Identification, Quantification and Kinetic Study of Carotenoids and Lipids in Rhodotorula toruloides CBS 14 Cultivated on Wheat Straw Hydrolysate
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrolysate Preparation
2.2. Rhodotorula toruloides Cultivation
2.2.1. Inoculum Preparation
2.2.2. Fermentation Experiments
2.3. Analytical Techniques
2.3.1. Optical Density (OD600)
2.3.2. Cell Dry Weight Determination
2.3.3. Sugars and Acetic acid Analysis
2.3.4. Lipid Extraction
2.3.5. Fatty Acid Profile
2.3.6. Lipid Profile Analysis
2.4. Carotenoid Extraction
2.5. Total Carotenoid Quantification Using Spectrophotometry
2.6. Carotenoid Identification and Quantification Using UHPLC-PDA
2.6.1. Chromatographic Method Conditions
2.6.2. Instrument Performance
2.7. Effect of Saponification on Total Carotenoids
2.8. Statistical Analysis
3. Results
3.1. Establishing Carotenoid Analysis Method
3.1.1. Characterization of Calibration Curves of the Two Carotenoid Analysis Methods
3.1.2. Relative Recovery and Matrix Effect
3.1.3. Saponification Effect
3.1.4. Carotenoid Content in R. toruloides
3.2. Kinetics of Carotenoid and Lipid Formation in Bioreactor Cultivation
3.2.1. Cell Growth, Cell Dry Weight, Sugar, and Lipid Content Analysis
3.2.2. Lipid Composition and Classes
3.2.3. Carotenoid Kinetics and Quantification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef]
- Shapaval, V.; Brandenburg, J.; Blomqvist, J.; Tafintseva, V.; Passoth, V.; Sandgren, M.; Kohler, A. Biochemical profiling, prediction of total lipid content and fatty acid profile in oleaginous yeasts by FTIR spectroscopy. Biotechnol. Biofuels 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Vicente, G.; Bautista, L.F.; Gutiérrez, F.J.; Rodríguez, R.A.; Martínez, V.; Rodríguez-Frómeta, R.A.; Ruiz-Vázquez, R.M.; Torres-Martínez, S.; Garre, V. Direct transformation of fungal biomass from submerged cultures into biodiesel. Energy Fuels 2010, 24, 3173–3178. [Google Scholar] [CrossRef]
- Abeln, F.; Chuck, C.J. The history, state of the art and future prospects for oleaginous yeast research. Microb. Cell Factories 2021, 20, 221. [Google Scholar] [CrossRef]
- Dai, C.-c.; Tao, J.; Xie, F.; Dai, Y.-j.; Zhao, M. Biodiesel generation from oleaginous yeast Rhodotorula glutinis with xylose assimilating capacity. Afr. J. Biotechnol. 2007, 6, 2130–2134. [Google Scholar]
- Blomqvist, J.; Pickova, J.; Tilami, S.K.; Sampels, S.; Mikkelsen, N.; Brandenburg, J.; Sandgren, M.; Passoth, V. Oleaginous yeast as a component in fish feed. Sci. Rep. 2018, 8, 15945. [Google Scholar] [CrossRef]
- Schulze, I.; Hansen, S.; Großhans, S.; Rudszuck, T.; Ochsenreither, K.; Syldatk, C.; Neumann, A. Characterization of newly isolated oleaginous yeasts-Cryptococcus podzolicus, Trichosporon porosum and Pichia segobiensis. AMB Express 2014, 4, 24. [Google Scholar] [CrossRef]
