Dynamic Regulation of Transporter Expression to Increase L-Threonine Production Using L-Threonine Biosensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Construction of Strains and Plasmids
2.3. Characterization of the L-Threonine-Sensing Promoters
2.4. Culture Medium and L-Threonine Fermentation
Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
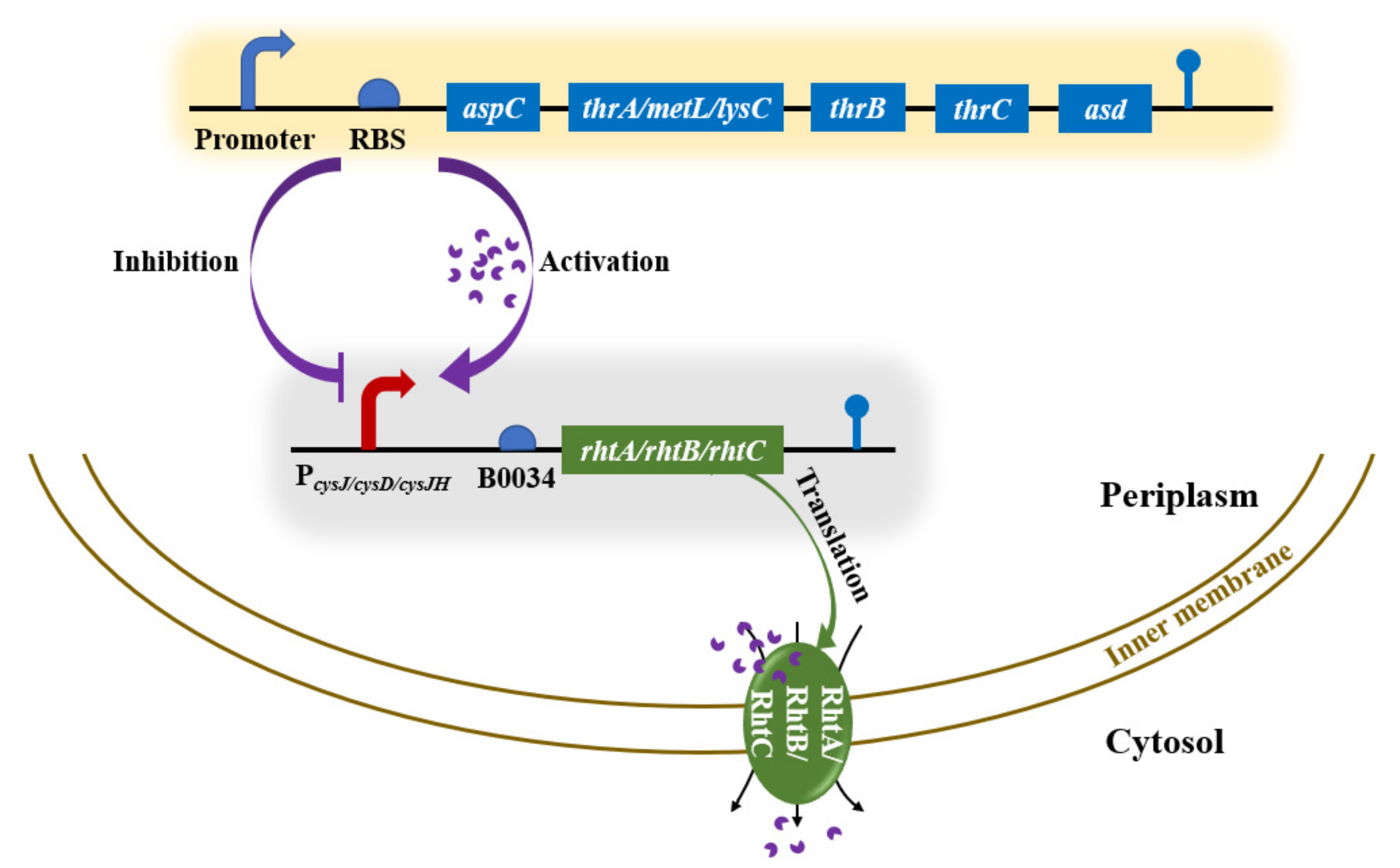
3.1. Simulating Dynamic rhtA Regulation with IPTG Induction
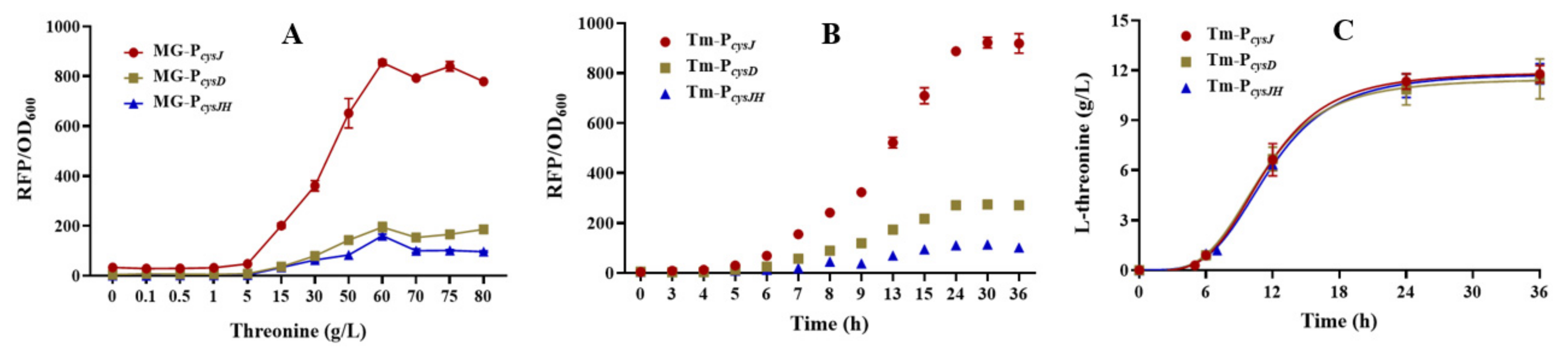
3.2. Characterizing the L-Threonine-Sensing Promoters
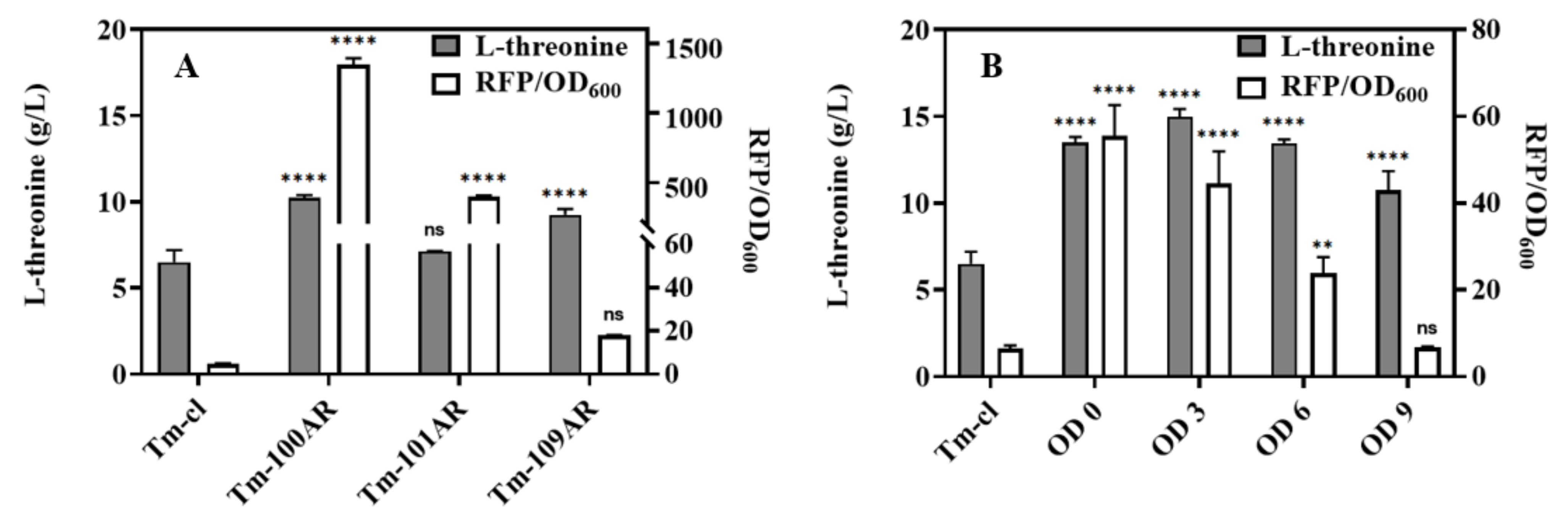
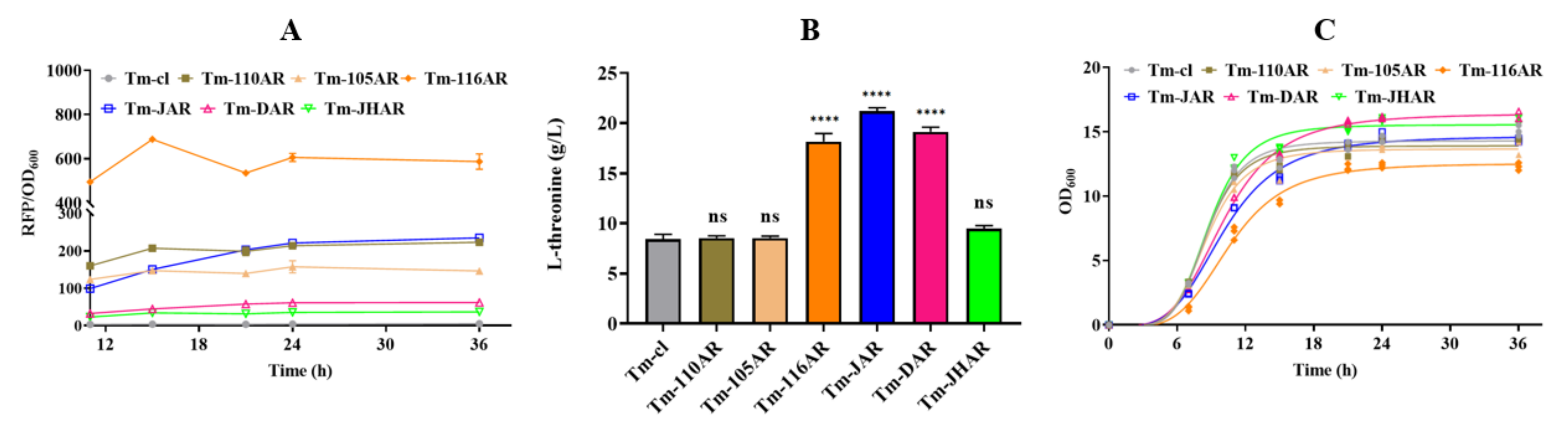
3.3. Improving L-Threonine Production through the Dynamic Regulation of rhtA Expression
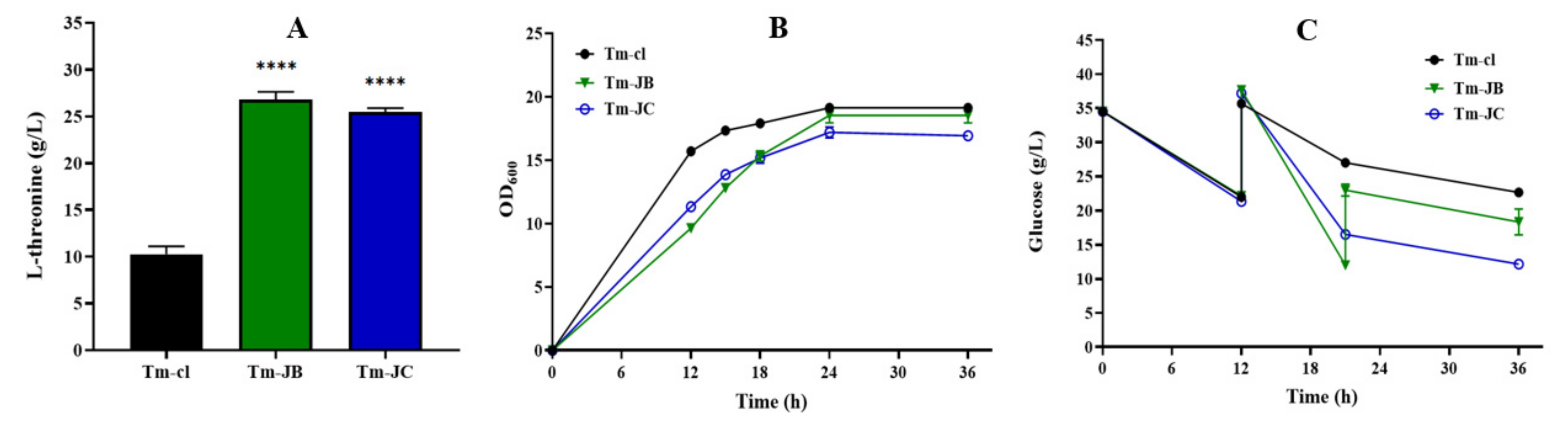
3.4. Regulating rhtB and rhtC to Further Improve L-Threonine Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, H.; Zhao, Y.; Wu, F.; Ouyang, P.; Chen, J.; Jiang, X.; Ye, J.; Chen, G.Q. Engineering Halomonas bluephagenesis for L-Threonine production. Metab. Eng. 2020, 60, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Wendisch, V.F. Metabolic engineering advances and prospects for amino acid production. Metab. Eng. 2020, 58, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Quinn, P.J.; Wang, X. Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for the production of L-threonine. Biotechnol. Adv. 2011, 29, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, J.; Ma, W.; Yang, J.; Zhang, H.; Zhao, L.; Chen, S.; Zhang, S.; Hu, X.; Li, Y.; et al. Rebalancing microbial carbon distribution for L-threonine maximization using a thermal switch system. Metab. Eng. 2020, 61, 33–46. [Google Scholar] [CrossRef]
