 2020 Statistiques De Capacité Renouvelable 2020 Estadísticas De Capacidad Renovable 2020. 2020. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2020/Mar/IRENA_RE_Capacity_Statistics_2020.pdf (accessed on 8 April 2022).
- International Renewable Energy Agency. 2050 ENERGY TRANSFORMATION E D I T I O N : 2 0 2 0 GLOBAL RENEWABLES OUTLOOK. 2020. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2020/Apr/IRENA_Global_Renewables_Outlook_2020.pdf (accessed on 8 April 2022).
- Janssen, J.L.L.C.C.; Weeda, M.; Detz, R.J.; van der Zwaan, B. Country-specific cost projections for renewable hydrogen production through off-grid electricity systems. Appl. Energy 2022, 309, 118398. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Shanmugam, S.; Sekar, M.; Mathimani, T.; Incharoensakdi, A.; Kim, S.H.; Parthiban, A.; Edwin Geo, V.; Brindhadevi, K.; Pugazhendhi, A. Insights on biological hydrogen production routes and potential microorganisms for high hydrogen yield. Fuel 2021, 291, 120136. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, D.; Li, X.; Yang, Q.; Xu, Q.; Ni, B.J.; Wang, Q.; Liu, X. Towards hydrogen production from waste activated sludge: Principles, challenges and perspectives. Renew. Sustain. Energy Rev. 2021, 135, 110283. [Google Scholar] [CrossRef]
- Park, J.H.; Chandrasekhar, K.; Jeon, B.H.; Jang, M.; Liu, Y.; Kim, S.H. State-of-the-art technologies for continuous high-rate biohydrogen production. Bioresour. Technol. 2021, 320, 124304. [Google Scholar] [CrossRef]
- Yukesh Kannah, R.; Kavitha, S.; Preethi; Parthiba Karthikeyan, O.; Kumar, G.; Dai-Viet, N.V.; Rajesh Banu, J. Techno-economic assessment of various hydrogen production methods—A review. Bioresour. Technol. 2021, 319, 124175. [Google Scholar] [CrossRef]
- Habashy, M.M.; Ong, E.S.; Abdeldayem, O.M.; Al-Sakkari, E.G.; Rene, E.R. Food Waste: A Promising Source of Sustainable Biohydrogen Fuel. Trends Biotechnol. 2021, 39, 1274–1288. [Google Scholar] [CrossRef]
- Aydin, M.I.; Karaca, A.E.; Qureshy, A.M.M.I.; Dincer, I. A comparative review on clean hydrogen production from wastewaters. J. Environ. Manag. 2021, 279, 111793. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Wang, J. Review and comparison of various hydrogen production methods based on costs and life cycle impact assessment indicators. Int. J. Hydrogen Energy 2021, 46, 38612–38635. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Pal, A. An overview of conventional and non-conventional hydrogen production methods. Mater. Today Proc. 2021, 46, 5353–5359. [Google Scholar] [CrossRef]
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-hydrogen: A review of main routes production, processes evaluation and techno-economical assessment. Biomass Bioenergy 2021, 144, 105920. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Duman, A.C.; Güler, Ö. Techno-economic analysis of off-grid PV/wind/fuel cell hybrid system combinations with a comparison of regularly and seasonally occupied households. Sustain. Cities Soc. 2018, 42, 107–126. [Google Scholar] [CrossRef]
- Dawood, F.; Shafiullah, G.M.; Anda, M. Stand-Alone Microgrid with 100% Renewable Energy: A Case Study with Hybrid Solar PV-Battery-Hydrogen. Sustainability 2020, 12, 2047. [Google Scholar] [CrossRef] [Green Version]
- Nascimento da Silva, G.; Rochedo, P.R.R.; Szklo, A. Renewable hydrogen production to deal with wind power surpluses and mitigate carbon dioxide emissions from oil refineries. Appl. Energy 2022, 311, 118631. [Google Scholar] [CrossRef]
