Biotransformation of Agricultural By-Products into Biovanillin through Solid-State Fermentation (SSF) and Optimization of Different Parameters Using Response Surface Methodology (RSM)
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Collection of Substrates
2.3. Culture Maintenance and Inoculum Preparation
2.4. Extraction and Quantification of Ferulic Acid from Agricultural By-Products
2.5. Selection of Best Substrate for Biovanillin Production by SSF
2.6. Optimization of Biovanillin for Maximum Production
2.7. Extraction of Biovanillin and Quantitative Estimation
2.8. Crystallization and Characterization of Biovanilin
2.9. Statistical Design
3. Results
3.1. Recovery of Ferulic Acid by Different Agricultural By-Products
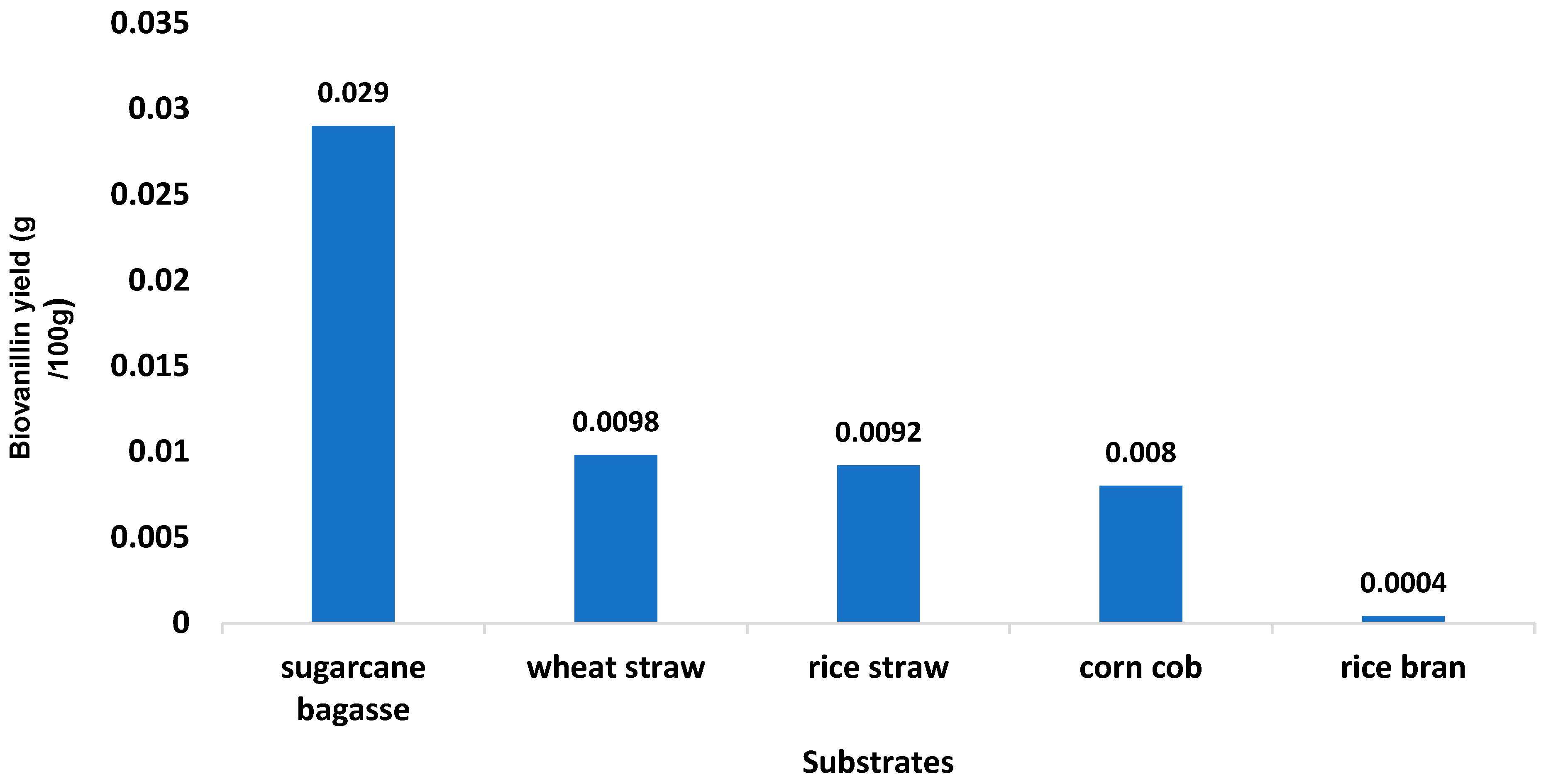
3.2. Selection of The Best Biovanillin-Producing Substrate
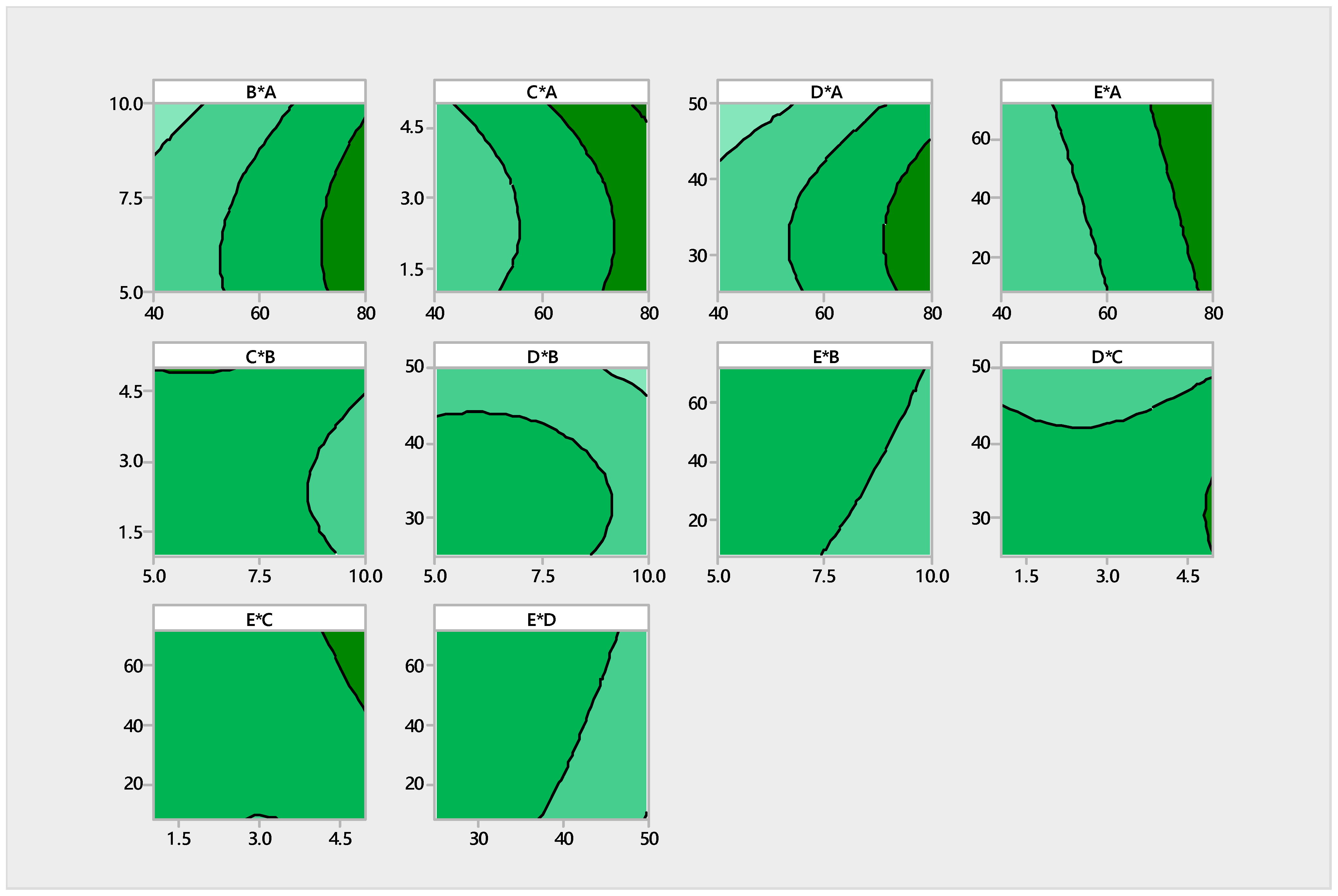
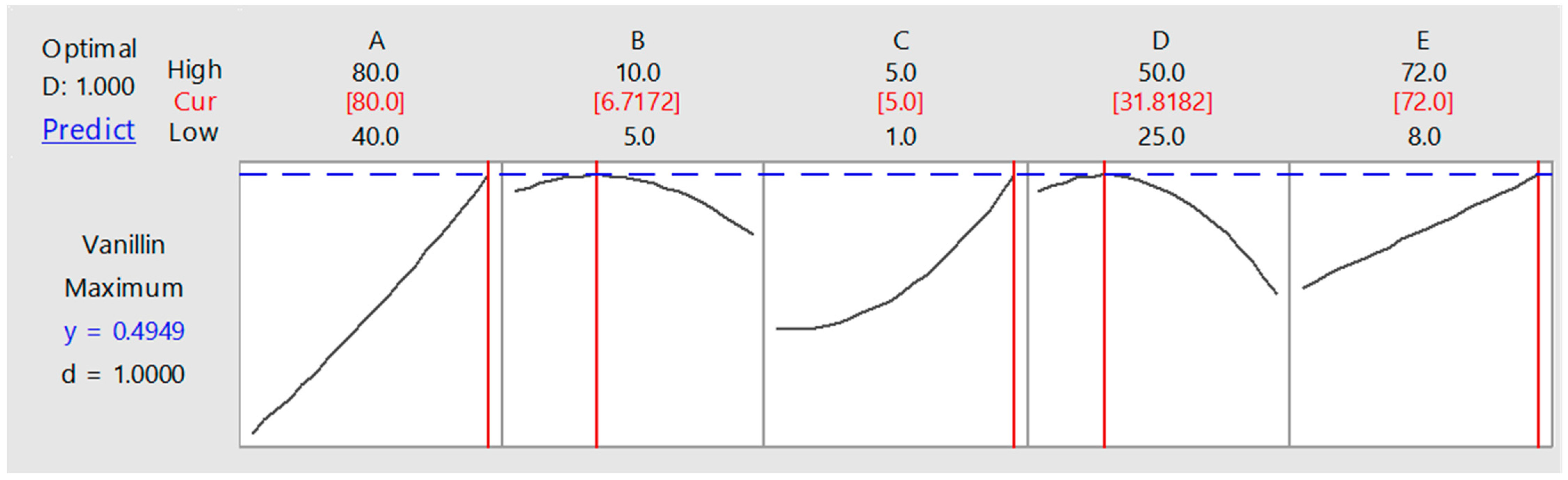
3.3. Optimization of Process Parameters for Biovanillin Production through RSM
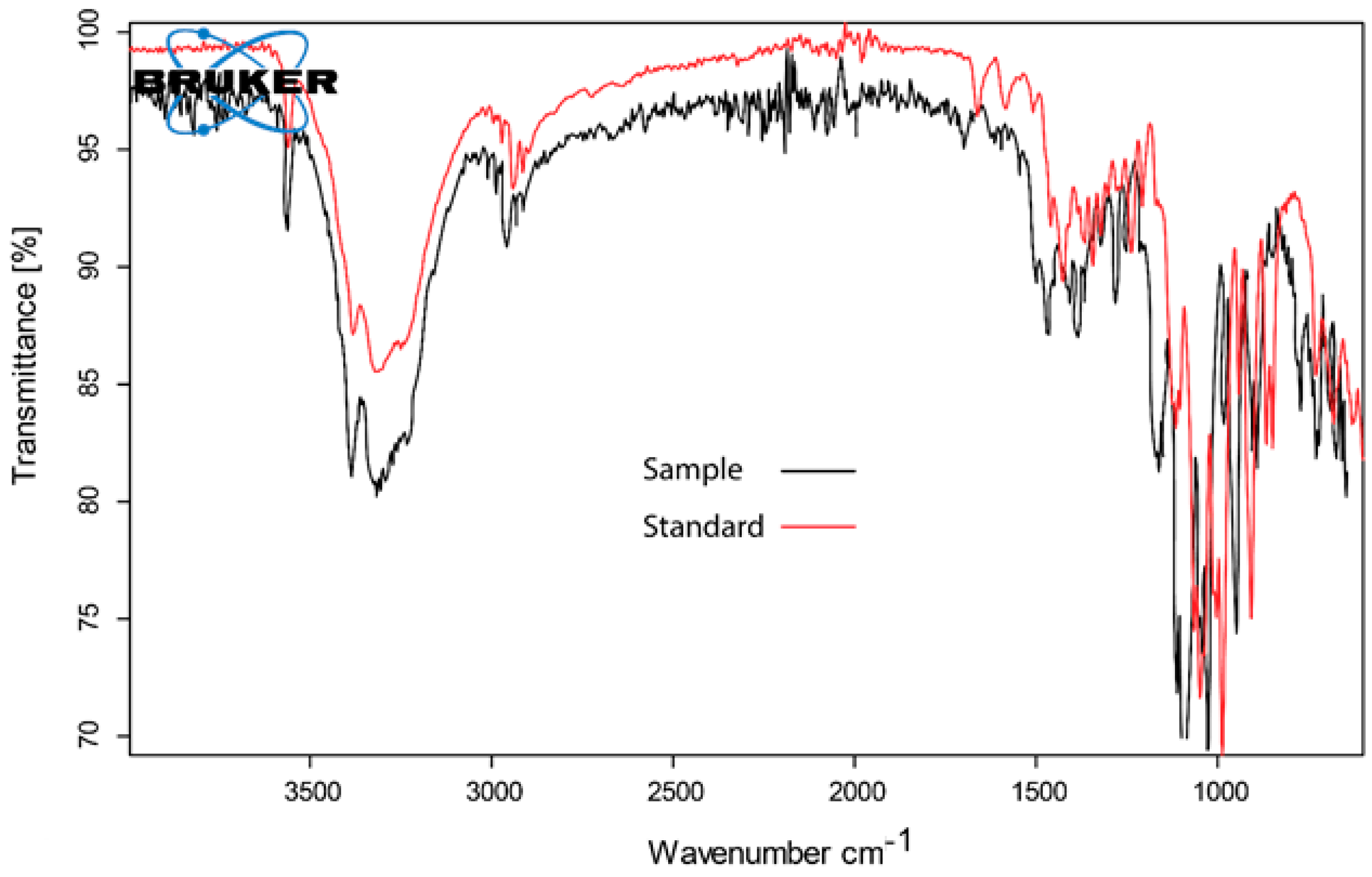
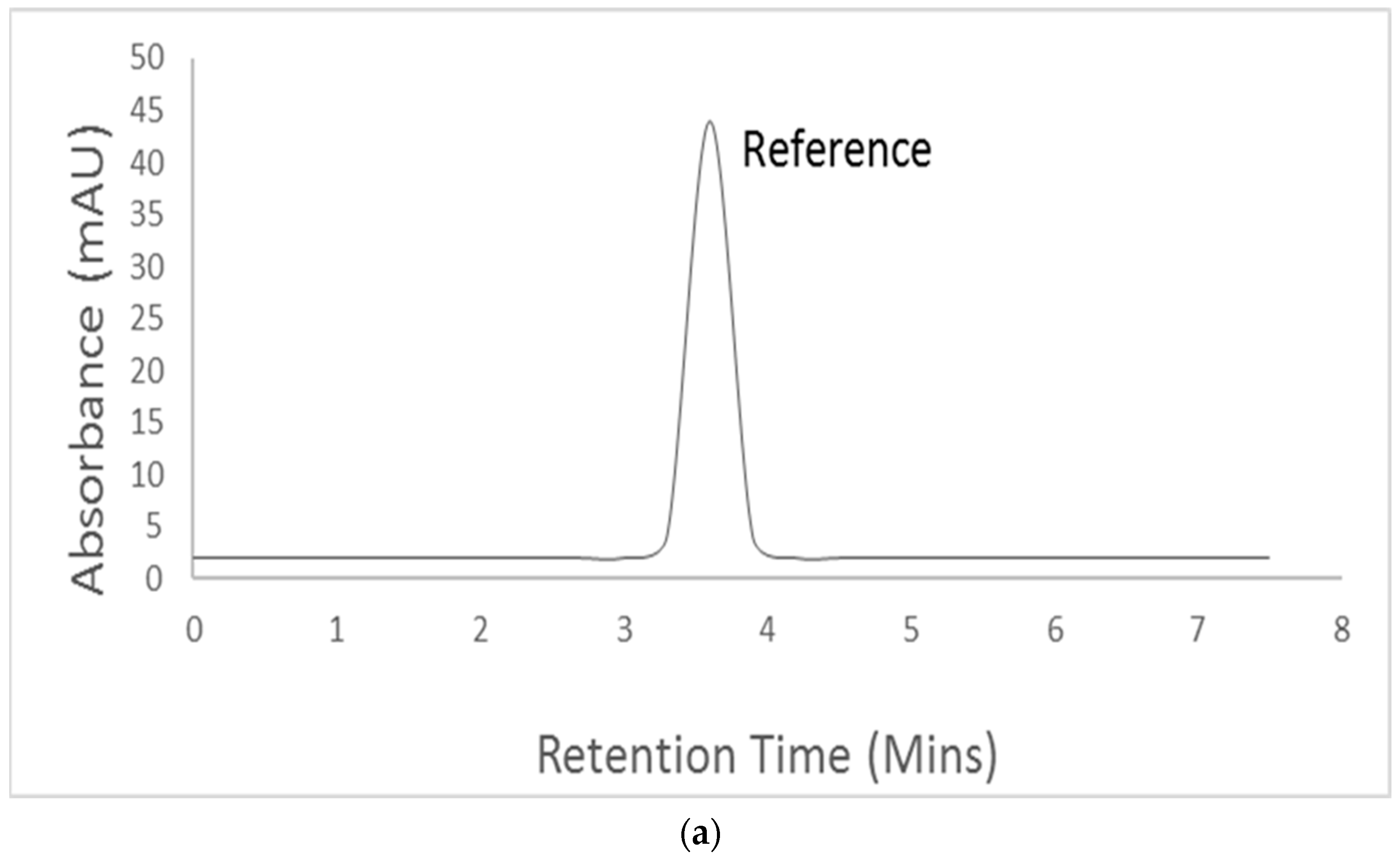
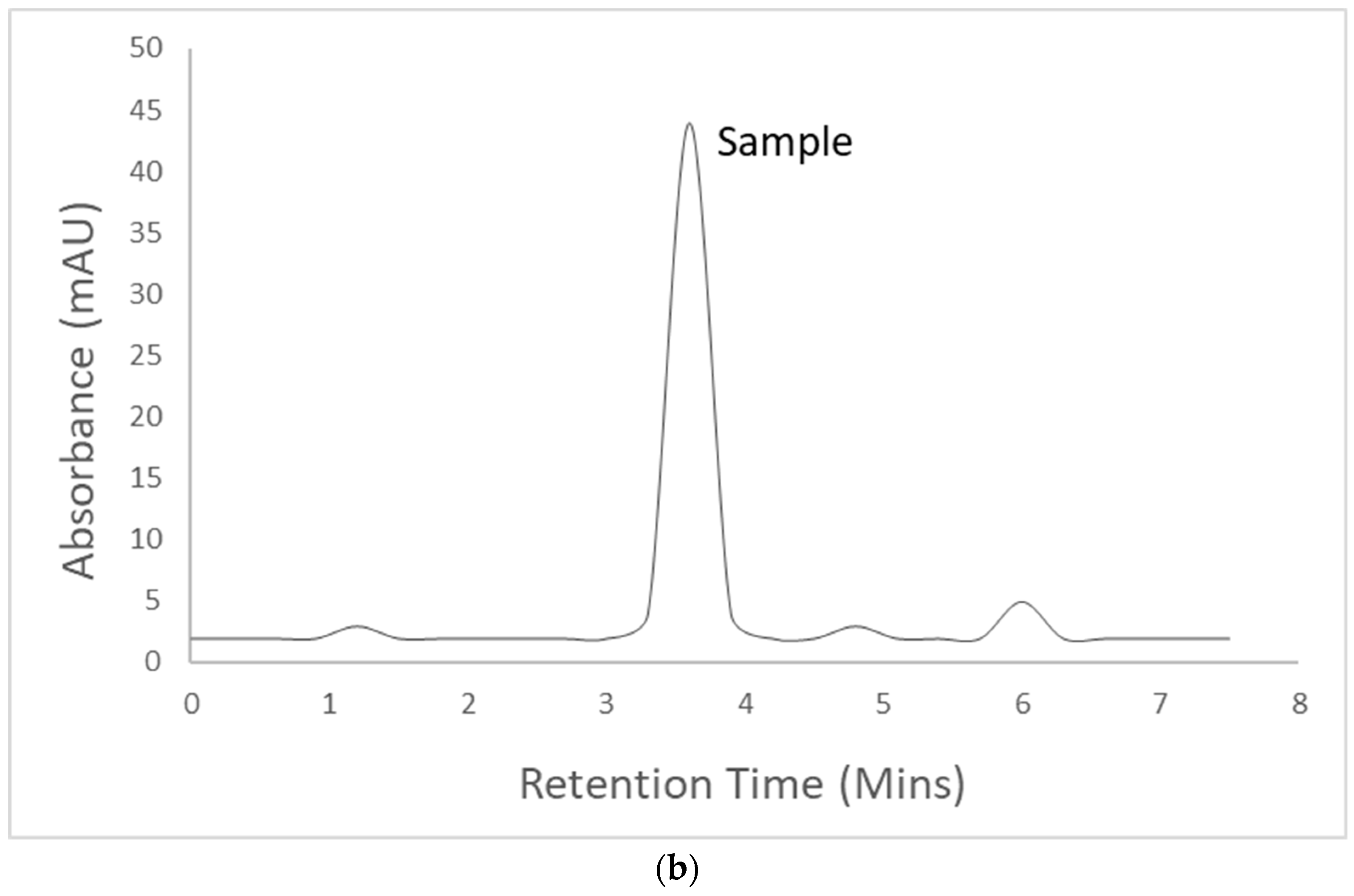
3.4. Identification and Quantitative Assessment of Biovanillin
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chakraborty, D.; Selvam, A.; Kaur, B.; Wong, J.W.C.; Karthikeyan, O.P. Application of recombinant Pediococcus acidilactici BD16 (fcs+/ech+) for biotransformation of agrowaste to vanillin. Appl. Microbiol. Biotechnol. 2017, 101, 5615–5626. [Google Scholar] [CrossRef] [PubMed]
- Priefert, H.; Rabenhorst, J.; Steinbüchel, A. Biotechnological production of vanillin. Appl. Microbiol. Biotechnol. 2001, 56, 296–314. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.; Rai, D.C.; Ramyaa Lakshmi, T.S.; Srivastava, S.K.; Tripathi, A.D. A comprehensive review on vanillin: Its microbial synthesis, isolation and recovery. Food Biotechnol. 2021, 35, 22–49. [Google Scholar] [CrossRef]