- Lee, K.H.; Park, J.H.; Kim, T.Y.; Kim, H.U.; Lee, S.Y. Systems metabolic engineering of Escherichia coli for L-threonine production. Mol. Syst. Biol. 2007, 3, 149. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, Y.; Yang, J.; Fang, Y.; Zhu, L.; Ding, Z.; Wang, C.; Ma, W.; Hu, X.; Wang, X. Expression regulation of multiple key genes to improve L-threonine in Escherichia coli. Microb. Cell Factories 2020, 19, 46. [Google Scholar] [CrossRef] [Green Version]
- Diesveld, R.; Tietze, N.; Fürst, O.; Reth, A.; Bathe, B.; Sahm, H.; Eggeling, L. Activity of exporters of Escherichia coli in Corynebacterium glutamicum, and their use to increase L-threonine production. J. Mol. Microbiol. Biotechnol. 2009, 16, 198–207. [Google Scholar] [CrossRef]
- Kruse, D.; Krämer, R.; Eggeling, L.; Rieping, M.; Pfefferle, W.; Tchieu, J.H.; Chung, Y.J.; Saier, M.H., Jr.; Burkovski, A. Influence of threonine exporters on threonine production in Escherichia coli. Appl. Microbiol. Biotechnol. 2002, 59, 205–210. [Google Scholar]
- Zhu, Y.; Zhou, C.; Wang, Y.; Li, C. Transporter Engineering for Microbial Manufacturing. Biotechnol. J. 2020, 15, e1900494. [Google Scholar] [CrossRef]
- van der Hoek, S.A.; Borodina, I. Transporter engineering in microbial cell factories: The ins, the outs, and the in-betweens. Curr. Opin. Biotechnol. 2020, 66, 186–194. [Google Scholar] [CrossRef]
- Pereira, R.; Wei, Y.; Mohamed, E.; Radi, M.; Malina, C.; Herrgård, M.J.; Feist, A.M.; Nielsen, J.; Chen, Y. Adaptive laboratory evolution of tolerance to dicarboxylic acids in Saccharomyces cerevisiae. Metab. Eng. 2019, 56, 130–141. [Google Scholar] [CrossRef]
- Gubellini, F.; Verdon, G.; Karpowich, N.K.; Luff, J.D.; Boël, G.; Gauthier, N.; Handelman, S.K.; Ades, S.E.; Hunt, J.F. Physiological response to membrane protein overexpression in E. coli. Mol. Cell. Proteom. MCP 2011, 10, M111.007930. [Google Scholar] [CrossRef] [Green Version]