- Granger, L.-M.; Perlot, P.; Goma, G.; Pareilleux, A. Effect of various nutrient limitations on fatty acid production by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 1993, 38, 784–789. [Google Scholar] [CrossRef]
- Beopoulos, A.; Cescut, J.; Haddouche, R.; Uribelarrea, J.-L.; Molina-Jouve, C.; Nicaud, J.-M. Yarrowia lipolytica as a model for bio-oil production. Prog. Lipid Res. 2009, 48, 375–387. [Google Scholar] [CrossRef]
- Takaku, H.; Matsuzawa, T.; Yaoi, K.; Yamazaki, H. Lipid metabolism of the oleaginous yeast Lipomyces starkeyi. Appl. Microbiol. Biotechnol. 2020, 104, 6141–6148. [Google Scholar] [CrossRef]
- Kaczor, A.; Baranska, M. Carotenoids: Nutrition, Analysis and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Rapoport, A.; Guzhova, I.; Bernetti, L.; Buzzini, P.; Kieliszek, M.; Kot, A.M. Carotenoids and some other pigments from fungi and yeasts. Metabolites 2021, 11, 92. [Google Scholar] [CrossRef]
- Ungureanu, C.; Marchal, L.; Chirvase, A.A.; Foucault, A. Centrifugal partition extraction, a new method for direct metabolites recovery from culture broth: Case study of torularhodin recovery from Rhodotorula rubra. Bioresour. Technol. 2013, 132, 406–409. [Google Scholar] [CrossRef]
- Brandenburg, J.; Poppele, I.; Blomqvist, J.; Puke, M.; Pickova, J.; Sandgren, M.; Rapoport, A.; Vedernikovs, N.; Passoth, V. Bioethanol and lipid production from the enzymatic hydrolysate of wheat straw after furfural extraction. Appl. Microbiol. Biotechnol. 2018, 102, 6269–6277. [Google Scholar] [CrossRef]
- Passoth, V.; Sandgren, M. Biofuel production from straw hydrolysates: Current achievements and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5105–5116. [Google Scholar] [CrossRef]
- Chmielarz, M.; Blomqvist, J.; Sampels, S.; Sandgren, M.; Passoth, V. Microbial lipid production from crude glycerol and hemicellulosic hydrolysate with oleaginous yeasts. Biotechnol. Biofuels 2021, 14, 65. [Google Scholar] [CrossRef]
- Frengova, G.I.; Beshkova, D.M. Carotenoids from Rhodotorula and Phaffia: Yeasts of biotechnological importance. J. Ind. Microbiol. Biotechnol. 2009, 36, 163. [Google Scholar] [CrossRef]
- Buzzini, P.; Innocenti, M.; Turchetti, B.; Libkind, D.; van Broock, M.; Mulinacci, N. Carotenoid profiles of yeasts belonging to the genera Rhodotorula, Rhodosporidium, Sporobolomyces, and Sporidiobolus. Can. J. Microbiol. 2007, 53, 1024–1031. [Google Scholar] [CrossRef]
- Perrier, V.; Dubreucq, E.; Galzy, P. Fatty acid and carotenoid composition of Rhodotorula strains. Arch. Microbiol. 1995, 164, 173–179. [Google Scholar] [CrossRef]
