Effect of Intermittent Fasting, Probiotic-Fermented Camel Milk, and Probiotic-Fermented Camel Milk Incorporating Sukkari Date on Diet-Induced Obesity in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Camel Milk and Other Ingredients
2.2. Preparation of Probiotic-Enriched FCM and FCM-SD
2.3. Animals and Experimental Design
2.4. Blood Samples and Organs’ Relative Weight
2.5. Biochemical Examinations
2.5.1. Adiponectin and Leptin
2.5.2. Determination of Lipid Profile and Fasting Blood Glucose Level
2.5.3. Oxidative Stress Biomarkers
2.5.4. Histopathological Examinations
2.6. Data Analysis
3. Results
3.1. Effect of IF, FCM, and FCM-D on Weight Change and Relative Organ Weight
3.2. Effect of IF, FCM, and FCM-D on Lipid Profile and Atherogenic Index
3.3. Effect of IF, FCM, and FCM-D on Lipid Profile and Atherogenic Index
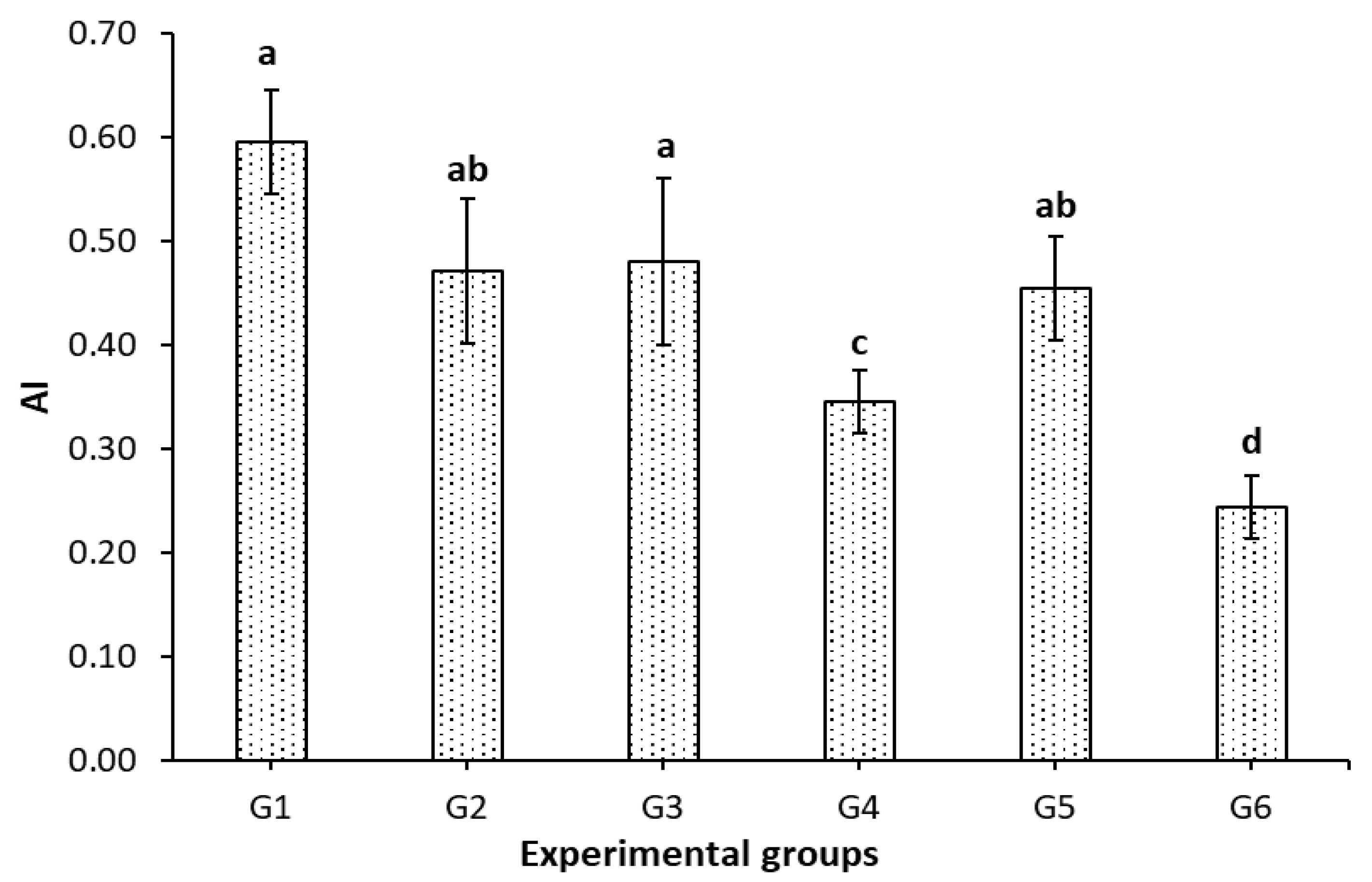
3.4. Effect of IF, FCM, and FCM-D on Atherogenic Index
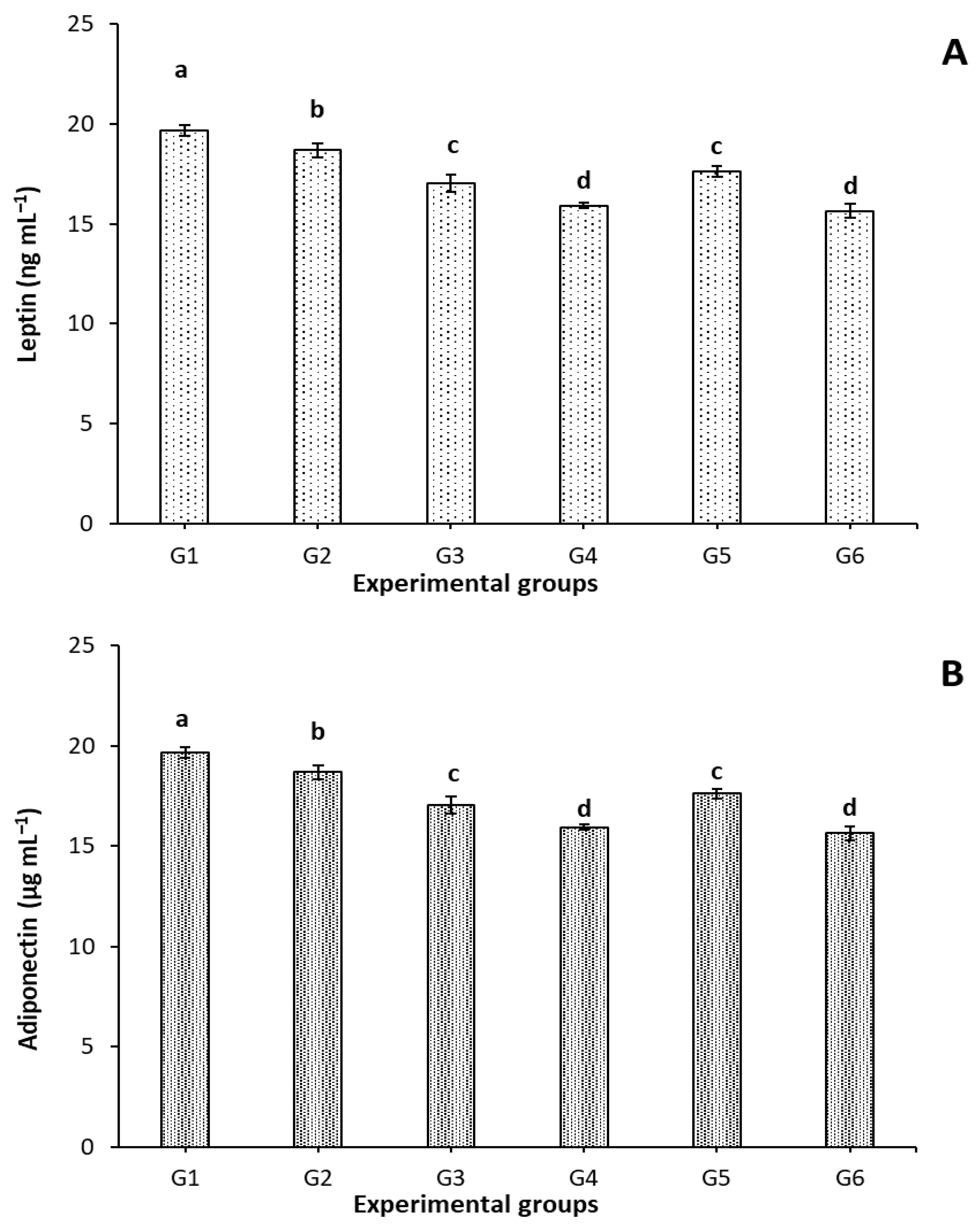
3.5. Effect of FCM, FCM-D, and IF on Leptin and Adiponectin Levels
3.6. Effect of FCM, FCM-D, and IF on Oxidative Stress Biomarkers
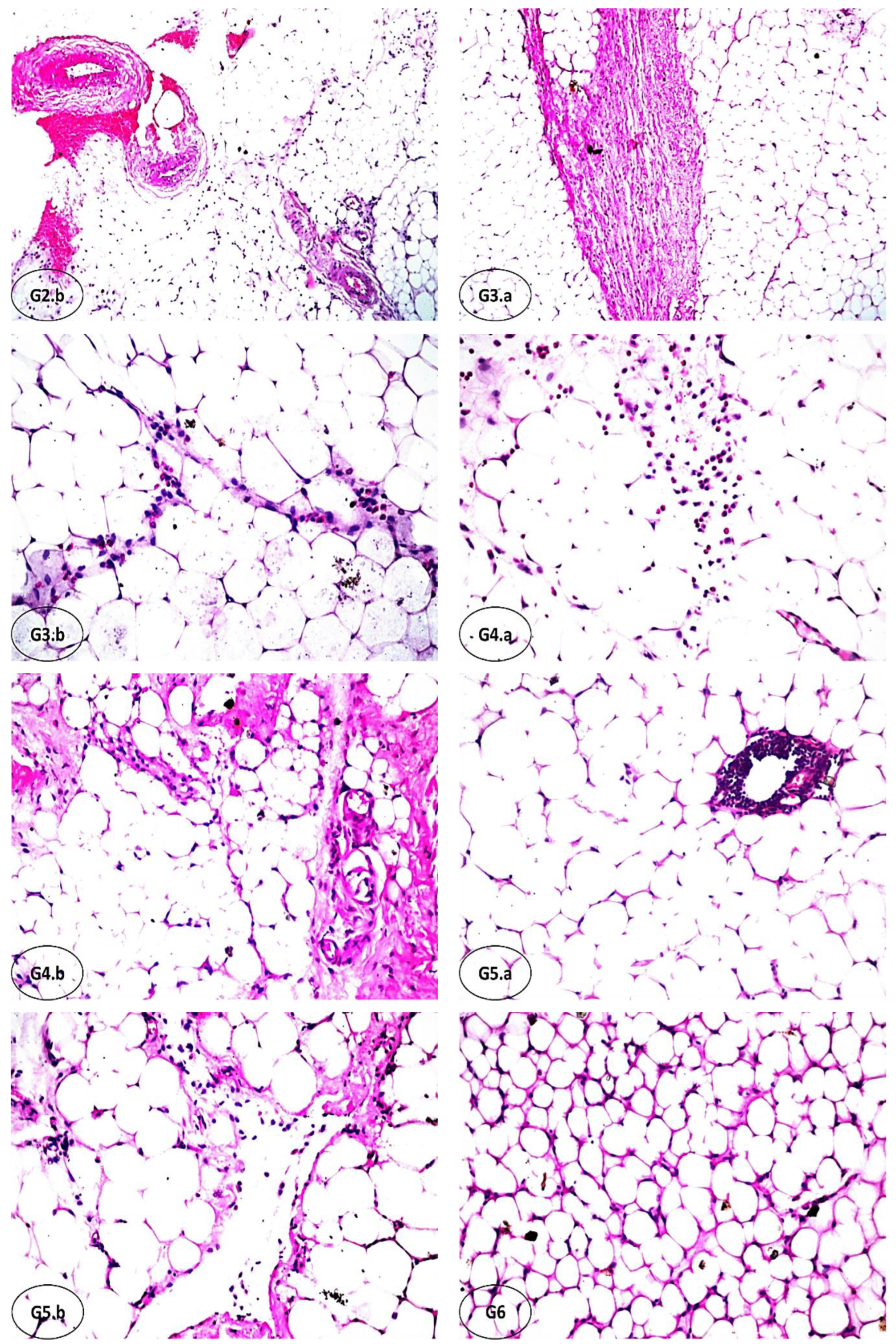