- Ramachandra Rao, S.; Ravishankar, G.A. Vanilla flavour: Production by conventional and biotechnological routes. J. Sci. Food Agric. 2000, 80, 289–304. [Google Scholar] [CrossRef]
- Arya, S.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Vanillin: A review on the therapeutic prospects of a popular flavouring molecule. Adv. Tradit. Med. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Waliszewski, K.N.; Pardio, V.T.; Ovando, S.L. A simple and rapid HPLC technique for vanillin determination in alcohol extract. Food Chem. 2007, 101, 1059–1062. [Google Scholar] [CrossRef]
- Li, T.; Rosazza, J.P. Biocatalytic synthesis of vanillin. Appl. Environ. Microbiol. 2000, 66, 684–687. [Google Scholar] [CrossRef]
- Gallage, N.J.; Møller, B.L. Vanillin–biotransformation and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Mol. Plant 2015, 8, 40–57. [Google Scholar] [CrossRef]
- Couto, S.R.; Sanromán, M.A. Application of solid-state fermentation to food industry—A review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- Galadima, A.I.; Salleh, M.M.; Hussin, H.; Shiong, C.C.; Yahaya, A.; Mohamad, S.E.; Aziz, S.A.; Yusof, N.N.M.; Al-Junid, A.F.M. Improvement of biovanillin production with two-stage pH control strategy from lemongrass leaves hydrolysates using Phanerochaete chrysosporium ATCC 24725 in batch culture. Biomass Conv. Biorefin. 2020, 1–10. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, S.; Chaudhry, N.; Munir, S.; Sana, H. Effect of torrefaction conditions on the physicochemical characterization of agricultural waste (sugarcane bagasse). Waste Manag. 2019, 88, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-k.; Daugulis, A.J. Effect of biotransformation conditions on vanillin production by Amycolatopsis sp. ATCC 39116 through an analysis of competing by-product formation. Bioprocess Biosys. Eng. 2014, 37, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, A.; Mizani, M.; Honarvar, M.; Faghihian, H.; Gerami, A. Extraction of ferulic acid from sugar beet pulp by alkaline hydrolysis and organic solvent methods. J. Food Meas. Charact. 2016, 10, 42–47. [Google Scholar] [CrossRef]