- Flieger, K.; Knabe, N.; Toepel, J. Development of an improved carotenoid extraction method to characterize the carotenoid composition under oxidative stress and cold temperature in the rock inhabiting fungus Knufia petricola A95. J. Fungi 2018, 4, 124. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, H.; Du, C.; Zhang, W.; Qian, H. Tentative Identification of Torulene Cis/trans Geometrical Isomers Isolated from Sporidiobolus pararoseus by High-Performance Liquid Chromatography–Diode Array Detection–Mass Spectrometry and Preparation by Column Chromatography. Anal. Sci. 2013, 29, 997–1002. [Google Scholar] [CrossRef]
- Weber, R.W.; Anke, H.; Davoli, P. Simple method for the extraction and reversed-phase high-performance liquid chromatographic analysis of carotenoid pigments from red yeasts (Basidiomycota, Fungi). J. Chromatogr. A 2007, 1145, 118–122. [Google Scholar] [CrossRef]
- Freitas, C.; Nobre, B.; Gouveia, L.; Roseiro, J.; Reis, A.; da Silva, T.L. New at-line flow cytometric protocols for determining carotenoid content and cell viability during Rhodosporidium toruloides NCYC 921 batch growth. Process Biochem. 2014, 49, 554–562. [Google Scholar] [CrossRef]
- Schüler, L.M.; Gangadhar, K.N.; Duarte, P.; Placines, C.; Molina-Márquez, A.M.; Léon-Bañares, R.; Sousa, V.S.; Varela, J.; Barreira, L. Improvement of carotenoid extraction from a recently isolated, robust microalga, Tetraselmis sp. CTP4 (chlorophyta). Bioprocess Biosyst. Eng. 2020, 43, 785–796. [Google Scholar] [CrossRef]
- de Quirós, A.R.-B.; Costa, H.S. Analysis of carotenoids in vegetable and plasma samples: A review. J. Food Compos. Anal. 2006, 19, 97–111. [Google Scholar] [CrossRef]
- Granado, F.; Olmedilla, B.; Blanco, I.; Rojas-Hidalgo, E. Carotenoid composition in raw and cooked Spanish vegetables. J. Agric. Food Chem. 1992, 40, 2135–2140. [Google Scholar] [CrossRef]
- Kalra, R.; Conlan, X.A.; Goel, M. Fungi as a potential source of pigments: Harnessing filamentous fungi. Front. Chem. 2020, 8, 369. [Google Scholar] [CrossRef]
- Buzzini, P. An optimization study of carotenoid production by Rhodotorula glutinis DBVPG 3853 from substrates containing concentrated rectified grape must as the sole carbohydrate source. J. Ind. Microbiol. Biotechnol. 2000, 24, 41–45. [Google Scholar] [CrossRef]
- Ochsenreither, K.; Glück, C.; Stressler, T.; Fischer, L.; Syldatk, C. Production strategies and applications of microbial single cell oils. Front. Microbiol. 2016, 7, 1539. [Google Scholar] [CrossRef]
- Brandenburg, J.; Blomqvist, J.; Pickova, J.; Bonturi, N.; Sandgren, M.; Passoth, V. Lipid production from hemicellulose with Lipomyces starkeyi in a pH regulated fed-batch cultivation. Yeast 2016, 33, 451–462. [Google Scholar] [CrossRef]