3.7. Effect of FCM, FCM-D, and IF on Histopathological Alterations of Adipose Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pi-Sunyer, X. The Medical Risks of Obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Aliss, E.M.; Sutaih, R.H.; Kamfar, H.Z.; Alagha, A.E.; Marzouki, Z.M. Physical Activity Pattern and its Relationship with Overweight and Obesity in Saudi Children. Int. J. Pediatr. Adolesc. Med. 2020, 7, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Kolotkin, R.L.; Andersen, J.R. A Systematic Review of Reviews: Exploring the Relationship Between Obesity, Weight Loss and Health-Related Quality of Life. Clin. Obes. 2017, 7, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Berhe, T.; Seifu, E.; Ipsen, R.; Kurtu, M.Y.; Hansen, E.B. Processing Challenges and Opportunities of Camel Dairy Products. Int. J. Food Sci. 2017, 2017, 9061757. [Google Scholar] [CrossRef]
- Elagamy, E.I. Effect of Heat Treatment on Camel Milk Proteins with Respect to Antimicrobial Factors: A Comparison with Cows’ and Buffalo Milk Proteins. Food Chem. 2000, 68, 227–232. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, W.; Wang, B.; Zhang, F.; Shao, Y. Influence of Bactrian Camel MILK on the Gut Microbiota. J. Dairy Sci. 2018, 101, 5758–5769. [Google Scholar] [CrossRef]