- Saeed, S.; Raza, S.Q.; Zafar, S.S.; Mujahid, H.; Irfan, M.; Mehmood, T. Microbial conversion of pomegranate peels to biovanillin using submerged fermentation and process optimization through statistical design. Biomass Conver. Biorefin. 2022, 1–10. [Google Scholar] [CrossRef]
- Tilay, A.; Bule, M.; Kishenkumar, J.; Annapure, U. Preparation of ferulic acid from agricultural wastes: Its improved extraction and purification. J. Agric. Food Chem. 2008, 56, 7644–7648. [Google Scholar] [CrossRef]
- Barberousse, H.; Roiseux, O.; Robert, C.; Paquot, M.; Deroanne, C.; Blecker, C. Analytical methodologies for quantification of ferulic acid and its oligomers. J. Sci. Food Agric. 2008, 88, 1494–1511. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmed, S.; Waseem, R.; Saeed, S.; Ahmed, W.; Irfan, M.; Ullah, A. Valorization of fruit peels into biovanillin and statistical optimization of process using Enterobacter hormaechei through solid-state fermentation. Fermentation 2022, 8, 40. [Google Scholar] [CrossRef]
- Chakraborty, D.; Kaur, B.; Obulisamy, K.; Selvam, A.; Wong, J.W. Agrowaste to vanillin conversion by a natural Pediococcus acidilactici strain BD16. Environ. Technol. 2017, 38, 1823–1834. [Google Scholar] [CrossRef]
- Saeed, S.; Baig, U.U.R.; Tayyab, M.; Altaf, I.; Irfan, M.; Raza, S.Q.; Nadeem, F.; Mehmood, T. Valorization of banana peels waste into biovanillin and optimization of process parameters using submerged fermentation. Biocatal. Agric. Biotechnol. 2021, 36, 102154. [Google Scholar] [CrossRef]
- Ou, S.; Zhang, J.; Wang, Y.; Zhang, N. Production of feruloyl esterase from Aspergillus niger by solid-state fermentation on different carbon sources. Enzym. Res. 2011, 2011, 848939. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.X.; Bolton, J.L.; Leary, G.J. Determination of ferulic and p-coumaric acids in wheat straw and the amounts released by mild acid and alkaline peroxide treatment. J. Agric. Food Chem. 1998, 46, 5283–5288. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Maswada, H.F.; El-Sayed, M.; Ahmed, M.E. Phenolic compounds and antioxidant activity of rice straw extract. Int. Lett. Nat. Sci. 2017, 64, 1–9. [Google Scholar] [CrossRef]