- Wagner, S.; Baars, L.; Ytterberg, A.J.; Klussmeier, A.; Wagner, C.S.; Nord, O.; Nygren, P.A.; van Wijk, K.J.; de Gier, J.W. Consequences of membrane protein overexpression in Escherichia coli. Mol. Cell. Proteom. MCP 2007, 6, 1527–1550. [Google Scholar] [CrossRef] [Green Version]
- Boyarskiy, S.; Davis López, S.; Kong, N.; Tullman-Ercek, D. Transcriptional feedback regulation of efflux protein expression for increased tolerance to and production of n-butanol. Metab. Eng. 2016, 33, 130–137. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Hu, Y.; Zhu, Z.; Siewers, V.; Nielsen, J. Engineering 1-Alkene Biosynthesis and Secretion by Dynamic Regulation in Yeast. ACS Synth. Biol. 2018, 7, 584–590. [Google Scholar] [CrossRef]
- Su, Y.; Guo, Q.Q.; Wang, S.; Zhang, X.; Wang, J. Effects of betaine supplementation on L-threonine fed-batch fermentation by Escherichia coli. Bioprocess Biosyst. Eng. 2018, 41, 1509–1518. [Google Scholar] [CrossRef]
- Xu, P.; Li, L.; Zhang, F.; Stephanopoulos, G.; Koffas, M. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proc. Natl. Acad. Sci. USA 2014, 111, 11299–11304. [Google Scholar] [CrossRef] [Green Version]
- Hossain, G.S.; Saini, M.; Miyake, R.; Ling, H.; Chang, M.W. Genetic Biosensor Design for Natural Product Biosynthesis in Microorganisms. Trends Biotechnol. 2020, 38, 797–810. [Google Scholar] [CrossRef]
- Lange, C.; Mustafi, N.; Frunzke, J.; Kennerknecht, N.; Wessel, M.; Bott, M.; Wendisch, V.F. Lrp of Corynebacterium glutamicum controls expression of the brnFE operon encoding the export system for L-methionine and branched-chain amino acids. J. Biotechnol. 2012, 158, 231–241. [Google Scholar] [CrossRef]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A., 3rd; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Zhang, X.; Yang, P.; Liang, Q.; Qi, Q. A novel whole-phase succinate fermentation strategy with high volumetric productivity in engineered Escherichia coli. Bioresour. Technol. 2013, 149, 333–340. [Google Scholar] [CrossRef]