- Al-Juboori, A.; Mohammed, M.; Rashid, J.; Kurian, J.; El Refaey, S. Nutritional and Medicinal Value of Camel (Camelus dromedarius) Milk; WIT Press: Billerica, MA, USA, 2013; Volume 170, pp. 221–232. [Google Scholar]
- Sharma, C.; Singh, C. Therapeutic Value of Camel Milk–A Review. Adv. J. Pharm. Life Sci. Res. 2014, 2, 7–13. [Google Scholar]
- Salwa, M.Q.; Lina, A.F.K. Antigenotoxic and Anticytotoxic Effect of Camel Milk in Mice Treated with Cisplatin. Saudi J. Biol. Sci. 2010, 17, 159–166. [Google Scholar] [CrossRef][Green Version]
- El Agamy, E.S.I.; Ruppanner, R.; Ismail, A.; Champagne, C.P.; Assaf, R. Antibacterial and Antiviral Activity of Camel Milk Protective Proteins. J. Dairy Res. 1992, 59, 169–175. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Serikbayeva, A.; Loiseau, G.; Narmuratova, M.; Faye, B. Lactoferrin of Camel Milk of Kazakhstan; 2005; Volume 362, pp. 158–167. Available online: https://agritrop.cirad.fr/525438/ (accessed on 18 October 2022).