- Enshaeieh, M.; Abdoli, A.; Madani, M.; Bayat, M. Recycling of lignocellulosic waste materials to produce high-value products: Single cell oil and xylitol. Int. J. Environ. Sci. Technol. 2015, 12, 837–846. [Google Scholar] [CrossRef][Green Version]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Appelqvist, L.-Å. Rapid methods of lipid extraction and fatty acid methyl ester preparation for seed and leaf tissue with special remarks on preventing the accumulation of lipid contaminants. Ark. Kemi 1968, 28, 551–570. [Google Scholar]
- Olsen, R.; Henderson, R. The rapid analysis of neutral and polar marine lipids using double-development HPTLC and scanning densitometry. J. Exp. Mar. Biol. Ecol. 1989, 129, 189–197. [Google Scholar] [CrossRef]
- Reif, C.; Arrigoni, E.; Schärer, H.; Nyström, L.; Hurrell, R.F. Carotenoid database of commonly eaten Swiss vegetables and their estimated contribution to carotenoid intake. J. Food Compos. Anal. 2013, 29, 64–72. [Google Scholar] [CrossRef]
- Davey, M.W.; Keulemans, J.; Swennen, R. Methods for the efficient quantification of fruit provitamin A contents. J. Chromatogr. A 2006, 1136, 176–184. [Google Scholar] [CrossRef]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 spectrophotometric methods for carotenoid determination in frequently consumed fruits and vegetables. J. Food Sci. 2010, 75, C55–C61. [Google Scholar] [CrossRef]
- Vilaro, F.; Perez-Hedo, M.; Eras, J.; Canela, R.; Eizaguirre, M. UHPLC-MS Analysis of Juvenile Hormone II in Mediterranean Corn Borer (Sesamia nonagrioides) Hemolymph Using Various Ionization Techniques. J. Agric. Food Chem. 2012, 60, 3020–3025. [Google Scholar] [CrossRef]
- Ligor, M.; Kováčová, J.; Gadzała-Kopciuch, R.; Studzińska, S.; Bocian, S.; Lehotay, J.; Buszewski, B. Study of RP HPLC retention behaviours in analysis of carotenoids. Chromatographia 2014, 77, 1047–1057. [Google Scholar] [CrossRef]
- Gosetti, F.; Mazzucco, E.; Zampieri, D.; Gennaro, M.C. Signal suppression/enhancement in high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 3929–3937. [Google Scholar] [CrossRef]
- Khachik, F.; Beecher, G.R.; Whittaker, N.F. Separation, identification, and quantification of the major carotenoid and chlorophyll constituents in extracts of several green vegetables by liquid chromatography. J. Agric. Food Chem. 1986, 34, 603–616. [Google Scholar] [CrossRef]
- Nierenberg, D.W.; Nann, S.L. A method for determining concentrations of retinol, tocopherol, and five carotenoids in human plasma and tissue samples. Am. J. Clin. Nutr. 1992, 56, 417–426. [Google Scholar] [CrossRef]
- Kimura, M.; Rodriguez-Amaya, D.B.; Godoy, H.T. Assessment of the saponification step in the quantitative determination of carotenoids and provitamins A. Food Chem. 1990, 35, 187–195. [Google Scholar] [CrossRef]
- Lietz, G.; Henry, C. A modified method to minimise losses of carotenoids and tocopherols during HPLC analysis of red palm oil. Food Chem. 1997, 60, 109–117. [Google Scholar] [CrossRef]