- Lau, T.; Harbourne, N.; Oruña-Concha, M.J. Optimization of enzyme-assisted extraction of ferulic acid from sweet corn cob by response surface methodology. J. Sci. Food Agric. 2020, 100, 1479–1485. [Google Scholar] [CrossRef]
- Webber, D.M.; Hettiarachchy, N.S.; Li, R.; Horax, R.; Theivendran, S. Phenolic profile and antioxidant activity of extracts prepared from fermented heat-stabilized defatted rice bran. J. Food Sci. 2014, 79, H2383–H2391. [Google Scholar] [CrossRef]
- Rosazza, J.; Huang, Z.; Dostal, L.; Volm, T.; Rousseau, B. Biocatalytic transformations of ferulic acid: An abundant aromatic natural product. J. Ind. Microbiol. Biotechnol. 1995, 15, 457–471. [Google Scholar] [CrossRef]
- Berger, R.G. Biotechnology as a source of natural volatile flavours. Curr. Opin. Food Sci. 2015, 1, 38–43. [Google Scholar] [CrossRef]
- Cerqueira, D.A.; Rodrigues Filho, G.; da Silva Meireles, C. Optimization of sugarcane bagasse cellulose acetylation. Carbohydr. Polym. 2007, 69, 579–582. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, M. A novel anaerobic co-culture system for bio-hydrogen production from sugarcane bagasse. Bioresour. Technol. 2013, 144, 623–631. [Google Scholar] [CrossRef]
- Guilherme, A.d.A.; Dantas, P.; Soares, J.; Dos Santos, E.; Fernandes, F.; De Macedo, G. Pretreatments and enzymatic hydrolysis of sugarcane bagasse aiming at the enhancement of the yield of glucose and xylose. Braz. J. Chem. Eng. 2017, 34, 937–947. [Google Scholar] [CrossRef]
- Mazhar, B.; Jahan, N.; Ali, N.M.; Andleeb, S.; Ali, S. Production of vanillin by a novel bacterium from waste residues of rice bran oil. Punjab Univ. J. Zool. 2017, 32, 137–142. [Google Scholar]