- Aljutaily, T. Evaluating the Nutritional and Immune Potentiating Characteristics of Unfermented and Fermented Turmeric Camel Milk in Cyclophosphamide-Induced Immunosuppression in Rats. Antioxidants 2022, 11, 792. [Google Scholar] [CrossRef]
- Aljutaily, T.; Huarte, E.; Martinez-Monteagudo, S.; Gonzalez-Hernandez, J.L.; Rovai, M.; Sergeev, I.N. Probiotic-Enriched Milk and Dairy Products Increase the Gut Microbiota Diversity: A Comparative Study. Nutr. Res. 2020, 82, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Aryana, K.J.; Olson, D.W. A 100-Year Review: Yogurt and Other Cultured Dairy Products. J. Dairy Sci. 2017, 100, 9987–10013. [Google Scholar] [CrossRef] [PubMed]
- Ebringer, L.; Ferenčík, M.; Krajčovič, J. Beneficial Health Effects of Milk and Fermented Dairy Products. Folia Microbiol. 2008, 53, 378–394. [Google Scholar] [CrossRef]
- Senok, A.C. Probiotics in the Arabian Gulf Region. Food Nutr. Res. 2009, 53, 1842. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial Ecology: Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A Core Gut Microbiome in Obese and Lean Twins. Nature 2009, 457, U480–U487. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced Dietary Intake of Carbohydrates by Obese Subjects Results in Decreased Concentrations of Butyrate and Butyrate-Producing Bacteria in Feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef]
- Walters, W.A.; Xu, Z.; Knight, R. Meta-Analyses of Human Gut Microbes Associated with Obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Udayappan, S.D.; Hartstra, A.V.; Dallinga-Thie, G.M.; Nieuwdorp, M. Intestinal Microbiota and Faecal Transplantation as Treatment Modality for Insulin Resistance and Type 2 Diabetes Mellitus. Clin. Exp. Immunol. 2014, 177, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Hamed, H.; Chaari, F.; Ghannoudi, Z.; ElFeki, A.; Ellouz, S.C.; Gargouri, A. Beneficial Effects of Fermented Camel Milk by Lactococcus lactis Subsp cemoris on Cardiotoxicity Induced by Carbon Tetrachloride in Mice. Biomed. Pharm. 2018, 97, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Hudson, J.A.; Korpela, R.; de los Reyes-Gavilán, C.G. Impact on Human Health of Microorganisms Present in Fermented Dairy Products: An Overview. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Ewis, S.A.; Abdel-Rahman, M.S. Effect of Metformin on Glutathione and Magnesium in Normal and Streptozotocin-Induced Diabetic Rats. J. Appl. Toxicol. 1995, 15, 387–390. [Google Scholar] [CrossRef] [PubMed]
- May, J.M. How Does Ascorbic Acid Prevent Endothelial Dysfunction? Free Radic. Biol. Med. 2000, 28, 1421–1429. [Google Scholar] [CrossRef]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef]
- Ziaee, V.; Razaei, M.; Ahmadinejad, Z.; Shaikh, H.; Yousefi, R.; Yarmohammadi, L.; Bozorgi, F.; Behjati, M.J. The Changes of Metabolic Profile and Weight During Ramadan Fasting. Singap. Med. J. 2006, 47, 409–414. [Google Scholar]
- Mindikoglu, A.L.; Opekun, A.R.; Gagan, S.K.; Devaraj, S. Impact of Time-Restricted Feeding and Dawn-to-Sunset Fasting on Circadian Rhythm, Obesity, Metabolic Syndrome, and Nonalcoholic Fatty Liver Disease. Gastroenterol. Res. Pract. 2017, 2017, 3932491. [Google Scholar] [CrossRef]