- Livshits, V.A.; Zakataeva, N.P.; Aleshin, V.V.; Vitushkina, M.V. Identification and characterization of the new gene rhtA involved in threonine and homoserine efflux in Escherichia coli. Res. Microbiol. 2003, 154, 123–135. [Google Scholar] [CrossRef]
- Lee, J.H.; Sung, B.H.; Kim, M.S.; Blattner, F.R.; Yoon, B.H.; Kim, J.H.; Kim, S.C. Metabolic engineering of a reduced-genome strain of Escherichia coli for L-threonine production. Microb. Cell Factories 2009, 8, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Jung, S.C.; Bui, L.M.; Kang, K.H.; Song, J.J.; Kim, S.C. Improved production of L-threonine in Escherichia coli by use of a DNA scaffold system. Appl. Environ. Microbiol. 2013, 79, 774–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Zhang, H.; Wang, X.; Han, G.; Ma, W.; Hu, X.; Li, Y. Transcriptomic analysis of an l-threonine-producing Escherichia coli TWF001. Biotechnol. Appl. Biochem. 2020, 67, 414–429. [Google Scholar] [PubMed]
- Reinscheid, D.J.; Kronemeyer, W.; Eggeling, L.; Eikmanns, B.J.; Sahm, H. Stable Expression of hom-1-thrB in Corynebacterium glutamicum and Its Effect on the Carbon Flux to Threonine and Related Amino Acids. Appl. Environ. Microbiol. 1994, 60, 126–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dyk, T.K. Bacterial efflux transport in biotechnology. Adv. Appl. Microbiol. 2008, 63, 231–247. [Google Scholar]
- Xu, S.; Zhang, L.; Zhou, S.; Deng, Y. Biosensor-Based Multigene Pathway Optimization for Enhancing the Production of Glycolate. Appl. Environ. Microbiol. 2021, 87, e0011321. [Google Scholar] [CrossRef]