- Soares, M.; Christen, P.; Pandey, A.; Soccol, C.R. Fruity flavour production by Ceratocystis fimbriata grown on coffee husk in solid-state fermentation. Process Biochem. 2000, 35, 857–861. [Google Scholar] [CrossRef]
- Gasson, M.J.; Kitamura, Y.; McLauchlan, W.R.; Narbad, A.; Parr, A.J.; Parsons, E.L.H.; Payne, J.; Rhodes, M.J.; Walton, N.J. Metabolism of ferulic acid to vanillin: A bacterial gene of the enoyl-SCoA hydratase/isomerase superfamily encodes an enzyme for the hydration and cleavage of a hydroxycinnamic acid SCoA thioester. J. Biol. Chem. 1998, 273, 4163–4170. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Chakraborty, D. Statistical media and process optimization for biotransformation of rice bran to vanillin using Pediococcus acidilactici. Indian J. Exp. Biol. 2013, 51, 935–943. [Google Scholar] [PubMed]
- Barghini, P.; Di Gioia, D.; Fava, F.; Ruzzi, M. Vanillin production using metabolically engineered Escherichia coli under non-growing conditions. Microb. Cell Fact. 2007, 6, 13. [Google Scholar] [CrossRef]
- Saeed, S.; Hashmi, A.S.; Tayyab, M.; Awan, A.R.; Anjum, A.A.; Firyal, S. Hyperproduction of alginate by mutated strain of Azotobacter vinelandii through submerged fermentation. Pak. J. Zool. 2016, 48, 1585–1589. [Google Scholar]
- Saeed, S.; Mehmood, T.; Irfan, M. Statistical optimization of cultural parameters for the optimized production of alginic acid using apple (Malus domestica) peels through solid-state fermentation. Biomass Conver. Biorefin. 2020, 1–9. [Google Scholar] [CrossRef]
- Yan, L.; Chen, P.; Zhang, S.; Li, S.; Yan, X.; Wang, N.; Liang, N.; Li, H. Biotransformation of ferulic acid to vanillin in the packed bed-stirred fermenters. Sci. Rep. 2016, 6, 34644. [Google Scholar] [CrossRef]
- Chen, P.; Yan, L.; Wu, Z.; Li, S.; Bai, Z.; Yan, X.; Wang, N.; Liang, N.; Li, H. A microbial transformation using Bacillus subtilis B7-S to produce natural vanillin from ferulic acid. Sci. Rep. 2016, 6, 20400. [Google Scholar] [CrossRef]