- Hajek, P.; Myers, K.; Dhanji, A.R.; West, O.; McRobbie, H. Weight Change During and After Ramadan Fasting. J. Public Health 2012, 34, 377–381. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.P.; et al. Insight Into the Prebiotic Concept: Lessons from an Exploratory, Double Blind Intervention Study With Inulin-Type Fructans in Obese Women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global Nutrition Transition and the Pandemic of Obesity in Developing Countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Zibaee, S.; Hosseini, S.M.; Yousefi, M.; Taghipour, A.; Kiani, M.A.; Noras, M.R. Nutritional and Therapeutic Characteristics of Camel Milk in Children: A Systematic Review. Electron. Physician 2015, 7, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Davati, N.; Tabatabaee Yazdi, F.; Zibaee, S.; Shahidi, F.; Edalatian, M.R. Study of Lactic Acid Bacteria Community From Raw Milk of Iranian One Humped Camel and Evaluation of Their Probiotic Properties. Jundishapur. J. Microbiol. 2015, 8, e16750. [Google Scholar] [CrossRef]
- Rahman, I.E.A.; Dirar, H.A.; Osman, M.A. Microbiological and Biochemical Changes and Sensory Evaluation of Camel Milk Fermented by Selected Bacterial Starter Cultures. Afr. J. Food Sci. 2009, 3, 398–405. [Google Scholar]
- Aljutaily, T.; Barakat, H.; Moustafa, M.M.; Rehan, M. Incorporation of Sukkari Date in Probiotic-Enriched Fermented Camel Milk Improves the Nutritional, Physicochemical, and Organoleptical Characteristics. Fermentation 2021, 8, 5. [Google Scholar] [CrossRef]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Yun, S.-I.; Park, H.-O. Effects of Lactobacillus Gasseri BNR17 on Body Weight and Adipose Tissue Mass in Diet-Induced Overweight Rats. J. Microbiol. 2010, 48, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; van Harmelen, K.; Duran-Sandoval, D.; van Dijk, T.H.; Grefhorst, A.; Abdelkarim, M.; Caron, S.; Torpier, G.; Fruchart, J.-C.; Gonzalez, F.J. The Farnesoid X Receptor Modulates Adiposity and Peripheral Insulin Sensitivity in Mice. J. Biol. Chem. 2006, 281, 11039–11049. [Google Scholar] [CrossRef] [PubMed]
- Hamad, E.M.; Sato, M.; Uzu, K.; Yoshida, T.; Higashi, S.; Kawakami, H.; Kadooka, Y.; Matsuyama, H.; Abd El-Gawad, I.A.; Imaizumi, K. Milk Fermented by Lactobacillus Gasseri Sbt2055 Influences Adipocyte Size Via Inhibition of Dietary Fat Absorption in Zucker Rats. Br. J. Nutr. 2008, 101, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Nwagha, U.; Ikekpeazu, E.; Ejezie, F.; Neboh, E.; Maduka, I. Atherogenic Index of Plasma as Useful Predictor of Cardiovascular Risk Among Postmenopausal Women in Enugu, Nigeria. Afr. Health Sci. 2010, 10, 248–252. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E. Improved Method for the Determination of Blood Glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase In Vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Bancroft, J.; Suvarna, S.; Layton, C. Bancroft’s Theory and Practice of Histological Techniques, 7th, ed.; Churchill Livingstone: London, UK; Elsevier Ltd.: New York, NY, USA, 2013. [Google Scholar]
- Steel, R.G. Pinciples and Procedures of Statistics A Biometrical Approach, 3rd ed.; McGraw-Hill: Boston, MA, USA, 1997. [Google Scholar]
- Shabana; Shahid, S.U.; Irfan, U. The Gut Microbiota and its Potential Role in Obesity. Future Microbiol. 2018, 13, 589–603. [Google Scholar] [CrossRef]
- Fallah, Z.; Feizi, A.; Hashemipour, M.; Namazi, N.; Azarbayejani, L.; Kelishadi, R. Effect of Fermented Camel Milk on Obesity Measures and Blood Pressure of Adolescents With Metabolic Syndrome. J. Pediatr. Rev. 2019, 7, 181–189. [Google Scholar] [CrossRef]