- Mazumder, M.; McMillen, D.R. Design and characterization of a dual-mode promoter with activation and repression capability for tuning gene expression in yeast. Nucleic Acids Res. 2014, 42, 9514–9522. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, Q.; Zheng, P.; Zhang, Z.; Liu, Y.; Sun, C.; Cao, G.; Zhou, W.; Wang, X.; Zhang, D.; et al. Developing a high-throughput screening method for threonine overproduction based on an artificial promoter. Microb. Cell Factories 2015, 14, 121. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.Z.; Prather, K.L. Dynamic pathway regulation: Recent advances and methods of construction. Curr. Opin. Chem. Biol. 2017, 41, 28–35. [Google Scholar] [CrossRef]
- Zhou, S.; Yuan, S.F.; Nair, P.H.; Alper, H.S.; Deng, Y.; Zhou, J. Development of a growth coupled and multi-layered dynamic regulation network balancing malonyl-CoA node to enhance (2S)-naringenin biosynthesis in Escherichia coli. Metab. Eng. 2021, 67, 41–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, M.; Zhao, G.; Zhang, W.; Li, Y.; Lin, B.; Li, Y.; Xu, Q.; Chen, N.; Zhang, C. High-level production of l-homoserine using a non-induced, non-auxotrophic Escherichia coli chassis through metabolic engineering. Bioresour. Technol. 2021, 327, 124814. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Wu, J.; Mu, S.; Wu, Z.; Jin, J.M.; Tang, S.Y. Dynamic control of toxic natural product biosynthesis by an artificial regulatory circuit. Metab. Eng. 2020, 57, 239–246. [Google Scholar] [CrossRef]