- Barba, A.O.; Hurtado, M.C.; Mata, M.S.; Ruiz, V.F.; De Tejada, M.L.S. Application of a UV–vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Pham, K.D.; Shida, Y.; Miyata, A.; Takamizawa, T.; Suzuki, Y.; Ara, S.; Yamazaki, H.; Masaki, K.; Mori, K.; Aburatani, S. Effect of light on carotenoid and lipid production in the oleaginous yeast Rhodosporidium toruloides. Biosci. Biotechnol. Biochem. 2020, 84, 1501–1512. [Google Scholar] [CrossRef]
- Nam, H.; Cho, S.; Rhee, J.S. High-performance liquid chromatographic analysis of major carotenoids from Rhodotorula glutinis. J. Chromatogr. A 1988, 448, 445–447. [Google Scholar] [CrossRef]
- Ratledge, C.; Wynn, J.P. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2002; Volume 51, pp. 1–52. [Google Scholar]
- Zhou, W.; Wang, Y.; Zhang, J.; Zhao, M.; Tang, M.; Zhou, W.; Gong, Z. A metabolic model of Lipomyces starkeyi for predicting lipogenesis potential from diverse low-cost substrates. Biotechnol. Biofuels 2021, 14, 148. [Google Scholar] [CrossRef]
- Cescut, J.; Fillaudeau, L.; Molina-Jouve, C.; Uribelarrea, J.-L. Carbon accumulation in Rhodotorula glutinis induced by nitrogen limitation. Biotechnol. Biofuels 2014, 7, 164. [Google Scholar] [CrossRef][Green Version]
- Maina, S.; Pateraki, C.; Kopsahelis, N.; Paramithiotis, S.; Drosinos, E.H.; Papanikolaou, S.; Koutinas, A. Microbial oil production from various carbon sources by newly isolated oleaginous yeasts. Eng. Life Sci. 2017, 17, 333–344. [Google Scholar] [CrossRef]
- Suutari, M.; Rintamäki, A.; Laakso, S. The effect of temperature on lipid classes and their fatty acid profiles in Lipomyces starkeyi. J. Am. Oil Chem. Soc. 1996, 73, 1071–1073. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol. J. Healthc. Nutr. Technol. 2006, 1, 420–439. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Enser, M.; Fisher, A.; Nute, G.; Sheard, P.; Richardson, R.; Hughes, S.; Whittington, F. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Sandager, L.; Gustavsson, M.H.; Ståhl, U.; Dahlqvist, A.; Wiberg, E.; Banas, A.; Lenman, M.; Ronne, H.; Stymne, S. Storage lipid synthesis is non-essential in yeast. J. Biol. Chem. 2002, 277, 6478–6482. [Google Scholar] [CrossRef] [PubMed]
- Tronchoni, J.; Rozès, N.; Querol, A.; Guillamón, J.M. Lipid composition of wine strains of Saccharomyces kudriavzevii and Saccharomyces cerevisiae grown at low temperature. Int. J. Food Microbiol. 2012, 155, 191–198. [Google Scholar] [CrossRef]
- Patel, A.; Arora, N.; Mehtani, J.; Pruthi, V.; Pruthi, P.A. Assessment of fuel properties on the basis of fatty acid profiles of oleaginous yeast for potential biodiesel production. Renew. Sustain. Energy Rev. 2017, 77, 604–616. [Google Scholar] [CrossRef]
- Eisenberg, T.; Büttner, S. Lipids and cell death in yeast. FEMS Yeast Res. 2014, 14, 179–197. [Google Scholar] [CrossRef]