- Qatanani, M.; Lazar, M.A. Mechanisms of Obesity-associated Insulin Resistance: Many Choices on the Menu. Genes Dev. 2007, 21, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Hong, N.; Kim, K.-w.; Cho, S.j.; Lee, M.; Lee, Y.-h.; Lee, Y.-h.; Kang, E.S.; Cha, B.-S.; Lee, B.-W. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1645. [Google Scholar] [CrossRef] [PubMed]
- Tay, A.; Pringle, H.; Penning, E.; Plank, L.D.; Murphy, R. PROFAST: A Randomized Trial Assessing the Effects of Intermittent Fasting and Lacticaseibacillus rhamnosus Probiotic among People with Prediabetes. Nutrients 2020, 12, 3530. [Google Scholar] [CrossRef] [PubMed]
- Fallah, Z.; Feizi, A.; Hashemipour, M.; Kelishadi, R. Effect of fermented camel milk on glucose metabolism, insulin resistance, and inflammatory biomarkers of adolescents with metabolic syndrome: A double-blind, randomized, crossover trial. J. Res. Med. Sci. 2018, 23, 32. [Google Scholar]
- Deng, Y.; Liu, W.; Wang, J.; Yu, J.; Yang, L.Q. Intermittent Fasting Improves Lipid Metabolism Through Changes in Gut Microbiota in Diet-Induced Obese Mice. Med. Sci. Monit. 2020, 26, 1643–3750. [Google Scholar] [CrossRef] [PubMed]
- Morales-Suarez-Varela, M.; Collado Sánchez, E.; Peraita-Costa, I.; Llopis-Morales, A.; Soriano, J.M. Intermittent Fasting and the Possible Benefits in Obesity, Diabetes, and Multiple Sclerosis: A Systematic Review of Randomized Clinical Trials. Nutrients 2021, 13, 3179. [Google Scholar] [CrossRef] [PubMed]
- Beltowski, J. Leptin and Atherosclerosis. Atherosclerosis 2006, 189, 47–60. [Google Scholar] [CrossRef]
- Ibars, M.; Ardid-Ruiz, A.; Suárez, M.; Muguerza, B.; Bladé, C.; Aragonès, G. Proanthocyanidins Potentiate Hypothalamic Leptin/STAT3 Signalling and Pomc Gene Expression in Rats with Diet-Induced Obesity. Int. J. Obes. 2017, 41, 129–136. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Fenton, J.I.; Hord, N.G.; Lavigne, J.A.; Perkins, S.N.; Hursting, S.D. Leptin, Insulin-Like Growth Factor-1, and Insulin-Like Growth Factor-2 Are Mitogens in ApcMin/+ but not Apc+/+ Colonic Epithelial Cell Lines. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Bråkenhielm, E.; Veitonmäki, N.; Cao, R.; Kihara, S.; Matsuzawa, Y.; Zhivotovsky, B.; Funahashi, T.; Cao, Y. Adiponectin-Induced Antiangiogenesis and Antitumor Activity Involve Caspase-Mediated Endothelial Cell Apoptosis. Proc. Natl. Acad. Sci. USA 2004, 101, 2476–2481. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.; Petridou, E.; Dessypris, N.; Chavelas, C.; Dalamaga, M.; Alexe, D.M.; Papadiamantis, Y.; Markopoulos, C.; Spanos, E.; Chrousos, G.; et al. Adiponectin and Breast Cancer Risk. J. Clin. Endocr. Met. 2004, 89, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.h.; Wolfe, R.R. Fatty Acid and Glycerol Kinetics in Septic Patients and in Patients with Gastrointestinal Cancer. The Response to Glucose Infusion and Parenteral Feeding. Ann. Surg. 1987, 205, 368–376. [Google Scholar] [CrossRef]
- Morshedi, M.; Valenlia, K.B.; Hosseinifard, E.S.; Shahabi, P.; Abbasi, M.M.; Ghorbani, M.; Barzegari, A.; Sadigh-Eteghad, S.; Saghafi-Asl, M. Beneficial Psychological Effects of Novel Psychobiotics in Diabetic Rats: The Interaction Among the Gut, Blood and Amygdala. J. Nutr. Biochem. 2018, 57, 145–152. [Google Scholar] [CrossRef]