- Zakataeva, N.P.; Aleshin, V.V.; Tokmakova, I.L.; Troshin, P.V.; Livshits, V.A. The novel transmembrane Escherichia coli proteins involved in the amino acid efflux. FEBS Lett. 1999, 452, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wei, H.; Wang, T.; Xu, Q.; Zhang, C.; Fan, X.; Ma, Q.; Chen, N.; Xie, X. Current status on metabolic engineering for the production of l-aspartate family amino acids and derivatives. Bioresour. Technol. 2017, 245, 1588–1602. [Google Scholar] [CrossRef]
- Lerner, C.G.; Inouye, M. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 1990, 18, 4631. [Google Scholar] [CrossRef] [Green Version]
| Titer (g/L) | Titer Increase (%) | Yield (g/g) | Productivity (g/L/h) | |
|---|---|---|---|---|
| Tm-cl | 10.26 (±0.68) | —— | 0.402 (±0.006) | 0.285 (±0.019) |
| Tm-JB | 26.78 (±0.67) | 161.01 (±6.61) | 0.627 (±0.023) | 0.743 (±0.018) |
| Tm-JC | 25.44 (±0.31) | 147.95 (±3.02) | 0.665 (±0.010) | 0.706 (±0.008) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Hao, R.; Jin, X.; Li, X.; Qi, Q.; Liang, Q. Dynamic Regulation of Transporter Expression to Increase L-Threonine Production Using L-Threonine Biosensors. Fermentation 2022, 8, 250. https://doi.org/10.3390/fermentation8060250
Wang S, Hao R, Jin X, Li X, Qi Q, Liang Q. Dynamic Regulation of Transporter Expression to Increase L-Threonine Production Using L-Threonine Biosensors. Fermentation. 2022; 8(6):250. https://doi.org/10.3390/fermentation8060250
Chicago/Turabian StyleWang, Sumeng, Ruxin Hao, Xin Jin, Xiaomeng Li, Qingsheng Qi, and Quanfeng Liang. 2022. "Dynamic Regulation of Transporter Expression to Increase L-Threonine Production Using L-Threonine Biosensors" Fermentation 8, no. 6: 250. https://doi.org/10.3390/fermentation8060250
APA StyleWang, S., Hao, R., Jin, X., Li, X., Qi, Q., & Liang, Q. (2022). Dynamic Regulation of Transporter Expression to Increase L-Threonine Production Using L-Threonine Biosensors. Fermentation, 8(6), 250. https://doi.org/10.3390/fermentation8060250