- Rashid, J.; Samat, N.; Mohtar, W.; Yusoff, W. Optimization of temperature, moisture content and inoculum size in solid state fermentation to enhance mannanase production by Aspergillus terreus SUK-1 using RSM. Pak. J. Biol. Sci. 2011, 14, 533–539. [Google Scholar] [CrossRef]
- Nema, A.; Patnala, S.H.; Mandari, V.; Kota, S.; Devarai, S.K. Production and optimization of lipase using Aspergillus niger MTCC 872 by solid-state fermentation. Bull. Natl. Res. Cent. 2019, 43, 82. [Google Scholar] [CrossRef]
| Sr. No. | Substrate | Ferulic Acid (g/100 g) |
|---|---|---|
| 1 | Sugarcane bagasse | 0.94 |
| 2 | Wheat straw | 0.48 |
| 3 | Rice straw | 0.39 |
| 4 | Corn cob | 0.169 |
| 5 | Rice bran | 0.0046 |
| Codes | Independent Parameters | Unit | Low Level | High Level |
|---|---|---|---|---|
| A | Moisture content | % | 40 | 80 |
| B | Inoculum size | mL | 1 | 5 |
| C | pH | - | 5 | 10 |
| D | Temperature | °C | 25 | 50 |
| E | Incubation time | hours | 8 | 72 |
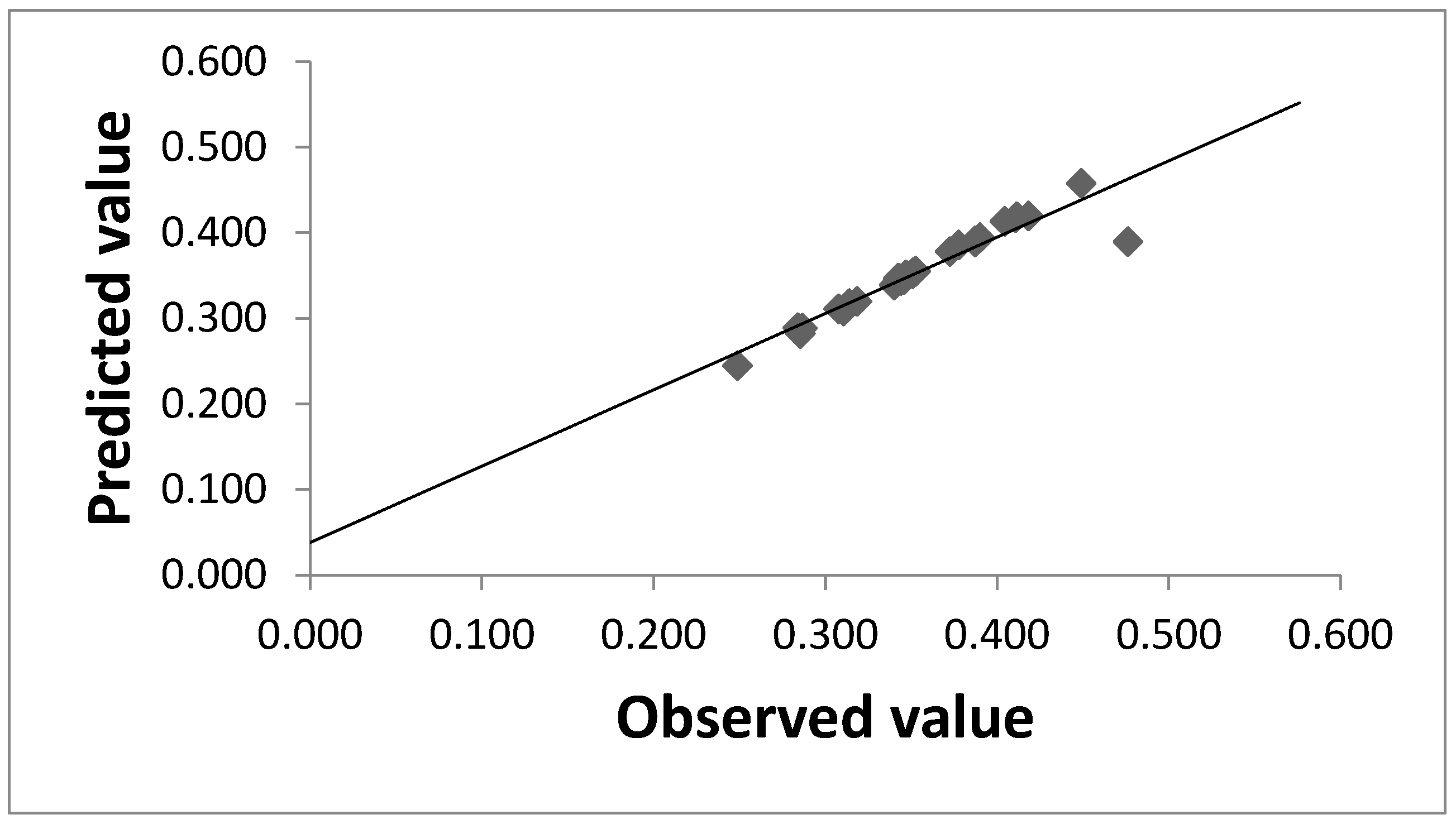
| Sr. No. | Moisture Content (%) | pH | Inoculum Size (mL) | Temperature (°C) | Incubation Time (hours) | Vanillin Yield (g/100 g) | Predicted | Residuals |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | 10 | 5 | 50 | 72 | 0.284 | 0.289 | −0.005 |
| 2 | 60 | 7.5 | 3 | 37.5 | 24 | 0.347 | 0.348 | −0.001 |
| 3 | 60 | 5 | 2 | 45 | 24 | 0.346 | 0.345 | 0.000 |
| 4 | 80 | 10 | 1 | 50 | 24 | 0.351 | 0.353 | −0.003 |
| 5 | 60 | 7.5 | 2 | 37.5 | 48 | 0.344 | 0.346 | −0.002 |
| 6 | 70 | 6.5 | 3 | 30 | 12 | 0.387 | 0.389 | −0.002 |
| 7 | 50 | 7.5 | 3 | 25 | 72 | 0.347 | 0.351 | −0.004 |
| 8 | 50 | 9 | 4 | 37.5 | 24 | 0.318 | 0.320 | −0.002 |
| 9 | 60 | 7.5 | 3 | 37.5 | 12 | 0.345 | 0.345 | 0.000 |
| 10 | 80 | 5 | 1 | 50 | 48 | 0.387 | 0.391 | −0.003 |
| 11 | 40 | 5 | 5 | 50 | 60 | 0.314 | 0.317 | −0.003 |
| 12 | 40 | 10 | 5 | 30 | 48 | 0.308 | 0.311 | −0.003 |
| 13 | 50 | 10 | 1 | 25 | 32 | 0.310 | 0.309 | 0.002 |
| 14 | 40 | 5 | 4 | 25 | 60 | 0.342 | 0.344 | −0.002 |