- Tehlivets, O.; Scheuringer, K.; Kohlwein, S.D. Fatty acid synthesis and elongation in yeast. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2007, 1771, 255–270. [Google Scholar] [CrossRef]
- Zinser, E.; Paltauf, F.; Daum, G. Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J. Bacteriol. 1993, 175, 2853–2858. [Google Scholar] [CrossRef]
- Tkacova, J.; Caplova, J.; Klempova, T.; Certik, M. Correlation between lipid and carotenoid synthesis in torularhodin-producing Rhodotorula glutinis. Ann. Microbiol. 2017, 67, 541–551. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Gientka, I.; Kieliszek, M.; Bryś, J. Torulene and torularhodin: “New” fungal carotenoids for industry? Microb. Cell Factories 2018, 17, 49. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Bryś, J. Simultaneous production of lipids and carotenoids by the red yeast Rhodotorula from waste glycerol fraction and potato wastewater. Appl. Biochem. Biotechnol. 2019, 189, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Eren, A.T. Carotenoids production by the yeast Rhodotorula mucilaginosa: Use of agricultural wastes as a carbon source. Process Biochem. 2005, 40, 2985–2991. [Google Scholar] [CrossRef]

| Carotenoid | RT, min | λmax, nm | Slope | Intercept | R2 | RSD, % | LOD, ng/mL | LOQ, ng/mL |
|---|---|---|---|---|---|---|---|---|
| UHPLC-PDA-method | ||||||||
| Torularhodin | 16.41 | 450 | 400.01 | 64,556 | 0.999 | 2 | 0.97 | 2.95 |
| (E/Z)-Torulene | 23.49 | 478 | 223.29 | 177,705 | 0.999 | 15 | 14.5 | 44.1 |
| β-carotene | 32.16 | 450 | 2331.4 | 877,044 | 0.993 | 12 | 0.26 | 0.72 |
| γ-carotene | 25.75 | 462 | 216.81 | 92,861 | 0.999 | 14 | 6.86 | 20.8 |
| Spectrophotometric method | ||||||||
| β-carotene | – | 450 | 1.23 | 0.03 | 0.999 | n.d | n.d | n.d |
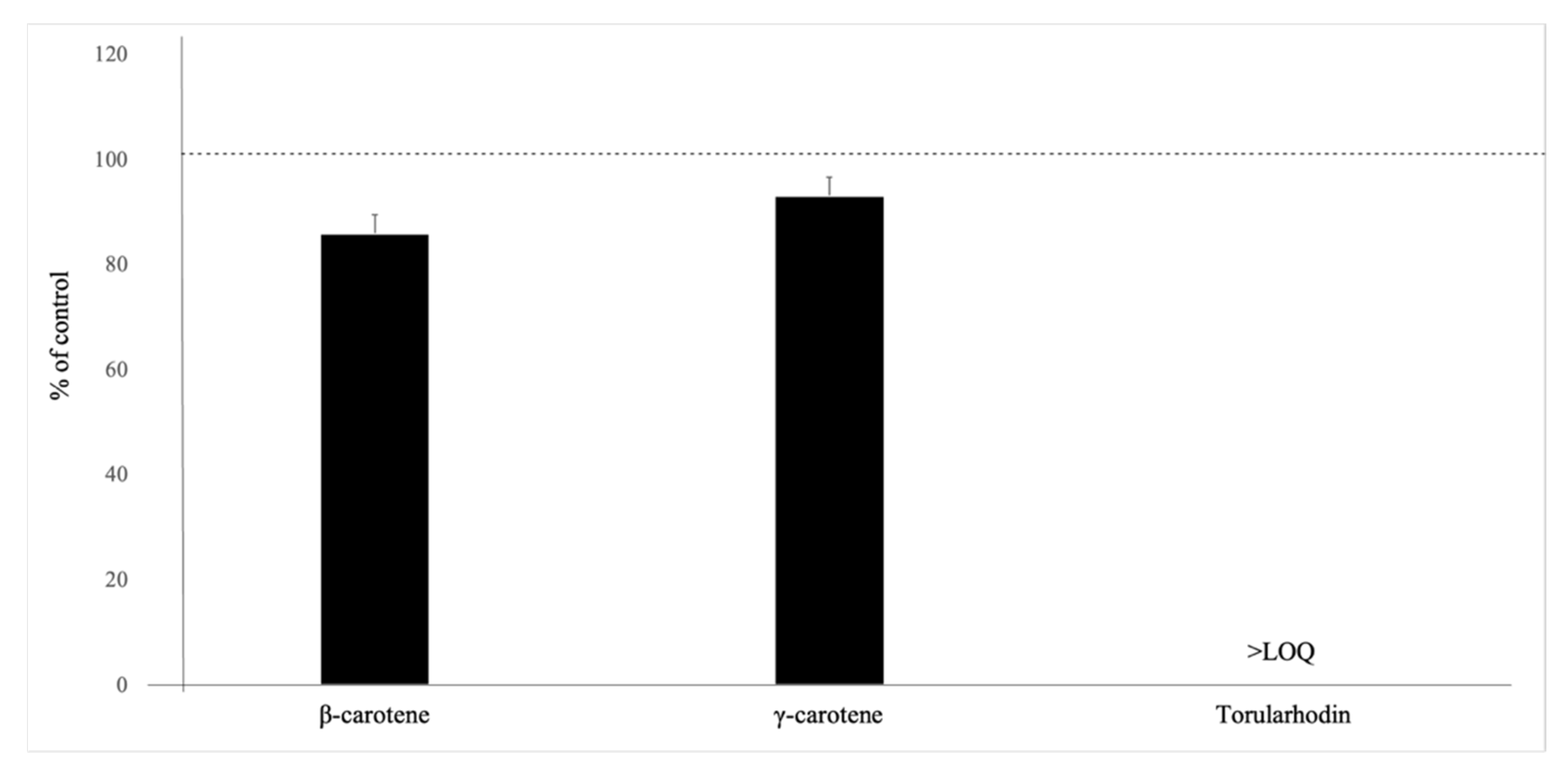
| Carotenoids | Relative Recovery, % | Matrix Effect, % |
|---|---|---|
| Torularhodin | 4.2 ± 0.04 | −18 ± 0.15 |
| (E/Z)-Torulene | <LOQ | n.a. |
| β-carotene | 65 ± 0.04 | −31 ± 0.16 |
| γ-carotene | 72 ± 0.06 | 12 ± 0.07 |
| UHPLC-PDA Saponified mg/100 g d.w. | Spectrophotometer Saponified mg β-EQ/100 g d.w. | Spectrophotometer Unsaponified mg β-EQ/100 g d.w. | |
|---|---|---|---|
| Torularhodin | 0.09 ± 0.028 | n.a. | n.a. |
| (E/Z)-Torulene | <LOQ | – | – |
| β-carotene | 1.48 ± 0.18 | – | – |
| γ-carotene | 0.42 ± 0.06 | – | – |
| Total carotenoids | 1.99 ± 0.27 a | 3.09 ± 0.78 ab | 4.02 ± 0.53 b |
| Cultivation Time (h) | Fatty Acid Profile (%) of the Total Fatty Acids | |||||
|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C17:1 | C18:1 (n-9) | C18:2 (n-6) | C18:3 (n-3) | |
| 0 | 14.7 ± 0.40 | 2.79 ± 0.27 | 0.47 ± 0.04 | 55.8 ± 0.54 | 13.3 ± 0.35 | 7.22 ± 0.36 |
| 24 | 13.2 ± 1.18 | 1.68 ± 0.67 | 0.30 ± 0.10 | 49.1 ± 11.23 | 22.9 ± 14.00 | 7.53 ± 0.45 |
| 48 | 14.8 ± 1.42 | 3.13 ± 2.60 | 0.17 ± 0.06 | 45.0 ± 16.82 | 26.0 ± 15.30 | 6.18 ± 1.59 |