- Blythe, J.; Ruggiero, M.; Pacini, S. Case Report: Intermittent Fasting and Probiotic Yogurt Consumption are Associated with Reduction of Serum Alpha-N-Acetylgalactosaminidase and Increased Urinary Excretion of Lipophilic Toxicants. Madr. J. Immunol. 2017, 1, 23–27. [Google Scholar] [CrossRef]
- Feingold, K.R.; Doerrler, W.; Dinarello, C.A.; Fiers, W.; Grunfeld, C. Stimulation of Lipolysis in Cultured Fat Cells by Tumor Necrosis Factor, Interleukin-1, and the Interferons is Blocked by Inhibition of Prostaglandin Synthesis. Endocrinology 1992, 130, 10–16. [Google Scholar] [CrossRef]
- Suganami, T.; Nishida, J.; Ogawa, Y. A Paracrine Loop Between Adipocytes and Macrophages Aggravates Inflammatory Changes: Role of Free Fatty Acids and Tumor Necrosis Factor A. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2062–2068. [Google Scholar] [CrossRef]
- de Souza Teixeira, A.A.; Lira, F.S.; Pimentel, G.D.; de Souza, C.O.; Batatinha, H.; Biondo, L.A.; Yamashita, A.S.; Junior, E.A.L.; Neto, J.C.R. Aerobic Exercise Modulates The Free Fatty Acids and Inflammatory Response During Obesity and Cancer Cachexia. Crit. Rev. Eukaryot. 2016, 26, 187–198. [Google Scholar] [CrossRef]
- Dirat, B.; Bochet, L.; Escourrou, G.; Valet, P.; Muller, C. Unraveling the Obesity and Breast Cancer Links: A Role for Cancer-Associated Adipocytes? Endocr. Dev. 2010, 19, 45–52. [Google Scholar] [CrossRef]
- Uysal, K.T.; Wiesbrock, S.M.; Marino, M.W.; Hotamisligil, G.S. Protection from Obesity-Induced Insulin Resistance in Mice Lacking TNF-α Function. Nature 1997, 389, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, A.; Schölmerich, J. Innate Immunity and Adipose Tissue Biology. Trends Immunol. 2010, 31, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Tsukumo, D.M.L.; Carvalho-Filho, M.A.; Carvalheira, J.B.C.; Prada, P.c.O.; Hirabara, S.M.; Schenka, A.A.; Araújo, E.P.; Vassallo, J.; Curi, R.; Velloso, L.c.A.; et al. Loss-of-Function Mutation in Toll-Like Receptor 4 Prevents Diet-Induced Obesity and Insulin Resistance. Diabetes 2007, 56, 1986–1998. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Ali Redha, A.; Valizadenia, H.; Siddiqui, S.A.; Maqsood, S. A State-of-Art Review on Camel Milk Proteins as An Emerging Source of Bioactive Peptides with Diverse Nutraceutical Properties. Food Chem. 2022, 373, 131444. [Google Scholar] [CrossRef] [PubMed]
- Narmaki, E.; Borazjani, M.; Ataie-Jafari, A.; Hariri, N.; Doost, A.H.; Qorbani, M.; Saidpour, A. The combined effects of probiotics and restricted calorie diet on the anthropometric indices, eating behavior, and hormone levels of obese women with food addiction: A randomized clinical trial. Nutr. Neurosci. 2022, 25, 963–975. [Google Scholar] [CrossRef] [PubMed]

| Group | Initial Body Weight of Obese Rats | % Body Weight Change | Relative Organs Weight | ||
|---|---|---|---|---|---|
| Liver | Kidneys | Spleen | |||
| G1 | 247.31 ± 11.24 | 22.41 ± 3.8 a | 3.47 ± 0.29 a | 0.59 ± 0.02 a | 0.30 ± 0.04 a |
| G2 | 233.64 ± 7.98 | 15.58 ± 5.86 b | 3.46 ± 0.36 ab | 0.65 ± 0.05 a | 0.38 ± 0.06 a |
| G3 | 251.27 ± 11.57 | 5.58 ± 1.79 c | 3.07 ± 0.14 abc | 0.63 ± 0.02 a | 0.32 ± 0.02 a |
| G4 | 235.24 ± 15.47 | 0.92 ± 5.96 cd | 2.84 ± 0.14 bc | 0.68 ± 0.05 a | 0.37 ± 0.04 a |
| G5 | 241.35 ± 5.78 | 3.93 ± 3.34 c | 2.82 ± 0.11 c | 0.63 ± 0.02 a | 0.31 ± 0.02 a |
| G6 | 239.78 ± 10.24 | −5.45 ± 12.53 d | 2.53 ± 0.08 c | 0.61 ± 0.02 a | 0.31 ± 0.02 a |
| Group | NFBG (mg dL−1) | FBG (mg dL−1) | ||||
|---|---|---|---|---|---|---|