| 15 | 40 | 10 | 1 | 50 | 24 | 0.249 | 0.245 | 0.004 |
| 16 | 60 | 7.5 | 3 | 45 | 60 | 0.342 | 0.347 | −0.004 |
| 17 | 80 | 10 | 1 | 25 | 48 | 0.390 | 0.394 | −0.004 |
| 18 | 80 | 6.5 | 5 | 50 | 48 | 0.404 | 0.414 | −0.009 |
| 19 | 80 | 5 | 5 | 25 | 48 | 0.449 | 0.458 | −0.009 |
| 20 | 40 | 7.5 | 3 | 50 | 72 | 0.286 | 0.289 | −0.002 |
| 21 | 60 | 9 | 4 | 30 | 12 | 0.352 | 0.354 | −0.002 |
| 22 | 80 | 5 | 5 | 50 | 32 | 0.411 | 0.419 | −0.008 |
| 23 | * 70 | 7.5 | 4 | 37.5 | 48 | 0.476 | 0.390 | 0.086 |
| 24 | 40 | 5 | 5 | 25 | 8 | 0.340 | 0.339 | 0.001 |
| 25 | 80 | 10 | 3 | 45 | 60 | 0.377 | 0.386 | −0.008 |
| 26 | 60 | 7.5 | 3 | 25 | 72 | 0.372 | 0.378 | −0.005 |
| 27 | 60 | 6.5 | 3 | 40 | 40 | 0.352 | 0.355 | −0.003 |
| 28 | 40 | 5 | 1 | 50 | 50 | 0.285 | 0.282 | 0.003 |
| 29 | 80 | 5 | 1 | 25 | 25 | 0.418 | 0.420 | −0.001 |
| 30 | 60 | 7.5 | 3 | 37.5 | 8 | 0.344 | 0.344 | 0.000 |
| 31 | 60 | 7.5 | 4 | 37.5 | 8 | 0.351 | 0.352 | −0.001 |
| 32 | 80 | 10 | 5 | 25 | 12 | 0.411 | 0.418 | −0.007 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 20 | 0.068491 | 0.003425 | 6.10 | 0.002 |
| Linear | 0.047890 | 0.009578 | 17.07 | 0.000 | |
| A | 1 | 0.035115 | 0.035115 | 62.58 | 0.000 |
| B | 1 | 0.003483 | 0.003483 | 6.21 | 0.030 |
| C | 1 | 0.000854 | 0.000854 | 1.52 | 0.243 |
| D | 1 | 0.004401 | 0.004401 | 7.84 | 0.017 |
| E | 1 | 0.000454 | 0.000454 | 0.81 | 0.388 |
| Square | 5 | 0.001433 | 0.000287 | 0.51 | 0.763 |
| A*A | 1 | 0.000022 | 0.000022 | 0.04 | 0.846 |
| B*B | 1 | 0.000294 | 0.000294 | 0.52 | 0.485 |
| C*C | 1 | 0.000277 | 0.000277 | 0.49 | 0.497 |
| D*D | 1 | 0.000224 | 0.000224 | 0.40 | 0.540 |
| E*E | 1 | 0.000000 | 0.000000 | 0.00 | 0.999 |
| 2-Way Interaction | 10 | 0.000497 | 0.000050 | 0.09 | 1.000 |
| A*B | 1 | 0.000176 | 0.000176 | 0.31 | 0.586 |
| A*C | 1 | 0.000120 | 0.000120 | 0.21 | 0.653 |
| A*D | 1 | 0.000011 | 0.000011 | 0.02 | 0.889 |
| A*E | 1 | 0.000001 | 0.000001 | 0.00 | 0.975 |
| B*C | 1 | 0.000012 | 0.000012 | 0.02 | 0.886 |