- Nazir, H.; Muthuswamy, N.; Louis, C.; Jose, S.; Prakash, J.; Buan, M.E.M.; Flox, C.; Chavan, S.; Shi, X.; Kauranen, P.; et al. Is the H2 economy realizable in the foreseeable future? Part III: H2 usage technologies, applications, and challenges and opportunities. Int. J. Hydrogen Energy 2020, 45, 28217–28239. [Google Scholar] [CrossRef]
- Arora, A.; Zantye, M.S.; Hasan, M.M.F. Sustainable hydrogen manufacturing via renewable-integrated intensified process for refueling stations. Appl. Energy 2022, 311, 118667. [Google Scholar] [CrossRef]
- Symes, D.; Maillard, J.G.; Courtney, J.; Watton, J.; Meadowcroft, A.; Chandan, A.S.; Gurley, L.; Priestly, R.; Serdaroglu, G. Development of a hydrogen fuelling infrastructure in the Northeast U.S.A. Int. J. Hydrogen Energy 2014, 39, 7460–7466. [Google Scholar] [CrossRef]
- Schlund, D.; Schönfisch, M. Analysing the impact of a renewable hydrogen quota on the European electricity and natural gas markets. Appl. Energy 2021, 304, 117666. [Google Scholar] [CrossRef]
- Dolci, F.; Thomas, D.; Hilliard, S.; Guerra, C.F.; Hancke, R.; Ito, H.; Jegoux, M.; Kreeft, G.; Leaver, J.; Newborough, M.; et al. Incentives and legal barriers for power-to-hydrogen pathways: An international snapshot. Int. J. Hydrogen Energy 2019, 44, 11394–11401. [Google Scholar] [CrossRef]
- Veluswamy, G.K.; Shah, K.; Ball, A.S.; Guwy, A.J.; Dinsdale, R.M. A techno-economic case for volatile fatty acid production for increased sustainability in the wastewater treatment industry. Environ. Sci. Water Res. Technol. 2021, 7, 927–941. [Google Scholar] [CrossRef]
- Bahreini, G.; Elbeshbishy, E.; Jimenez, J.; Santoro, D.; Nakhla, G. Integrated fermentation and anaerobic digestion of primary sludges for simultaneous resource and energy recovery: Impact of volatile fatty acids recovery. Waste Manag. 2020, 118, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Moscariello, C.; Matassa, S.; Pirozzi, F.; Esposito, G.; Papirio, S. Valorisation of industrial hemp (Cannabis sativa L.) biomass residues through acidogenic fermentation and co-fermentation for volatile fatty acids production. Bioresour. Technol. 2022, 355, 127289. [Google Scholar] [CrossRef] [PubMed]
- Huq, N.A.; Hafenstine, G.R.; Huo, X.; Nguyen, H.; Tifft, S.M.; Conklin, D.R.; Stück, D.; Stunkel, J.; Yang, Z.; Heyne, J.S.; et al. Toward net-zero sustainable aviation fuel with wet waste–derived volatile fatty acids. Proc. Natl. Acad. Sci. USA 2021, 118, e2023008118. [Google Scholar] [CrossRef]
- Patterson, T.; Massanet-Nicolau, J.; Jones, R.; Boldrin, A.; Valentino, F.; Dinsdale, R.; Guwy, A. Utilizing grass for the biological production of polyhydroxyalkanoates (PHAs) via green biorefining: Material and energy flows. J. Ind. Ecol. 2020, 25, 802–815. [Google Scholar] [CrossRef]
- Kumar, G.; Ponnusamy, V.K.; Bhosale, R.R.; Shobana, S.; Yoon, J.-J.; Bhatia, S.K.; Rajesh Banu, J.; Kim, S.-H. A review on the conversion of volatile fatty acids to polyhydroxyalkanoates using dark fermentative effluents from hydrogen production. Bioresour. Technol. 2019, 287, 121427. [Google Scholar] [CrossRef]
- Policastro, G.; Giugliano, M.; Luongo, V.; Napolitano, R.; Fabbricino, M. Enhancing photo fermentative hydrogen production using ethanol rich dark fermentation effluents. Int. J. Hydrogen Energy 2022, 47, 117–126. [Google Scholar] [CrossRef]
- Cheng, J.; Li, H.; Ding, L.; Zhou, J.; Song, W.; Li, Y.-Y.; Lin, R. Improving hydrogen and methane co-generation in cascading dark fermentation and anaerobic digestion: The effect of magnetite nanoparticles on microbial electron transfer and syntrophism. Chem. Eng. J. 2020, 397, 125394. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).