| Week 2 | Week 4 | Week 6 | Week 2 | Week 4 | Week 6 | |
| G1 | 141.63 ± 2.44 aA | 138.88 ± 2.46 aA | 133.38 ± 2.32 aB | 109.25 ± 2.51 abcA | 106.50 ± 2.04 aA | 94.25 ± 6.08 aC |
| G2 | 142.63 ± 1.77 aA | 127.63 ± 1.77 bB | 125.00 ± 2.02 bB | 113.75 ± 2.48 aA | 98.75 ± 2.48 bB | 90.63 ± 2.57 aC |
| G3 | 140.38 ± 4.71 abA | 129.38 ± 4.71 bB | 122.25 ± 2.07 bC | 108.50 ± 3.72 bcA | 97.50 ± 3.72 bB | 85.00 ± 3.64 bC |
| G4 | 138.13 ± 2.66 abA | 124.88 ± 3.88 bB | 117.13 ± 2.97 cC | 110.00 ± 3.24 abcA | 90.00 ± 3.24 cB | 77.25 ± 3.03 dC |
| G5 | 137.88 ± 3.88 abA | 128.13 ± 2.89 bB | 126.13 ± 2.39 bB | 106.00 ± 1.31 cA | 98.00 ± 1.31 bB | 80.50 ± 3.62 cC |
| G6 | 136.13 ± 2.89 bA | 118.13 ± 2.66 cB | 116.13 ± 2.68 cB | 111.50 ± 1.48 abA | 96.50 ± 1.48 bB | 73.50 ± 1.74 dB |
| Group | Mg dL−1 | |||||
|---|---|---|---|---|---|---|
| FBG | TG | CHO | HDL-c | LDL-c | VLDL-c | |
| G1 | 128.82 ± 6.51 a | 148.61 ± 9.72 ab | 154.41 ± 11.77 a | 37.75 ± 2.35 c | 86.93 ± 13.49 a | 29.72 ± 1.94 a |
| G2 | 104.95 ± 2.85 b | 120.83 ± 6.44 c | 131.20 ± 5.07 b | 40.83 ± 3.53 bc | 66.20 ± 7.19 ab | 24.17 ± 1.29 bc |
| G3 | 105.40 ± 3.99 b | 130.93 ± 7.50 bc | 129.39 ± 6.64 b | 43.33 ± 2.16 abc | 59.87 ± 6.86 b | 26.19 ± 1.50 ab |
| G4 | 90.66 ± 2.68 c | 105.28 ± 4.86 cd | 112.73 ± 8.63 bc | 47.50 ± 3.45 ab | 44.18 ± 9.04 b | 21.06 ± 0.97 cd |
| G5 | 97.64 ± 4.31 bc | 121.93 ± 9.67 c | 125.94 ± 4.95 b | 42.75 ± 2.68 bc | 58.81 ± 7.11 b | 24.39 ± 1.93 bc |
| G6 | 77.18 ± 2.89 d | 89.01 ± 4.42 d | 98.03 ± 5.34 c | 50.75 ± 2.36 a | 29.48 ± 6.86 c | 17.80 ± 0.88 d |
| Group | Antioxidant Biomarkers | |||
|---|---|---|---|---|
| GSH (µg dL−1) | MDA (nmol mL−1) | CAT (U L−1) | SOD (U L−1) | |
| G1 | 71.40 ± 7.33 d | 14.40 ± 0.86 a | 103.53 ± 2.95 c | 87.98 ± 1.29 d |
| G2 | 73.25 ± 9.00 d | 13.40 ± 0.55 a | 115.70 ± 6.62 bc | 93.5 ± 3.86 cd |
| G3 | 87.13 ± 4.05 c | 10.20 ± 0.70 b | 103.78 ± 7.38 c | 98.46 ± 1.42 c |
| G4 | 109.31 ± 11.06 ab | 8.84 ± 0.48 bc | 153.4 ± 4.83 a | 116.45 ± 2.38 b |
| G5 | 102.62 ± 5.33 bc | 9.42 ± 0.73 b | 121.12 ± 4.37 b | 96.34 ± 1.76 c |
| G6 | 126.25 ± 3.94 a | 7.48 ± 0.37 c | 156.87 ± 6.85 a | 123.96 ± 2.48 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljutaily, T.; Rehan, M.; Moustafa, M.M.A.; Barakat, H. Effect of Intermittent Fasting, Probiotic-Fermented Camel Milk, and Probiotic-Fermented Camel Milk Incorporating Sukkari Date on Diet-Induced Obesity in Rats. Fermentation 2022, 8, 619. https://doi.org/10.3390/fermentation8110619
Aljutaily T, Rehan M, Moustafa MMA, Barakat H. Effect of Intermittent Fasting, Probiotic-Fermented Camel Milk, and Probiotic-Fermented Camel Milk Incorporating Sukkari Date on Diet-Induced Obesity in Rats. Fermentation. 2022; 8(11):619. https://doi.org/10.3390/fermentation8110619
Chicago/Turabian StyleAljutaily, Thamer, Medhat Rehan, Mahmoud M. A. Moustafa, and Hassan Barakat. 2022. "Effect of Intermittent Fasting, Probiotic-Fermented Camel Milk, and Probiotic-Fermented Camel Milk Incorporating Sukkari Date on Diet-Induced Obesity in Rats" Fermentation 8, no. 11: 619. https://doi.org/10.3390/fermentation8110619
APA StyleAljutaily, T., Rehan, M., Moustafa, M. M. A., & Barakat, H. (2022). Effect of Intermittent Fasting, Probiotic-Fermented Camel Milk, and Probiotic-Fermented Camel Milk Incorporating Sukkari Date on Diet-Induced Obesity in Rats. Fermentation, 8(11), 619. https://doi.org/10.3390/fermentation8110619