| B*D | 1 | 0.000012 | 0.000012 | 0.02 | 0.886 |
| B*E | 1 | 0.000034 | 0.000034 | 0.06 | 0.809 |
| C*D | 1 | 0.000129 | 0.000129 | 0.23 | 0.641 |
| C*E | 1 | 0.000178 | 0.000178 | 0.32 | 0.584 |
| D*E | 1 | 0.000047 | 0.000047 | 0.08 | 0.778 |
| Error | 11 | 0.006172 | 0.000561 | ||
| Total | 31 | 0.074663 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehmood, T.; Saleem, F.; Javed, S.; Nawaz, S.; Sultan, A.; Safdar, A.; Ullah, A.; Waseem, R.; Saeed, S.; Abbas, M.; et al. Biotransformation of Agricultural By-Products into Biovanillin through Solid-State Fermentation (SSF) and Optimization of Different Parameters Using Response Surface Methodology (RSM). Fermentation 2022, 8, 206. https://doi.org/10.3390/fermentation8050206
Mehmood T, Saleem F, Javed S, Nawaz S, Sultan A, Safdar A, Ullah A, Waseem R, Saeed S, Abbas M, et al. Biotransformation of Agricultural By-Products into Biovanillin through Solid-State Fermentation (SSF) and Optimization of Different Parameters Using Response Surface Methodology (RSM). Fermentation. 2022; 8(5):206. https://doi.org/10.3390/fermentation8050206
Chicago/Turabian StyleMehmood, Tahir, Fozia Saleem, Sadia Javed, Sadia Nawaz, Aeysha Sultan, Ambreen Safdar, Azmat Ullah, Rida Waseem, Shagufta Saeed, Mateen Abbas, and et al. 2022. "Biotransformation of Agricultural By-Products into Biovanillin through Solid-State Fermentation (SSF) and Optimization of Different Parameters Using Response Surface Methodology (RSM)" Fermentation 8, no. 5: 206. https://doi.org/10.3390/fermentation8050206
APA StyleMehmood, T., Saleem, F., Javed, S., Nawaz, S., Sultan, A., Safdar, A., Ullah, A., Waseem, R., Saeed, S., Abbas, M., Bilal, M., Ahmad, M. M., & Firyal, S. (2022). Biotransformation of Agricultural By-Products into Biovanillin through Solid-State Fermentation (SSF) and Optimization of Different Parameters Using Response Surface Methodology (RSM). Fermentation, 8(5), 206. https://doi.org/10.3390/fermentation8050206