| 72 | 23.7 ± 3.83 | 4.24 ± 0.84 | 0.47 ± 0.06 | 38.8 ± 2.31 | 24.2 ± 6.09 | 3.55 ± 0.78 |
| 96 | 26.6 ± 1.66 | 4.70 ± 0.44 | 0.36 ± 0.03 | 40.3 ± 1.73 | 20.4 ± 1.31 | 2.93 ± 0.87 |
| Cultivation Time (h) | Lipid Class Expressed as % of Total Lipid Classes | |||||
|---|---|---|---|---|---|---|
| Phospholipids | MAG | DAG | Sterols | FFAs | TAG | |
| 0 | 12.6 ± 0.47 | 5.09 ± 0.24 | 3.60 ± 0.71 | 8.54 ± 0.51 | 37.2 ± 0.32 | 33.5 ± 0.60 |
| 24 | 12.1 ± 0.92 | 4.99 ± 1.39 | 3.90 ± 0.94 | 9.12 ± 0.64 | 41.8 ± 4.42 | 28.2 ± 3.37 |
| 48 | 13.8 ± 4.72 | 4.90 ± 1.00 | 4.02 ± 0.75 | 8.68 ± 0.93 | 38.3 ± 6.14 | 30.3 ± 13.2 |
| 72 | 7.40 ± 0.34 | 4.42 ± 0.26 | 3.95 ± 0.77 | 6.92 ± 0.38 | 27.9 ± 5.01 | 49.4 ± 6.66 |
| 96 | 8.05 ± 1.20 | 4.02 ± 0.63 | 2.54 ± 0.42 | 6.80 ± 0.42 | 20.5 ± 1.58 | 58.1 ± 3.32 |
| Cultivation Time (h) | Quantity of Carotenoids (mg/100 g d.w.) | |||
|---|---|---|---|---|
| β-Carotene | γ-Carotene | Torularhodin | Total | |
| 0 | 0.15 ± 0.002 | 0.01 ± 0.0005 | 0.0003 ± 0.0001 | 0.16 ± 0.001 |
| 24 | 0.22 ± 0.02 | 0.03 ± 0.03 | 0.004 ± 0.004 | 0.25 ± 0.03 |
| 48 | 0.45 ± 0.17 | 0.12 ± 0.11 | 0.01 ± 0.01 | 0.58 ± 0.24 |
| 72 | 0.85 ± 0.16 | 0.46 ± 0.16 | 0.03 ± 0.01 | 1.33 ± 0.27 |
| 96 | 1.47 ± 0.14 | 1.32 ± 0.14 | 0.06 ± 0.003 | 2.85 ± 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagaraj, Y.N.; Burkina, V.; Okmane, L.; Blomqvist, J.; Rapoport, A.; Sandgren, M.; Pickova, J.; Sampels, S.; Passoth, V. Identification, Quantification and Kinetic Study of Carotenoids and Lipids in Rhodotorula toruloides CBS 14 Cultivated on Wheat Straw Hydrolysate. Fermentation 2022, 8, 300. https://doi.org/10.3390/fermentation8070300
Nagaraj YN, Burkina V, Okmane L, Blomqvist J, Rapoport A, Sandgren M, Pickova J, Sampels S, Passoth V. Identification, Quantification and Kinetic Study of Carotenoids and Lipids in Rhodotorula toruloides CBS 14 Cultivated on Wheat Straw Hydrolysate. Fermentation. 2022; 8(7):300. https://doi.org/10.3390/fermentation8070300
Chicago/Turabian StyleNagaraj, Yashaswini Nagavara, Viktoriia Burkina, Laura Okmane, Johanna Blomqvist, Alexander Rapoport, Mats Sandgren, Jana Pickova, Sabine Sampels, and Volkmar Passoth. 2022. "Identification, Quantification and Kinetic Study of Carotenoids and Lipids in Rhodotorula toruloides CBS 14 Cultivated on Wheat Straw Hydrolysate" Fermentation 8, no. 7: 300. https://doi.org/10.3390/fermentation8070300
APA StyleNagaraj, Y. N., Burkina, V., Okmane, L., Blomqvist, J., Rapoport, A., Sandgren, M., Pickova, J., Sampels, S., & Passoth, V. (2022). Identification, Quantification and Kinetic Study of Carotenoids and Lipids in Rhodotorula toruloides CBS 14 Cultivated on Wheat Straw Hydrolysate. Fermentation, 8(7), 300. https://doi.org/10.3390/fermentation8070300








